Abstract
Summary
Background and objectives
Children with IgA nephropathy showing diffuse (>80%) mesangial proliferation are at high risk for end-stage renal failure (ESRF). A previous controlled trial showed that combination therapy consisting of prednisolone, azathioprine, heparin-warfarin, and dipyridamole early in the course of disease reduces immunologic renal injury and prevents the progression of sclerosed glomeruli. The objective of this study was to evaluate the long-term effectiveness of combination therapy in children with IgA nephropathy showing diffuse mesangial proliferation.
Design, setting, participants, & measurements
A secondary analysis of a multicenter, randomized, controlled trial involving 78 children with IgA nephropathy who received either 2-year combination therapy or heparin-warfarin and dipyridamole (control) therapy was conducted.
Results
The median duration of observation was 10 years (range, 0.5 to 18). Two of 40 patients (5%) who received combination therapy and five of 34 patients (14.7%) who received control therapy developed ESRF. A Kaplan-Meier plot of renal survival showed that the outcomes of patients in the combined therapy group were better than those in the control therapy group (log-rank P = 0.03). The 10-year renal survival probability of each group was 97.1% (95% confidence interval, 81.4 to 99.6%) and 84.8% (95% confidence interval, 55.4 to 95.5%), respectively. The Cox proportional hazards model showed that the 2-year combination therapy was significantly associated with renal survival in both univariate and multivariate analyses.
Conclusions
Two-year combination therapy not only ameliorated the activity of the acute phase of nephritis but also improved the long-term outcome of severe childhood IgA nephropathy.
Introduction
IgA nephropathy is the most common variety of primary glomerulonephritis in the world today. Long-term follow-up studies have revealed that the disease progresses to renal failure in 20 to 50% of adult patients over 20 years (1). Variability in and severity of glomerular manifestations, as well as a long disease duration, have made it difficult to determine optimal treatments for patients with IgA nephropathy (1,2). A study of 241 Japanese pediatric patients revealed that 11% of the patients exhibited ESRF within 15 years (3). Children with diffuse (> 80%) mesangial proliferation on renal biopsy were reported to be at high risk for renal insufficiency, and 17% of these patients progressed to ESRF (4).
We previously conducted a multicenter randomized controlled trial (RCT) for childhood IgA nephropathy (5). We compared combination therapy consisting of prednisolone, azathioprine, heparin-warfarin, and dipyridamole with control therapy consisting of heparin-warfarin and dipyridamole for newly diagnosed childhood IgA nephropathy showing diffuse mesangial proliferation. In this trial, the 2-year combination therapy significantly reduced urinary protein excretion and improved pathologic findings such as crescents and mesangial proliferations without increasing the ratio of sclerosed glomeruli. In contrast, the control therapy did not reduce urinary protein excretion nor improve pathologic findings and led to an increase in the ratio of sclerosed glomeruli. We concluded that this 2-year combination therapy is a suitable treatment for children with IgA nephropathy showing diffuse mesangial proliferation. However, whether the combination therapy can improve the long-term prognosis compared with the control therapy remains uncertain. Here, we report the long-term outcome of patients who were included in this trial and elucidate the effectiveness of the combination therapy in childhood IgA nephropathy with diffuse mesangial proliferation.
Study Population and Methods
Patients
Originally, this study was a prospective controlled RCT involving 20 Japanese pediatric renal centers (the Japanese Pediatric IgA Nephropathy Treatment Study Group) (5). The study protocol was in accordance with the standards of the ethics committee at each center, and all of the patients' parents gave informed consent. The follow-up study protocol was approved by the regional research ethics vetting boards.
The entry and exclusion criteria of this trial have been reported in detail elsewhere (5). Briefly, between 1990 and 1993, 78 eligible children less than 15 years old with biopsy-proven IgA nephropathy showing diffuse mesangial proliferation were randomized to either the 2-year combination therapy (n = 40) or the control therapy (n = 38). The patients given the combination therapy received prednisolone, azathioprine, heparin-warfarin, and dipyridamole for 24 months. Prednisolone was given orally at a dose of 2 mg/kg per day (maximum, 80 mg/d) in three divided doses for 4 weeks, followed by 2 mg/kg given as a single dose in the morning of every other day for 4 weeks, 1.5 mg/kg every other day for 4 weeks, and 1 mg/kg every other day for 21 months. Azathioprine was given orally at a dose of 2 mg/kg per day as a single morning dose for 24 months. Heparin was given by continuous intravenous infusion in sufficient doses to keep the partial thromboplastin time at 60 seconds for 28 days. This was followed by oral warfarin given as a single morning dose to maintain the thrombotest at 30 to 50% for 23 months. Dipyridamole was given orally at a dose of 5 mg/kg per day (400 mg/d) in three divided doses for 24 months. The patients given the control therapy received only heparin-warfarin and dipyridamole for 24 months, and the treatment protocols for these medications were the same as those for the combination therapy. Diffuse mesangial proliferation was defined as >80% of glomeruli showing mesangial proliferation (more than three cells per peripheral mesangial area) on the basis of the World Health Organization criteria (6). Therapies used after the completion of the 2-year treatment periods were not restricted. We investigated the long-term outcome of children by reviewing hospital medical records or by telephone contact with the patients or their family members. The renal outcome at the last observation was graded as no proteinuria, mild proteinuria, heavy proteinuria, renal insufficiency, or ESRF.
Outcome Definitions
The primary endpoint was the development of ESRF that required renal replacement therapy. Renal insufficiency was defined as an estimated GFR (eGFR) of <60 ml/min per 1.73 m2. eGFR was calculated using the Schwartz formula (7) for less than 18 years of age and the Cockroft-Gault formula (8) for more than 18 years of age. Proteinuria was evaluated using the urinary protein amount per day (g/1.73 m2 per day) during the initial 2-year study period; after that, the early morning urinary protein/creatinine ratio (uP/Cr) was used. Heavy, mild, and no proteinuria were defined as ≥1.0, 0.2 to 1.0 and <0.1 g/1.73 m2 per day, or ≥1.0, 0.2 to 1.0 and <0.2 g/g, respectively.
Statistical Analyses
The data were analyzed with JMP version 8.0 (SAS Institute Japan Ltd., Tokyo, Japan). We used the Mann-Whitney U test for continuous variables and Fisher's exact test for categorical variables. Renal survival curves were calculated using the Kaplan-Meier method, and comparisons were made with a log-rank test. For multivariate analysis, we utilized the Cox proportional hazard model in a stepwise fashion, entering and eliminating variables with a P value of 0.20 in the model. Statistical significance was established at P < 0.05.
Results
Baseline Characteristics
The baseline characteristics of each group are shown in Table 1. The clinical and pathologic findings in the two groups were similar with the exception of urinary protein excretion, which was higher in the combination therapy group than in the heparin-warfarin and dipyridamole therapy group.
Table 1.
Clinical and pathological findings at the start of initial treatments
| Combination Therapy (n = 40) | Heparin-Warfarin and Dipyridamole Therapy (n = 38) | P | |
|---|---|---|---|
| Gender (Men/Women) | 22/18 | 29/9 | 0.06 |
| Age at diagnosis (years) | 12.2 ± 3.0 | 11.6 ± 2.3 | 0.10 |
| Initial presentation (Chance/Gross hematuria) | 34/6 | 30/8 | 0.56 |
| Creatinine clearance (ml/min per 1.73 m2) | 144 ± 52 | 152 ± 47 | 0.35 |
| Serum albumin (g/dl) | 3.73 ± 0.65 | 3.94 ± 0.59 | 0.36 |
| Serum cholesterol (mg/dl) | 210 ± 116 | 194 ± 47 | 0.13 |
| Serum IgA (mg/dl) | 290 ± 115 | 276 ± 98 | 0.75 |
| Urinary protein excretion (g/1.73 m2/day) | 2.09 ± 1.78 | 1.35 ± 1.26 | 0.02 |
| Hematuriaa | 2.9 ± 0.8 | 2.6 ± 0.9 | 0.16 |
| Glomeruli | |||
| showing sclerosis (%) | 5.7 ± 8.6 | 4.1 ± 5.8 | 0.65 |
| showing crescents (%) | 23.6 ± 20.7 | 21.2 ± 17.9 | 0.68 |
| showing capsular adhesions (%) | 9.6 ± 10.0 | 7.0 ± 7.1 | 0.40 |
| showing mesangial proliferations (%) | 91.7 ± 7.4 | 90.2 ± 7.0 | 0.11 |
| Intensity of mesangial IgA depositsb | 2.2 ± 0.6 | 2.3 ± 0.5 | 0.72 |
The plus-minus values are the means ± SD.
Hematuria was quantified using dipsticks, and macrohematuria was quantified as 4.
The intensity of deposits on immunofluorescence microscopy was graded semiquantitatively on a scale from 0 to 3+: 0, no; 1+, slight; 2+, moderate; 3+, intense.
Clinical and pathologic data at the end of the initial 2-year treatments are shown in Table 2. Four patients in the heparin-warfarin and dipyridamole therapy group were lost to follow-up during the 2-year study period. The mean 24-hour urinary protein excretion and the ratio of patients with heavy proteinuria (≥1.0 g/1.73 m2 per day) were significantly lower in the combination therapy group than in the heparin-warfarin and dipyridamole therapy group. On the other hand, the ratio of patients with no proteinuria (<0.2 g/1.73 m2 per day) was significantly higher in the combination therapy group. The value of serum IgA and the intensity of hematuria were significantly lower in the combination therapy group. In terms of pathologic findings, the mean percentages of glomeruli showing global sclerosis and mesangial proliferation were lower in the combination therapy group than in the heparin-warfarin and dipyridamole therapy group, although this was not statistically significant. The intensity of mesangial IgA deposits was significantly lower in the combination therapy group than in the control group.
Table 2.
Clinical and pathological findings of at the end of initial 2-year treatments
| Combination Therapy (n = 40) | Heparin-Warfarin and Dipyridamole Therapy (n = 34) | P | |
|---|---|---|---|
| Creatinine clearance (ml/min per 1.73 m2) | 147 ± 33 | 145 ± 44 | 0.89 |
| Serum IgA (mg/dl) | 229 ± 87 | 281 ± 92 | 0.03 |
| Urinary protein excretion (g/1.73 m2 per day) | 0.28 ± 0.36 | 1.07 ± 1.57 | 0.01 |
| Patients with heavy proteinuria (≥1.0 g/1.73 m2 per day) | 2 (5.0%) | 10 (23.5%) | 0.009 |
| Patient without proteinuria (<0.2 g/1.73 m2 per day) | 20 (50.0%) | 6 (17.6%) | 0.007 |
| Hematuriaa | 0.5 ± 1.0 | 1.5 ± 1.1 | 0.0002 |
| Glomeruli | |||
| showing sclerosis (%) | 5.0 ± 6.9 | 16.4 ± 23.0 | 0.07 |
| showing crescents (%) | 0.4 ± 1.1 | 4.4 ± 10.3 | 0.16 |
| showing capsular adhesions (%) | 8.1 ± 11.2 | 6.4 ± 10.4 | 0.86 |
| showing mesangial proliferations (%) | 30.7 ± 26.7 | 57.5 ± 36.4 | 0.07 |
| Intensity of mesangial IgA depositsb | 1.3 ± 1.1 | 2.2 ± 0.6 | 0.02 |
The plus-minus values are the means ± SD.
Hematuria was quantified using dipsticks, and macrohematuria was quantified as 4.
The intensity of deposits on immunofluorescence microscopy was graded semiquantitatively on a scale from 0 to 3+: 0, no; 1+, slight; 2+, moderate; 3+, intense.
The treatments used after the initial 2-year study period are shown in Table 3. Because they were not controlled, there were some differences between the two groups. On the basis of the outcome of this RCT, more patients received the combination therapy in the heparin-warfarin and dipyridamole therapy group than in the combination therapy group. On the other hand, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), which were restricted in the initial 2-year study period, were more often used in the combination therapy group than in the control group.
Table 3.
Treatments used after the initial 2-year study period
| Combination Therapy (n = 40) | Heparin-Warfarin and Dipyridamole Therapy (n = 34) | |
|---|---|---|
| Combination | ||
| with ACEI or ARB | 0 (0.0%) | 2 (5.9%) |
| without ACEI or ARB | 0 (0.0%) | 5 (14.7%) |
| Steroid and immunosuppressant | ||
| with ACEI or ARB | 3 (7.5%) | 0 (0.0%) |
| without ACEI or ARB | 0 (0.0%) | 1 (2.9%) |
| Steroid | ||
| with ACEI or ARB | 6 (15.0%) | 1 (2.9%) |
| without ACEI or ARB | 1 (2.5%) | 0 (0.0%) |
| ACEI or ARB (partly with anti-platelets and/or warfarin and/or Sairei-to) | 11 (27.5%) | 3 (8.8%) |
| Anti-platelets and/or warfarin and/or Sairei-to | 6 (15.0%) | 4 (11.8%) |
| Warfarin alone | 0 (0.0%) | 1 (2.9%) |
| Sairei-to alone | 6 (15.0%) | 4 (11.8%) |
| No treatment | 7 (17.5%) | 9 (26.5%) |
| Unknown | 0 (0.0%) | 4 (11.8%) |
Combination treatment included prednisolone, azathioprine, heparin-warfarin, and dipyridamole. The immunosuppressant used was azathioprine or mizoribine. Sairei-to is a Chinese herbal medicine.
Renal Survival
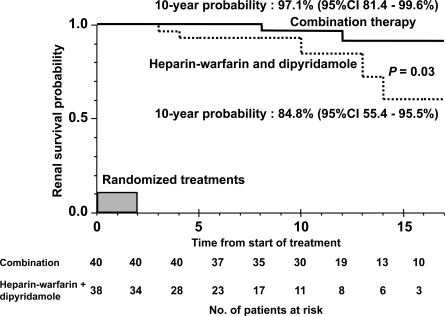
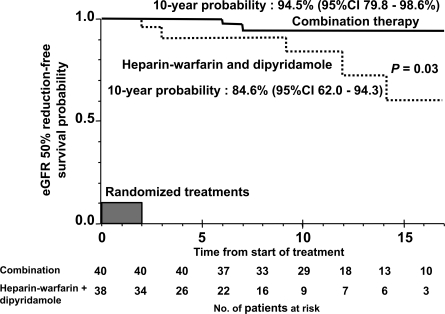
The median duration of observation was 10 years (range, 0.5 to 18) for the total study population, 11.5 years (range, 4 to 18) for the combination therapy group, and 7 years (range, 0.5 to 17) in the heparin-warfarin and dipyridamole therapy group. The outcome in each group at the last observation is shown in Table 4. Two of 40 patients (5%) who received combination therapy and five of 34 patients (14.7%) who received heparin-warfarin and dipyridamole therapy developed ESRF. Figure 1 shows a comparison of probabilities for renal survival between the two groups. The probability for renal survival in the combination therapy group was significantly better than that of the dipyridamole and heparin-warfarin therapy group (P = 0.03); the 10-year renal survival probability of each group was 97.1% (95% confidence interval [CI], 81.4 to 99.6%) and 84.8% (95% CI, 55.4 to 95.5%), respectively. We also analyzed baseline (at diagnosis) eGFR 50% reduction-free survival (Figure 2). The results were similar to the renal survival probability.
Table 4.
Renal outcome at the last observation
| Combination Therapy (n = 40) | Heparin-Warfarin and Dipyridamole Therapy (n = 34) | |
|---|---|---|
| No proteinuria (uP/Cr <0.2 g/g) | 24 (60.0%) | 18 (52.9%) |
| Mild proteinuria (≤0.2 uP/Cr <1.0 g/g) | 12 (30.0%) | 6 (17.6%) |
| Heavy proteinuria (uP/Cr ≥1.0 g/g) | 1 (2.5%) | 4 (11.8%) |
| Renal insufficiency (eGFR <60 ml/min per 1.73 m2) | 1 (2.5%) | 1 (2.9%) |
| ESRF (requiring renal replacement therapy) | 2 (5.0%) | 5 (14.7%) |
Figure 1.
Kaplan-Meier plot of renal survival stratified by the groups of initial 2-year treatments in children with IgA nephropathy showing diffuse mesangial proliferation.
Figure 2.
Kaplan-Meier plot of eGFR 50% reduction-free survival stratified by the groups of initial 2-year treatments in children with IgA nephropathy showing diffuse mesangial proliferation.
Predicted Risk for ESRF
We evaluated predicted risk factors using univariate and multivariate analyses using the Cox proportional hazard model for ESRF. The initial 2-year treatments (combination or control) and ratios of glomeruli showing global sclerosis at diagnosis were included as significant risk factors in both the univariate and multivariate analyses for ESRF (Table 5). The urinary protein excretion at diagnosis was significantly included as a risk factor in the multivariate analysis for ESRF (Table 5). The ratios of glomeruli showing crescents and mesangial proliferations at diagnosis were not selected as significant risk factors for ESRF with a multivariate Cox model in a stepwise fashion.
Table 5.
Predicted risk factors for ESRF
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Initial treatment (combination or control) | 0.2 | 0.0 to 0.9 | 0.03 | 0.03 | 0.0 to 0.3 | 0.002 |
| Urinary protein excretion at diagnosis (g/1.73 m2 per day) | 2.3 | 1.0 to 5.0 | 0.06 | 2.7 | 1.1 to 6.5 | 0.03 |
| Glomeruli showing global sclerosis at diagnosis (%) | 1.1 | 1.0 to 1.1 | 0.04 | 1.1 | 1.0 to 1.2 | 0.02 |
HR, hazard ratio.
Proteinuria at the End of the Initial 2-Year Treatments and Outcome
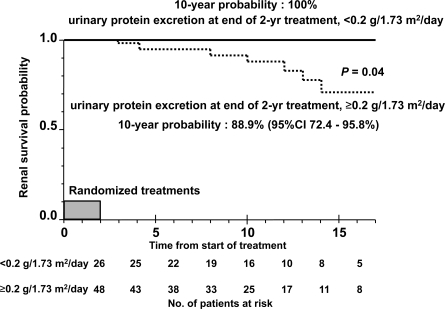
Because the importance of a change in proteinuria with treatment has been emphasized in IgA nephropathy, we compared the long-term outcomes of patients with proteinuria at the end of the initial 2-year treatment with those without proteinuria. The probability of renal survival in patients with proteinuria was significantly worse than in those without proteinuria (P = 0.04). The 10-year renal survival probability after the start of initial treatment in each group was 88.9% (95% CI, 72.4 to 95.8%) and 100%, respectively (Figure 3).
Figure 3.
Kaplan-Meier plot of renal survival stratified by urinary protein excretion at the end of initial 2-year treatments in children with IgA nephropathy showing diffuse mesangial proliferation. Normal urinary protein excretion is defined as less than 0.2 g/1.73 m2 per day.
Discussion
Our previous two RCTs and clinical trial showed the short-term effectiveness of combination therapy in childhood IgA nephropathy (5,9,10). To the best of our knowledge, no well-designed RCTs of childhood IgA nephropathy have been performed with the exception of those that we conducted. However, the long-term effectiveness of combination therapy has not been determined. This study is the first long-term follow-up study of such a RCT. This prolonged follow-up study of a previous RCT for children with IgA nephropathy showing diffuse mesangial proliferation demonstrated a significant superiority in renal survival in patients who received combination therapy compared with those who received control therapy. Because of the variable rate of progression to renal failure and the probable multifactorial pathogenesis of IgA nephropathy (11), it is desirable to evaluate the effectiveness of any IgA nephropathy treatment by a prospective controlled trial. However, although the ultimate endpoint in any clinical trial of progressive IgA nephropathy is the development of chronic renal insufficiency, most pediatric patients do not develop chronic renal insufficiency during the study period (12). Therefore, the data in this study provide us with unique and valuable information about IgA nephropathy in children.
Kaplan-Meier analysis, the log-rank test, and the multivariate Cox model clearly indicated the significant long-term effectiveness of the combination therapy in this study. Even though this was a prospective observational study, factors after the initial RCT period were difficult to control between the two groups. Therefore, proper analyses of long-term follow-up data are important to evaluate disease outcome and treatment effectiveness (13,14). Valid statistical evaluation using a multivariate Cox model was thought to work effectively in this study.
This study also reveals the importance of a change in proteinuria in IgA nephropathy with the 2-year treatment. We observed an 87% reduction in proteinuria from baseline in the combination therapy group compared with only a 21% reduction in the dipyridamole and heparin-warfarin group at the end of the initial 2-year treatments. The proportion of patients without proteinuria was significantly high, whereas the proportion with heavy proteinuria was significantly low at the end of the initial 2-year treatment in the combination group. The probability of renal survival in patients without proteinuria at the end of the initial 2-year treatment was 100% during the entire long-term follow-up period. These findings are likely to be pathophysiological grounds for the long-term outcome in this study. Severe proteinuria at diagnosis and/or during follow-up is a strong predictor in adult IgA nephropathy according to the most accurate studies of the literature (13). Our previous study also supported this finding, even in child IgA nephropathy (4). Interestingly, a recent review reported that mean proteinuria during follow-up was a powerful independent prognostic predictor, but proteinuria at baseline was not significantly related to renal progression (15). Actually, in a substantial number of reports, presenting proteinuria has not been found to be a significant long-term predictor (14,16–19). Coppo et al. (17) found that presenting proteinuria was NS as an independent prognostic value in their trial when examined by a multivariate Cox model. Our recent analysis in 500 children with IgA nephropathy supported this result (14). There is a possibility that the proteinuria level at diagnosis can be improved by appropriate treatments; thus, it may not be an absolute prognostic factor.
Corticosteroids have been widely used to treat moderate to severe IgA nephropathy. The current cumulative evidence suggests that corticosteroids have statistically significant effects on protecting renal function and reducing proteinuria in adult patients with IgA nephropathy (20–28). In a notable study, an Italian prospective RCT demonstrated that a 6-month course of steroid treatment protected against deterioration of renal function during follow-up (20). The long-term follow-up data of the trial showed that corticosteroids significantly reduced proteinuria and protected against renal function deterioration (21). This study has provided further evidence for the use of corticosteroids in childhood IgA nephropathy.
The role of immunosuppressive drugs in the treatment of IgA nephropathy remains controversial. Goumenos et al. (29) retrospectively reported that treatment with prednisolone and azathioprine appeared to be beneficial in slowing the progression of severe adult IgA nephropathy. We conducted another multicenter prospective RCT comparing combination therapy with prednisolone monotherapy for severe childhood IgA nephropathy (9). The results of this study showed that patients who received prednisolone monotherapy had more sclerosed glomeruli than did those who received combination therapy, although proteinuria was reduced in both groups. We believe that immunosuppressants play an important role in combination therapy, as do corticosteroids, especially in preventing the progression of glomerular sclerosis, which was a significant outcome factor in the present follow-up study.
The natural course of childhood IgA nephropathy showing diffuse mesangial proliferation was reported to be 83% of renal survival at 5 years (4). The disease course of the dipyridamole and heparin-warfarin therapy group was almost identical to the natural course of childhood IgA nephropathy showing diffuse mesangial proliferation. We believe that the 2-year combination therapy decreased protein excretion, suppressed acute inflammation of glomeruli, reduced IgA production, and minimized the abnormal immune response after IgA deposition, which contributed to the prevention of glomerular sclerosis and the preservation of long-term renal survival (5,30).
A limitation of this study was the impossibility of treatment control after the initial 2-year study period. On the basis of the short-term outcome of this RCT, more patients received the combination therapy in the control therapy group than in the combination therapy group. On the other hand, because the use of ACEIs, which was restricted in the initial 2-year study period, has become clearly effective even in childhood IgA nephropathy (31), there was a tendency toward using it more often in the combination therapy group than in the control group. This may have affected the long-term outcome in this study. In particular, the former may have reduced the difference in long-term outcome between the two groups. However, such a situation seems to be ethically justifiable.
In our experience renal function is reversible in eGFR of 89 to 60 ml/min per 1.73 m2, whereas it is irreversible in eGFR of <60 ml/min per 1.73 m2 in children with IgA nephropathy (4). Therefore, we have used eGFR of <60 ml/min per 1.73 m2 as a definition for renal insufficiency.
Conclusions
In conclusion, the 2-year combination therapy consisting of prednisolone, azathioprine, dipyridamole, and heparin-warfarin not only ameliorated the acute phase of nephritis, it also improved the long-term prognosis of childhood IgA nephropathy with diffuse mesangial proliferation. The long-term effectiveness of the combination therapy seems to be based on the reduction of urinary protein excretion and the suppression of glomerular sclerosis progression.
Disclosures
None.
Acknowledgments
The authors thank all participants and attending physicians for their contributions.
The Japanese Pediatric IgA Nephropathy Treatment Study Group Coordinating committee: H. Ito (Tokyo), N. Yoshikawa (Wakayama). Statistics committee: T. Kawamura (Nagoya), M. Saito (Tokyo). Investigators: S. Sasaki, Y. Takekoshi, H. Tochimaru, S. Hoshii (Sapporo); Y. Kondo (Sendai); Y. Ikezumi (Niigata); Y. Owada (Tochigi); K. Tamura (Mito); K. Kawamura (Sakura); T. Igarashi, F. Niimura, M. Awazu, M. Honda, M. Ikeda, H. Hataya, K. Ishikura, S. Ito (Tokyo); K. Nakanishi (Wakayama); K. Yamaoka, K. Nakagawa, (Osaka); H. Kamituji, M. Nakajima (Nara); K. Iijima, R. Tanaka, K. Nozu (Kobe); T. Hisano, Y. Kaku (Fukuoka); S. Hattori, H. Nakazato, A. Furuse (Kumamoto); M. Ninomiya (Kagoshima); Y. Otsuka (Saga).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Appel GB, Waldman M: The IgA nephropathy treatment dilemma. Kidney Int 69: 1939–1944, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Barratt J, Feehally J: Treatment of IgA nephropathy. Kidney Int 69: 1934–1938, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Yoshikawa N, Tanaka R, Iijima K: Pathophysiology and treatment of IgA nephropathy in children. Pediatr Nephrol 16: 446–457, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Yoshikawa N, Ito H, Nakamura H: Prognostic indicators in childhood IgA nephropathy. Nephron 60: 60–67, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Yoshikawa N, Ito H, Sakai T, Takekoshi Y, Honda M, Awazu M, Ito K, Iitaka K, Koitabashi Y, Yamaoka K, Nakagawa K, Nakamura H, Matsuyama S, Seino Y, Takeda N, Hattori S, Ninomiya M. for the Japanese Pediatric IgA Nephropathy Treatment Group: A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. J Am Soc Nephrol 10: 101–109, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Churg J, Bernstein J, Glassock RJ: Renal Disease: Classification and Atlas of Glomerular Diseases, 2nd Ed., Tokyo, Japan, Igakushoin Medical Publishers, 1995, pp. 86–88 [Google Scholar]
- 7. Schwartz GJ, Haycock GB, Edelmann CM, Jr., Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 8. Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 9. Yoshikawa N, Honda M, Iijima K, Awazu M, Hattori S, Nakanishi K, Ito H. for the Japanese Pediatric IgA Nephropathy Treatment Study Group: Steroid treatment for severe childhood IgA nephropathy: A randomized controlled trial. Clin J Am Soc Nephrol 1: 511–517, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Yoshikawa N, Nakanishi K, Ishikura K, Hataya H, Iijima K, Honda M, Japanese Pediatric IgA Nephropathy Treatment Study Group: Combination therapy with mizoribine for severe childhood IgA nephropathy: A pilot study. Pediatr Nephrol 23: 757–763, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Nakanishi K, Yoshikawa N: Immunoglobulin A nephropathy. In: Pediatric Nephrology, 6th Ed., edited by Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Heidelberg, New York, Springer, pp. 757–781, 2009 [Google Scholar]
- 12. Wyatt RJ, Hogg RJ: Evidence-based assessment of treatment options for children with IgA nephropathies. Pediatr Nephrol 16: 156–167, 2001 [DOI] [PubMed] [Google Scholar]
- 13. D'Amico G: Natural History of Idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Yata N, Nakanishi K, Shima Y, Togawa H, Obana M, Sako M, Nozu K, Tanaka R, Iijima K, Yoshikawa N: Improved renal survival in Japanese children with IgA nephropathy. Pediatr Nephrol 23: 905–912, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coppo R, D'Amico G: Factors predicting progression of IgA nephropathies. J Nephrol 18: 503–512, 2005 [PubMed] [Google Scholar]
- 16. Praga M, Gutiérrez E, González E, Morales E, Hernández E: Treatment of IgA nephropathy with ACE inhibitors: A randomized and controlled trial. J Am Soc Nephrol 14: 1578–1583, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Coppo R, Peruzzi L, Amore A, Piccoli A, Cochat P, Stone R, Kirschstein M, Linné T: IgACE: A placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol 18: 1880–1888, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Rauta V, Finne P, Fagerudd J, Rosenlof K, Tornroth T, Gronhagen-Riska C: Factors associated with progression of IgA nephropathy are related to renal function: A model for estimating risk of progression in mild disease. Clin Nephrol 58: 85–94, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Bartosik LP, Lajoie G, Sugar L, Cattran DC: Predicting progression in IgA nephropathy. Am J Kidney Dis 38: 728–735, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, Locatelli F: Corticosteroids in IgA nephropathy: A randomized controlled trial. Lancet 353: 883–887, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Manno C, Torres DD, Rossini M, Pesce F, Schena FP: Randomized controlled clinical trial of corticosteroids plus ACE inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 24: 3694–3701, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H: Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 53: 26–32, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Julian BA, Barker C: Alternate-day prednisone therapy in IgA nephropathy. Preliminary analysis of a prospective, randomized, controlled trial. Contrib Nephrol 104: 198–206, 1993 [PubMed] [Google Scholar]
- 25. Lai KN, Lai FM, Ho CP, Chan KW: Corticosteroid therapy in IgA nephropathy with nephrotic syndrome: A long-term controlled trial. Clin Nephrol 26: 174–180, 1986 [PubMed] [Google Scholar]
- 26. Katafuchi R, Ikeda K, Mizumasa T, Tanaka H, Ando T, Yanase T, Masutani K, Kubo M, Fujimi S: Controlled, prospective trial of steroid treatment in IgA nephropathy: A limitation of low-dose prednisolone therapy. Am J Kidney Dis 41: 972–983, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Shoji T, Nakanishi I, Suzuki A, Hayashi T, Togawa M, Okada N, Imai E, Hori M, Tsubakihara Y: Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am J Kidney Dis 35: 194–201, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Cheng J, Zhang X, Zhang W, He Q, Tao X, Chen J: Efficacy and safety of glucocorticoids therapy for IgA nephropathy: A meta-analysis of randomized controlled trials. Am J Nephrol 30: 315–322, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Goumenos D, Ahuja M, Shortland JR, Brown CB: Can immunosuppressive drugs slow the progression of IgA nephropathy? Nephrol Dial Transplant 10: 1173–1181, 1995 [PubMed] [Google Scholar]
- 30. Shima Y, Nakanishi K, Kamei K, Togawa H, Nozu K, Tanaka R, Sasaki S, Iijima K, Yoshikawa N: Disappearance of glomerular IgA deposits in childhood IgA nephropathy showing diffuse mesangial proliferation after 2 years of combination/prednisolone therapy. Nephrol Dial Transplant 26: 163–169, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Nakanishi K, Iijima K, Ishikura K, Hataya H, Awazu M, Sako M, Honda M, Yoshikawa N, The Japanese Pediatric IgA Nephropathy Treatment Study Group: Efficacy and safety of lisinopril for mild childhood IgA nephropathy: A pilot study. Pediatr Nephrol 24: 845–849, 2009 [DOI] [PubMed] [Google Scholar]