Abstract
Summary
Background and objectives
It is unclear how to optimally care for chronic kidney disease (CKD). This study compares a new coordinated model to usual care for CKD.
Design, setting, participants, & measurements
A randomized trial in nephrology clinics and the community included 474 patients with median estimated GFR (eGFR) 42 ml/min per 1.73 m2 identified by laboratory-based case finding compared care coordinated by a general practitioner (controls) with care by a nurse-coordinated team including a nephrologist (intervention) for a median (interquartile range [IQR]) of 742 days. 32% were diabetic, 60% had cardiovascular disease, and proteinuria was minimal. Guided by protocols, the intervention team targeted risk factors for adverse kidney and cardiovascular outcomes. Serial eGFR and clinical events were tracked.
Results
The average decline in eGFR over 20 months was −1.9 ml/min per 1.73 m2. eGFR declined by ≥4 ml/min per 1.73 m2 within 20 months in 28 (17%) intervention patients versus 23 (13.9%) control patients. Control of BP, LDL, and diabetes were comparable across groups. In the intervention group there was a trend to greater use of renin-angiotensin blockers and more use of statins in those with initial LDL >2.5 mmol/L. Treatment was rarely required for anemia, acidosis, or disordered mineral metabolism. Clinical events occurred in 5.2% per year.
Conclusions
Patients with stage 3/4 CKD identified through community laboratories largely had nonprogressive kidney disease but had cardiovascular risk. Over a median of 24 months, the nurse-coordinated team did not affect rate of GFR decline or control of most risk factors compared with usual care.
Introduction
Chronic kidney disease (CKD) is associated with end-stage kidney disease as well as cardiovascular events and premature death (1–6). Interventions such as BP control, renin-angiotensin-aldosterone (RAAS) blockade (7,8), and treatment of dyslipidemia (9) have been shown to modify disease outcomes in CKD, but studies suggest a need for improved care in CKD (10–12). The optimal approach to CKD care is unclear. In the United Kingdom, an emphasis is on electronic records to detect CKD at the primary care level linked to guidelines selecting patients for referral to specialized kidney care teams (13). Other systems, particularly in Canada, suggest a role for specialized multidisciplinary clinics in CKD care (14–16). Similarly, there have been suggestions to involve pharmacists in CKD care (17), and an ongoing trial compares nurse practitioners with physicians in management of patients with CKD (18). Finally, disease-management strategies have been proposed, especially in the United States and in the context of managed care (19,20), but there remains great variability in delivery of CKD care including at the interface between nephrology and primary care in the United States (21).
Key elements in managing chronic disease include an organized approach using evidence-based therapies, supporting self management, examination of trends to determine whether patients meet treatment targets, and communication among providers. We hypothesized that by incorporating these elements, a model of CKD care involving a nurse as a primary caregiver, but supported by medical protocols and a nephrologist, might be superior to usual care. To test the effectiveness of such an intervention, we conducted a pilot randomized controlled trial.
Materials and Methods
We conducted a randomized, unblinded, pilot clinical trial in five urban centers in Canada. Patients with elevated serum creatinine levels were identified by community laboratories, and their family physicians were then asked to consider referring the patient to the study. This approach was used to minimize recruitment of patients already under the care of a nephrologist, and in fact only 4% of those recruited were already receiving nephrology care. Eligible patients were aged 40 to 75 yrs and had documented CKD with an estimated GFR (eGFR) between 25 and 60 ml/min per 1.73 m2. Patients were excluded if they had any of the following: likely to die within 6 months; recently unstable/advanced cardiovascular disease; current treatment for malignancy; receiving immunotherapy for kidney disease; on dialysis or with an organ transplant either currently or likely within 6 months; already enrolled in a disease management program for kidney or cardiovascular disease or another interventional clinical trial; or resident of a location too distant to attend study visits.
All of the patients received the usual care, and half were randomized to additional nurse-coordinated care focused on risk factor modification. The nurse followed medical protocols and worked in close collaboration with a nephrologist. Randomization was masked and stratified by site and clinical status (diabetes, nondiabetic with proteinuria, or nondiabetic without proteinuria). All of the participants provided informed consent, and the study was approved by ethics review boards at each site. The aims of this pilot trial were to assess recruitment and the application of the intervention as well as achievement of surrogate endpoint targets. These targets included: BP <130/80 mmHg; use of RAAS blockers; minimization of proteinuria; LDL <2.5 mmol/L; use of anti-platelet agents in those with a history of ischemic disease or diabetes; Hba1c of ≤7.0% in diabetics; serum bicarbonate >22 mmol/L; serum phosphate <1.8 mmol/L; hemoglobin >105 g/L; and iron saturation >0.2. The change in kidney function was tracked by serum creatinine every 4 months. Major clinical kidney and cardiovascular adverse events were predefined, and their occurrence was judged by a blinded assessment team.
Study Visits and Measurements
After randomization, all of the trial participants were seen every 4 months. For intervention-group patients, the visits included clinical care. For controls, the visits only assessed outcomes. At each visit, any adverse clinical outcomes were noted. Current drugs and all health care resources used since the prior visit were recorded. Serum was sent to a central laboratory for measurement of creatinine. eGFR was calculated using the Modification of Diet in Renal Disease formula for standardized creatinine levels (22). Central laboratory values were not available to guide care. At baseline and annually, all of the participants had height, weight, and BP recorded. Blood and urine samples were sent to local laboratories for complete blood count, chemistry, Hba1c, lipid profile, ferritin, iron saturation, and parathyroid hormone. Serum creatinine levels were measured locally in intervention-group patients only. Local laboratory results were made available to each patient's family doctor. Additional laboratory data were obtained at any point during the trial if requested by each patient's own health care provider. At several points during the trial, the nurses and nephrologists completed logs of trial-related activities.
Care Provided to Each Trial Group
All of the patients received whatever usual care that their health care providers felt indicated. Usual care meant care delivered by a family doctor providing assessments and treatments for their patients as they saw fit. The family doctors could consult specialists or involve allied health personnel if necessary. Intervention group participants had additional clinical care delivered by the study nurse and nephrologist guided by protocols aimed at achieving the targets noted above but focused on the needs of the individual. Such care was coordinated with the usual care being provided by the family doctors. Most intervention-group patients were seen for additional interim study visits to address identified clinical issues. Protocols allowed for both pharmacologic and nonpharmacologic interventions. There was emphasis on patient self-management and working collaboratively. Details of the nature of the care provided have been described (23).
Study staff only intervened in the clinical care of controls if they became aware of a serious or life-threatening clinical problem not already being managed. Recommendations for management did not accompany laboratory data sent to physicians caring for controls.
Outcome Measures
The main focus of this pilot study was on achievement of treatment targets for surrogate outcomes, but “quality of life” as measured by the KDQOL-SF (24), the WHOQOL-BREF (25), and the HUI Mark 3 (26) together with resource utilization were also recorded. The effect on quality of life and the cost utility of the intervention are reported elsewhere. Satisfaction with care in the experimental group only was measured using the Client Satisfaction Questionnaire 8 (27).
Sample Size Estimation
One goal of this study was to determine whether 500 patients could be recruited across five sites within 12 to 18 months. This sample size was also chosen to achieve specific confidence interval (CI) widths around possible estimates of clinical endpoint event rates (e.g. estimate 4%, 95% CI, 2.5% to 6.1%).
Analysis
The characteristics of the study groups are presented as proportions, median (interquartile range [IQR]), or means (SD) as appropriate. Comparison of proportions was by χ2. The means were compared by t tests, and the medians were compared by a median test. Generalized estimating equations were used to compare groups at baseline and over time in terms of the proportion meeting treatment targets. The groups were compared over time adjusting for baseline BP using a general linear model for repeated measures. Similar methods were used to compare groups in terms of LDL, cholesterol, and eGFR over time. All of the analyses were completed using SAS (version 9.1.3) or SPSS (version 15).
Results
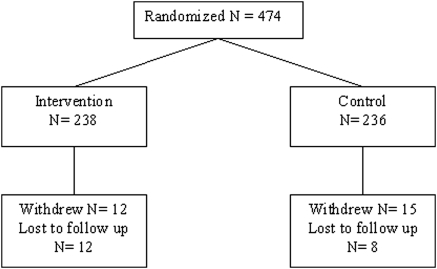
The trial ran from May 2005 to June 2008. Figure 1 shows the distribution of trial participants. Median (IQR) follow-up time was 742 days (614 to 854 days) for the 474 participants. Twenty (4.2%) were lost to follow-up, and 27 (5.7%) withdrew, of which five (1%) withdrew after they developed cancer or another serious health condition. Table 1 shows baseline characteristics of trial participants. The participants were largely Caucasian seniors living independently, and a little over half were female. Baseline eGFR centered around 42 ml/min per 1.73 m2. Proteinuria was minimal with only 19 patients in total (six intervention and 13 control) having proteinuria of >1 g/d. Almost one-third had diabetes mellitus, and 59.7% had a history of cardiovascular disease. There were few current smokers. Baseline BP tended to be higher in the control group. Delivering care to the intervention group took an average of 12 minutes of nephrologist time and 187 minutes of nursing time per working day.
Figure 1.
Disposition of trial participants.
Table 1.
Baseline characteristics of the trial population
| Median (IQR) |
P Values for the Difference | ||
|---|---|---|---|
| Experimental Intervention (n = 238) | Standard Care Control (n = 236) | ||
| Age (years) | 67 (62, 72) | 67 (61, 72) | 0.85 |
| Baseline serum creatinine (μmol/L) | 127 (112, 145) | 128 (114, 143) | 0.85 |
| Baseline eGFR (ml/min per 1.73 m2) | 42 (40, 46) | 42 (37, 46) | 0.78 |
| Weight (kg) | 83 (72, 96) | 82 (72, 91) | 0.33 |
| Systolic BP | 128 (116, 140) | 132 (120, 144) | 0.001 |
| Diastolic BP | 74 (66, 80) | 74 (68, 81) | 0.96 |
| Proteinuria (g/day) | 0.11 (0.07, 0.2) | 0.12 (0.08, 0.22) | 0.06 |
| LDL cholesterol (mmol/L) | 2.6 (2.1, 3.3) | 2.7 (2.1, 3.5) | 0.07 |
| Hba1c among diabetics (%) | 6.9 (6.4, 7.9) | 7.1 (6.3, 7.6) | 0.58 |
| Hemoglobin (g/L) | 136 (125, 144) | 134 (126, 144) | 0.33 |
| Number (%) | Number (%) | ||
| Female | 131 (55) | 132 (56) | 0.85 |
| Caucasian | 223 (94) | 224 (95) | 0.28 |
| Retired | 144 (61) | 158 (67) | 0.15 |
| Working | 58 (24) | 55 (23) | 0.42 |
| Post-secondary school education | 96 (40) | 100 (42) | 0.90 |
| Married/living as married | 167 (70) | 154 (65) | 0.37 |
| Living in own home, no hired assistance | 224 (94) | 219 (93) | 0.77 |
| Current smoker | 18 (8) | 18 (8) | 1.0 |
| Systolic BP >130 mmHg | 84 (36) | 120 (51) | 0.001 |
| Systolic BP >140 mmHg | 61 (26) | 81 (35) | 0.05 |
| Diastolic BP >80 mmHg | 40 (17) | 60 (26) | 0.03 |
| Diastolic BP >90 mmHg | 10 (4) | 11 (5) | 0.98 |
| Diabetes mellitus | 73 (31) | 76 (33) | 0.65 |
| Angina | 19 (8) | 28 (12) | 0.21 |
| History of myocardial infarction | 39 (17) | 33 (14) | 0.55 |
| History of PTCA | 26 (11) | 20 (9) | 0.46 |
| History of CABG | 25 (11) | 19 (8) | 0.45 |
| History of heart failure | 13 (6) | 9 (4) | 0.53 |
| History of cardiac arrhythmia | 32 (14) | 32 (14) | 1.0 |
| History of cerebrovascular event | 10 (4) | 15 (7) | 0.40 |
| History of hypertension | 182 (78) | 178 (77) | 0.87 |
| History of chronic lung disease | 46 (20) | 49 (21) | 0.78 |
| History of cancer | 34 (15) | 40 (17) | 0.50 |
| Taking an ACE inhibitor or ARB | 165 (70) | 156 (66) | 0.51 |
| Taking a statin | 118 (25) | 103 (22) | 0.23 |
PTCA, percutaneous transluminal coronary angioplast; CABG, coronary artery bypass graft; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker. Continuous variables are presented as medians (intraquartile range). Binary variables are presented as numbers (percentages).
Rate of Change of Kidney Function
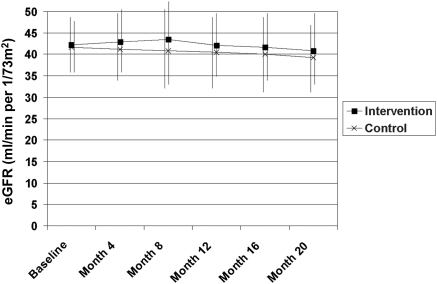
310 patients had at least 20 months of follow-up with eGFR estimates every 4 months. In a general linear model for repeated measures adjusted for baseline eGFR, mean eGFR was slightly higher in the intervention group (P = 0.009, difference in marginal mean 1.4 ml/min per 1.73 m2 [95% CI, 0.36 to 2.5]). Much of the difference related to an increase in eGFR in the intervention group at months 4 and 8, with both groups showing a similar rate of decline after that (Figure 2). This pattern could not be explained by differences in use of nonsteroidal anti-inflammatory drugs (9.1% of intervention and 6.4% of controls) or diuretics (25.4% of intervention versus 24.1% of controls) at 4 months. eGFR declined by ≥4 ml/min per 1.73 m2 from baseline to 20 months in 28 (17%) of the intervention group versus 23 (13.9%) of controls (P = 0.43). Overall the average decline in eGFR over 20 months was −1.9 ml/min per 1.73 m2 (95% CI, −1.2 to −2.6).
Figure 2.
Mean ± SD estimated GFR from baseline to 20-month follow-up by trial group.
Achievement of Clinical and Treatment Targets (Table 2)
Table 2.
Achievement of clinical and treatment targets comparing trial groups over time
| Time | Experimental Intervention Number (%) | Standard Care Control Number (%) | P | |
|---|---|---|---|---|
| BP ≤130/80 | Baseline | 139/236 (59) | 101/235 (43) | 0.03a |
| 12 months | 134/218 (61.5) | 100/218 (45.9) | 0.47b | |
| 24 months | 81/128 (63.2) | 64/136 (47) | 0.76c | |
| LDL <2.5 mmol/L | Baseline | 99/230 (43) | 81/220 (36.8) | 0.41a |
| 12 months | 97/206 (47.1) | 99/214 (46.3) | <0.001b | |
| 24 months | 78/122 (63.9) | 76/128 (59.4) | 0.74c | |
| On RAAS blocker | Baseline | 165/236 (70) | 156/235 (66) | 0.49a |
| 12 months | 165/219 (75) | 146/220 (66) | 0.92b | |
| 24 months | 102/130 (78) | 92/140 (66) | 0.06c | |
| Hba1c ≤7.0% in diabetics | Baseline | 38/68 (55.9) | 36/74 (48.6) | 0.58a |
| 12 months | 50/70 (71.4) | 52/77 (67.5) | <0.001b | |
| 24 months | 40/49 (81.6) | 43/52 (82.7) | 0.76c | |
| Hemoglobin ≥105 g/L | Baseline | 229/235 (97.4) | 232/234 (99.1) | 0.81a |
| 12 months | 208/214 (97.2) | 203/214 (94.8) | 0.07b | |
| 24 months | 125/128 (97.7) | 130/136 (95.6) | 0.64c | |
| Iron saturation ≥0.2 | Baseline | 169/225 (75.1) | 160/226 (70.8) | 0.28a |
| 12 months | 154/210 (73.3) | 155/210 (73.8) | 0.24b | |
| 24 months | 95/128 (74.2) | 95/124 (76.6) | 0.31c | |
| Serum phosphate <1.8 mmol/L | Baseline | 235/235 (100) | 233/233 (100) | NA |
| 12 months | 211/211 (100) | 218/218 (100) | NA | |
| 24 months | 125/126 (99.2) | 126/127 (99.2) | NA | |
| Bicarbonate ≥22 mmol/L | Baseline | 225/234 (96.1) | 230/234 (98.3) | 0.18a |
| 12 months | 209/215 (97.2) | 212/214 (99.1) | 0.68b | |
| 24 months | 124/127 (97.6) | 124/127 (97.6) | 0.37c |
All of the P values are from generalized estimating equations. NA, statistical analysis is not applicable as target was almost uniformly met. RAAS, renin-angiotensin-aldosterone.
Comparison at baseline.
Comparison over time within group.
Comparison between groups over time adjusted for baseline.
BP Management.
BP was lower in the intervention group at baseline, but the proportion meeting treatment targets did not significantly change over time in either group. At baseline, the mean number of anti-hypertensive medications taken was similar in the control and intervention groups (mean, 2.2 versus 2.3). Adjusting for baseline number of anti-hypertensive drugs in a Poisson regression, the number of such drugs prescribed was only higher by an average of 0.1 drugs (P < 0.01) throughout 24 months of follow-up in the intervention group.
Use of RAAS Blockade.
At baseline, 165 patients (70%) in the intervention group and 156 (66%) in the control group used RAAS blockade. This proportion was higher in diabetics (64 [88%] versus 70 [91%]). At 24 months 78% of intervention patients versus 66% of controls were on RAAS blockers (P = 0.06 for group comparison over time).
Lipid Management.
Among all patients, the proportion meeting LDL targets at baseline was nonsignificantly higher in the intervention group, whereas the proportion meeting targets rose comparably over time in each study group. Among those with baseline LDL >2.5 mmol/L, a similar proportion in each group were already treated with a lipid-lowering agent (intervention 39% versus controls 35%), whereas at each time point after baseline, this subgroup was more likely to be taking lipid-lowering therapy if they were in the intervention group (at month 12, 66% versus 42%, P = 0.0003, and at month 24, 84% versus 51%, P = 0.0003). Among those with baseline LDL >2.5 mmol/L, there was a nonsignificant trend to greater involvement of a dietitian by 12 months in the intervention group (21% versus 13% in controls, P = 0.09). Nearly all patients taking lipid-lowering therapy at baseline in each group remained on such therapy at later time points (at month 12, 97% of intervention versus 98% of controls, and at month 24, 99% versus 92%).
Management of Iron and Anemia.
The vast majority of patients in each group met hemoglobin targets, and there was no significant difference in this proportion over time or between groups. Erythropoiesis-stimulating agents were used in between one and five patients in each group at any time. The proportion meeting targets for iron saturation was comparable over time and between groups. Among people with baseline iron saturation <0.2, oral iron supplements were more likely to be prescribed in the intervention group by 12 months (35% versus 14% for controls, P = 0.005).
Management of Diabetes.
The proportion of diabetics meeting Hba1c target increased over time but comparably across study groups. A similar proportion of diabetics in each trial group reported a visit to a dietitian (23% intervention versus 25% control, P = 0.8) or a nurse educator (16% versus 18%, P = 0.75) within 12 months of enrollment.
Management of Mineral Metabolism.
Almost all of the trial participants met serum phosphate targets throughout the study. During the trial, phosphate binders were taken by 2% to 5% of patients, and vitamin D was taken by 10 to 15% at any given time with no difference between groups.
Management of Acidosis.
Serum bicarbonate was at target in the vast majority of patients at all time points and did not differ over time or across groups.
Use of Anti-platelet Therapy.
Among those with diabetes or cardiovascular disease, for whom anti-platelet therapy might be indicated (28), this therapy was prescribed to 95 (80%) in the intervention group and 88 (77%) of controls at 12-month follow-up (P = 0.54). The same pattern was seen at other time points.
Smoking Cessation.
Less than 8% of trial participants reported being current smokers at trial entry. There was no apparent difference in the quit rates between intervention and control groups.
Satisfaction with Care
Intervention-group patients were extremely satisfied with their care. With a maximum possible score of 32, the median (IQR) score was constant at 31 (29,30) at 8, 16, and 24 months.
Clinical Endpoints
As shown in Table 3, there were 48 clinical endpoints, half in each trial group. In the intervention group, one person had an amputation followed by cardiac death, another doubled serum creatinine and required dialysis, whereas a third had acute coronary syndrome and was hospitalized for heart failure before suffering a noncardiac death. Among controls, one person had two legs amputated, two people were hospitalized twice for heart failure, whereas another had three such events. Overall the annual incidence of clinical endpoints was 5.2% (95% CI, 3.8% to 6.7%).
Table 3.
Distribution of clinical endpoints by study group
| Experimental Intervention (n = 238) | Standard Care Control (n = 236) | |
|---|---|---|
| Cardiovascular death | 2 (0.8) | 2 (0.8) |
| Other death | 5 (2.1) | 0 (0.0) |
| Myocardial infarction | 5 (2.1) | 4 (1.7) |
| Acute coronary syndrome | 1 (0.4) | 2 (0.8) |
| Congestive heart failure | 5 (2.1) | 8 (3.4) |
| Stroke | 1 (0.4) | 1 (0.4) |
| Amputation above ankle | 2 (0.8) | 2 (0.8) |
| Dialysis | 2 (0.8) | 1 (0.4) |
| Doubled serum creatinine | 1 (0.4) | 4 (1.7) |
| Total cases with ≥1 event | 19 (8.0) | 19 (8.0) |
| Total events | 24 | 24 |
| Event rate per year (%) | 5.3 | 5.2 |
The proportions are presented as numbers (percentages).
Discussion
This trial was designed to test a nurse-coordinated model of care in people with CKD identified from the community. The vast majority of the care time was provided by the nurses. The care model had a similar effect on control of cardiovascular risk factors as care by family doctors. Some drugs were used more frequently in eligible patients. There was a trend to greater use of RAAS blockers in the intervention group over time, and intervention-group patients with high LDL or low iron saturation were more likely to receive treatment than similar controls.
The recruitment mechanism was intended to enroll a group of people with CKD that would be more representative of those in the general community rather than the referred populations already receiving care from nephrology teams. Although reduction of proteinuria was a treatment target, very few patients with significant proteinuria were entered in the trial. The slowly progressive nature of the kidney disease in these patients identified from the community using laboratory-based case findings supports the argument that the majority of stage 3 patients who have nonproteinuric CKD do not need care by a nephrologist (31). The observed rate of loss of kidney function was close to that documented in studies of community dwelling adults aged greater than 50 years (29). Chan et al. (32) recently reported a similar trial in patients with type 2 diabetes and nephropathy who had a higher risk of progression of kidney disease. Although care process was improved, the study was not powerful enough to determine whether there would be differences in kidney outcomes. Significant anemia, hyperphosphatemia, and hyperparathyroidism were not common in our trial, largely because of the relatively preserved level of kidney function.
Even though this study population had a low rate of kidney disease progression, they had a significant risk for adverse cardiovascular events (5.2% per annum) despite the relatively well-controlled traditional cardiovascular risk-factor profile of the population at trial entry. The results of this trial did not show any difference in clinical cardio-renal endpoints between trial groups, but this pilot study was not powered for this outcome. At about 5% per annum, the rate of cardiovascular events seen was comparable with that in other populations with CKD (5). The preponderance of cardiovascular events over kidney events is similar to that seen in unselected populations with CKD (5). Indeed, the relative likelihood of reaching end-stage kidney disease only approaches that of cardiovascular events in selected populations with more advanced stages of kidney failure under the care of nephrology teams (2).
As documented elsewhere, the intervention teams applied the model of chronic disease care as designed (23). Intervention group participants expressed a high degree of satisfaction with the care received, whereas this was not measured in the controls. The nature of the interventions actually used lie within the scope of practice of most generalist physicians and advanced practice nurses or nurse practitioners. As such, any effort to apply this model of chronic disease care to populations with CKD similar to that seen in this trial population should probably be focused at the level of the primary health care team (30). Indeed, a very similar intervention based in primary care was recently reported as having positive effects on other chronic diseases (33). Specialized nephrology teams do not appear necessary to apply the interventions used in this trial, and such teams might be better to concentrate on care of those with more advanced and progressive kidney disease.
The trial has a number of limitations. First, it targeted CKD patients not referred to nephrologists and thus more likely to have nonprogressive kidney disease. Second, the recruitment process may have led to bias in that the family physicians may have selected their “best” patients to refer to the trial, knowing that their prior and ongoing care for these patients would be under scrutiny. BP control was better than often seen in populations with hypertension and CKD (12). Average LDL levels were also not that high, and diabetic control was excellent. The proportion of current smokers was low. Consequently, there was less room for the intervention to make an effect in comparison with usual care (3). Third, a bias caused by contamination in the control group may be a further factor reducing the difference between groups in the use of therapies and achievement of surrogate endpoints (such as LDL targets). For ethical reasons, the results of the annual laboratory tests done for outcome assessment were shared with the physicians caring for controls. This may have triggered some interventions, such as the increased use of statins, which might not have occurred if these physicians would not have ordered the laboratory tests in the first place.
Three conclusions can be reached: (1) CKD patients identified through community laboratories usually have nonprogressive kidney disease and do not necessarily require specialized nephrology care; (2) these patients have a substantial risk of cardiovascular events despite good management of traditional risk factors; and (3) in this particular trial, despite its limitations, the nurse-coordinated model of care had similar effects on control of risk factors as usual care provided by a family doctor and was associated with greater use of some drugs in eligible patients. Given the limitations, the model should be further assessed.
Disclosures
None.
Acknowledgments
This study was supported by a New Emerging Team grant co-funded by the Canadian Institutes for Health Research, the Kidney Foundation of Canada, the Heart and Stroke Foundation of Canada, and the Canadian Diabetes Association and by unrestricted grants from Amgen Canada, Ortho Biotech, and Merck Frosst Canada. Drs. Barrett and Singer and Mr. Ayers had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors acknowledge the assistance of Mr. Tyler Wish with some of the analyses. The trial was registered at ClinicalTrials.gov NCT00231803.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Nurse-Coordinated Care in CKD: Time for Translation into Practice?” on pages 1229–1231.
References
- 1. Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives: A position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Levin A, Djurdjev O, Beaulieu M, Er L: Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis 52: 661–671, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Evans M, Fryzek JP, Elinder CG, Cohen SS, McLaughlin JK, Nyren O, Fored CM: The natural history of chronic renal failure: Results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis 46: 863–870, 2005 [DOI] [PubMed] [Google Scholar]
- 4. John R, Webb M, Young A, Stevens PE: Unreferred chronic kidney disease: A longitudinal study. Am J Kidney Dis 43: 825–835, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Li S, Foley RN, Collins AJ: Anemia and cardiovascular disease, hospitalization, end stage renal disease, and death in older patients with chronic kidney disease. Int Urol Nephrol 37: 395–402, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S: Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med 134: 629–636, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Strippoli GF, Navaneethan SD, Johnson DW, Perkovic V, Pellegrini F, Nicolucci A, Craig JC: Effects of statins in patients with chronic kidney disease: Meta-analysis and meta-regression of randomised controlled trials. BMJ 336: 645–651, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nissenson AR, Collins AJ, Hurley J, Petersen H, Pereira BJ, Steinberg EP: Opportunities for improving the care of patients with chronic renal insufficiency: Current practice patterns. J Am Soc Nephrol 12: 1713–1720, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Tonelli M, Bohm C, Pandeya S, Gill J, Levin A, Kiberd BA: Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency. Am J Kidney Dis 37: 484–489, 2001 [PubMed] [Google Scholar]
- 12. Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS: Cardiovascular disease risk factors in chronic kidney disease: Overall burden and rates of treatment and control. Arch Intern Med 166: 1884–1891, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Klebe B, Farmer C, Cooley R, de Lusignan S, Middleton R, O'Donoghue D, New J, Stevens P: Kidney disease management in UK primary care: Guidelines, incentives and information technology. Family Practice 24: 330–335, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Curtis BM, Ravani P, Malberti F, Kennett F, Taylor PA, Djurdjev O, Levin A: The short- and long-term impact of multi-disciplinary clinics in addition to standard nephrology care on patient outcomes. Nephrol Dial Transplant 20: 147–154, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Goldstein M, Yassa T, Dacouris N, McFarlane P: Multidisciplinary predialysis care and morbidity and mortality of patients on dialysis. Am J Kidney Dis 44: 706–714, 2004 [PubMed] [Google Scholar]
- 16. Hemmelgarn BR, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Walsh M, Culleton BF: Association between multidisciplinary care and survival for elderly patients with chronic kidney disease. J Am Soc Nephrol 18: 993–999, 2007 [DOI] [PubMed] [Google Scholar]
- 17. St Peter WL: Introduction: Chronic kidney disease: A burgeoning health epidemic. J Manag Care Pharm 13[9 Suppl D]: S2–S5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Zuilen AD, Wetzels JF, Bots ML, Van Blankestijn PJ: MASTERPLAN Study Group. MASTERPLAN: Study of the role of nurse practitioners in a multifactorial intervention to reduce cardiovascular risk in chronic kidney disease patients. J Nephrol 21: 261–267, 2008 [PubMed] [Google Scholar]
- 19. Rastogi A, Linden A, Nissenson AR: Disease management in chronic kidney disease. Adv Chronic Kidney Dis 15: 19–28, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Thorp ML, Eastman L, Smith DH, Johnson ES: Managing the burden of chronic kidney disease. Dis Manag 9: 115–121, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Rettig RA, Vargas RB, Norris KC, Nissenson AR: Chronic kidney disease: A quiet revolution in nephrology. Six case studies. 2010. RAND Technical report. Available at: http://www.rand.org/pubs/technical_reports/TR826/ Accessed February 16, 2011 [PMC free article] [PubMed]
- 22. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Chronic Kidney Disease Epidemiology Collaboration: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Molzahn AE, Pelletier Hibbert M, Gaudet D, Starzomski R, Barrett B, Morgan J: Managing chronic kidney disease in a nurse run clinic: The CanPREVENT experience. CJNR 40: 96–113, 2008 [PubMed] [Google Scholar]
- 24. Hays RD, Kallich JD, Mapes DL, Coons SJ, Amin N, Carter WB, Kamberg CJ: Kidney Disease Quality of Life Short Form (KDQOL-SF), version 1.3: A Manual for Use and Scoring. Santa Monica, CA, RAND, 1997 [Google Scholar]
- 25. WHOQOL Group: Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychological Medicine 28: 551–558, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Furlong W, Feeney D, Torrance GW, Barr R: The Health Utilities Index (HUI) system for assessing health related quality of life in clinical studies. Ann Intern Med 33: 375–384, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Attkisson CC, Greenfield TK: The UCSF Client satisfaction scales: I. The Client Satisfaction Questionnaire-8. In: The use and psychological testing for treatment planning and outcomes assessment, 3rd Ed., edited by Maruish ME, Mahway NJ, Lawrence Erlbaum Associates, 2004, pp 799–811 [Google Scholar]
- 28. Canadian Diabetes Association Clinical Practice Guideline Expert Committee: Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 32[Suppl 1]: S102–S106, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Granerus G, Aurell M: Reference values for 51Cr-EDTA clearance as a measure of glomerular filtration rate. Scand J Clin Lab Invest 41: 611–616, 1981 [DOI] [PubMed] [Google Scholar]
- 30. Richards N, Harris K, Whitfield M, O'Donoghue D, Lewis R, Mansell M, Thomas S, Townend J, Eames M, Marcelli D: Primary care-based disease management of chronic kidney disease (CKD), based on estimated glomerular filtration rate (eGFR) reporting, improves patient outcomes. Nephrol Dial Transplant 23: 549–555, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Castro AF, Coresh J: CKD surveillance using laboratory data from the population-based National Health and Nutrition Examination Survey (NHANES). Am J Kidney Dis 53[3 Suppl 3]: S46–S55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan JC, So WY, Yeung CY, Ko GT, Lau IT, Tsang MW, Lau KP, Siu SC, Li JK, Yeung VT, Leung WY, Tong PC: SURE Study Group. Effects of structured versus usual care on renal endpoint in type 2 diabetes: The SURE study: A randomized multicenter translational study. Diabetes Care 32: 977–982, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Katon WJ, Lin EHB, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D: Collaborative care for patients with depression and chronic illness. N Engl J Med 363: 2611–2620, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]