Abstract
Summary
Background and objectives
There are still controversies whether peritoneal dialysis (PD) with icodextrin preserves residual renal and peritoneal membrane functions in patients with diabetes. However, there are no randomized controlled and long-term clinical trials in newly started PD patients with diabetic nephropathy.
Design, setting, participants, & measurements
Forty-one patients with diabetic nephropathy with ESRD were enrolled and randomly assigned to the glucose group (GLU) treated with 8 L of 1.5% or 2.5% glucose or an icodextrin group (ICO) treated with 1.5 or 2.0 L of 7.5% icodextrin-containing solutions. Technique failure, body fluid management, glucose and lipid metabolism, and residual renal and peritoneal functions and were evaluated over 2 years.
Results
The technique survival rate was 71.4% in ICO and 45.0% in GLU, with most of the technique failure due to volume overload. ICO showed significantly better cumulative technique survival. Net ultrafiltration volume was significantly higher in ICO throughout the study period. There were no beneficial effects of icodextrin on hemoglobin A1c, glycoalbumin, and lipid profile at 24 months. Urine volume and residual renal function declined faster in ICO, but there were no significant differences between the two groups. For peritoneal function, no differences were observed in dialysis-to-plasma creatinine ratios during the observation.
Conclusions
In PD therapy for diabetic nephropathy, the use of icodextrin-containing solutions has a beneficial effect on technique survival, but there are no apparent benefits or disadvantages in residual renal and peritoneal functions compared with conventional PD with glucose solution.
Introduction
Peritoneal dialysis (PD) has become the major alternative modality to hemodialysis (HD) through the continuous technical improvements since its introduction in 1975. Indeed, PD provides several advantages over HD for ESRD patients with diabetes (1). Both dialysis modalities are suitable for most patients with diabetes; however, the application of PD is not the first-line therapy in many countries, including Japan (2), where PD had only a 3.4% penetration rate at the end of 2009. In the 1990s, the advent of a glucose polymer (i.e., icodextrin) in Europe as a replacement for the traditional glucose in PD solutions enabled the long dwell and sustained ultrafiltration (UF) in continuous ambulatory PD and automated PD (3,4). The effectiveness of icodextrin as an osmotic agent has been demonstrated because the gradient can be maintained at adequate UF values for 8 to 12 hours (5).
Glucose-based PD solutions have been linked to many adverse metabolic consequences of systemic glucose absorption, including hyperglycemia, hyperinsulinemia, hyperlipidemia, obesity, and suppressed appetite. Icodextrin-based solutions are used in PD for better UF and to decrease peritoneal glucose exposure (3,6). Icodextrin may benefit patients with diabetes by facilitating the achievement of better glycemic control and blunting the hyperinsulinemic response to carbohydrate absorption. In such a group of patients, icodextrin has been reported to improve BP (7), delayed gastric emptying (8), and quality of life (9).
Fluid overload is another common problem in ESRD patients, especially in PD patients with diabetes. Fluid overload may be associated with a higher prevalence of cardiovascular complications and the subsequent withdrawal from PD therapy. Icodextrin solutions alter the body fluid overload by reducing the extracellular fluid volume in patients on PD. ESRD patients with diabetes on continuous cyclic PD achieved better UF with icodextrin than nondiabetic patients (10). In addition to advantages in UF, residual renal function (RRF) was preserved and superior to the conventional glucose solution at 6 months (9) and 1 year (11) in randomized controlled trials (RCTs) of nondiabetic ESRD patients. In addition, the use of icodextrin for 2 years preserved UF capacity and the dialysis-to-plasma (D/P) creatinine ratio in nondiabetic ESRD patients (9).
Thus, these clinical observations suggest the superiority of icodextrin in comparison to glucose solution, but no randomized controlled and long-term clinical trials of icodextrin have been performed in incident or newly started PD patients with diabetes. To measure the beneficial effects of icodextrin on technique failure, body fluid management, glucose and lipid metabolism, and residual renal and peritoneal functions of PD therapy, RCTs were designed and performed in the patients with diabetic nephropathy who were newly introduced to PD therapy.
Materials and Methods
Patients and Study Design
We conducted this prospective, randomized, controlled, open-label multicenter clinical trial at Okayama University Hospital and 22 affiliate hospitals. Patients were eligible and included in the study if they were at end-stage renal failure because of diabetic nephropathy and were newly started on PD (continuous ambulatory and automatic PD) as a first renal replacement therapy. The diagnosis of diabetic nephropathy was made based on the diagnosis of type 1 or type 2 diabetes >10 years ago confirmed by medical records and the presence of current diabetic retinopathy. Exclusion criteria were age <18 years or >80 years; urine volume <400 ml/d; urinary tract obstruction due to neoplasm; neurogenic bladder; pregnancy; and previous renal replacement therapies including PD, HD, and renal transplantation.
Study Protocol
A total of 41 new PD patients from Okayama University Hospital and its affiliate hospitals were enrolled from May 2005 to April 2007. They were randomly assigned to a control group (GLU; n = 20, 13 men and 7 women) treated with a maximum of 8 L of daily 1.5% or 2.5% Dianeal PD-2 or PD-4 (Baxter) or an icodextrin group (ICO; n = 21, 14 men and 7 women) treated with a maximum of 6 L of daily 1.5% or 2.5% Dianeal PD-2 or PD-4 in association with an overnight or daytime dwell of 2 or 1.5 L of 7.5% icodextrin-containing solution (Extraneal, Baxter). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and approved by the Institutional Review Board of Okayama University Hospital (#050302) and by all of the appropriate institutional review boards. The protocol was registered in the University hospital Medical Information Network Clinical Trials Registry (ID: UMIN000001040). All patients gave written, informed consent to participate in the study.
Clinical Efficacy and Outcomes
Primary outcome was the rate at 2 years of PD technical survival. The PD technical survival is defined by the discontinuation of PD therapy because of volume overload for >4 weeks under the use of 2.5% Dianeal and fulfilled the following criteria: the elevation of systolic BP >40 mmHg or diastolic BP >20 mmHg, >3 kg increase in body weight within 2 weeks, intractable generalized edema, and >8% increase in cardiothoracic ratio (CTR). Secondary outcomes were rate of decline in RRF and status of lipid and glucose metabolism. Clinical indices including markers of body fluid status such as body weight, BP, CTR, and UF volume were examined. Metabolic profiles were investigated by serum levels of hemoglobin A1c, glycoalbumin, LDL-cholesterol, HDL-cholesterol, and triglycerides. RRF was measured with daily urine volume, renal creatinine clearance, and weekly Kt/V that were measured at 1 (baseline), 3, 6, 12, 18, and 24 months from the initiation of PD. Plasma human atrial natriuretic peptide (ANP) levels were measured at 1, 3, 12, and 24 months. A peritoneal equilibration test (PET) was performed at 1, 12, and 24 months. D/P creatinine was evaluated at 4 hours (12). Weekly Kt/V urea was calculated from a 24-hour collection of dialysate and urine. The RRF was calculated as the average of residual renal creatinine and urea clearances.
Total Glucose Exposure and PET
The total daily exposure to glucose was calculated from the dialysis regime. The product of the volume and glucose concentration for each exchange was calculated. For example, for an individual who was using 6 L of daily exchanges (4 L × 1.5% and 2 L × 2.5%), total glucose exposure would be 60 + 50 = 110 g/d. A standard PET was used to measure peritoneal transport status (12). Briefly, a standard 4-hour dwell period was used with a 2.5% glucose concentration and 2-L volume exchange. D/P creatinine was measured at the completion of the 4-hour dwell period.
Statistical Analyses
Data are presented as mean and SD for continuous variables and as proportions for categorical variables. Poisson models were used to analyze hospitalizations and peritonitis. Statistical analysis was made with PASW Statistics 18 (SPSS, Inc., Chicago, IL). PD technique survival curves, survival probabilities, and estimated mean survival times were generated according to the Kaplan–Meier method combined with between-group comparison using the log rank test. The changes over time were compared using a paired t test and the differences between the groups were compared using an independent t test and Fisher's exact test. Values were presented as mean ± SD, and P values below 0.05 were considered significant.
Results
Baseline Characteristics
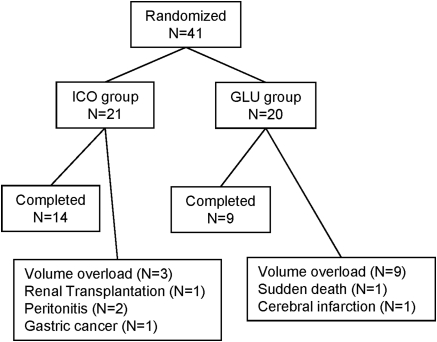
Forty-one patients were enrolled in the study: 21 patients were randomized to ICO and 20 were randomized to GLU (Figure 1). There were no differences in gender (7 of 21 were women [33%] versus 7 of 20 [35%]) or age (55.9 ± 11.16 versus 56.5 ± 9.86 years) between ICO and GLU, respectively. At baseline, both groups were similar in all of the analyzed variables, including demographic and clinical characteristics, comorbidities, laboratory measurements, and dialysis efficacy (Table 1). The patients were categorized by PET into four groups: high (H, n = 3), high-average (HA, n = 10), low average (LA, n = 6), and low transporters (L, n = 2) in ICO; and H (n = 1), HA (n = 9), LA (n = 7), and L (n = 3) in GLU.
Figure 1.
The flow of study subjects in the RCT.
Table 1.
Baseline characteristics of patients
| Characteristic | ICO | GLU | P |
|---|---|---|---|
| Number of patients | 21 | 20 | – |
| Men:women | 14:7 | 13:7 | 0.91 |
| Age (years) | 55.90 ± 11.16 | 56.50 ± 9.86 | 0.86 |
| Body weight (kg) | 67.46 ± 11.55 | 63.02 ± 9.86 | 0.19 |
| Systolic BP (mmHg) | 156.95 ± 21.36 | 147.60 ± 25.78 | 0.21 |
| Diastolic BP (mmHg) | 82.67 ± 13.67 | 82.80 ± 13.38 | 0.97 |
| CTR (%) | 53.26 ± 6.67 | 50.33 ± 4.34 | 0.11 |
| Urine volume (ml/day) | 1169.42 ± 451.85 | 1039.35 ± 472.54 | 0.34 |
| ANP (pg/ml) | 74.58 ± 44.19 | 66.72 ± 50.91 | 0.61 |
| Renal creatinine clearance (ml/min) | 7.04 ± 3.05 | 8.52 ± 5.17 | 0.31 |
| D/P creatinine | 0.66 ± 0.13 | 0.62 ± 0.13 | 0.30 |
| Blood urea nitrogen (mg/dl) | 62.89 ± 16.83 | 66.41 ± 20.28 | 0.55 |
| Creatinine (mg/dl) | 8.13 ± 1.86 | 7.64 ± 1.84 | 0.40 |
| Albumin (g/dl) | 3.14 ± 0.49 | 3.03 ± 0.73 | 0.58 |
| Hemoglobin A1c (%) | 5.72 ± 0.75 | 5.67 ± 1.07 | 0.87 |
| Glycoalbumin (%) | 18.64 ± 4.72 | 17.20 ± 4.57 | 0.33 |
| Total cholesterol (mg/dl) | 181.90 ± 45.28 | 189.35 ± 56.59 | 0.64 |
| LDL-cholesterol (mg/dl) | 112.39 ± 33.95 | 108.99 ± 42.27 | 0.79 |
| HDL-cholesterol (mg/dl) | 40.33 ± 14.40 | 46.46 ± 14.64 | 0.20 |
Adverse Events and Outcomes
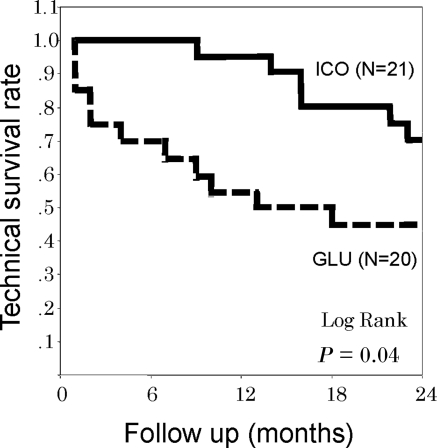
At the 24-month follow-up, the technique survival rate was 71.4% in ICO and 45.0% in GLU. ICO showed significantly better cumulative technique survival (log-rank test, P = 0.0365) (Figure 2). For the reason of discontinuation of PD therapy, 9 of 11 patients in GLU and 3 of 6 patients in ICO withdrew because of uncontrolled fluid volume excess caused by insufficient UF. They were switched to other renal replacement therapies, such as HD and renal transplantation. For adverse events, recurrent peritonitis and gastric cancer were observed in ICO and sudden death of unknown cause and cerebral infarction were observed in GLU (Figure 1).
Figure 2.
Cumulative technique survival curves in ICO and GLU.
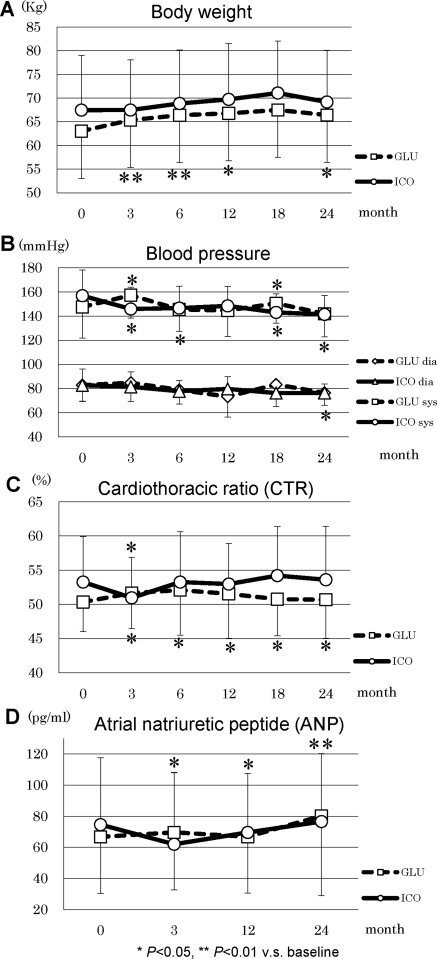
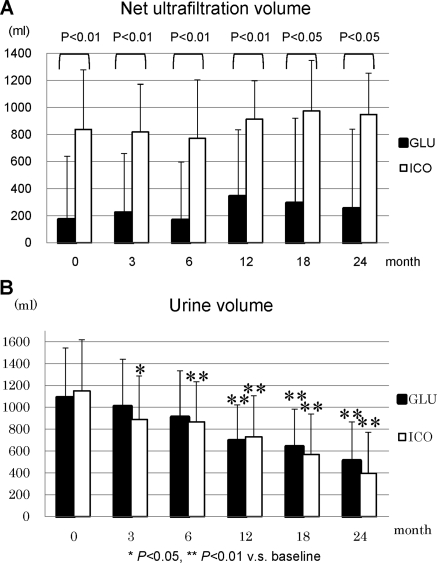
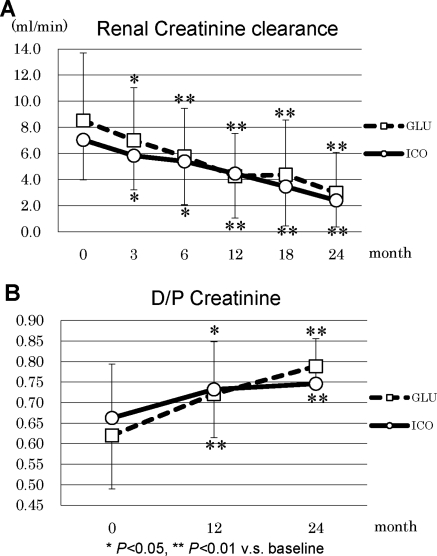
During the study period, the patients in GLU had incremental changes in body weight, whereas body weight was unaltered in ICO (Figure 3A). BP was well controlled in ICO during the observation period, and ICO demonstrated significantly lower BP (141.4 ± 15.6 mmHg) at the end of the study compared with baseline (157.0 ± 21.4 mmHg, P = 0.012) (Figure 3B). Dosages of antihypertensive agents were increased in 65% of GLU and 33% of ICO patients, whereas they were reduced in 5% of GLU and 24% of ICO patients. CTR significantly increased at 3 months in GLU (51.7 ± 5.2%) compared with baseline (50.3 ± 4.3%, P = 0.049), and it remained elevated during the observation period. In addition, ANP significantly increased throughout the observation period in GLU (Figure 3, C and D). The net UF volume was significantly higher in ICO over the entire observation period compared with GLU (Figure 4A) and it was maintained at comparable levels with baseline. Urine volumes were 1086 ± 457 ml/d in GLU and 1149 ± 469 ml/d in ICO; they progressively decreased during the observation period to 508 ± 357 ml/d in GLU and 395 ± 377 ml/d in ICO (Figure 4B). No statistical difference between the two groups was observed in the urine volume, although both groups demonstrated a significant decline at 24 months in comparison to the respective baseline values. Renal creatinine clearance was also found to be significantly decreased in ICO and GLU from 3 months in comparison to the baseline (Figure 5A). Furthermore, D/P creatinine significantly increased in ICO (0.75 ± 0.11) and GLU (0.79 ± 0.05) compared with baseline, ICO (0.66 ± 0.13), and GLU (0.62 ± 0.13), respectively. D/P creatinine progressively increased in ICO and GLU, and there were no differences between the groups (Figure 5B).
Figure 3.
Parameters of body fluid management in ICO and GLU: (A) body weight, (B) BP, (C) CTR, and (D) ANP.
Figure 4.
(A) Net UF and (B) urine volume in ICO and GLU.
Figure 5.
(A) Renal creatinine clearance and (B) D/P creatinine in ICO and GLU.
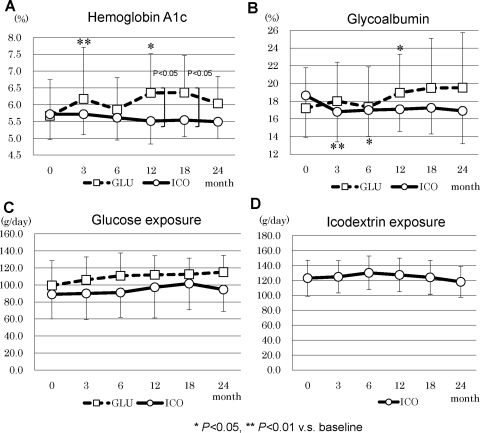
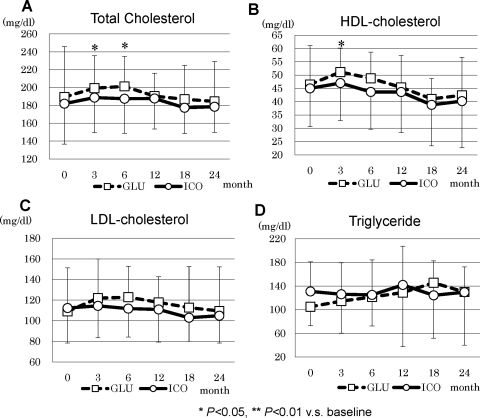
Hemoglobin A1c increased in GLU at 3 and 12 months and was significantly higher than in ICO at 12 and 18 months (Figure 6A). However, at the end of this study, no difference was observed between the two groups. Glycoalbumin significantly decreased in ICO from 3 to 6 months and increased in GLU at 12 months compared with the respective baseline values, but there were no statistical differences in later periods of the study (Figure 6A). There were no differences in daily glucose exposure between ICO and GLU during the entire study period (Figure 6, C and D). In lipid metabolism, total cholesterol increased at 3 and 6 months and HDL-cholesterol increased at 3 months in GLU (Figure 7A and B). No statistical difference between the two groups was observed in LDL-cholesterol and triglycerides (Figure 7, C and D).
Figure 6.
Glucose metabolism and daily glucose exposure in ICO and GLU: (A) hemoglobin A1c, (B) glycoalbumin, (C) glucose exposure, and (D) icodextrin exposure.
Figure 7.
Lipid profile in ICO and GLU: (A) total cholesterol, (B) HDL-cholesterol, (C) LDL-cholesterol, and (D) triglycerides.
Discussion
ESRD patients with diabetic nephropathy also frequently have nephrotic syndrome, congestive heart failure, and increased permeability of the blood vessels, which are all tightly linked to difficulties in body fluid management. Especially in PD therapy, withdrawal and technique failure are mainly caused by volume overload and excess of body fluid. In the RCT presented here, stable and superior UF was observed in ICO throughout the 2-year research period, which considerably contributed to the higher technique survival rate of PD therapy. In our research protocol, we prohibited the use of 4.25% glucose solution in both groups because it demonstrated unfavorable effects on the metabolic management and long-term maintenance of peritoneal membrane functions reported in previous studies (13). The technique failure rates in GLU in the study presented here were higher compared with the previous RCT trial, in which the use of 4.25% glucose solution was allowed (13). The lower technique failure rate in the previous RCT may also be because the recruited patients already stably received previous PD therapy for 19.6 and 17.6 months in GLU and ICO, respectively (13). The use of icodextrin demonstrated the beneficial effect on UF and body fluid status (e.g., body weight, BP, CTR, and ANP levels). Previous reports demonstrated the beneficial effects of icodextrin in BP and body fluid control (13,14), and indeed nine patients in GLU faced with UF failure discontinued PD because of uncontrolled BP, edema, and increase in CTR. The next important research question is whether higher UF in ICO reduces the urine volume and RRF. Urine volume and renal creatinine clearance significantly declined in earlier periods in ICO compared with GLU; however, there were no statistical differences between both groups throughout the 2-year observation period. The results presented here suggest that the use of icodextrin sustained the RRF for 2 years almost comparable to a glucose-based dialysis solution and we did not find beneficial effects of icodextrin on RRF. Certainly, the superior UF may inevitably reduce the urine volume if dialysis therapy goes beyond 2 years.
Many clinical research studies have emphasized the beneficial effects of icodextrin on glucose and lipid metabolism, although controversial results were reported (15,16). The inhibition of body weight gain and fat accumulation and improvement of glucose and lipid metabolism were reported in previous studies (1,15,17,18). In our RCT, the beneficial effect on glucose metabolism demonstrated by hemoglobin A1c and glycoalbumin was observed only at 3 and 6 months, and we did not see such beneficial effects in later periods. In addition, we did not see any beneficial effects on lipid profiles throughout the research period. This may be due to the total amount of the glucose exposure to PD patients. Because the use of 4.25% glucose solution was not allowed in both groups, total glucose exposure was not statistically different between the two groups.
The final research question is whether the direct beneficial effects of ICO on the peritoneal membrane and its functions were clinically relevant. In an animal study, we investigated the effects of daily injections of 4.25% glucose solution or 7.5% icodextrin for 8 weeks in 5/6-nephrectomized diabetic rats (19). The animals exposed to a high glucose solution demonstrated neovascular formation; widening of the submethothelial zone; and prominent immune staining of vascular endothelial growth factor, basic fibroblast growth factor, and advanced glycation endproduct and its receptors. In contrast to the animal study, there have been concerns that icodextrin may induce the local peritoneal inflammation demonstrated by elevated hyaluronan (20), IL-6 (21,22), and TNF-α (23). Although cohort studies or RCTs have yet to be reported, there are concerns that icodextrin might be an independent risk factor for the development of encapsulating peritoneal sclerosis (24–26). In clinical settings, icodextrin is always used in combination with a glucose-based solution; therefore, it may be difficult to interpret the data. In the study presented here, we did not demonstrate the differences in peritoneal functions such as Kt/V and D/P creatinine throughout the observation period. Considering the benefits and disadvantages of icodextrin on peritoneal membrane functions, there are still many questions whether icodextrin is proinflammatory or anti-inflammatory, proangiogenic or antiaigiogenic, beneficial in preserving the peritoneal membrane function or not, and an independent risk for encapsulating peritoneal sclerosis or not; this needs to be addressed in future studies.
We clearly demonstrated that icodextrin increases technique survival rate in PD patients with diabetic nephropathy by improving body fluid management, and it is beneficial in clinical practice in the treatment of ESRD patients with diabetes. However, the limitation of our study is the relatively small number of enrolled patients to draw the conclusion that icodextrin has further advantages in RRF and glucose and lipid metabolism; therefore, future larger randomized clinical trials are required.
In conclusion, we investigated ESRD patients with diabetic nephropathy who newly started PD as a first renal replacement therapy. They were randomly assigned to ICO and GLU and were treated for 2 years. At 24 months of follow-up, the technique survival rate was 71.4% for ICO and 45.0% for GLU. The net UF volume was significantly higher in ICO over the entire observation period compared with GLU. There were no differences in RRF and glucose and lipid metabolism in the PD patients with diabetes who completed the 2-year observation period. The use of an icodextrin solution in PD patients is primarily beneficial in increasing technique survival by improving body fluid management.
Disclosures
None.
Acknowledgments
Members of Okayama Kidney Disease Study Group (OKDIGO): Makoto Hiramatsu, Keisuke Maruyama, and Tsuneto Onbe (Okayama Saiseikai General Hospital, Okayama); Masaki Fukushima (Kurashiki Central Hospital); Yasuaki Mino (Ochiai General Hospital); Kosuke Ota and Kosuke Fukuoka (Okayama Medical Center); Kazuhi Taniai and Shigeru Morioka (Okayama Central Hospital); Masafumi Taki and Tatsuya Matsubara (Shigei Medical Research Hospital); Masahiko Kushiro (Tottori Municipal Hospital); Shigeaki Nishimura (Ehime Prefectural Central Hospital); Takashi Sekikawa and Hirohiko Sato (Matsuyama Shimin Hospital); Hisanori Morimoto (Mitoyo General Hospital); Naoyuki Gamou and Takashi Kihara (Sumitomo Besshi Hospital); Yoshinori Tsuchiyama (Kochi Health Science Center); Hidetaka Kawasaki (Fuchu General Hospital); Ishii Keita and Kyoji Hirata (Chugoku Central Hospital); Isao Kumagai and Shinji Fukuda (Teraoka Memorial Hospital); Masami Hashimoto (Onomichi Municipal Hospital); Yasushi Yamasaki, Hiroshi Nakazono, and Yuki Takazawa (Hiroshima City Hospital); Kazuhiro Ujike and Hiroo Hashimoto (Innoshima General Hospital); Tsutomu Hiromasa (Japan Research Cross Society Himeji Hospital); Tetsuya Onoda (Kobe Ekisaikai Hospital); and Yoshihiko Yamamoto (Kagawa Rosai Hospital).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Kuriyama S: Peritoneal dialysis in patients with diabetes: Are the benefits greater than the disadvantages? Perit Dial Int 27[Suppl 2]: S190–S195, 2007 [PubMed] [Google Scholar]
- 2. Masakane I, Tsubakihara Y, Akiba T, Watanabe Y, Iseki K: The most recent trends of peritoneal dialysis in Japan. Perit Dial Int 28[Suppl 3]: S27–S31, 2008 [PubMed] [Google Scholar]
- 3. Mistry CD, Gokal R, Peers E: A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis. Kidney Int 46: 496–503, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Mistry CD, Mallick NP, Gokal R: Ultrafiltration with an isosmotic solution during long peritoneal dialysis exchanges. Lancet 2: 178–182, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Frampton JE, Plosker GL: Icodextrin: A review of its use in peritoneal dialysis. Drugs 63: 2079–2105, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Wolfson M, Piraino B, Hamburger RJ, Morton AR: A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis 40: 1055–1065, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Woodrow G, Oldroyd B, Stables G, Gibson J, Turney JH, Brownjohn AM: Effects of icodextrin in automated peritoneal dialysis on blood pressure and bioelectrical impedance analysis. Nephrol Dial Transplant 15: 862–866, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Van V, Schoonjans RS, Struijk DG, Verbanck JJ, Vanholder RC, Van B, Lefebvre RA, De V, Lameire NH: Influence of dialysate on gastric emptying time in peritoneal dialysis patients. Perit Dial Int 22: 32–38, 2002 [PubMed] [Google Scholar]
- 9. Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimburger O, Simonsen O, Davenport A, Tranaeus A, Divino Filho JC: Icodextrin improves the fluid status of peritoneal dialysis patients: Results of a double-blind randomized controlled trial. J Am Soc Nephrol 14: 2338–2344, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Jeloka TK, Oreopoulos DG: Is ultrafiltration with icodextrin higher in diabetics than in nondiabetics? Perit Dial Int 26: 729–730, 2006 [PubMed] [Google Scholar]
- 11. Adachi Y, Nakagawa Y, Nishio A: Icodextrin preserves residual renal function in patients treated with automated peritoneal dialysis. Perit Dial Int 26: 405–407, 2006 [PubMed] [Google Scholar]
- 12. Twardowski ZJ: Clinical value of standardized equilibration tests in CAPD patients. Blood Purif 7: 95–108, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Paniagua R, Ventura MD, Avila-Diaz M, Cisneros A, Vicente-Martinez M, Furlong MD, Garcia-Gonzalez Z, Villanueva D, Orihuela O, Prado-Uribe MD, Alcantara G, Amato D: Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int 29: 422–432, 2009 [PubMed] [Google Scholar]
- 14. Paniagua R, Orihuela O, Ventura MD, Avila-Diaz M, Cisneros A, Vicente-Martinez M, Furlong MD, Garcia-Gonzalez Z, Villanueva D, Prado-Uribe MD, Alcantara G, Amato D: Echocardiographic, electrocardiographic and blood pressure changes induced by icodextrin solution in diabetic patients on peritoneal dialysis. Kidney Int Suppl: S125–S130, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Babazono T, Nakamoto H, Kasai K, Kuriyama S, Sugimoto T, Nakayama M, Hamada C, Furuya R, Hasegawa H, Kasahara M, Moriishi M, Tomo T, Miyazaki M, Sato M, Yorioka N, Kawaguchi Y: Effects of icodextrin on glycemic and lipid profiles in diabetic patients undergoing peritoneal dialysis. Am J Nephrol 27: 409–415, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Marshall J, Jennings P, Scott A, Fluck RJ, McIntyre CW: Glycemic control in diabetic CAPD patients assessed by continuous glucose monitoring system (CGMS). Kidney Int 64: 1480–1486, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Martikainen T, Teppo AM, Gronhagen-Riska C, Ekstrand A: Benefit of glucose-free dialysis solutions on glucose and lipid metabolism in peritoneal dialysis patients. Blood Purif 23: 303–310, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Cho KH, Do JY, Park JW, Yoon KW: Effect of icodextrin dialysis solution on body weight and fat accumulation over time in CAPD patients. Nephrol Dial Transplant 25: 593–599, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Nakao A, Nakao K, Takatori Y, Kojo S, Inoue J, Akagi S, Sugiyama H, Wada J, Makino H: Effects of icodextrin peritoneal dialysis solution on the peritoneal membrane in the STZ-induced diabetic rat model with partial nephrectomy. Nephrol Dial Transplant 25: 1479–1488, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Parikova A, Zweers MM, Struijk DG, Krediet RT: Peritoneal effluent markers of inflammation in patients treated with icodextrin-based and glucose-based dialysis solutions. Adv Perit Dial 19: 186–190, 2003 [PubMed] [Google Scholar]
- 21. Martikainen T, Ekstrand A, Honkanen E, Teppo AM, Gronhagen-Riska C: Do interleukin-6, hyaluronan, soluble intercellular adhesion molecule-1 and cancer antigen 125 in dialysate predict changes in peritoneal function? A 1-year follow-up study. Scand J Urol Nephrol 39: 410–416, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Moriishi M, Kawanishi H: Icodextrin and intraperitoneal inflammation. Perit Dial Int 28[Suppl 3]: S96–S100, 2008 [PubMed] [Google Scholar]
- 23. Martikainen TA, Teppo AM, Gronhagen-Riska C, Ekstrand AV: Glucose-free dialysis solutions: Inductors of inflammation or preservers of peritoneal membrane? Perit Dial Int 25: 453–460, 2005 [PubMed] [Google Scholar]
- 24. Trigka K, Dousdampanis P, Chu M, Khan S, Ahmad M, Bargman JM, Oreopoulos DG: Encapsulating peritoneal sclerosis: A single-center experience and review of the literature. Int Urol Nephrol 2010 [DOI] [PubMed] [Google Scholar]
- 25. Krediet RT: Effects of icodextrin on the peritoneal membrane. Nephrol Dial Transplant 25: 1373–1375, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Kawanishi H, Shintaku S, Shishida M, Morrishi M, Tsuchiya S, Dohi K: A case of encapsulating peritoneal sclerosis suspected to result from the use of icodextrin peritoneal solution. Adv Perit Dial 25: 45–49, 2009 [PubMed] [Google Scholar]