Abstract
Summary
Background and objectives
Commonly sleep is disrupted and physical activity is restricted among patients with CKD and those on long-term dialysis. However, few studies have assessed patients longitudinally.
Design, setting, participants, & measurements
We compared the prevalence of sleep disturbances measured both subjectively using a questionnaire and objectively using actimetry among patients with CKD (n = 145), those on hemodialysis (n = 116), and people without kidney disease (n = 19). Activity level during the day was measured using actimetry, and patients were then followed for up to 2 years.
Results
Compared with people without kidney disease, patients with CKD not on dialysis had disruption of sleep that was independent of several risk factors. However, disrupted sleep was correlated with neither estimated GFR in cross-sectional nor longitudinal assessment. Those on hemodialysis had sleep disruption that was of much greater severity than that found among those with CKD not on dialysis. Furthermore, missing or shortening the prescribed duration of dialysis was associated with greater severity of sleep disturbance in cross-sectional but not in longitudinal assessment. Day-time activity declined both in duration and intensity from controls to CKD to hemodialysis.
Conclusions
The loss of kidney function is related to both reduced duration and intensity of day time physical activity. Although patients with CKD have disrupted sleep, this is independent of estimated GFR. However, compared with those with CKD, dialysis patients have more severely disrupted sleep; this is related to missing dialysis. Thus, shortening patients' dialysis may reduce their sleep.
Introduction
Sleep disturbance is common and bothersome to patients with chronic kidney disease (CKD) including those on long-term dialysis. The natural history of sleep disturbance in these patients has not been adequately studied. Although at least two studies have compared the quality of sleep among patients with CKD and those on dialysis, neither of these studies had a control group nor had longitudinal follow-up (1,2). Therefore, it remains unclear how sleep disturbance is affected by the progression of CKD.
CKD progresses to dialysis in many, and dialysis is also recognized to have a large burden of sleep disturbance. However, it remains unclear how dialysis dose affects sleep disturbance. Nocturnal dialysis has been reported to markedly ameliorate sleep disturbance in these individuals (3), by mechanisms that remain unclear but include improvement in pharyngeal wall edema and sensitization of chemoreceptors (3,4). However, the vast majority of patients are treated not by long-duration nocturnal dialysis, but by dialysis three times a week. Patients often shorten or skip dialysis treatments, provoking variable degrees of uremia that vary among individuals and within individuals over time. The effect of shortening or missing dialysis treatments on sleep among dialysis patients and within a patient over time also remains unclear. These questions are important, because if missed or shortened dialysis treatments are associated with poor sleep quality then counseling patients to modify this behavior can potentially realize a significant benefit in their quality of life.
This study was both cross-sectional and longitudinal. The purpose of the cross-sectional study was to compare objective and subjective measures of sleep as well as the duration and intensity of day time physical activity among people with CKD, those undergoing hemodialysis, and a normal control group. The purpose of the longitudinal follow-up was two-fold. Among patients with CKD, the purpose was to examine the effect of progression of CKD on sleep. Among patients on dialysis, the purpose was to examine the association of missed or shortened dialysis treatments on sleep within individuals over time.
Materials and Methods
Subjects
We studied three groups of people: control patients, those with chronic kidney disease, and those on hemodialysis. All of the patients were participating in a study to assess BP and sleep, and activity assessment was an add-on assessment. The characteristics of these subjects are described below.
Control Patients.
Veterans between the ages of 18 and 90 years who did not have hypertension, diabetes mellitus, or chronic kidney disease were studied. The presence of chronic kidney disease was assessed by albuminuria and estimated GFR. Those with morbid obesity (body mass index of ≥40 kg/m2) were excluded.
Patients with Chronic Kidney Disease.
Veterans between the ages of 18 and 90 years with CKD defined as having an estimated GFR (eGFR) of <60 ml/min per 1.73 m2, the presence of proteinuria (spot urine/protein ratio of >200 mg/g creatinine), or the presence of structural kidney disease (e.g. adult polycystic kidney disease) were recruited. We excluded those patients who met any of the following characteristics: morbid obesity, eGFR of ≤15 ml/min per 1.73 m2, hospitalization within the preceding 2 months, seated clinic BP of ≥140/90 mmHg, or substantial cardiac arrhythmia (defined as six or more irregular heart beats per minute). Patients who progressed to ≤15 ml/min per 1.73 m2 during the longitudinal follow-up were not excluded.
Patients on Hemodialysis.
We studied patients, both veterans and nonveterans, with end-stage renal disease who had been on hemodialysis for at least 3 months. We excluded patients with morbid obesity as defined above or with atrial fibrillation.
Actigraphy
Sleep and wake times were recorded in a diary for the entire duration of actigraphy. Activity monitoring was performed using an actigraph (Actiwatch 64; Mini Mitter, Bend, OR), a watch-sized device worn on the dominant wrist for up to 7 days in controls and in patients with CKD but only for approximately 2 days in those on hemodialysis. Among hemodialysis patients, on each of the 2 days spanning the entire interdialytic interval, we carried out ambulatory BP monitoring. Ambulatory BP monitoring was also performed for 1 of the 7 days of actimetry among the nonhemodialysis patients. The internal clocks of the ambulatory BP monitor and actigraph were synchronized, and activity was assessed in 15-second epochs throughout the 24-hour period. Therefore, ambulatory BP recording times and actigraphy times were synchronized. Actimetry data were exported to a custom-designed relational database and analyzed for the entire duration of recording.
Sleep Assessment with Actigraphy
The following definitions were used to assess sleep and activity.
Rest per Day.
Cumulative period of the rest interval reported by the patient on each day recorded in the diary.
Active Time per Day.
Minutes of the day reported by the patient to be awake and out of bed.
Sleep per Day.
Duration in minutes where the patient was asleep as judged by actigraphy scores. When a weighted average of the epoch of activity in question and 16 surrounding epochs had a total activity count of <20, the patient was said to be asleep. When total activity counts was 20 or more the patient was said to be awake.
Wake during Rest per Day.
Duration in minutes where the patient is labeled as awake during the rest interval. The percentage of time awake was calculated as the fraction of total awake time to duration in bed × 100%.
Wake but Resting.
Duration in minutes where the patient stated that he was resting, but actimetry never detected the onset of sleep.
Sleep Onset Latency.
Contiguous immobile time of 10 minutes or more were required for sleep onset. The time elapsed from the diary recorded time in bed to sleep onset was taken as sleep onset latency.
Snooze Time.
The time elapsed between sleep end time and the end time of the given rest interval (when the patient gets out of bed) as reported by the subject.
Wake after Sleep Onset (WASO).
The number of minutes between sleep onset and wake time scored as wake. WASO periods also included one of the following two definitions: (1) night sleep, between 10 p.m. and 8 a.m., with awake states of less than 2 hours with at least 45 minutes of sleep flanking the awake state or (2) night sleep, between 12 a.m. and 5 a.m., with awake state of less than 3 hours with at least 45 minutes of sleep flanking the awake state.
Sleep Efficiency.
The percentage of scored total sleep time to the duration in bed. We computed this with the following formula: (rest per day − (onset latency + snooze time + WASO + wake but resting)) × 100%/rest per day.
Fragmentation Index.
Calculated as the percentage of minutes scored as mobile (any activity during rest period) + percentage of immobile bouts that were 60 seconds in length/total number of immobile bouts. Thus, this metric gives an index of movement during rest.
Activity Classification
To determine the level of activity, we first calculated the total duration of waking period. The levels of activity counts of <80 were computed, and duration of any count <80 was taken as resting (level 0). Duration of activity between 80 and 160 counts/epoch (level 1), 160 and 320 counts/epoch (level 2), and >320 counts/epoch (level 3) were taken as evidence of increasingly vigorous activity. Visual inspection of a wheelchair-bound patient for each day for 2 weeks revealed nearly all counts per epoch to be <160, suggesting a generally sedentary existence. Similarly, inspection of plots of activity in patients who exercised frequently showed bursts of activity >320 count/epoch, suggesting more vigorous activity. Although categorizing the level of activity reduces the power of the study, we feel that these are more appropriate because they present more conservative estimates.
Subjective Assessment of Sleep
To assess the subjective quality of sleep, the Kidney Disease Quality of Life (KDQOL) sleep subscale was administered to all individuals. This instrument asks four questions: (1) How do you rate your sleep overall? (2) Do you awaken during the night and have trouble falling asleep again? (3) Do you get the amount of sleep that you need? and (4) Do you have trouble staying awake during the day? Each of the four questions that we classify as overall sleep rating, sleep maintenance, sleep sufficiency, and day-time somnolence was scaled from 0 to 100, with 100 being the best possible score. The sleep quality KDQOL score was also rated from 0 to 100 and was the composite of the four answers, 100 being the best quality of sleep.
Other Definitions
Cardiovascular Disease.
Patient reported history of myocardial infarction, stroke, or cardiac revascularization procedure was taken as evidence of cardiovascular disease.
Missed Dialysis.
We collected dialysis run records over a period ranging from 2 weeks to 30 days before the placement of the actigraph. From each dialysis run, we determined the number of minutes of dialysis prescribed and the number of minutes of dialysis actually received. From this, we computed the number of minutes of missed dialysis and were able to compute a percentage of dialysis missed as a number of minutes of missed dialysis/number of minutes of prescribed time on dialysis. The numbers are reported as mean numbers of minutes per session or percentages.
Follow-up
Patients with CKD were studied at baseline and after 2 years using the same protocol. Those with ESRD on hemodialysis were studied at baseline and 3, 6, and 12 months. A few patients were followed for 2 years.
Statistical Methods
Graphical methods were used to visualize all of the data before analysis. Assessment of sleep was made both while wearing and while not wearing an ambulatory BP monitor in all patients except those on hemodialysis. Among those on hemodialysis, sleep was assessed only while wearing an ambulatory BP monitor. We have previously reported that wearing an ambulatory BP monitor can by itself influence sleep (5); therefore we did not analyze the effect of wearing an ambulatory BP monitor on sleep again in this study. Because hemodialysis patients had actigraphy performed only while wearing the monitor, we report results found only when subjects were wearing an ambulatory BP monitor. For reporting adjusted analyses (as in Table 3), a mixed effects linear intercept model was used. The fixed effects were age, sex, race, body mass index, diabetes, and cardiovascular disease. The random effect was the subject. Maximal likelihood estimation was used. Marginal means are reported along with 95% confidence interval and P values.
Table 3.
Adjusted objective and subjective sleep parameters in participants
| Sleep Characteristic | Control | CKD − Control | P (CKD versus control) | HD − Control | P (HD versus control) | HD − CKD | P (HD versus CKD) |
|---|---|---|---|---|---|---|---|
| Rest per day (min) | 443.0 (392.1, 493.8) | −1.3 (−47.4, 44.7) | 1 | −2.8 (−55.7, 50.0) | 0.9 | −1.5 (−43.5, 40.6) | 0.9 |
| Sleep onset latency (min/day) | 23.0 (−1.3, 47.3) | 15.2 (−7.0, 37.4) | 0.2 | 50.2 (25.0, 75.5) | <0.0001 | 35.0 (15.1, 54.9) | <0.001 |
| Snooze time (min/day) | 23.0 (9.4, 36.7) | 8.2 (−4.1, 20.4) | 0.2 | 17.6 (3.4, 31.8) | 0.02 | 9.4 (−2.0, 20.9) | 0.1 |
| Wake but rest (min/day) | 1.3 (−9.8, 12.4) | 2.2 (−7.8, 12.3) | 0.7 | 13.7 (2.1, 25.2) | 0.02 | 11.4 (2.3, 20.6) | 0.01 |
| Active time (min/day) | 791.0 (726.0, 855.9) | 37.3 (−20.5, 95.2) | 0.2 | 36.0 (−31.7, 103.7) | 0.3 | −1.3 (−56.4, 53.8) | 1 |
| Sleep (min/day) | 358.3 (312.5, 404.2) | −32.5 (−74.5, 9.4) | 0.1 | −73.9 (−121.5, −26.3) | <0.01 | −41.3 (−78.6, −4.1) | 0.03 |
| Sleep/rest time (%) | 81.2 (74.4, 87.9) | −6.7 (−13.0, −0.5) | 0.04 | −15.5 (−22.5, −8.5) | <0.0001 | −8.8 (−14.2, −3.4) | <0.01 |
| WASO (min/day) | 63.1 (42.7, 83.5) | 7.9 (−10.6, 26.4) | 0.4 | 10.9 (−10.3, 32.1) | 0.3 | 3.0 (−13.9, 19.9) | 0.7 |
| Sleep efficiency (%) | 75.6 (67.2, 83.9) | −8.6 (−16.3, −0.9) | 0.03 | −21.0 (−29.7, −12.3) | <0.0001 | −12.4 (−19.1, −5.6) | <0.001 |
| Fragmentation Index (%) | 16.1 (10.2, 22.0) | 8.4 (3.0, 13.9) | <0.01 | 17.3 (11.2, 23.5) | <0.0001 | 8.9 (4.2, 13.6) | <0.001 |
| Subjective quality of sleep | |||||||
| overall sleep quality | 56.8 (43.7, 69.9) | −5.7 (−18.1, 6.7) | 0.4 | −11.1 (−25.0, 2.8) | 0.1 | −5.4 (−15.5, 4.8) | 0.3 |
| sleep maintenance | 58.2 (41.6, 74.8) | −2.8 (−18.5, 12.8) | 0.7 | −8.1 (−25.7, 9.4) | 0.4 | −5.3 (−18.2, 7.5) | 0.4 |
| sleep sufficiency | 50.4 (33.8, 67.0) | −5.8 (−21.3, 9.8) | 0.5 | −7.8 (−25.3, 9.7) | 0.4 | −2.1 (−15.0, 10.8) | 0.8 |
| daytime somnolence | 63.8 (48.6, 78.9) | −5.6 (−20.0, 8.7) | 0.4 | 6.6 (−9.5, 22.7) | 0.4 | 12.2 (0.4, 24.0) | 0.04 |
| sleep quality (KDQOL score) | 56.7 (45.6, 67.9) | −4.6 (−15.2, 6.1) | 0.4 | −5.1 (−17.0, 6.8) | 0.4 | −0.5 (−9.2, 8.2) | 0.9 |
HD, hemodialysis.
A random coefficient model was used to describe the longitudinal changes on sleep variables as in Table 5. Here the effect of missing dialysis over time was modeled with each of the two continuous variables and their interactions. Other fixed variables were the adjustment variables reported above and their interactions with time. The random effects included subjects and time using an unstructured covariance matrix and maximal likelihood estimation.
Table 5.
Estimates of sleep parameters per percent missed or shortened dialysis
| Sleep Characteristic | Cross-sectional Estimate (per 10% Missed Dialysis) | P | Longitudinal Estimate (per 10% Missed Dialysis) | P |
|---|---|---|---|---|
| Rest (min/day) | −7.6 (−29.8, 14.6) | 0.5 | 0.7 (−2.4, 3.7) | 0.7 |
| Sleep (min/day) | −23.1 (−39.8, −6.3) | <0.01 | 1.0 (−1.2, 3.2) | 0.4 |
| Sleeping during rest (%) | −4.1 (−6.7, −1.4) | <0.01 | 0.2 (−0.1, 0.5) | 0.3 |
| Sleep efficiency (%) | −5.1 (−8.2, −1.9) | <0.01 | 0.3 (−0.1, 0.7) | 0.1 |
| Fragmentation index (%) | 3.4 (0.9, 5.8) | <0.01 | −0.1 (−0.4, 0.2) | 0.4 |
| Sleep quality (KDQOL score) | −5.5 (−8.4, −2.5) | <0.001 | 0.6 (0.1, 1.0) | <0.01 |
Results
Table 1 shows the baseline characteristics of the study participants. By definition, the control group did not have hypertension, diabetes mellitus, or kidney disease. Dialysis patients were younger, were more often female and black, and had less cardiovascular disease compared with CKD patients. A greater number of black patients and less cardiovascular disease is expected from what is known of the epidemiology of ESRD.
Table 1.
Baseline characteristics of participants
| Clinical Characteristic | Control | CKD | HD | Total | P |
|---|---|---|---|---|---|
| n | 19 (7%) | 145 (52%) | 116 (41%) | 280 (100%) | |
| Gender | 17 (89%) | 141 (97%) | 73 (63%) | 231 (83%) | <0.0001 |
| Race | <0.0001 | ||||
| white | 15 (79%) | 121 (83%) | 15 (13%) | 151 (54%) | |
| black | 3 (16%) | 16 (11%) | 99 (85%) | 118 (42%) | |
| other | 1 (5%) | 8 (6%) | 2 (2%) | 11 (4%) | |
| Age (years) | 60.1 ± 10.0 | 69.0 ± 10.6 | 51.5 ± 11.4 | 61.1 ± 13.7 | <0.0001 |
| Height (cm) | 178.3 ± 6.9 | 174.3 ± 8.6 | 172.1 ± 10.1 | 173.8 ± 9.2 | 0.02 |
| Weight (kg) | 87.7 ± 15.2 | 93.2 ± 16.8 | 81.7 ± 19.2 | 88.3 ± 18.4 | <0.0001 |
| Body mass index (kg/m2) | 27.5 ± 4.0 | 30.6 ± 4.7 | 27.5 ± 6.0 | 29.2 ± 5.4 | <0.0001 |
| Cardiovascular disease | 0 (0%) | 83 (57%) | 28 (24%) | 111 (40%) | <0.0001 |
| Diabetes mellitus | 0 (0%) | 86 (59%) | 46 (40%) | 132 (47%) | <0.0001 |
| Albumin (g/dl) | 4.0 ± 0.3 | 3.7 ± 0.4 | 3.6 ± 0.5 | 3.7 ± 0.4 | <0.01 |
| Hemoglobin (g/dl) | 14.7 ± 0.9 | 13.2 ± 1.7 | 12.0 ± 1.4 | 12.8 ± 1.7 | <0.0001 |
| eGFR (ml/min per 1.73 m2) | 77.6 ± 7.9 | 43.9 ± 14.3 | NA | 47.8 ± 17.4 | <0.0001 |
HD, hemodialysis.
Objective Assessment of Sleep
Table 2 shows the crude differences among groups in both subjective and objective parameters of sleep and activity. All three groups rested similar amounts per day. However, compared with controls, who slept 366 minutes, those with CKD slept 338 minutes, and those on dialysis slept 290 minutes only. Compared with 11% of normal controls who slept <5 hours per day, 31% of those with CKD and 53% on hemodialysis slept <5 hours. Sleep onset increased from controls to CKD and increased even more among hemodialysis patients. Sleep efficiency averaged 74.1% in controls, 67.6% in CKD, and 54.4% among hemodialysis patients. Fragmentation of sleep increased progressively from controls to hemodialysis patients. Subjective assessment of sleep was similar among groups except for day-time somnolence that was greater among those with CKD.
Table 2.
Sleep and activity characteristics of participants
| Sleep Characteristic | Control | CKD | HD | Total | P |
|---|---|---|---|---|---|
| n | 19 (7%) | 148 (53%) | 114 (41%) | 281 (100%) | |
| Rest per day (min) | 465 ± 79 | 457 ± 119 | 458 ± 151 | 458 ± 131 | 1 |
| Sleep onset latency (min/day) | 28 ± 27 | 41 ± 53 | 78 ± 73 | 55 ± 64 | <0.0001 |
| Snooze time (min/day) | 23 ± 23 | 33 ± 48 | 38 ± 40 | 34 ± 44 | 0.3 |
| Wake but rest per day (min) | 0 ± 0 | 1 ± 5 | 12 ± 55 | 5 ± 36 | 0.03 |
| Active time per day (min) | 667 ± 280 | 836 ± 210 | 861 ± 195 | 835 ± 214 | <0.01 |
| Sleep per day (min) | 366 ± 67 | 338 ± 96 | 290 ± 112 | 320 ± 105 | <0.001 |
| Sleep/rest time (%) | 78.8 ± 7.9 | 74.8 ± 12.6 | 63.3 ± 17.6 | 70.4 ± 15.8 | <0.0001 |
| WASO (min/day) | 70 ± 31 | 74 ± 47 | 79 ± 67 | 76 ± 55 | 0.7 |
| Naps (number/day) | 0.2 ± 0.3 | 0.4 ± 0.6 | 0.4 ± 0.6 | 0.4 ± 0.6 | 0.4 |
| Sleep <5 h/night | 2 (11%) | 46 (31%) | 60 (53%) | 108 (38%) | <0.0001 |
| Sleep efficiency (%) | 74.1 ± 8.9 | 67.6 ± 16.8 | 54.4 ± 21.0 | 62.7 ± 19.5 | <0.0001 |
| Time mobile during sleep/day (min) | 57.1 ± 29.8 | 86.6 ± 57.6 | 126.4 ± 84.8 | 100.8 ± 72.1 | <0.0001 |
| Fragmentation index | 16.8 ± 6.9 | 24.7 ± 10.7 | 34.1 ± 16.2 | 28.0 ± 14.1 | <0.0001 |
| Subjective quality of sleep | |||||
| overall sleep quality | 60.6 ± 28.9 | 59.2 ± 25.3 | 55.8 ± 24.8 | 57.9 ± 25.3 | 0.6 |
| sleep maintenance | 65.0 ± 33.1 | 61.9 ± 28.2 | 55.8 ± 33.6 | 59.7 ± 30.8 | 0.3 |
| sleep sufficiency | 52.5 ± 39.2 | 51.8 ± 30.6 | 44.7 ± 30.1 | 49.0 ± 31.1 | 0.2 |
| daytime somnolence | 71.3 ± 37.9 | 64.7 ± 28.7 | 73.8 ± 25.7 | 68.8 ± 28.4 | 0.05 |
| sleep quality (KDQOL score) | 62.3 ± 25.6 | 59.4 ± 21.2 | 57.5 ± 19.1 | 58.8 ± 20.7 | 0.6 |
HD, hemodialysis.
Table 3 shows differences among groups in sleep parameters after adjustments. Adjustments for the following clinically relevant variables were performed: age, sex, race, body mass index, diabetes mellitus, and cardiovascular disease. All of the analyses report the results recorded while patients were wearing an ambulatory BP monitor. The results shown in Table 3 are modeled results for a 60-year-old white man with a body mass index of 30 g/m2 with diabetes and cardiovascular disease while wearing an ambulatory BP monitor. Compared with the control group, patients with CKD not on dialysis slept 6.7% less while trying to sleep, had lower sleep efficiency, and had more sleep fragmentation. Compared with the control group, patients on hemodialysis had the following impairments: increased sleep onset latency, increased snooze time, increased self-reported wake time in bed, reduced length of sleep, increased awake state during sleep, reduced sleep efficiency, and increased sleep fragmentation. Compared with the CKD group, hemodialysis patients had the following impairments: increased sleep onset latency, increased minutes spent in bed awake, >40 minutes less sleep, reduced percentage of time sleep, increased minutes awake, increased percent of time awake, reduced sleep efficiency, and increased sleep fragmentation.
Adjusted differences between groups—normal controls, CKD, and hemodialysis patients—in perception of sleep were less obvious. All groups of patients rated their sleep as similarly poor. Day-time somnolence was significantly different among groups, with CKD patients being more somnolent and hemodialysis patients less compared with controls.
Day-time Activity
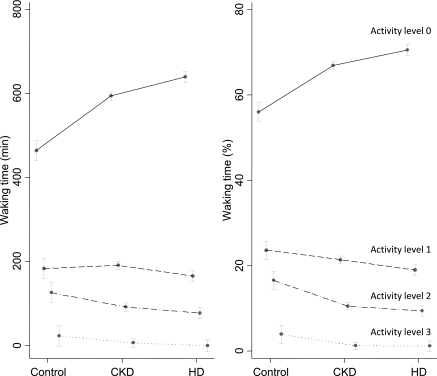
Table 4 shows the level of day-time physical activity. Although active time was different among groups increasing from control to hemodialysis patients, the distribution of day-time activity in increasing levels of physical activity from rest (level 0) to mild (level 1), moderate (level 2), and strenuous activity (level 3) was different. Among controls, these levels were 56, 24, 16, and 4%, respectively. For CKD patients, the percentages of time spent in these respective strata were 66, 22, 11, and 1%. For hemodialysis patients, these percentages were 71, 19, 9, and 1%, making this the least active group. Adjusted for age, sex, race, body mass index, diabetes, and cardiovascular disease, the corresponding levels of day-time activity are shown in Figure 1 for the three groups. For both waking minutes and percentages of waking minutes, there was a significant group × activity level interaction (P < 0.0001). Patients with CKD tended to rest more and were less physically active compared with controls. Patients on hemodialysis were the least physically active.
Table 4.
Activity characteristics of participants
| Activity duration and intensity | Control | CKD | HD | Total | P for difference among groups |
|---|---|---|---|---|---|
| Active time per day (min) | 798 ± 203 | 898 ± 173 | 904 ± 151 | 894 ± 168 | 0.04 |
| Wake at activity level 0 (min/day) | 456 ± 187 | 595 ± 167 | 644 ± 167 | 605 ± 174 | <0.0001 |
| Wake at activity level 1 (min/day) | 191 ± 58 | 195 ± 79 | 167 ± 79 | 183 ± 78 | 0.02 |
| Wake at activity level 2 (min/day) | 125 ± 71 | 97 ± 66 | 83 ± 68 | 93 ± 68 | 0.02 |
| Wake at activity level 3 (min/day) | 26 ± 22 | 11 ± 15 | 10 ± 14 | 12 ± 16 | <0.001 |
| Wake at activity level 0 (%/day) | 55.6 ± 14.7 | 66.4 ± 13.6 | 71.3 ± 14.6 | 67.7 ± 14.6 | <0.0001 |
| Wake at activity level 1 (%/day) | 24.4 ± 5.4 | 21.7 ± 7.5 | 18.5 ± 8.0 | 20.6 ± 7.8 | <0.001 |
| Wake at activity level 2 (%/day) | 16.3 ± 9.0 | 10.8 ± 6.9 | 9.1 ± 7.0 | 10.5 ± 7.3 | <0.001 |
| Wake at activity level 3 (%/day) | 3.7 ± 3.5 | 1.2 ± 1.6 | 1.1 ± 1.5 | 1.3 ± 1.9 | <0.0001 |
Figure 1.
The figure shows the levels of day-time activity by patient group. The raw minutes spent in each activity are shown on the left, and percentages are shown on the right. These values are adjusted for age, sex, race, body mass index, diabetes, and cardiovascular disease. The solid line represents the activity level of zero or rest; the dotted lines show increasing levels of activity. For both graphs (waking minutes and percentages of waking minutes), there was a significant group × activity level interaction (P < 0.0001). In other words, patients with CKD tended to rest more and were less physically active compared with controls. Patients on hemodialysis were the least physically active.
Effect of Progression of Kidney Disease and Sleep Quality
Of the 149 patients with CKD, 27 (18.1%) had measurements at 2 years along with actimetry. The estimated GFR at baseline was 43 ± 14 ml/min per 1.73 m2, which fell nonsignificantly (P = 0.21) by 4.8 ml/min per 1.73 m2 at follow-up. The effect of GFR and its progression on various parameters of sleep quality did not reveal any linear pattern. Furthermore, no cogent patterns emerged that related sleep quality to stages of CKD or to the change in stages of CKD (data not shown).
Effect of Missing Dialysis over Time and Sleep Quality
The effect of skipping or shortening dialysis and change in this behavior over time on various parameters of sleep quality is shown in Table 5. Skipping dialysis or cutting it short was not associated with fewer hours spent in bed, but each 10% shorter dialysis was associated with the following disturbances: 23.1 fewer minutes of sleep, 4.1% fewer minutes of the time in bed that were spent sleeping, 5.1% lower sleep efficiency, 3.4% greater sleep fragmentation, and 5.5% reduction in overall subjective sleep quality. We had 229 measurements, of which 113 (49%) were following the baseline. Of the postbaseline measurements available in 72 (62%) patients, 31 (43%) were at 3 months, 52 (72%) were at 6 months, 18 (25%) were at 12 months, and 12 (17%) were at 24 months. Within individuals over time, cutting dialysis short was not associated with worsening parameters of objective sleep but somewhat surprisingly was associated with slightly improved subjective perception of sleep quality. There was no measurable effect of shortening dialysis on day-time physical activity scores (data not shown).
Discussion
Our study shows that among free-living dialysis patients, sleep is greatly disturbed. Compared with people without kidney disease, patients with CKD not on dialysis also had disruption of sleep that was independent of several risk factors. However, disrupted sleep was not correlated with estimated GFR. Those on hemodialysis had sleep disruption that was of much greater severity than that found among those with CKD not on dialysis. Furthermore, missing or shortening the prescribed duration of dialysis was associated with greater burden of sleep disturbance. Day-time activity declined both in duration and intensity from controls to CKD to hemodialysis. These differences were statistically significant and clinically relevant despite adjustments for demographic and clinical differences between the groups.
Although several authors have pointed out the poor subjective quality of sleep among patients with CKD (2,6–11), there are only a handful of reports of formal polysomnographic studies in patients with CKD. Among the first was a report in 1993 by Pressman et al. (12), who used polysomnograpy to study eight patients with ESRD who had sleep disturbance. All had sleep apnea that resulted in severe sleep fragmentation. Upon treatment with continuous positive airway pressure, subjective and objective measures of sleep improved. Unruh et al. (13) reported that hemodialysis patients are significantly more likely than patients without CKD to have the following sleep disturbances: short sleep (odds ratio, 3.27), decreased sleep efficiency (odds ratio, 5.5), more difficulty getting back to sleep (odds ratio, 2.25), and waking up too early (odds ratio, 2.39). There was no association between polysomnography sleep time and self-reported sleep time. The latter is confirmed by our study. Parker et al. (1) compared 16 hemodialysis patients with eight CKD patients. Although a normal control group was absent, when compared with normative data, both groups had reduced total sleep time and reduced sleep efficiency. Among hemodialysis patients, the following abnormalities were noted: less total sleep time, increased wake after sleep onset, lower sleep efficiency, higher periodic limb movement index, and longer latencies to sleep onset and rapid eye movement sleep. Our study confirms some of these disorders.
The results of cross-sectional survey and the longitudinal study yielded results on sleep parameters that were somewhat surprising. Whereas the cross-sectional survey demonstrated the expected relationships between kidney disease and sleep, the results of longitudinal study were different and in most instances did not find the relationships expected from the cross-sectional study. The differences between the cross-sectional and longitudinal studies may be because of several reasons. First, there may not be a cause-and-effect relationship between kidney failure and sleep. The cross-sectional association may exist because of common conditions or diseases that cause both sleep disturbances and also CKD. Although this may be true, it is unlikely because we found a relationship between the severity of sleep disturbances when transitioning from CKD to hemodialysis. Second, the changes in missed dialysis or estimated GFR within individuals may not be sufficiently large to detect a relationship between these parameters and sleep quality. In other words, we may not have enough power to detect this relationship. Thus, either a longer follow-up to observe a greater decline in GFR or a more drastic change in behavior, such as missing fewer dialysis treatments, may be required before changes in sleep disturbances are apparent.
Because missing dialysis invariably leads to both volume and solute/toxin excess, it is difficult to say which of the two is the culprit. Whereas we did not study the mechanism by which missing dialysis can lead to sleep disturbances, it is possible that residual uremia may blunt the chemoreceptor sensitivity and cause central sleep apnea. It is equally likely that assumption of a supine posture may aggravate pharyngeal edema and lead to obstructive sleep apnea; this problem may be aggravated in those with volume excess. Some studies that have performed polysomnography and magnetic resonance imaging confirm this notion (4,14). Factors that are associated with shortened dialysis such as psychosocial or medical may also be associated with sleep apnea. Therefore, causality cannot be established in this study. However, given the fact that long-term dialysis patients dialyzed three times weekly have remarkable improvement in sleep disorders when switched to nocturnal dialysis suggests that a cause and effect relationship between uremia and sleep disorders may exist (3).
There are several strengths of our study. First, we measured sleep not only by questionnaires but also objectively using actigraphy. We demonstrate that actigraphy can serve as an attractive, noninvasive tool to study sleep and activity. Another portable tool to study sleep outside the sleep laboratory is nocturnal oximetry. Second, we followed these patients longitudinally to provide estimates of factors on individual level sleep estimates. There are, however, some limitations to our study. Although we objectively measured sleep and rest, we did not perform polysomnography in these patients, so we cannot ascertain the true prevalence of sleep disordered breathing. The sample size of the control group was relatively small; therefore the assessment of sleep in these individuals may be made with less precision. In contrast to approximately 1 week of recording among patients with CKD and normal controls, we had only 2 days of recordings among those on hemodialysis. Thus, estimates among hemodialysis patients may be less precise.
In conclusion, patients with CKD not on dialysis have some disruption of sleep. However, those on hemodialysis have major sleep disturbances. Among those on hemodialysis, missing dialysis is associated with greater burden of sleep disturbance. Thus, disrupted sleep may be a sign of undertreated uremia among dialysis patients. Because sleep disturbances (such as periodic limb movements during sleep) are associated with increased long-term mortality (15), it is possible that improving sleep may improve not only the well being of the patients but their survival. Therapeutic interventions aimed at decreasing sedentary behavior during the day are warranted to examine whether they also improve sleep quality. Further research investigating the relative roles of uremic toxin versus volume may reveal how we can improve sleep disturbances among patients on long-term dialysis.
Disclosures.
None.
Acknowledgments
Supported by grants from VA Merit Review and NIH 5RO1-062030-07.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Parker KP, Bliwise DL, Bailey JL, Rye DB: Polysomnographic measures of nocturnal sleep in patients on chronic, intermittent daytime haemodialysis vs those with chronic kidney disease. Nephrol Dial Transplant 20: 1422–1428, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Kurella M, Luan J, Lash JP, Chertow GM: Self-assessed sleep quality in chronic kidney disease. Int Urol Nephrol 37: 159–165, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Hanly PJ, Pierratos A: Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med 344: 102–107, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Beecroft JM, Hoffstein V, Pierratos A, Chan CT, McFarlane P, Hanly PJ: Nocturnal haemodialysis increases pharyngeal size in patients with sleep apnoea and end-stage renal disease. Nephrol Dial Transplant 23: 673–679, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Agarwal R, Light RP: The effect of measuring ambulatory blood pressure on nighttime sleep and daytime activity: Implications for dipping. Clin J Am Soc Nephrol 5: 281–285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iliescu EA, Yeates KE, Holland DC: Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transplant 19: 95–99, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Cohen SD, Patel SS, Khetpal P, Peterson RA, Kimmel PL: Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol 2: 919–925, 2007 [DOI] [PubMed] [Google Scholar]
- 8. De Santo RM, Bartiromo M, Cesare MC, Di Iorio BR: Sleeping disorders in early chronic kidney disease. Semin Nephrol 26: 64–67, 2006 [DOI] [PubMed] [Google Scholar]
- 9. De Santo RM, Bilancio G, Santoro D, Vecchi ML, Perna A, De Santo NG, Cirillo M: A longitudinal study of sleep disorders in early-stage chronic kidney disease. J Ren Nutr 20: S59–S63, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Chiu YL, Chuang YF, Fang KC, Liu SK, Chen HY, Yang JY, Pai MF, Peng YS, Wu KD, Tsai TJ: Higher systemic inflammation is associated with poorer sleep quality in stable haemodialysis patients. Nephrol Dial Transplant 24: 247–251, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Unruh ML, Buysse DJ, Dew MA, Evans IV, Wu AW, Fink NE, Powe NR, Meyer KB: Sleep quality and its correlates in the first year of dialysis. Clin J Am Soc Nephrol 1: 802–810, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Pressman MR, Benz RL, Schleifer CR, Peterson DD: Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int 43: 1134–1139, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Chami H, Budhiraja R, Punjabi NM, Buysse D, Newman AB: Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: Comparison with matched controls from the sleep heart health study. Am J Kidney Dis 52: 305–313, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang SC, Lam B, Lai AS, Pang CB, Tso WK, Khong PL, Ip MS, Lai KN: Improvement in sleep apnea during nocturnal peritoneal dialysis is associated with reduced airway congestion and better uremic clearance. Clin J Am Soc Nephrol 4: 410–418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benz RL, Pressman MR, Hovick ET, Peterson DD: Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis 35: 1052–1060, 2000 [DOI] [PubMed] [Google Scholar]