Abstract
Due to their capability of modifying chromatin structure and thereby regulating gene transcription, histone deacetylases (HDACs) have been reported to play important roles in osteogenesis and considered a promising potential therapeutic target for bone diseases, including osteoporosis. We showed that the novel marine-derived HDAC inhibitor largazole exhibits in vitro and in vivo osteogenic activity. Largazole significantly induced the expression of ALP and OPN. The osteogenic activity of largazole was mediated through the increased expression of Runx2 and BMPs. Importantly, largazole showed in vivo bone-forming efficacy in the mouse calvarial bone formation assay and the rabbit calvarial bone fracture healing model. The dual action of largazole to stimulate bone formation and inhibit bone resorption would be a useful feature in drug development for bone-related disorders.
Keywords: Largazole, osteogenic activity, histone deacetylases, Runx2, bone morphogenetic protein
The cyclic depsipeptide largazole (Figure 1A), isolated from a cyanobacterium of the genus Symploca by Luesch and co-workers, is a marine natural product with novel chemical scaffold.1 Largazole possesses highly differential growth-inhibitory activity, preferentially targeting transformed over nontransformed cells. The intriguing structure and biological activity of largazole have attracted strong interest from the synthetic chemistry community to establish synthetic routes to largazole and to investigate its potential as a cancer therapeutic. Luesch and Hong reported the first total synthesis of largazole, identified histone deacetylases (HDACs) as the molecular targets, and demonstrated antitumor activity in a xenograft mouse model.2,3 Following our initial report, additional total and formal syntheses, further studies on its HDAC inhibition, and structure−activity relationship studies have appeared in the literature.4−17
Figure 1.
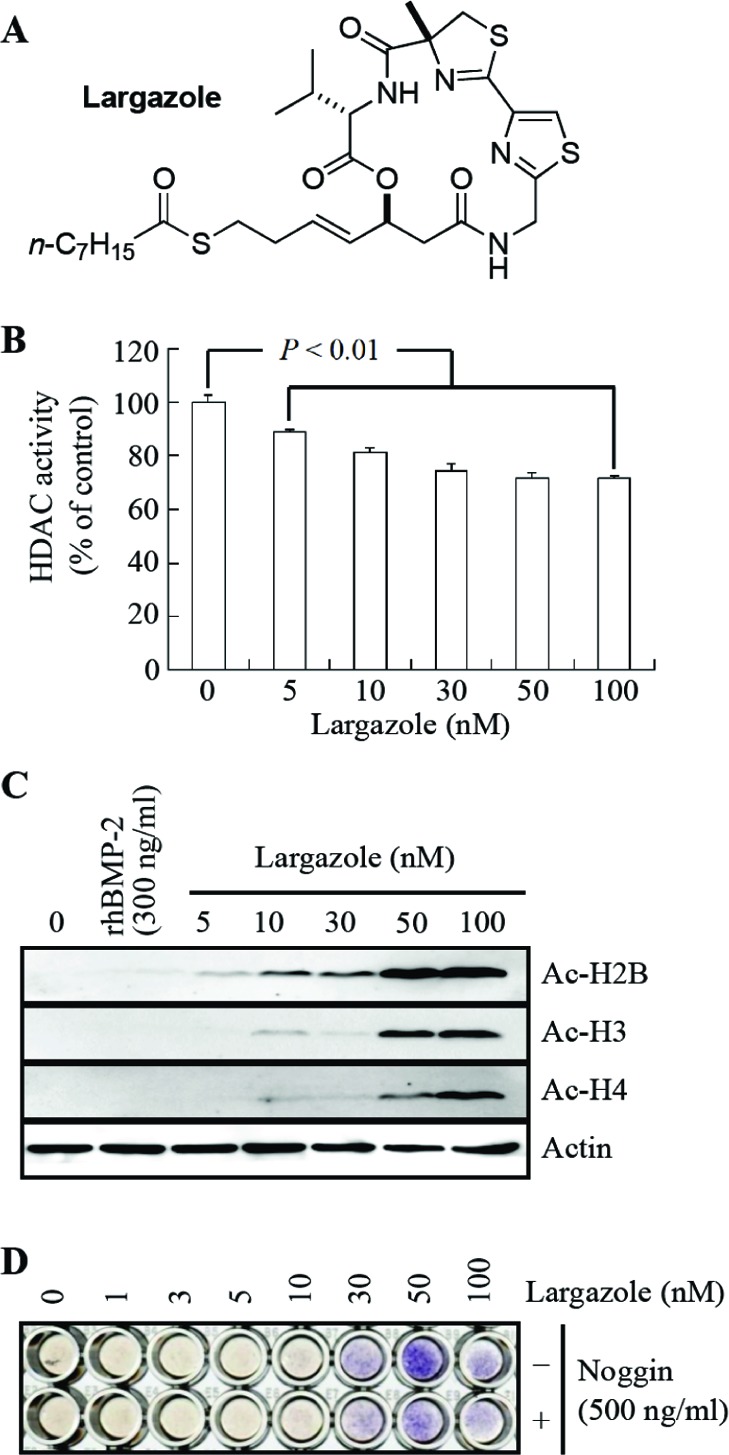
Largazole exhibited HDAC inhibitory and osteogenic activities in C2C12 cells. (A) Structure of largazole. (B) Effect of largazole on the total HDAC activity in C2C12 cells. Cells (4 × 103 cells/well) were cultured in a 96-well plate for 24 h and pretreated with largazole for 1 h before incubation with deacetylase Lysyl substrate of the HDAC Fluorescent Activity Assay kit. HDAC activity was measured according to the manufacturer’s protocol. (C) Effect of largazole on the levels of hyperacetylated histones in C2C12 cells. Cells (2 × 106 cells/plate) were cultured in a 100-mm plate for 24 h and treated with largazole. After 24 h, protein extracts were prepared and Western blot analysis was performed. Actin was used as a loading control. (D) Effect of largazole on osteoblast differentiation in C2C12 cells. The effect of largazole on osteoblast differentiation was evaluated by ALP staining. Cells (4 × 103 cells/well) were cultured in a 96-well plate for 24 h and treated with largazole for 6 days. Medium was changed every 3 days. Noggin (a specific BMP inhibitor) was cotreated with largazole.
Osteogenesis is a complex process associated with dramatic changes in gene expression.18 Due to their capability of modifying chromatin structure and thereby modulating gene transcription, HDACs have been reported to play important roles in osteogenesis and considered a promising potential therapeutic target for bone diseases, including osteoporosis.19 Several HDAC inhibitors, including trichostatin A,20 sodium butyrate,20 and valproic acid,21 have been tested for their osteogenic activity, but none have been shown effective in in vivo models for further development as potential therapeutics for bone-related diseases. Moreover, it has been reported that long-term treatment with valproic acid increases the incidence of osteoporosis and bone fracture.22,23 Herein, we report the in vitro and in vivo osteogenic activity and the significant potential of largazole in bone formation, repair, and regeneration.
Murine pluripotent mesenchymal precursor C2C12 cells were used as an in vitro model of osteogenesis. The C2C12 cells have been known to be committed into osteoblasts with the induction of several osteogenic bone morphogenetic protein (BMP) isoforms such as BMP-2, 4, 6, 7, and 9.24 Before evaluating the osteogenic activity of largazole, we examined the effect of largazole on the viability of C2C12 cells and found that largazole did not exhibit a significant level of cytotoxicity up to 100 nM (see the Supporting Information for details).
Next, we examined the effects of largazole on the inhibition of HDAC activity and the accumulation of acetylated histones in C2C12 cells to confirm its HDAC inhibitory activity. Largazole decreased the total HDAC activity in C2C12 cells (Figure 1B)25 and significantly increased the levels of acetylated histones in a dose-dependent manner (Figure 1C).
The effect of largazole on the commitment of C2C12 cells into osteoblasts was determined by the expression level of alkaline phosphatase (ALP), an early phase marker of osteoblast differentiation. Up to 50 nM, largazole increased the expression of ALP in a dose-dependent manner (Figure 1D). In addition, largazole significantly induced the expression of ALP and osteopontin (OPN) at the transcript level (see the Supporting Information for details).
Since Runx2 has been shown to be critical in osteogenesis and regulates the expression of bone-specific genes such as ALP and OPN,26 we examined the effect of largazole on the activation and mRNA expression of Runx2 using the p6×osteoblast-specific cis-acting element 2-luciferase reporter27 and real-time PCR, respectively (Figure 2). Relative to the DMSO control, largazole at 50 nM significantly increased the activity and mRNA level of Runx2 by 29-fold and 3-fold, respectively (Figure 2A and B). Since HDACs have been identified as corepressor proteins to affect Runx2 activity,28 these results suggested that inhibition of HDACs by largazole increases Runx2 activation, potentiates osteoblast differentiation, and increases bone formation.
Figure 2.
Effect of largazole on the Runx2 activation and mRNA induction of Runx2 and BMPs in C2C12 cells. (A) Runx2 activation was measured by luciferase reporter assay using 6×OSE2-luc plasmid-transfected C2C12 cells. The cells (4 × 103 cells/well) were cultured in a 96-well plate for 1 day and incubated with DMEM containing 5% FBS in the presence or absence of largazole for 1 day before luciferase activity assay. (B) The mRNA levels were evaluated by quantitative real-time PCR in cells (1 × 106 cells/60-mm plate) treated with largazole for 6 days. rhBMP-2 (300 ng/mL) was used as the reference of osteoblastogenesis inducer (bar labels: a, P < 0.05; b, P < 0.01; c, P < 0.001).
From the data showing that the increased expression of ALP induced by largazole was inhibited by noggin, a specific inhibitor of BMPs (see Figure 1D), we hypothesized that the osteogenic activity of largazole could result from its potential to increase the expression of BMPs. To test this hypothesis, we evaluated the effect of largazole on the mRNA expression levels of BMPs (Figure 2B). Largazole significantly induced the expressions of BMP-2, 4, 6, 7, and 9, supporting our hypothesis that the osteogenic activity of largazole is mediated through the increase of the expression and/or activity of Runx2 and BMPs. Recently, BMP-2 and BMP-7, with strong osteogenic activity, have been approved for clinical applications, including spinal fusion, fracture healing, and dental tissue engineering.29,30 Therefore, anabolic agents such as largazole that stimulate BMP expression or the BMP-signaling pathway would be useful for the treatment of osteoblast-related diseases by bone formation or regeneration.31,32 In addition, we found that largazole inhibits the formation of multinucleated osteoclasts (see the Supporting Information for details), suggesting that largazole might elicit the dual action to stimulate bone formation and suppress bone resorption.
Next, we examined the in vivo osteogenic activity of largazole using two independent in vivo models: the mouse calvarial bone formation assay and the rabbit calvarial bone fracture healing model.
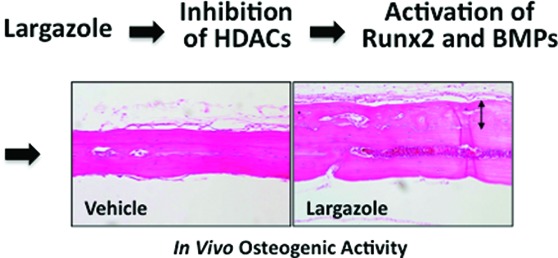
In the mouse calvarial bone formation assay, when collagen sponges soaked with largazole were implanted in calvarial bones, largazole induced woven bone formation over the periosteum of the calvarial bones (Figure 3). The woven bone formation at the lower concentration (10 μM, Figure 3B) was more significant than that at the higher concentration (50 μM, Figure 3C). This data suggested that largazole may show the biphasic effect which has been observed with other osteogenic compounds.33−35 Further studies are required to determine the optimal dose for the highest in vivo efficacy.
Figure 3.

Largazole exhibited bone-forming activity in mouse calvaria. Collagen sponges containing 5 μL of PBS vehicle (A) or largazole (B, 10 μM; C, 50 μM) were placed onto mouse calvarial bones. After a 3- week implantation, the mice were sacrificied. Calvarial bones were removed, fixed, decalcified, embedded in paraffin, and sectioned. Sections were stained with H&E and photographed at 200× magnification. Arrows indicated the region of newly forming woven bone.
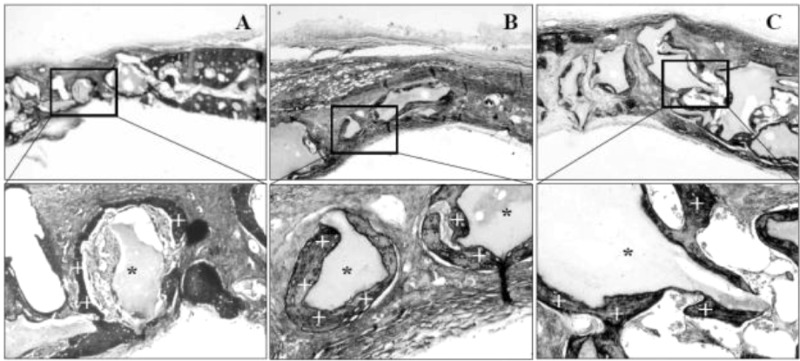
We further evaluated the in vivo bone-forming activity of largazole in the rabbit calvarial bone fracture healing model (Figure 4). In this study, macroporous biphasic calcium phosphate (MBCP) was used as a basic scaffold to induce bone formation. While an incomplete bone formation was observed with MBCPs alone (Figure 4A), newly formed bones in direct contact with MBCPs mixed with largazole were observed (Figure 4B and C). Since there is a significantly unmet clinical need for small molecules to improve the biological properties of MBCP and its osteoconductivity, this data clearly shows the great potential of largazole for improvement of the property of bone graft substitutes in bone defect reconstruction.
Figure 4.
Largazole exhibited bone-regenerating activity in rabbit calvaria. Bone graft substitute (10 mg), MBCP (indicated by a black asterisk (*); Biomatlante, France), was mixed with 50 μL of PBS (A) or largazole (B, 100 nM; C, 250 nM), and defects were filled with these mixtures. Rabbits were sacrificed at 4 weeks after surgery. The bone (indicated by a white plus (+)) grows into and around bone graft substitute. Box images in the top were magnified in the bottom.
In summary, we showed that the HDAC inhibitor largazole exhibits in vitro and in vivo osteogenic activity. Largazole significantly induced the expression of ALP and OPN. The osteogenic activity of largazole was mediated through the increased expression of Runx2 and BMPs. More importantly, largazole showed in vivo bone-forming efficacy in the mouse calvarial bone formation assay and the rabbit calvarial bone fracture healing model. The dual action of largazole to stimulate bone formation and inhibit bone resorption would be a useful feature in drug development for bone-related disorders. Taken together, largazole shows great clinical potential as a novel treatment for bone-related diseases in addition to its already proven potential as an antitumor agent.3
Acknowledgments
We thank Professors Hyun-Mo Ryoo and Zang Hee Lee (Department of Cell and Developmental Biology, School of Dentistry and Dental Research Institute, Seoul National University, Seoul, Korea) for the p6×OSE2-luc reporter vector and helpful discussion.
Supporting Information Available
General experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
This work has been supported by grants from the Korea Healthcare Technology R&D Project, the Ministry of Health, Welfare & Family Affairs, Republic of Korea (A084242 to S.L. and S.H.K.), and the National Institutes of Health (R01CA138544-01 to J.H. and H.L.).
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Taori K.; Paul V. J.; Luesch H. Structure and Activity of Largazole, a Potent Antiproliferative Agent from the Floridian Marine Cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008, 130, 1806–1807. [DOI] [PubMed] [Google Scholar]
- Ying Y.; Taori K.; Kim H.; Hong J.; Luesch H. Total Synthesis and Molecular Target of Largazole, a Histone Deacetylase Inhibitor. J. Am. Chem. Soc. 2008, 130, 8455–8459. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Salvador L. A.; Byeon S.; Ying Y.; Kwan J. C.; Law B. K.; Hong J.; Luesch H. Anticolon Cancer Activity of Largazole, a Marine-Derived Tunable Histone Deacetylase Inhibitor. J. Pharmacol. Exp. Ther. 2010, 335, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers A.; West N.; Taunton J.; Schreiber S. L.; Bradner J. E.; Williams R. M. Total Synthesis and Biological Mode of Action of Largazole: A Potent Class I Histone Deacetylase Inhibitor. J. Am. Chem. Soc. 2008, 130, 11219–11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiser T.; Kamena F.; Cramer N. Synthesis and Biological Activity of Largazole and Derivatives. Angew. Chem., Int. Ed. 2008, 47, 6483–6485. [DOI] [PubMed] [Google Scholar]
- Nasveschuk C. G.; Ungermannova D.; Liu X.; Phillips A. J. A Concise Total Synthesis of Largazole, Solution Structure, and Some Preliminary Structure Activity Relationships. Org. Lett. 2008, 10, 3595–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Kulkarni S. Enantioselective Total Synthesis of (+)-Largazole, a Potent Inhibitor of Histone Deacetylase. Org. Lett. 2008, 10, 3907–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y.; Liu Y.; Byeon S. R.; Kim H.; Luesch H.; Hong J. Synthesis and Activity of Largazole Analogues with Linker and Macrocycle Modification. Org. Lett. 2008, 10, 4021–4024. [DOI] [PubMed] [Google Scholar]
- Ren Q.; Dai L.; Zhang H.; Tan W.; Xu Z.; Ye T. Total Synthesis of Largazole. Synlett 2008, 2379–2383. [Google Scholar]
- Numajiri Y.; Takahashi T.; Takagi M.; Shin-ya K.; Doi T. Total Synthesis of Largazole and Its Biological Evaluation. Synlett 2008, 2483–2486. [Google Scholar]
- Bowers A. A.; Greshock T. J.; West N.; Estiu G.; Schreiber S. L.; Wiest O.; Williams R. M.; Bradner J. E. Synthesis and Conformation-Activity Relationships of the Peptide Isosteres of FK228 and Largazole. J. Am. Chem. Soc. 2009, 131, 2900–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers A. A.; West N.; Newkirk T. L.; Troutman-Youngman A. E.; Schreiber S. L.; Wiest O.; Bradner J. E.; Williams R. M. Synthesis and Histone Deacetylase Inhibitory Activity of Largazole Analogs: Alteration of the Zinc-Binding Domain and Macrocyclic Scaffold. Org. Lett. 2009, 11, 1301–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.; Gao A.-H.; Li J.; Nan F.-J. Synthesis and Biological Evaluation of C7-Demethyl Largazole Analogues. ChemMedChem 2009, 4, 1269–1272. [DOI] [PubMed] [Google Scholar]
- Wang B.; Forsyth C. J. Total Synthesis of Largazole−Devolution of a Novel Synthetic Strategy. Synthesis 2009, 2873–2880. [Google Scholar]
- Souto J. A.; Vaz E.; Lepore I.; Pöppler A.-C.; Franci G.; Álvarez R.; Altucci L.; de Lera Á. R. Synthesis and Biological Characterization of the Histone Deacetylase Inhibitor Largazole and C7-Modified Analogues. J. Med. Chem. 2010, 53, 4654–4667. [DOI] [PubMed] [Google Scholar]
- Zeng X.; Yin B.; Hu Z.; Liao C.; Liu J.; Li S.; Li Z.; Nicklaus M. C.; Zhou G.; Jiang S. Total Synthesis and Biological Evaluation of Largazole and Derivatives with Promising Selectivity for Cancers Cells. Org. Lett. 2010, 12, 1368–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q.; Wang L.-P.; Jiao X.-Z.; Liu X.-Y.; Wu Q.; Xie P. Concise Total Synthesis of Largazole. J. Asian Nat. Prod. Res. 2010, 12, 940–949. [DOI] [PubMed] [Google Scholar]
- Katagiri T.; Takahashi N. Regulatory Mechanisms of Osteoblast and Osteoclast Differentiation. Oral Dis. 2002, 8, 147–159. [DOI] [PubMed] [Google Scholar]
- Jensen E. D.; Nair A. K.; Westendorf J. J. Histone Deacetylase Co-Repressor Complex Control of Runx2 and Bone Formation. Crit. Rev. Eukaryot. Gene Expr. 2007, 17, 187–196. [DOI] [PubMed] [Google Scholar]
- Schroeder T. M.; Westendorf J. J. Histone Deacetylase Inhibitors Promote Osteoblast Maturation. J. Bone Miner. Res. 2005, 20, 2254–2263. [DOI] [PubMed] [Google Scholar]
- Cho H. H.; Park H. T.; Kim Y. J.; Bae Y. C.; Suh K. T.; Jung J. S. Induction of Osteogenic Differentiation of Human Mesenchymal Stem Cells by Histone Deacetylase Inhibitors. J. Cell Biochem. 2005, 96, 533–542. [DOI] [PubMed] [Google Scholar]
- Guo C. Y.; Ronen G. M.; Atkinson S. A. Long-Term Valproate and Lamotrigine Treatment May Be a Marker for Reduced Growth and Bone Mass in Children with Epilepsy. Epilepsia 2001, 42, 1141–1147. [DOI] [PubMed] [Google Scholar]
- Boluk A.; Guzelipek M.; Savli H.; Temel I.; Ozişik H. I.; Kaygusuz A. The Effect of Valproate on Bone Mineral Density in Adult Epileptic Patients. Pharmacol. Res. 2004, 50, 93–97. [DOI] [PubMed] [Google Scholar]
- Cheng H.; Jiang W.; Phillips F. M.; Haydon R. C.; Peng Y.; Zhou L.; Luu H. H.; An N.; Breyer B.; Vanichakarn P.; Szatkowski J. P.; Park J. Y.; He T. C. Osteogenic Activity of the 14 Types of Human Bone Morphogenetic Proteins (BMPs). J. Bone Joint Surg. Am. 2003, 85A, 1544–1552. [DOI] [PubMed] [Google Scholar]
- Largazole reduced the expressions of HDACs 2, 3, 4, and 5 but not the expression of HDAC 1 (see the Supporting Information for details).
- Franceschi R. T.; Xiao G. Regulation of the Osteoblast-Specific Transcription Factor, Runx2: Responsiveness to Multiple Signal Transduction Pathways. J. Cell Biochem. 2003, 88, 446–454. [DOI] [PubMed] [Google Scholar]
- Kim H. J.; Park H. D.; Kim J. H.; Cho J. Y.; Choi J. Y.; Kim J. K.; Kim H. J.; Shin H. I.; Ryoo H. M. Establishment and Characterization of a Stable Cell Line to Evaluate Cellular Runx2 Activity. J. Cell Biochem. 2004, 9, 1239–1247. [DOI] [PubMed] [Google Scholar]
- Westendorf J. J. Transcriptional Co-Repressors of Runx2. J. Cell Biochem. 2006, 98, 54–64. [DOI] [PubMed] [Google Scholar]
- McKay W. F.; Peckham S. M.; Badura J. M. A Comprehensive Clinical Review of Recombinant Human Bone Morphogenetic Protein-2 (INFUSE Bone Graft). Int. Orthop. 2007, 31, 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. P.; Vaccaro A. R.; Hall J. A.; Whang P. G.; Friel B. C.; McKee M. D. Clinical Applications of BMP-7/OP-1 in Fractures, Nonunions and Spinal Fusion. Int. Orthop. 2007, 31, 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M.; Reddi A. H. The Application of Bone Morphogenetic Proteins to Dental Tissue Engineering. Nat. Biotechnol. 2003, 21, 1025–1032. [DOI] [PubMed] [Google Scholar]
- Seeherman H.; Wozney J. M. Delivery of Bone Morphogenetic Proteins for Orthopedic Tissue Regeneration. Cytokine Growth Factor Rev. 2005, 16, 329–345. [DOI] [PubMed] [Google Scholar]
- Yeo H.; Beck L. H.; McDonald J. M.; Zayzafoon M. Cyclosporin A Elicits Dose-Dependent Biphasic Effects on Osteoblast Differentiation and Bone Formation. Bone 2007, 40, 1502–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J. G.; Kim Y. S.; Min Y. K.; Kim S. H. Low Concentration of 3-Carene Stimulates the Differentiation of Mouse Osteoblastic MC3T3-E1 Subclone 4 Cells. Phytother. Res. 2008, 22, 18–22. [DOI] [PubMed] [Google Scholar]
- Lee S. U.; Shin H. K.; Min Y. K.; Kim S. H. Emodin Accelerates Osteoblast Differentiation through Phosphatidylinositol 3-Kinase Activation and Bone Morphogenetic Protein-2 Gene Expression. Int. Immunopharmacol. 2008, 8, 741–747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.