Abstract
Summary
Background and objectives
Potential cost and effectiveness of a nephrologist/nurse–based multifaceted intervention for stage 3 to 4 chronic kidney disease are not known. This study examines the cost-effectiveness of a chronic disease management model for chronic kidney disease.
Design, setting, participants, & measurements
Cost and cost-effectiveness were prospectively gathered alongside a multicenter trial. The Canadian Prevention of Renal and Cardiovascular Endpoints Trial (CanPREVENT) randomized 236 patients to receive usual care (controls) and another 238 patients to multifaceted nurse/nephrologist–supported care that targeted factors associated with development of kidney and cardiovascular disease (intervention). Cost and outcomes over 2 years were examined to determine the incremental cost-effectiveness of the intervention. Base-case analysis included disease-related costs, and sensitivity analysis included all costs.
Results
Consideration of all costs produced statistically significant differences. A lower number of days in hospital explained most of the cost difference. For both base-case and sensitivity analyses with all costs included, the intervention group required fewer resources and had higher quality of life. The direction of the results was unchanged to inclusion of various types of costs, consideration of payer or societal perspective, changes to the discount rate, and levels of GFR.
Conclusions
The nephrologist/nurse–based multifaceted intervention represents good value for money because it reduces costs without reducing quality of life for patients with chronic kidney disease.
Introduction
The prevalence of chronic kidney disease (CKD) in Canada is between 5.0 and 6.75% (1,2), which corresponds to approximately 1.7 to 2.2 million Canadians having CKD; renal impairment in CKD progresses from stages 1 and 2 (slight impairment or mild disease) to almost 50% having stages 3 to 5 (moderate, severe, or failure). Associated with disease progression are increased rates of cardiovascular complications such as myocardial infarction, stroke, and death. The direct medical expense to treat all Canadians with CKD without major comorbidities is at least $3 billion per year (3), and when comorbidities are present, the cost of care is 2 to 2.5 higher (4). Recent research has focused on slowing of the rate of progression of comorbidities related to CKD.
Clinical trials have proven the efficacy of several interventions in slowing the progression of CKD to ESRD and in prevention of advanced cardiovascular disease events, including mortality. Many of the interventions that are beneficial for CKD are also beneficial for cardiovascular disease (5–7). Unfortunately, multiple observational studies in the general population, primary care, and specialty practice settings have documented a care gap, whereby those with CKD have an underutilization of therapies with proven efficacy (8–10).
In addition to clinical underutilization, there is also uncertainty with respect to whether there are additional costs or savings when implementing early intervention programs (11–14). One study looked at stage 4 CKD patients who were under the care of a nephrologist (12), but this study involved later stage CKD with creatinine clearance of 20 ml/min or lower. However, even with stage 4 CKD, there was cost savings for early referral. A different cost-effectiveness analysis that included patients over 50 years of age with proteinuria indicated that early screening was cost-effective for ages 60 and over or cases with hypertension and not diabetes (13). However, neither of these studies included costs for cardiovascular outcomes, and each was based on cohort studies or literature evidence (11). A subsequent cost-effectiveness analysis based on a trial of early screening for albuminuria included patients with normal BP and cholesterol levels (14). In this analysis, patients with albuminuria were treated with an angiotensin-converting enzymes inhibitor and this was cost-effective to prevent cardiovascular events.
To reduce care gaps, care models that rely on physicians or nurses that closely track and manage chronic diseases after early screening have shown promise (15–17). To extend these findings and determine if similar management of chronic kidney disease is feasible, effective and cost-effective, a multicenter randomized control trial was conducted to compare the benefit of a chronic care model compared with usual care (18).
The Canadian Collaborative Group for the Prevention of Renal and Cardiovascular Endpoints Trial (CanPREVENT) consisted of 5 centers across Canada. The trial tested the hypothesis that a nurse-coordinated multirisk factor intervention clinic involving a nephrologist and focusing on applying evidence-based treatments in patients with CKD would reduce or delay the onset of advanced kidney and cardiovascular disease. The purpose of this report was to evaluate the cost-effectiveness of this intervention compared with usual care costs.
Materials and Methods
In the trial, patients with CKD were randomized to chronic disease management or usual care. The methods for the trial and the clinical efficacy have been reported elsewhere (18). In an effort to minimize referral biases, a laboratory-based case finding method was employed as described (18). Patients were recruited from 5 centers in Canada (located in Vancouver, BC; London, ON; Greenfield Park, QU; Halifax, NS; and St. John's, NF, and Labrador). Two years of follow-up data were used to conduct the economic analysis (last 2-year follow-up completed June 2008).
The Intervention Group
Study nurses and nephrologists worked with the patients' usual care providers to deliver care to patients in the intervention group. The nurse, together with the nephrologist, actively helped patients manage identifiable current or future health threats associated with progression of CKD and development of cardiovascular disease–related morbidity and mortality.
Goal-directed therapy consisted of the following:
Achievement of BP target (<130/80);
Use of angiotensin-converting enzymes inhibitors or angiotensin receptor blocker with dose increased to achieve BP target and minimize proteinuria;
Management of dyslipidemia primarily by statin (target LDL <2.6 mmol/L)
Treatment of renal anemia by erythropoietic drugs (target Hb 105 to 120 g/L) and iron as indicated;
Use of diet, phosphate binders, and activated vitamin D to maintain serum phosphate, calcium, and parathyroid hormone in target range;
Use of antiplatelet therapy if cardiovascular disease or diabetes;
β-Blockade with dose titration if postmyocardial infarction or heart failure;
Efforts to help smoking cessation;
Diabetes control with target Hba1c <7%;
Metabolic acidosis control;
Diet appropriate to the medical condition.
The trial was registered at ClinicalTrials.gov registration number: NCT00231803.
The nurse, as indicated by circumstances, initiated referral to dietitians, social workers, diabetes educators, and other professionals. In addition, the study nurse coordinated and communicated with other health care professionals interacting with the patient. The latter included the family doctor, specialist physicians (including the study nephrologist), other nurses (e.g., community nurses and diabetes educators), social workers, and other allied health professionals.
To document status and apply protocol-based interventions, patients in the intervention group were seen at least every 4 months by the study nurse. At each 4 monthly visit a clinical and laboratory assessment was done by the nurse. The nurse determined the frequency of extra visits, but at minimum each patient was seen every 4 months. Between visits the nurse provided telephone outreach support as required.
The Control Group
Patients in the control group received usual care from their primary care provider. After an explanation of their kidney status on study entry, the patients were advised to rely on their usual health care providers for all further clinical advice and care. The nephrologist assigned to the study clinic did not provide care, other than in an emergency, or on-call, to control group patients before ESRD. Usual care providers were free to refer controls for specialist care, including to nephrologists, which happened in the usual way for those few patients who progressed to ESRD. Care for patients in the control group was directed, delivered, and coordinated in the usual way by whatever health care professionals they saw and in all cases this included their family doctor. There were no limitations on the care that was delivered to the control group. Control group patients were seen at study clinics every 4 months for measurement purposes only. However, for ethical reasons, the patient's primary physician was notified of critical issues identified or abnormal laboratory findings on an annual basis.
Resource Utilization
Data on resource utilization was captured every 4 months with a nurse-initiated questionnaire, which documented the number of health care–related visits and types of visits that occurred since the last visit. Specific items that were collected included emergency room visits, hospitalizations, family physician and specialists visits, clinic visits, diagnostic tests and procedures, and care by other health care workers (e.g., home care) (see Table 1). In addition, societal costs, such as the time lost from work by the patient and caregiver and assistive time required for activities of health care, personal care, shopping, and transportation were captured. Differences in resource utilization between the two intervention groups were tested with χ2 tests with degrees of freedom of 1. The level of P that was considered statistically significant was reduced to 0.026 because of multiple testing adjustments with the Simes procedure (19).
Table 1.
Unit prices that were applied to resource utilization identified over 2 years
| Unit Costs | Cost | Source |
|---|---|---|
| Emergency room visits | $246.00 | 20 |
| Hospitalizations | ||
| medical stays | $907.00 | 20 |
| surgical stays | $1,887.00 | 20 |
| ICU stays | $2,337.00 | 20 |
| Physician services consultation fee (repeat consultation fee) | ||
| family physician | $56.10 ($42.35) | 21 |
| cardiology | $132.50 ($82.90) | 21 |
| endocrinology | $71.30 ($45.85) | 21 |
| gastroenterology | $132.50 ($82.90) | 21 |
| nephrology | $71.30 ($45.85) | 21 |
| otolaryngology | $71.30 ($45.85) | 21 |
| respirology | $132.50 ($82.90) | 21 |
| surgeon | $86.60 ($46.30) | 21 |
| Other health care professionals | ||
| occupational therapist | $132.50 ($30.60) | 21 |
| social worker | $110.00 | 22 |
| home care visit | $61.45 | 25 |
| walk-in clinic | $56.10 | 21 |
| pharmacist consultation | $50.00 | 23 |
| physiotherapist | $24.40 | 24 |
| other health care workers | $61.45 | b |
| Tests and procedures | ||
| x-ray | $82.50 | a |
| CT scans | $324.20 | a |
| MRI | $162.00 | a |
| ultrasound | $175.00 | a |
| ECG | $46.75 | a |
| stress test | $326.55 | a |
| angioplasty without stent | $3163.00 | a |
| angioplasty with stent | $3663.00 | a |
| pacemaker insertion | $2869.00 | a |
| fistula insertion | $1606.00 | a |
| central venous catheter insertion | $1840.00 | a |
| average hourly wage in Canada | $20.63 | 26 |
| Intervention program costs | ||
| nursing wage | $40.00 | 27 |
| nephrology time (per 20 minutes) | $45.85 | 21 |
ICU, intensive care unit; CT, computed tomography; MRI, magnetic resource imaging; ECG, electrocardiography.
Hospital case costing.
Average of other health care providers.
Unit Prices
The source of resource utilization was data from the 5 participating study centers. To estimate costs, the unit prices of the resources were applied from one set of sources (Table 1). The unit prices included the cost of emergency room visits, hospitalizations, tests, and procedures from a case costing center in Ontario (20). The unit prices of physician services, including family physician and specialists, were obtained from Ontario Schedule of Benefits for Physicians (21). The cost of social work came from the Ontario Association of Social Workers (22).The cost of occupational therapy came from the Ontario Schedule of Benefits (21). Walk-in clinic costs were evaluated at family physician fee (21), pharmacist consultation taken as the Ontario MedsCheck consultation fee (23), physiotherapist from Ontario Physiotherapists Association web site (24), and other health care workers taken as the average of the other allied health professionals. Other specific prices were obtained from public sources (25). For societal costs, the wage loss was valued at the average industrial wage for Canada on March 2009 (26). These costs reflect the value of the time lost for the patient and caregiver. All prices are reported in 2009 Canadian dollars.
The Cost of the Intervention Program
The costs of the intervention included the time spent by the nurse coordinator and the nephrologist managing the care of each patient. To capture the average time spent by the nurse and nephrologists, the nurse coordinator at each site and the nephrologist at each site completed timing logs to record all events relating to care for the study patients at several points during the trial. This included the extra time for meetings and communications (e-mail phone) relating to patient care, as well as in-person visits. The results of the time logs created an average time per visit for each nurse and an average time per week by each nephrologist. To estimate the cost per patient of nursing time, the number of visits for each patient (including protocol-driven visits) was multiplied by the average time per nurse visit, and then multiplied by the maximum wage that an Ontario registered nurse receives (27). To derive the cost of nephrologists' time, the average time per week that was recorded from the sampling logs was multiplied by 104 weeks (2 years), then multiplied by the 5 nephrologists in the study, and then this total time was valued at the rate of the physician fee for a 20-minute repeat consultation (21) This total cost was divided by the number of intervention patients to derive the nephrology time cost per patient.
Quality of Life
Information on quality of life was captured for each patient by administering a Health Utility Index version 3 (HUI-3) questionnaire at baseline and every 8 months. The raw question scores were converted to an overall health-related quality of life by using the HUI3 algorithm (28). To estimate total quality adjusted life years (QALYs) over 2 years for each patient, the value of HUI-3 at each time (0, 8, 16, and 24 months) was plotted and the area under the curve was derived via a trapezoid rule. From these measures the estimated number of QALYs that occurred for the patient during the 2-year period was generated. Incremental improvements in quality of life from each patient's baseline were used for the cost-effectiveness analysis. A minimally clinical important difference in the level of 0.05 for HUI-3 has been suggested (29).
Missing Data
After unit costs were applied to the resource utilization, the total cost that occurred for a completed questionnaire was estimated. Resource utilization and quality of life data were occasionally missing on visits attended by patients as well as missing with loss to follow-up. Consequently, estimates of total cost for each visit and missing HUI-3 values were imputed based on costs and utility at previous visits of the patient and other patients using multiple imputation regression methods in STATA version 11.0 SE command mvis. From the raw data, 10 different copies of imputed data were created and then the copies were pooled by simple averaging to create one final data set for analysis.
Cost-Effectiveness Analysis
The costs and utilities were estimated for a 2-year horizon, where costs and utility in the second year were discounted by 5% as per Canadian recommendations (30). First, the differences in costs and differences in QALYs were derived to present differences between the intervention group and usual care, and tests for significance for differences in means were conducted with gamma distributions. Then the difference in costs was jointly evaluated with differences in quality of life. The overall strategy was to first determine whether one strategy was dominant over the other (lower costs, more QALYs). In the absence of dominance, an incremental cost-effectiveness ratio was calculated and expressed as an incremental cost per QALY gained.
Probabilistic Analysis
To address uncertainty in cost and outcomes across both arms of the study, probabilistic analysis was conducted by bootstrapping cost and QALY pairs from each patient with 1000 replicates. The results of the replicates are presented in the cost-effectiveness plane. In addition, a cost-effectiveness acceptability curve (CEAC) was derived to estimate the probability of the intervention being cost-effective at different amounts of society's willingness to pay for health outcomes (i.e., cost per QALY gained) or otherwise known as thresholds.
Ten data sets were created and pooled for the probabilistic analysis. In particular, because the cost-effectiveness plane and CEAC are built on the probabilistic nature of the data, the uncertainty of the imputation process of the 10 data sets were averaged to produce one estimate.
Sensitivity Analysis
To address uncertainty that costs may or may not be disease-related, patients in consultation with their nurse coordinator were asked to identify whether they thought the resource use was related to the diseases of interest (renal or cardiac). The base-case analysis included these disease-related costs only and the sensitivity analysis included all costs incurred by the patient over the study period. In addition, the effect of the exclusion of the productivity costs to the cost-effectiveness analysis was investigated, as was the effect on cost-effectiveness from changing the discount rate. Another sensitivity analysis assessed the effect of baseline GFR on the cost-effectiveness results. Analysis was conducted by cutoff levels less than or greater than or equal to 40 and 45 ml/min.
Results
For CanPREVENT, there were 238 patients in the intervention group and 236 patients in the control group. Participants were largely Caucasian seniors, living independently, and a little over half were women (18). Baseline estimated GFR (eGFR) by MDRD equation (age, sex, and serum creatinine) centered around 42 ml/min per 1.73 m2. Proteinuria of >1 g/d existed in 19 patients in total (6 intervention and 13 control). Almost one third had diabetes mellitus and 59.7% had a history of cardiovascular disease. There were few current smokers. Baseline BP tended to be higher in the control group. At the end of the first year, 92% of the intervention patients and 93% of the control patients were followed. Average follow-up time was 21.1 months and 88% of all visits were completed, with 6% of the questionnaires not completed in the first year, 29% in the second year.
Resource Utilization
The resource utilization presented by the average annual resource used per patient is provided in Table 2. For the base-case analysis (disease-related costs only) there was no statistical difference in the number of emergency room visits, family physician visits, clinic visits, and tests and procedures. The number of patients that had at least one hospital admission was 48 (20%) in the intervention group and 69 (29%) in the control group (P = 0.02). The average length of stay for admissions was 6.1 days for patients in the intervention group and 9.6 days for patients in the control group (P = 0.08). The average length of stay in intensive care unit (ICU) was 2.4 days for the intervention group and 3.6 days for the control group (P = 0.09). There was for the intervention group a lower number of days spent in the hospital (intervention: 0.47 days; control: 0.58 days; P = 0.03), fewer days spent in ICU or cardiac care unit (CCU) (intervention: 0.03 days; control: 0.19 days; P < 0.01), fewer specialist visits (intervention: 1.48 visits; control: 1.89 visits; P < 0.01), a higher number of visits to other health care workers (intervention: 3.39 visits; control: 2.14 visits; P < 0.01), and less hours required for assistive care (intervention: 3.47 hours; control: 7.17 hours; P < 0.01). In addition, there was a protocol-driven increased number of visits to the study nurse that resulted on average in 3.66 visits per year (0.66 above protocol-driven visits) with the intervention versus 0.02 visits for the control group (P < 0.01).
Table 2.
Resource utilization based on the 2-year results of the CanPREVENT study, by all cause and related to disease for the intervention and control groups
| All Cause |
Related to Disease |
|||||
|---|---|---|---|---|---|---|
| Intervention | Control | P | Intervention | Control | P | |
| Annualized resource use per patient per year | ||||||
| emergency room visits | 0.68 | 0.58 | 0.07 | 0.17 | 0.14 | 0.37 |
| hospitalizations (n) | 0.16 | 0.21 | 0.08 | 0.05 | 0.06 | 0.58 |
| hospitalizations days | 1.12 | 2.60 | <0.01 | 0.47 | 0.58 | 0.03 |
| ICU days | 0.06 | 0.28 | <0.01 | 0.03 | 0.19 | <0.01 |
| family physician visits | 6.39 | 5.87 | <0.01 | 3.66 | 3.75 | 0.52 |
| clinic visits | ||||||
| outpatient | 4.35 | 4.26 | 0.55 | 4.34 | 4.25 | 0.58 |
| walk-in | 0.22 | 0.23 | 0.64 | 0.06 | 0.07 | 0.34 |
| health promotion | 0.06 | 0.10 | 0.06 | 0.03 | 0.07 | 0.01 |
| diabetic | 0.20 | 0.16 | 0.14 | 0.20 | 0.16 | 0.14 |
| free standing | 0.00 | 0.04 | <0.01 | 0.00 | 0.01 | 0.03 |
| multidisciplinary | 0.04 | 0.09 | <0.01 | 0.02 | 0.05 | 0.01 |
| total clinic visits | 4.86 | 4.87 | 0.94 | 4.64 | 4.62 | 0.89 |
| specialists visits | ||||||
| nephrologist | 0.28 | 0.30 | 0.60 | 0.27 | 0.30 | 0.38 |
| cardiology | 0.31 | 0.40 | 0.02 | 0.30 | 0.40 | 0.02 |
| endocrinologist | 0.13 | 0.12 | 0.88 | 0.12 | 0.09 | 0.36 |
| internist visits | 0.28 | 0.34 | 0.10 | 0.19 | 0.25 | 0.09 |
| surgeon visits | 0.40 | 0.59 | <0.01 | 0.07 | 0.18 | <0.01 |
| other specialists | 8.39 | 8.85 | 0.03 | 0.53 | 0.67 | 0.01 |
| total specialist visits | 9.78 | 10.62 | <0.01 | 1.48 | 1.89 | <0.01 |
| tests | ||||||
| x-rays | 0.69 | 0.92 | <0.01 | 0.15 | 0.19 | 0.18 |
| CT scans | 0.15 | 0.21 | 0.09 | 0.07 | 0.07 | 0.87 |
| MRI | 0.06 | 0.08 | 0.44 | 0.03 | 0.01 | 0.13 |
| ultrasound | 0.27 | 0.34 | 0.07 | 0.18 | 0.17 | 0.76 |
| ECG | 0.37 | 0.46 | 0.04 | 0.53 | 0.39 | 0.01 |
| stress test | 0.10 | 0.08 | 0.46 | 0.09 | 0.08 | 0.52 |
| angioplasty with stent | 0.00 | 0.01 | 0.08 | 0.00 | 0.01 | 0.08 |
| angioplasty without stent | 0.00 | 0.01 | 0.08 | 0.00 | 0.01 | 0.08 |
| pacemaker insertion | 0.00 | 0.00 | 0.32 | 0.00 | 0.00 | 0.32 |
| fistula | 0.00 | 0.00 | 0.32 | 0.00 | 0.00 | 0.32 |
| central vein catheter | 0.00 | 0.01 | 0.08 | 0.00 | 0.00 | 0.32 |
| total tests | 1.64 | 2.12 | <0.01 | 1.04 | 0.93 | 0.12 |
| Annualized resource use per case per year | ||||||
| other health care workers | ||||||
| social worker | 0.03 | 0.03 | 0.85 | 0.01 | 0.01 | 0.99 |
| dietician | 0.31 | 0.20 | <0.01 | 0.26 | 0.18 | 0.02 |
| diabetes clinic nurse | 0.17 | 0.15 | 0.34 | 0.17 | 0.15 | 0.39 |
| other nurse specialty | 0.15 | 0.38 | <0.01 | 0.09 | 0.22 | <0.01 |
| home care | 0.67 | 1.75 | <0.01 | 0.56 | 0.57 | 0.81 |
| pharmacist consulting | 0.06 | 0.05 | 0.74 | 0.03 | 0.03 | 0.82 |
| smoking cessation | 0.03 | 0.00 | <0.01 | 0.03 | 0.00 | <0.01 |
| weight loss program | 1.74 | 0.60 | <0.01 | 1.45 | 0.49 | <0.01 |
| physiotherapist | 0.95 | 1.59 | <0.01 | 0.14 | 0.03 | <0.01 |
| occupational therapist | 0.01 | 0.03 | 0.09 | 0.00 | 0.00 | 0.99 |
| other | 2.61 | 2.19 | <0.01 | 0.65 | 0.46 | <0.01 |
| total other health care workers | 6.73 | 6.97 | 0.20 | 3.39 | 2.14 | <0.01 |
| days lost from work | ||||||
| patient | 2.79 | 3.55 | <0.01 | 2.79 | 3.55 | <0.01 |
| caregiver | 0.29 | 0.47 | <0.01 | 0.29 | 0.47 | <0.01 |
| lost from work (total days) | 3.08 | 4.02 | <0.01 | 3.08 | 4.02 | <0.01 |
| hours assisted | ||||||
| health care activities | 1.18 | 1.88 | <0.01 | 0.83 | 0.24 | <0.01 |
| personal care activities | 1.38 | 7.08 | <0.01 | 0.64 | 0.74 | 0.08 |
| shopping | 12.83 | 20.02 | <0.01 | 2.25 | 4.90 | <0.01 |
| transportation | 2.88 | 7.93 | <0.01 | 0.52 | 2.82 | <0.01 |
| total hours assisted | 18.27 | 36.92 | <0.01 | 4.23 | 8.71 | <0.01 |
| program costs | ||||||
| study care nurse (no. visits per year) | 3.66 | 0.02 | <0.01 | 3.66 | 0.02 | <0.01 |
| nephrologists' minutes (per year) | 77.9 | 0 | 77.9 | 0 | ||
Level of significance adjusted to 0.026 because of multiple testing using the Simes Procedure (significance = α(m + 1)/2m, where m is the number of tests). ICU, intensive care unit; CT, computed tomography; MRI, magnetic resource imaging; ECG, electrocardiography.
Difference in the number of specialist visits resulted from the intervention group seeing fewer cardiologists, surgeons, and other specialists. Differences in the other health care specialists resulted from the intervention group seeing more weight loss program specialists, dieticians, and physiotherapists while seeing fewer other nurse specialists. Differences in hours lost by caregiver for the intervention resulted from fewer hours of assistance for shopping and transportation, but more hours of assistance with health care activities.
Similar patterns existed when considering all-cause resource utilization. There were fewer days in the hospital (intervention: 1.12 days; control: 2.60 days; P < 0.01) and fewer number of specialists' visits in the intervention group. The key differences between the base-case and the all-cause results are that, in the intervention group, there was more family physician visits but less difference in the number of other health care workers visits. Within the category “other health care workers”, the intervention group reported more visits to the dietician and weight loss program but fewer visits to other nurse specialists, home care, and physiotherapists.
Costs
Program Costs.
The cost of the intervention included extra visits with the study nurse and time spent by the nephrologist. For the study nurse visit the average time per visit was 69.3 minutes with a unit cost of $40.00 per hour applied based on the ONA wage schedule (27). On average, there were 3.66 scheduled and extra visits per year for the intervention group and 0.02 extra visits for the control group. Measurement only visits every 4 months are not applicable for controls. This represents an extra $293 per year per patient in nursing time.
For the nephrologist, the average extra time required to monitor all of the patients was 68.9 min/wk, which included patient follow-up (56.1 min/wk) and communication such as e-mail (12.8 min/wk). Applying this for 5 nephrologists (×5), then annualizing (×52 wk/yr), and then dividing by the number of intervention patients (÷238) represented 75.3 min/yr per patient. Applying the average fee from the Ontario schedule of $45.85 for a 20-minute repeat consultation represented a nephrologist time cost of $172.58 per patient per year. The total program cost (including protocol-driven visits) per patient per year was $466 ($293 + $173).
Costs by Different Categories.
Costs by different categories per patient are provided in Table 3. These costs are the raw resource utilization with the applied unit costs. The final costs for the cost-effectiveness analysis are higher as they include imputed values for patients that were lost to follow-up.
Table 3.
Cumulative costs by categories for patients in the intervention and control groups (presented as per patient costs over 2 years, not discounted, nonimputed)
| Disease-Related Costs |
All Costs |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention |
Control |
2-Year Differencea | Intervention |
Control |
2-Year Difference | |||||
| Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | |||
| Emergency | $48 | $63 | $52 | $59 | $1 | $149 | $194 | $136 | $154 | $54 |
| Hospitalization | $240 | $425 | $936 | $1244 | −$1,515 | $2762 | $2866 | $3604 | $4073 | −$2049 |
| Family physician | $172 | $192 | $196 | $166 | $2 | $314 | $308 | $302 | $260 | $61 |
| Nephrology | $25 | $10 | $14 | $25 | −$5 | $26 | $11 | $14 | $25 | −$3 |
| Cardiology | $32 | $45 | $43 | $55 | −$21 | $33 | $45 | $43 | $57 | −$21 |
| Endocrinology | $16 | $8 | $13 | $10 | $0 | $16 | $10 | $16 | $13 | −$3 |
| Internist | $26 | $20 | $31 | $27 | −$13 | $34 | $32 | $45 | $35 | −$13 |
| Surgeon | $8 | $9 | $10 | $31 | −$24 | $47 | $47 | $47 | $90 | −$43 |
| Other physician | $73 | $35 | $85 | $52 | −$29 | $304 | $209 | $281 | $290 | −$58 |
| Clinic | $257 | $257 | $260 | $244 | $11 | $271 | $268 | $273 | $258 | $7 |
| Tests and procedures | $110 | $128 | $173 | $197 | −$132 | $204 | $231 | $318 | $374 | −$258 |
| Other health care provider | $221 | $149 | $92 | $165 | $113 | $398 | $320 | $311 | $440 | −$33 |
| Societal costs | $688 | $347 | $766 | $745 | −$476 | $1035 | $530 | $1225 | $1176 | −$836 |
| Study nurse | $293 | $293 | $1 | $1 | $584 | $293 | $293 | $1 | $1 | $584 |
| Study nephrologists | $173 | $173 | $0 | $0 | $346 | $173 | $173 | $0 | $0 | $346 |
| Total cost | $2382 | $2154 | $2672 | $3021 | −$1157 | $6059 | $5537 | $6616 | $7246 | −$2266 |
Difference is calculated as intervention − control.
For the raw data, the categories with the largest significant differences in cost between the intervention and control group were the cost of hospitalization and costs of tests and procedures, which are higher in the control group, whereas in the base case other health care provider costs are higher in the intervention group. Societal costs are also higher in the intervention group.
Quality of Life
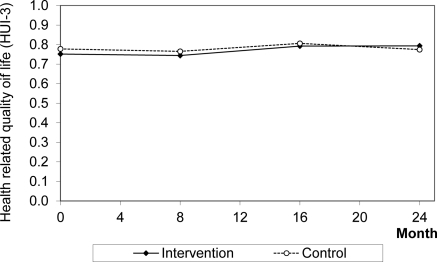
The trend in utility is presented in Figure 1. From baseline to 24 months, there was a statistical difference in change in HUI-3 level between the groups (intervention + 0.024, control − 0.021; P = 0.01). Over 2 years, when baseline HUI-3 was equalized between patient groups, the patients in the intervention group experienced 1.502 QALYs and the control group experienced 1.456 QALYs for a mean difference of 0.046 QALYs gained per patient by the intervention group (P = 0.21).
Figure 1.
Health-related quality of life (HUI-3) for intervention and control groups over the first 2 years of CanPREVENT. Abbreviation: HUI-3, Health Utility Index version 3.
Cost-Effectiveness Analysis
The results of the cost-effectiveness analysis of the mean values after imputation are reported in Table 4. For disease-related costs analysis, the incremental cost of the intervention was −$ 1109 and the incremental number of QALYs was 0.046, which indicates dominance (less costs on average, higher QALYs on average, although results were not signficant).
Table 4.
Incremental costs, incremental health-related quality of life (QALYs), and cost-effectiveness analysis
Probabilistic Analysis
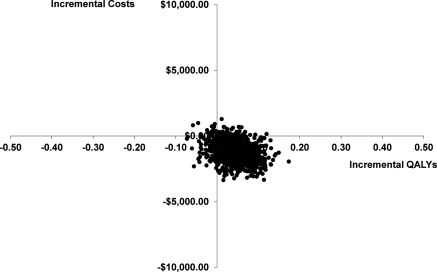
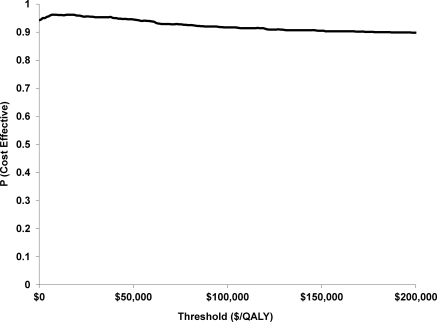
The probabilistic sensitivity analysis is presented in Figure 2 with most of the bootstrap replicates being the lower right dominant quadrant (less costly, more QALYs). To further represent this uncertainty, the CEAC is presented in Figure 3. From this figure, we can see the probability of being cost-effective at different thresholds of cost per QALY. For the base case the probability of being cost-effective at $10,000/QALY is 0.961, at $50,000/QALY is 0.945, and at $100,000/QALY is 0.917.
Figure 2.
Cost-effectiveness plane: Incremental costs versus incremental QALYs gained estimated with 1000 bootstrap replicates. Disease-specific costs. Abbreviation: QALYs, quality-adjusted life years.
Figure 3.
Cost-effectiveness acceptability curve: Probability of the intervention being cost-effective relative to the control group at different society willingness to pay thresholds: $/QALY. Disease-specific costs. The probability of being cost-effective on the vertical access incorporates the uncertainty of being cost-effective given the patient level observations from the study. At $10,000/QALY the percentage is 0.961, at $50,000/QALY it is 0.945, and at $100,000/QALY it is 0.917. This implies, if you are willing to pay $10,000 more for the intervention, then there is a 96.1% of an improvement in 1 QALY. At $0 willingness to pay for a QALY outcome (i.e., horizontal line at the origin in Figure 2), there is a greater than 90% chance that improvements in quality of life will have occurred. Abbreviation: QALYs, quality-adjusted life years.
Sensitivity Analysis
For the case of using all-cause costs, the dominance is even stronger (Table 4). The cost of the intervention was $11,739 and for the control group it was $14,180, for a difference of $2441 (P = 0.02). Further sensitivity analysis is to consider the cost-effectiveness from a health care payer perspective, which is to exclude productivity costs. From Table 2, we see that the difference in societal costs indicated as being disease related was $476, and for all costs the difference in societal costs was $836. Both numbers are small relative to the overall cost savings and would not change the economic result: the intervention is a dominant strategy versus the control. Further analysis was with a discount rate of 0%. The cost of the intervention rises to $4740 and the cost of the control rises to $5896 for a difference of $1156. This increased difference in cost (versus $1109) indicates that the benefits are not driven by first-year events but that the subsequent years continue to add further economic benefit.
The results based on different cutoff values of baseline GFR are reported in Table 5. Similar to the disease-cost analysis and the all-costs analysis, the results based on difference cutoff levels for GFR are similar. In all cutoffs, above and below 40 and 45 ml/min, the patients in the intervention group versus the control incurred less costs and more QALYs, although the results were not statistically significant.
Table 5.
Incremental costs, incremental health-related quality of life (QALYs), and cost-effectiveness analysis by level of GFR
| Costs | QALYs | Costs | QALYs | |
|---|---|---|---|---|
| GFR ≥ 45 (Intervention n = 79, Control n = 81) | GFR < 45 (Intervention n = 159, Control n = 155) | |||
| Cost-effectiveness results (disease-related costs) | ||||
| intervention | $3582 | 1.511 | $4986 | 1.446 |
| control | $6185 | 1.456 | $5738 | 1.440 |
| incrementala | −$2603 | 0.055 | −$753 | 0.006 |
| Cost-effectiveness results (all costs) | ||||
| intervention | $10,598 | 1.511 | $12,205 | 1.446 |
| control | $14,603 | 1.456 | $14,084 | 1.440 |
| incrementala | −$4005 | 0.055 | −$1880 | 0.006 |
| GFR ≥ 40 (Intervention n = 158, Control n = 146) | GFR < 40 (Intervention n = 80, Control n = 90) | |||
| Cost-effectiveness results (disease-related costs) | ||||
| intervention | $4389 | 1.536 | $4945 | 1.459 |
| control | $5304 | 1.498 | $7233 | 1.382 |
| incrementala | −$915 | 0.038 | −$2,288 | 0.077 |
| Cost-effectiveness results (all costs) | ||||
| intervention | $11,761 | 1.536 | $11,360 | 1.459 |
| control | $12,927 | 1.498 | $16,664 | 1.382 |
| incrementala | −$1166 | 0.038 | −$5304 | 0.077 |
Incremental, intervention minus control.
Less costly, more QALYs.
Discussion
A cost-effectiveness analysis was conducted based on the results of CanPREVENT, whose purpose was to evaluate the use of nurses and nephrologists in a multifaceted team to attempt to prevent kidney and associated cardiac events from occurring in the future by aggressively managing the intermediate modifiable biologic risk factors. On the basis of 2 years of the study for the base-case analysis that included disease costs only, the patients in the intervention group incurred less resource use than the patients in the control group. In addition, the intervention also provided 0.046 extra QALYs, although the difference was significant. The magnitude is similar to the suggested minimal clinically important difference for HUI-3 (0.05), but caution is required in this finding because the minimal important difference may be higher and is not yet validated for chronic kidney disease.
The dominant cost-effectiveness result was unchanged by the inclusion of all costs, removing the societal perspective costs of productivity losses for patient and caregiver, and to changing discount rates. In addition, assumptions were made in the analysis that favored the control group involving the cost of the intervention program. In particular, the cost of nephrology time was solely attributed to the intervention group; meanwhile, the time for checking blood work or communicating to the patients may have been for costs that should have been attributed to the control group. In addition, the maximum wage for the nurses was used. The results were also similar regardless of cutoff level of baseline GFR.
The strength of this analysis is the results are unaffected by different cost assumptions, and the change in costs over time follow a logical pattern from the implementation of the intervention to cost savings. In particular, for the intervention group, the cost estimates reported there was an increase in the number of visits to the study nurse, which lead to increased use of weight loss programs and dietician's visits. This in turn resulted in decreased visits to other nurse specialities, less visits to specialists such as cardiologists, and fewer days spent in hospital or in the ICU/CCU.
There are also limitations to the analysis. First, the cost-effectiveness analysis did not include the cost of drugs, although the clinical paper that reported levels of drug use did not detect large differences in drug use between the treatment groups. Specifically, there was a 0.1 tablet per day greater use for BP in the intervention group. A second limitation of the study was the short duration of the trial, only 24 months. However, both the difference in costs and quality of life was higher in the second year, with over 70% of the cost savings, and over 60% of the quality of life benefit occurring in the second year. If we extrapolate into the next few years, additional cost savings and quality of life improvement may occur. One potential long-term risk that may introduce future costs due to morbidity is the risk of stroke associated with use of high levels of erythropoietin, but in this study there was little difference between the groups in the use of such drugs and doses were not high. Another possible limitation is the use of the self-reported resource utilization questionnaires every 4 months that are administered by the nurse implementing the intervention in the study. In addition, we did not rely on other data such as patient hospital charts or administrative records. This may introduce an over- or under-representation of true resource utilization due to interviewer bias.
On the basis of the work for a large HMO in the United States (4), the rising costs associated with the progression of CKD is observed by increasing costs associated mostly with an increase in hospitalizations. In this analysis of the resource utilization of CanPREVENT, a statistical reduction in the rates of hospitalization was observed with the intervention program after only 2 years.
To satisfy the gap in the lack of economic evidence that multifaceted nondrug interventions are cost-effective in reducing or preventing renal and cardiac complications in CKD, CanPREVENT was conducted. From this study, the economic results of CanPREVENT indicate that using a study nurse in consultation with a nephrologist used fewer resources and had lower cost to health care and society when considering all costs while not reducing quality of life for patients with CKD.
Disclosures
None.
Acknowledgments
The study was supported by a New Emerging Team grant co-funded by the Canadian Institutes for Health Research, the Kidney Foundation of Canada, the Heart and Stroke Foundation of Canada, and the Canadian Diabetes Association and by unrestricted grants from Amgen Canada, Ortho Biotech, and Merck Frosst Canada. None of the funders had any role in the design, execution, interpretation, or reporting of the study.
The analysis was based alongside a trial that was registered at ClinicalTrials.gov NCT00231803. Ethics approval was received at the review board of each participating site.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Nurse-Coordinated Care in CKD: Time for Translation into Practice?” on pages 1229–1231.
References
- 1. Song G, Manns BJ, Culleton BF, Tonelli M, Quan H, Crowshoe L, Ghali WA, Svenson LW, Hemmelgarn BR: Prevalence of chronic kidney disease and survival among aboriginal people. J Am Soc Nephrol 18: 2953–2959, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Garg AX, Mamdani M, Juurlink DN, van Walraven C: Identifying individuals with a reduced GFR using ambulatory laboratory database surveillance. J Am Soc Nephrol 16: 1433–1439, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Canada Health Infoway. Available at http://www.infoway-inforoute.ca/lang-en/about-infoway/news/news-releases/149-online-tool-will-support-ontarians-with-chronic-kidney-disease-in-management-of-their-own-care Accessed January 8, 2010
- 4. Smith D, Gullion C, Nichols G, Keith D, Brown J: Cost of Medical Care for Chronic Kidney Disease and Comorbidity among Enrollees in a Large HMO Population. J Am Soc Nephrol 5: 1300–1306, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S: Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE Randomized Trial. Ann Intern Med 134: 707–709, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Strippoli GF, Navaneethan SD, Johnson DW, Perkovic V, Pellegrini F, Nicolucci A, Craig JC. Effects of statins in patients with chronic kidney disease: Meta-analysis andmeta-regression of randomised controlled trials. BMJ 336: 645–651, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nissenson AR, Collins AJ, Hurley J, Petersen H, Pereira BJ, Steinberg EP: Opportunities for improving the care of patients with chronic renal insufficiency: Current practice patterns. J Am Soc Nephrol 12: 1713–1720, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Tonelli M, Bohm C, Pandeya S, Gill J, Levin A, Kiberd BA: Cardiac risk factors and the use of cardioprotective medications in patients with renal insufficiency. Am J Kidney Dis 37: 484–489, 2001 [PubMed] [Google Scholar]
- 10. Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS: Cardiovascular disease risk factors in chronic kidney disease: Overall burden andrates of treatment and control. Arch Intern Med 166: 1884–1891, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, Fluck N, MacLeod A, McNamee P, Prescott G, Smith C. Early referral strategies for management of people with markers of renal disease: A systematic review of the evidence of clinical effectiveness, cost effectiveness and economic analysis. Health Technol Assess 14: 1–184, 2010 [DOI] [PubMed] [Google Scholar]
- 12. McLaughlin K, Manns B, Culleton B, Donaldson C, Taub K: An economic evaluation of early versus late referral of patients with progressive renal insufficiency. Am J Kidney Dis 38: 1122–1128, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR: Screening for proteinuria in US adults. A cost-effective analysis. JAMA 290: 3101–3114, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Atthobari J, Asselbergs FW, Boersma C, de VR, Hillege HL, van Gilst WH, Gansevoort RT, de Jong PE, de Jong-van den Berg LT, Postma MJ: Cost-effectiveness of screening for albuminuria with subsequent fosinopril treatment to prevent cardiovascular events: A pharmacoeconomic analysis linked to the Prevention of Renal and Vascular Endstage Disease (PREVEND) study and the Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVEND IT). Clin Ther 28: 432–444, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Philipneri MD, Rocca Rey LA, Schnitzler MA, Abbott KC, Brennan DC, Takemoto SK, Buchanan PM, Burroughs TE, Willoughby LM, Lentine KL: Delivery patterns of recommended chronic kidney disease care in clinical practice: Administrative claims-based analysis and systematic literature review. Clin Exp Nephrol 12: 41–52, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Patwardhan MB, Matchar DB, Samsa GP, Haley WE: Opportunities for improving management of advanced chronic kidney disease[b]. Am J Med Qual 23: 184–192, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Jones C, Roderick P, Harris S, Rogerson M: Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transplant 21: 2133–2143, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Barrett BJ, Garg AX, Goeree R, Levin A, Molzahn A, Rigatto C, Singer J, Soltys G, Soroka S, Ayers D, Parfrey PS: A nurse-coordinated model of care versus usual care for stage 3/4 chronic kidney disease in the community: A randomized controlled trial. Clin J Am Soc Nephrol 2011, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Statist Soc B 57: 289–300, 1995 [Google Scholar]
- 20. Ontario Ministry of Health and Long term Care; Ontario Case Costing Initiative. http://www.occp.com Accessed June 3, 2009
- 21. Ontario Ministry of Health and Long Term Care: Ontario Health Insurance (OHIP)Schedule of Benefits and Fees. Available at http://www.health.gov.on.ca/english/providers/program/ohip/sob/sob_mn.html Accessed June 3, 2009
- 22.Jeff Packer MSW and Associates. [Accessed June 2009]. Available at http://www.jeffpacker.com/policies.html.
- 23. Ontario Ministry of Health and Long Term care Medscheck Consult. Available at http://www.health.gov.on.ca/cs/medscheck/pub_new.html Accessed June 3, 2009
- 24.Ontario Physiotherapists Association. [Accessed June 3, 2009]. Available at http://www.opa.on.ca/IN_THE_NEWS_FSCO_060608.SHTML.
- 25. Health Canada: Provincial submission to the Canada Health Act Annual Report 2005–2006. Available at http://www.hc-sc.gc.ca/hcs-sss/medi-assur/cha-lcs/pt-plans-on-eng.php Accessed June 3, 2009
- 26. Statistics Canada Latest statistics (monthly). Available at http://www40.statcan.gc.ca/l01/cst01/media01-eng.htm?sdi=average%20weekly%20earnings Last accessed June 2009
- 27. Ontario Nursing Association Salary facts. Available at http://www.ona.org/node/68 Accessed June 3, 2009
- 28. Health Utilities Inc. Available at http://www.healthutilities.com/hui3.htm Accessed July 16, 2009
- 29. Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI(R)): Concepts, measurement properties and applications. Health Qual Life Outcomes 1: 54, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guidelines for the economic evaluation of health technologies: Canada [3rd Edition], Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health, 2006 [Google Scholar]