Abstract
Summary
Background and objectives
Vitamin D has gained attention for its pleiotropic effects in areas other than bone metabolism, and the effects of vitamin D in preventing respiratory infections have been reported as one of its immunomodulating properties. This study assessed the preventive effect of vitamin D receptor activator (VDRA) on respiratory infections in dialysis patients.
Design, setting, participants, & measurements
Maintained Japanese hemodialysis patients (n = 508) were observed for 5 years, and the incidence of hospitalization during this period because of acute respiratory infection (ARI) was recorded.
Results:
Of the 508 patients, 212 had taken oral VDRA at the start of the study, whereas 296 patients had not received it. During the 5-year follow-up period, 57 patients were hospitalized because of ARIs. Kaplan–Meier analysis revealed that the incidence of hospitalization because of respiratory infection was significantly lower in patients who had been treated with VDRA compared with patients who had not (log rank test; P = 0.02). The multivariate Cox proportional hazards model demonstrated that the patients who had taken oral VDRA were at a significantly lower risk of hospitalization because of respiratory disease (hazard ratio 0.47, 95% confidence interval 0.25 to 0.90).
Conclusions:
The findings of this study suggest that the administration of oral VDRA has a preventive effect on the incidence of ARIs in dialysis patients.
Introduction
The pleiotropic effects of vitamin D, with a wide range of immunomodulating (1), antitumor (2), renal protective (3,4), and cardiovascular (5–7) actions in addition to its effects on bone metabolism, have garnered attention in recent years. Immunomodulating actions such as its effectiveness in preventing autoimmune diseases (8) and diabetes mellitus (DM) as well as its prophylactic action have been reported (9). There have been various reports on its effectiveness in preventing respiratory infections, which is one of the prophylactic properties of vitamin D. Observational and epidemiologic studies have found that the lower the serum 25-hydroxyvitamin D (25(OH)D) concentration, the greater the incidence of upper respiratory infections and pneumonia (10–13).
Patients with chronic renal failure are typically vitamin D deficient (14,15). Recently, there has been a series of reports on the improved life expectancy of patients with chronic renal failure, including dialysis patients, who are treated with vitamin D receptor activator (VDRA) compared with those who are not (16–24). This improved prognosis seems to be mainly attributed to the preventive effects of VDRA against death due to cardiovascular disease, and a few reports have indicated its benefits in preventing death from infections (17,19,21). However, there are still no studies that have exclusively investigated the preventive effects of VDRA on respiratory infections in dialysis patients.
Considering the fact that death from infectious diseases is the second-most common cause of death in dialysis patients after cardiovascular death (25), taking countermeasures against infections is a matter of importance. The incidence of pneumonia among patients with chronic renal failure is reportedly 14 to 16 times higher than in the general population (26), and in many cases the patient's condition worsens, leading to death. In the study presented here, we focused on respiratory infections in particular, and we investigated whether the use of VDRA prevents their onset.
Materials and Methods
Study Population
Those included in the study were 762 hemodialysis patients who had been on maintenance dialysis on an outpatient basis at Inoue Hospital (Suita City, Osaka, Japan) in March 2000. Of them, patients who were transferred to another facility, transitioned to peritoneal dialysis, or underwent a kidney transplant as well as those patients who had inadequate characteristics at the start of the study were excluded from the study. The remaining 508 patients were observed for a period of 5 years. The baseline patient characteristics were assessed and measured based on the parameters shown in Table 1. Hemodialysis was performed 2 to 3 times a week, with each session lasting 3 to 5 hours. Patients were also searched for concomitant chronic respiratory diseases (CRDs; bronchial asthma, emphysema, chronic bronchitis, or interstitial pneumonia) and past history of cardiovascular diseases (CVDs; cerebral infarction, cerebral hemorrhage, or subarachnoid hemorrhage) as comorbidities considered likely to cause respiratory infection. CRD was diagnosed by a pulmonologist on the basis of patient symptoms or the data from an imaging test, respiratory function tests, etc. Past history of CVDs was assessed based on information obtained from patients and from medical records. Ejection fraction was also measured by using an echocardiogram to evaluate cardiac function with the aim of considering the effect of heart disease. Echocardiogram was performed using the SSA-260A system (Toshiba, Tochigi, Japan) with a 3.75-MHz probe. Evaluation of body type was performed using body mass index (body weight in kilograms/height in square meters) on the basis of dry weight.
Table 1.
Characteristics of cohort at baseline
| Mean ± SDa | Range | |
|---|---|---|
| Gender (male/female) | 309:199 | |
| Age (years) | 59.6 ± 11.3 | 26.2 to 94.9 |
| Duration of dialysis (years) | 10.0 ± 6.9 | 0.2 to 29.8 |
| Diabetes (yes/no) | 77:431 | |
| Smoking (smoker/nonsmoker) | 127:381 | |
| Body mass index (kg/m2) | 21.9 ± 3.0 | 15.3 to 35.2 |
| Corrected Ca (mg/dl) | 9.5 ± 0.9 | 6.6 to 12.1 |
| P (mg/dl) | 5.9 ± 1.4 | 2.9 to 11.1 |
| Intact PTH (pg/ml) | 245 ± 287 | 10 to 2351 |
| Alb (g/dl) | 3.8 ± 0.3 | 2.0 to 4.7 |
| Hemoglobin (mg/dl) | 9.6 ± 1.2 | 5.4 to 14 |
| Kt/V | 1.45 ± 0.30 | 0.63 to 3.76 |
| Ejection fraction (%) | 63.9 ± 10.0 | 15.0 to 90.0 |
| Past history of stroke (yes/no) | 49:459 | |
| Chronic pulmonary disease (yes/no) | 27:481 | |
| VDRA (users/nonusers) | 212:296 |
Unless otherwise noted. VDRA, vitamin D receptor activator; Alb, albumin; PTH, parathyroid hormone; Ca, calcium; P, phosphorus.
Biochemistry
Blood samples were collected from the patients at the start of the week before dialysis, and serum calcium (Ca), phosphorus (P), intact parathyroid hormone (PTH), albumin (Alb), and hemoglobin were measured according to standard methods. Serum Ca was corrected based on the Alb level; that is, corrected Ca (mg/dl) = measured total Ca (mg/dl) + (4.0 − serum Alb [g/dl]). Meanwhile, the dialysis dose was calculated by measuring Kt/V using the Daugirdas formula of Kt/V = −ln(Ce/Cs − 0.008 × t) + (4 − 3.5 × Ce/Cs) × ΔBW/BW, in which ln is the natural logarithm, Ce is postdialysis blood urea nitrogen, Cs is predialysis blood urea nitrogen, t is dialysis time, ΔBW is removed water volume, and BW is postdialysis body weight.
VDRA Preparation
VDRA was administered at the discretion of each supervising physician. The study population included patients who were treated with VDRA for secondary hyperparathyroidism and prevention of hypocalcemia. Because intravenous VDRA had not been approved in Japan when the study was initiated, only oral preparations were used in the study. At the start of the study, 212 patients were routinely administered oral VDRA. Of these, 129 patients were continuously given Alfacalcidol at a dose of 0.25 to 1.0 μg/d, with the mean dose of 0.366 μg/d. The remaining 83 patients were administered oral Calcitriol, of whom 29 patients received continuous administration at a dose of 0.25 to 0.5 μg/d, and the other 54 patients underwent oral VDRA pulse therapy at a dose of 1.0 to 3.0 μg three times a week for secondary hyperparathyroidism. The mean daily dose of Calcitriol was 0.469 μg.
Respiratory Infections
The incidence of hospitalization during the 5-year observation period because of acute respiratory infections (ARIs; e.g., pneumonia and bronchitis) was observed. Diagnosis of respiratory infections was made based on clinical symptoms and the data from the imaging test and the biochemical tests. Respiratory infection pathogens were evaluated using sputum cultures, serodiagnosis, etc. In patients in whom the infectious pathogens could not be determined by these tests, these pathogens were instead evaluated based on reactions to therapeutic drugs such as antibiotics. The number of patients who experienced respiratory infection during hospitalization for other diseases was not calculated.
Statistical Analyses
Data were expressed as mean ± SD. Statistically significant differences were analyzed using StatView version 5.0 (SAS Institute, Inc., Cary, NC) software. The differences between the two groups were calculated using the χ2 test or t test. The Kaplan–Meier method and Cox proportional hazards model were also used to investigate the influence of each factor on respiratory disease.
Results
The baseline patient characteristics are shown in Table 1. The study population consisted of 309 men and 199 women with a mean age of 59.6 years and a mean duration of dialysis of 10.0 years. Seventy-seven patients had underlying DM, whereas 431 patients were nondiabetic. Mean body mass index was 21.9, and mean serum Alb level was 3.8 g/dl. At the start of the study, 27 patients had a concomitant CRD and 49 patients had a history of stroke. VDRA had been orally administered to 212 patients.
Differences in Patient Characteristics between Treatment and Control Groups
The patient characteristics classified into two groups according to presence or absence of oral administration of VDRA at the start of the study (treatment group) and nonadministration (control group) are shown in Table 2. There was no significant difference between the two groups in terms of sex, age, vintage of dialysis, smoking habit, history of stroke, concomitant CRD, or serum Alb level. The number of patients with underlying DM was lower in the treatment group than in the control group. Although there was no difference in serum Ca or P values between the groups, intact PTH was significantly higher in the treatment group. Hemoglobin level was slightly lower in the treatment group than in the control group.
Table 2.
Comparison of characteristics between VDRA users and nonusers
| VDRAa |
Pb | ||
|---|---|---|---|
| Users | Nonusers | ||
| Gender (male/female) | 126:86 | 183:113 | 0.59 |
| Age (years) | 59.4 ± 11.2 | 59.6 ± 11.5 | 0.86 |
| Duration of dialysis (years) | 10.5 ± 7.1 | 9.6 ± 6.8 | 0.12 |
| Diabetes (yes/no) | 24:188 | 53:243 | 0.04 |
| Smoking (smoker/nonsmoker) | 48:164 | 79:217 | 0.30 |
| Body mass index (kg/m2) | 22.1 ± 3.0 | 21.9 ± 2.9 | 0.49 |
| Corrected Ca (mg/dl) | 9.6 ± 0.8 | 9.4 ± 0.9 | 0.08 |
| P (mg/dl) | 6.0 ± 1.2 | 5.8 ± 1.5 | 0.39 |
| Intact PTH (pg/ml) | 290 ± 306 | 212 ± 269 | <0.01 |
| Alb (g/dl) | 3.8 ± 0.4 | 3.8 ± 0.3 | 0.94 |
| Hemoglobin (mg/dl) | 9.5 ± 1.2 | 9.7 ± 1.2 | 0.05 |
| Kt/V | 1.46 ± 0.31 | 1.45 ± 0.29 | 0.80 |
| Ejection fraction (%) | 63.4 ± 10.6 | 64.2 ± 9.5 | 0.39 |
| Past history of stroke (yes/no) | 15:197 | 34:262 | 0.10 |
| Chronic pulmonary disease (yes/no) | 10:202 | 17:279 | 0.61 |
Values are presented as means +/− SDs unless otherwise noted.
Student t test, χ2 test.
During the 5-year observation period, 57 patients were hospitalized because of ARIs. A breakdown of these ARIs is shown in Table 3. Bacterial or bronchial pneumonia was diagnosed in 51 patients, and 3 of these patients had aspiration pneumonia. Furthermore, mycoplasma pneumonia was diagnosed in one patient, fungal pneumonia in another, and viral bronchitis or pneumonia, including that caused by influenza viruses, was diagnosed in four patients.
Table 3.
Hospitalization for acute pulmonary infection during follow-up period
| Number | |
|---|---|
| Bacterial pneumonia | 51 |
| Mycoplasma pneumonia | 1 |
| Fungal pneumonia | 1 |
| Influenza-virus pneumonia | 4 |
| Total | 57 |
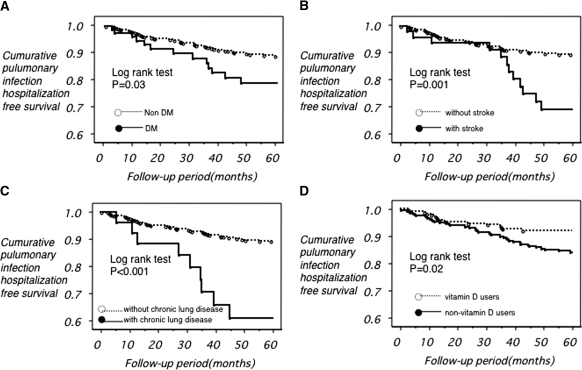
Whether or not the key related factors had any influence regarding hospitalization for respiratory infection was analyzed using the Kaplan–Meier method. The results are shown in Figure 1. Hospitalizations were significantly higher among patients with underlying DM compared with nondiabetic patients. In addition, patients with a history of stroke and those with a concomitant CRD had a significantly higher incidence of hospitalization because of respiratory infection compared with those without a history or complications. Meanwhile, the incidence of hospitalization because of respiratory infection was significantly greater in the control group than in the VDRA treatment group.
Figure 1.
Cumulative pulmonary infection hospitalization-free survival. The closed circles connected by the solid line and the opened circles connected by the dotted line represent the patients (a) with and without diabetes, (b) with and without stroke, and (c) with and without chronic lung disease, respectively. (d) The opened circles connected by the dotted line and the closed circles connected by the solid line represent the vitamin D user and nonuser, respectively.
In a univariate Cox proportional hazards model (Table 4), each factor (including age, vintage of dialysis, presence or absence of DM, serum Alb level, presence or absence of past history of stroke, presence or absence of CRD, and use or nonuse of VDRA) had a significant effect on the risk of hospitalization because of respiratory disease.
Table 4.
Univariate Cox proportional hazards models for hospitalization because of pulmonary infection
| Hazard Ratio | 95% CI | P | |
|---|---|---|---|
| Gender (male/female) | 1.12 | 0.66 to 1.89 | 0.68 |
| Age (years) | 1.07 | 1.05 to 1.10 | <0.001 |
| Vintage of dialysis (years) | 0.95 | 0.91 to 0.99 | 0.02 |
| Diabetes (yes/no) | 1.95 | 1.08 to 3.43 | 0.03 |
| Smoking (smoker/nonsmoker) | 1.64 | 0.93 to 2.90 | 0.09 |
| Body mass index (kg/m2) | 0.97 | 0.88 to 1.07 | 0.50 |
| Corrected Ca (mg/dl) | 0.91 | 0.67 to 1.22 | 0.52 |
| P (mg/dl) | 0.83 | 0.68 to 1.01 | 0.06 |
| Intact PTH (pg/ml) | 1.00 | 1.00 to 1.00 | 0.14 |
| Intact PTH (<300 pg/ml versus >300 pg/ml) | 1.33 | 0.74 to 2.40 | 0.35 |
| Alb (g/dl) | 0.31 | 0.16 to 0.61 | <0.001 |
| Hemoglobin (mg/dl) | 0.83 | 0.67 to 1.04 | 0.10 |
| Kt/V | 0.40 | 0.14 to 1.12 | 0.08 |
| Ejection fraction (%) | 0.15 | 0.01 to 1.88 | 0.14 |
| Past history of stroke (yes/no) | 2.80 | 1.48 to 5.29 | <0.01 |
| Chronic pulmonary disease (yes/no) | 3.89 | 1.91 to 7.94 | <0.001 |
| VDRA (users/nonusers) | 0.51 | 0.28 to 0.93 | 0.03 |
CI, confidence interval.
Next, multivariate analysis was performed using the Cox proportional hazards model on the factors that showed significant results in the univariate analysis as shown in Table 5. As a result, age (hazard ratio: 1.06; 95% confidence interval [CI]: 1.03 to 1.09), presence or absence of CRD (hazard ratio: 3.28; 95% CI: 1.58 to 6.82), serum Alb level (hazard ratio: 0.40; 95% CI: 0.17 to 0.94), and use or nonuse of VDRA (hazard ratio: 0.47; 95% CI: 0.25 to 0.90) were found to be independent effect factors. These findings suggest that advanced age, concomitant CRD, low serum Alb level, and nonuse of VDRA are all risk factors for hospitalization because of respiratory infection.
Table 5.
Multivariate Cox proportional hazards models for hospitalization because of pulmonary infection
| Hazard Ratio | 95% CI | P | |
|---|---|---|---|
| Age (years) | 1.06 | 1.03 to 1.09 | <0.001 |
| Vintage of dialysis (years) | 0.99 | 0.95 to 1.04 | 0.42 |
| Diabetes (yes/no) | 1.41 | 0.72 to 2.78 | 0.53 |
| Alb (g/dl) | 0.40 | 0.17 to 0.94 | 0.04 |
| Past history of stroke (yes/no) | 1.72 | 0.90 to 3.28 | 0.08 |
| Chronic pulmonary disease (yes/no) | 3.28 | 1.58 to 6.82 | 0.01 |
| VDRA (users/nonusers) | 0.47 | 0.25 to 0.90 | 0.02 |
Discussion
In the study presented here, it was demonstrated that the use of VDRA in hemodialysis patients significantly decreases the incidence of hospitalization for respiratory infection independent of other factors. Infectious disease is reportedly common among dialysis patients, and its causes have been assumed to be reduced immune function because of uremia, problems with biosynthesis of dialysis membranes and fluid, etc., infections associated with blood access, and deficiency of trace elements and vitamins, as well as aging—a common risk factor (27–32). Allon et al. carried out analysis in a prospective Hemodialysis (HEMO) Study to see whether the rates of hospitalization and infectious mortality decreased in response to increased dialysis dose (33). The study concluded that neither dialysis doses nor membranes were found to have any effect, whereas age, DM, Alb level, and comorbidities were all significant effect factors. These factors were also found to be effective in our study. There have also been many studies on infections relating to blood access (34). However, few observational studies indicate that infections in dialysis patients are triggered by trace element and vitamin deficiencies. Similarly, there have been no reports on the susceptibility to infection of dialysis patients with vitamin D deficiency.
There are many reports on the association of vitamin D and respiratory infections in patients without renal failure. Some observational studies have found that the lower the serum 25(OH)D concentration, the greater the incidence of upper respiratory infections and pneumonia (10–13). Meanwhile, other studies demonstrate a decline in the incidence of respiratory infections and influenza after administration of nutritional vitamin D (35–37). However, others indicated no significant difference in patients' use or nonuse of nutritional vitamin D (38,39). The study reports in which no significant difference was observed concluded that this finding may have been because the subjects' serum vitamin D concentrations were not overly low to begin with, so that administration of vitamin D preparations was unlikely to have any effect. It is well known that serum vitamin D concentration is typically low in patients with reduced kidney function (14,15), so it is presumed that the administration of vitamin D preparations is more effective in these patients than in those without renal insufficiency, and that significant differences are likely to appear between the two.
Recently, there have been many studies showing an association between VDRA and life expectancy in dialysis and other renal failure patients. Some of these reports indicate that life expectancy is better among patients who have taken oral or intravenous VDRA (16–24), whereas others showed poor life expectancy in patients with low serum vitamin D (25(OH)D or 1,25-dihydroxyvitamin D) concentrations (40–46). The reasons ascribed to this trend of better life expectancy among VDRA treatment population or high serum vitamin D population have tended to focus on its ability to reduce cardiovascular mortality. There are three reports that present a relationship between VDRA and infectious mortality. Teng et al. (18) reported in a 2-year observational study of dialysis patients that not only cardiovascular mortality but also infectious mortality was more prevalent in patients who had not taken intravenous VDRA compared with patients who had received VDRA. Meanwhile, Naves- Naves-Díaz et al. (17) reported that dialysis patients treated with oral VDRA showed reduced infectious, cardiovascular, and neoplastic mortality compared with patients who had not received VDRA. In addition, St. Peter et al. (20) reported in an assessment of patients according to dialysis duration that those patients with relatively short dialysis duration who had received intravenous VDRA had a lower incidence of infectious mortality than those who had not received VDRA. However, these reports comprise all types of infections and there are no reports focusing solely on respiratory infections.
There are several reports on how active vitamin D affects immune system response in dialysis patients. There have also been reports to the effect that administration of VDRA to dialysis patients restored cytokine release from reduced lymphocytes and conversely inhibited excessive cytokine release (47–49), and that it improved cell proliferation in patients with reduced lymphocyte counts (50,51). It has therefore been demonstrated that, in the adaptive immune system, not nutritional vitamin D but VDRA restores lowered immune response in dialysis patients to normal levels. Meanwhile, there have been few reports about the innate immune system of dialysis patients. Gombart et al. (52) demonstrated the increase in infectious mortality among dialysis patients with a low plasma level of the cathelicidin antimicrobial peptide related to innate immunity and that the level of not 25(OH)D but 1α,25-dihydroxyvitamin D is correlated with that of cathelicidin. We can infer from these reports that, even in dialysis patients, active vitamin D deficiency reduces innate immunity and increases the risk of infection.
There are several potential limitations to the study presented here. First, some variables in the baseline study were different between VDRA users and nonusers, such as the level of intact PTH and the proportion of diabetic subjects. However, the association between the use of VDRA and a lower risk of hospitalization remained significant after adjustment for these factors. Recently, Dukkipati et al. (53) reported that low PTH level is another facet of malnutrition and wasting that is associated with infection. In the study presented here, Alb levels were not significantly different between both groups and also there was no association between PTH levels and Alb levels (data not shown), suggesting that PTH might not be a simple surrogate marker of malnutrition. Second, we were unable to examine in detail the contribution of dosage to our results because two kinds of VDRAs (Alfacalcidol and Calcitriol) were administered to the patients. Moreover, injectable VDRA has been available since 2001 in Japan, but we were unable to obtain precise information on injectable VDRA status during the observational period. Therefore, a large-scale randomized controlled trial of dialysis patients using a single VDRA should be conducted to further observe the relationship between VDRA and respiratory infections. In addition, the effects of VDRA on infections other than those of a respiratory nature should be investigated.
In conclusion, it was demonstrated that VDRA may act to prevent respiratory infections in dialysis patients in our study. When considering the known preventive effects of VDRA on CVD and its antitumor effects, we believe that in dialysis patients, who are typically vitamin D-deficient, it is advisable to actively conduct VDRA replacement therapy while monitoring for increases in Ca and P levels.
Disclosures
None.
Acknowledgments
H.T. was supported by a grant from the Japan Dialysis Outcome Research Group (no. 003). H.K. is currently with the Department of Internal Medicine, Division of Endocrinology and Metabolism at Hyogo College of Medicine in Hyogo, Japan.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Veldman CM, Cantorna MT, DeLuca HF: Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys 374: 334–338, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Holick MF: Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79: 362–371, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D: Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68: 2823–2828, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Makibayashi K, Tatematsu M, Hirata M, Fukushima N, Kusano K, Ohashi S, Abe H, Kuze K, Fukatsu A, Kita T, Doi T: A vitamin D analog ameliorates glomerular injury on rat glomerulonephritis. Am J Pathol 158: 1733–1741, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zittermann A, Koerfer R: Protective and toxic effects of vitamin D on vascular calcification: Clinical implications. Mol Aspects Med 29: 423–432, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Park CW, Oh YS, Shin YS, Kim CM, Kim YS, Kim SY, Choi EJ, Chang YS, Bang BK: Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis 33: 73–81, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C: Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab 86: 1633–1637, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG; Iowa Women's Health Study: Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women's Health Study. Arthritis Rheum 50: 72–77, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Gysemans CA, Cardozo AK, Callewaert H, Giulietti A, Hulshagen L, Bouillon R, Eizirik DL, Mathieu C: 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: Implications for prevention of diabetes in nonobese diabetic mice. Endocrinology 146: 1956–1964, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Ginde AA, Mansbach JM, Camargo CA, Jr: Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 169: 384–390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki H, Ylikomi T: An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr 86: 714–717, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Wayse V, Yousafzai A, Mogale K, Filteau S: Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr 58: 563–567, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A: Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr 63: 473–477, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Ishimura E, Nishizawa Y, Inaba M, Matsumoto N, Emoto M, Kawagishi T, Shoji S, Okuno S, Kim M, Miki T, Morii H: Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int 55: 1019–1027, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Bhan I, Burnett-Bowie SA, Ye J, Tonelli M, Thadhani R: Clinical measures identify vitamin D deficiency in dialysis. Clin J Am Soc Nephrol 5: 460–467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, Inaba M, Fukumoto S, Ishimura E, Miki T, Tabata T, Nishizawa Y: Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant 19: 179–184, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Naves-Díaz M, Alvarez-Hernández D, Passlick-Deetjen J, Guinsburg A, Marelli C, Rodriguez-Puyol D, Cannata-Andía JB: Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int 74: 1070–1078, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo CA, Jr, Thadhani R: Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol 16: 1115–1125, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 20. St. Peter WL, Li S, Liu J, Gilbertson DT, Arneson TJ, Collins AJ: Effects of monthly dose and regular dosing of intravenous active vitamin D use on mortality among patients undergoing hemodialysis. Pharmacotherapy 29: 154–164, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Tentori F, Albert JM, Young EW, Blayney MJ, Robinson BM, Pisoni RL, Akiba T, Greenwood RN, Kimata N, Levin NW, Piera LM, Saran R, Wolfe RA, Port FK: The survival advantage for haemodialysis patients taking vitamin D is questioned: Findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 24: 963–972, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG: Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int 70: 1858–1865, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K: Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med 168: 397–403, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B: Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol 19: 1613–1619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. U.S. Renal Data System: 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2009 [Google Scholar]
- 26. Sarnak MJ, Jaber BL: Pulmonary infectious mortality among patients with end-stage renal disease. Chest 120: 1883–1887, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Powe NR, Jaar B, Furth SL, Hermann J, Briggs W: Septicemia in dialysis patients: Incidence, risk factors, and prognosis. Kidney Int 55: 1081–1090, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Hoen B, Paul-Dauphin A, Hestin D, Kessler M: A multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol 9: 869–876, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B: Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 3: 1526–1533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vanholder R, Van Loo A, Dhondt AM, De Smet R, Ringoir S: Influence of uraemia and haemodialysis on host defence and infection. Nephrol Dial Transplant 11: 593–598, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Hauser AB, Stinghen AE, Kato S, Bucharles S, Aita C, Yuzawa Y, Pecoits-Filho R: Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit Dial Int 28[Suppl 3]: S183–S187, 2008 [PubMed] [Google Scholar]
- 32. Dalrymple LS, Go AS: Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol 3: 1487–1493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allon M, Depner TA, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ; HEMO Study Group: Impact of dialysis dose and membrane on infection-related hospitalization and death: Results of the HEMO Study. J Am Soc Nephrol 14: 1863–1870, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Pastan S, Soucie JM, McClellan WM: Vascular access and increased risk of death among hemodialysis patients. Kidney Int 62: 620–626, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Linday LA, Shindledecker RD, Tapia-Mendoza J, Dolitsky JN: Effect of daily cod liver oil and a multivitamin-mineral supplement with selenium on upper respiratory tract pediatric visits by young, inner-city, Latino children: Randomized pediatric sites. Ann Otol Rhinol Laryngol 113: 891–901, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Rehman PK: Sub-clinical rickets and recurrent infection. J Trop Pediatr 40: 58, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H: Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 91: 1255–1260, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, Berbari N: A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect 137: 1396–1404, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Avenell A, Cook JA, Maclennan GS, Macpherson GC: Vitamin D supplementation to prevent infections: A sub-study of a randomised placebo-controlled trial in older people. Age Ageing 36: 574–577, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Inaguma D, Nagaya H, Hara K, Tatematsu M, Shinjo H, Suzuki S, Mishima T, Kurata K: Relationship between serum 1,25-dihydroxyvitamin D and mortality in patients with pre-dialysis chronic kidney disease. Clin Exp Nephrol 12: 126–131, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C: Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 75: 88–95, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Barreto DV, Barreto FC, Liabeuf S, Temmar M, Boitte F, Choukroun G, Fournier A, Massy ZA: Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol 4: 1128–1135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mehrotra R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, Martins D, Adler SG, Norris KC: Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int 76: 977–983, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Matias PJ, Ferreira C, Jorge C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A: 25-Hydroxyvitamin D3, arterial calcifications and cardiovascular risk markers in haemodialysis patients. Nephrol Dial Transplant 24: 611–618, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Wang AY, Lam CW, Sanderson JE, Wang M, Chan IH, Lui SF, Sea MM, Woo J: Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: A 3-y prospective cohort study. Am J Clin Nutr 87: 1631–1638, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Tabata T, Shoji T, Kikunami K, Matsushita Y, Inoue T, Tanaka S, Hino M, Miki T, Nishizawa Y, Morii H: In vivo effect of 1 alpha-hydroxyvitamin D3 on interleukin-2 production in hemodialysis patients. Nephron 50: 295–298, 1988 [DOI] [PubMed] [Google Scholar]
- 48. Panichi V, De Pietro S, Andreini B, Bianchi AM, Migliori M, Taccola D, Giovannini L, Tetta C, Palla R: Calcitriol modulates in vivo and in vitro cytokine production: A role for intracellular calcium. Kidney Int 54: 1463–1469, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Cohen ML, Douvdevani A, Chaimovitz C, Shany S: Regulation of TNF-alpha by 1alpha,25-dihydroxyvitamin D3 in human macrophages from CAPD patients. Kidney Int 59: 69–75, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Tabata T, Suzuki R, Kikunami K, Matsushita Y, Inoue T, Inoue T, Okamoto T, Miki T, Nishizawa Y, Morii H: The effect of 1 alpha-hydroxyvitamin D3 on cell-mediated immunity in hemodialyzed patients. J Clin Endocrinol Metab 63: 1218–1221, 1986 [DOI] [PubMed] [Google Scholar]
- 51. Alpdogan O, Ozgun S, Lawrence R, Ozener C, Akoglu E, Akoglu T: The effect of 1,25 dihydroxyvitamin D3 on lymphocyte transformation in patients with chronic renal failure. Intern Med 34: 240–242, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Jr, Koeffler HP, Thadhani R: Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis 48: 418–424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dukkipati R, Kovesdy CP, Colman S, Budoff MJ, Nissenson AR, Sprague SM, Kopple JD, Kalantar-Zadeh K: Association of relatively low serum parathyroid hormone with malnutrition-inflammation complex and survival in maintenance hemodialysis patients. J Ren Nutr 20: 243–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]