Abstract
Summary
Background and objectives
Lanthanum carbonate (LC) is a nonaluminum, noncalcium phosphate binder that is effective for hyperphosphatemia in dialysis patients. However, its efficacy and cost-effectiveness as second-line therapy have not been fully examined.
Design, setting, participants, & measurements
We first conducted a multicenter, open-label, 16-week clinical trial to examine the effect of additive LC in 116 hemodialysis patients who had uncontrolled hyperphosphatemia with conventional phosphorus-lowering therapy alone. Based on these clinical data, a state transition model was developed to evaluate the benefits and costs associated with LC as second-line therapy. Reduced risks for cardiovascular morbidity and mortality among patients treated with LC arise through more of the population achieving the target phosphorus levels. Uncertainty was explored through sensitivity analysis.
Results
After 16 weeks of additive LC treatment, mean serum phosphorus levels decreased from 7.30 ± 0.90 to 5.71 ± 1.32 mg/dl, without significant changes in serum calcium or intact parathyroid hormone levels. A subsequent cost-effectiveness analysis showed that compared with conventional treatment, additive LC incurred an average additional lifetime cost of $22,054 per person and conferred an additional 0.632 quality-adjusted life years (QALYs). This resulted in an incremental cost-effectiveness ratio of $34,896 per QALY gained. Applying a cost-effectiveness threshold of $50,000 per QALY, a probabilistic sensitivity analysis showed that additive LC had a 97.4% probability of being cost-effective compared with conventional treatment.
Conclusions
Our results indicate that the use of LC as second-line therapy would be cost-effective among hemodialysis patients with uncontrolled hyperphosphatemia in Japan.
Introduction
The number of patients with ESRD has grown exponentially worldwide (1), causing a progressive increase in the expenditure of dialysis treatment. The Medicare cost for ESRD in the US has reached $23.9 billion, 5.8% of the entire Medicare budget (1). In Japan, the total cost exceeds $13.0 billion, which accounts for approximately 4% of the total health expenditure (2). Chronic kidney disease-mineral and bone disorder (CKD-MBD) is a common complication of dialysis patients and is substantially related to the increased expenditure, because treatment for this disease itself and its related cardiovascular diseases (CVDs) and bone fractures causes a high economic burden. It is, therefore, important to assess the cost-effectiveness of new medications for CKD-MBD management (3).
Hyperphosphatemia is a key factor in CKD-MBD and is linked to cardiovascular and all-cause mortality in ESRD patients. Because dietary phosphorus restriction and phosphorus removal by dialysis modalities are not sufficient to control serum phosphorus levels within recommended ranges, treatment with oral phosphate binders is frequently required for these patients. However, despite the widespread use of phosphate binders, 30% to 40% of dialysis patients still show serum phosphorus levels >6 mg/dl, possibly resulting from limited use of these agents because of their toxicity and/or tolerability (4–6).
Lanthanum carbonate (LC) is a new nonaluminum, noncalcium phosphate binder with high efficacy, low pill burden, and low toxicity (7,8). However, the cost-effectiveness of LC has not been fully examined. Recently, we estimated the cost-effectiveness of LC as first-line therapy in hemodialysis patients in Japan and showed that LC is unlikely to be cost-effective compared with calcium carbonate (9). Thus, LC seems more suited as second-line therapy for patients with uncontrolled hyperphosphatemia. To the best of our knowledge, however, no extensive study investigated the clinical efficacy of LC as second-line therapy, and only one study examined the cost-effectiveness of LC as second-line therapy, but using data derived from a clinical trial of LC as first-line therapy (10).
Therefore, the aim of this study was to evaluate the clinical efficacy of LC as second-line therapy in hemodialysis patients in Japan and to assess the cost-effectiveness of additive LC treatment based on the clinical data.
Materials and Methods
Clinical Trial
Study population.
Study candidates were >18 years of age and had received hemodialysis for at least 3 months. The eligibility criteria were (1) serum phosphorus levels ≥6.0 mg/dl and (2) receiving calcium-based phosphate binders and/or sevelamer hydrochloride or could not receive these agents because of adverse effects. Hospitalized patients were excluded. The study was conducted in accordance with the Declaration of Helsinki principles, and informed consent was obtained from all patients before their enrollment. The study protocol was approved by the institutional review board at each study site. This study is registered with the Universal hospital Medical Information Network clinical trials registry, no. UMIN000002058.
Study design.
This was a multicenter, open-label, 16-week study conducted at seven dialysis centers in Japan between May and December 2009. All study subjects received LC at an initial dose of 750 mg/d. The doses were titrated at 2-week intervals to a maximum 2250 mg/d to achieve the Japanese target phosphorus ranges (3.0 to 6.0 mg/dl) (11). When serum phosphorus levels decreased below 6.0 mg/dl, reductions in the dosages of calcium-based phosphate binders or sevelamer hydrochloride were permitted. No restrictions were imposed on the choice of phosphate binders to be reduced or on the use of vitamin D sterols and cinacalcet hydrochloride. Dialysate calcium levels remained unchanged throughout the study.
Blood samples were collected at the start of the dialysis session after the longest interdialytic period. Serum calcium, phosphorus, and albumin levels were measured every 2 weeks and serum intact parathyroid hormone (PTH) levels every 4 weeks at local laboratories. Serum intact PTH levels were determined using an electrochemiluminescence immunoassay (Elecsys PTH; Roche Diagnostics, Mannheim, Germany). Serum calcium levels were corrected for albumin concentration using the original Payne's equation (12). Medication data were collected every 4 weeks. Safety was evaluated by reports of adverse events.
Statistical analysis.
Continuous variables are presented as means ± SD, means ± SEM, or median (interquartile range). Categorical variables are presented as percentages. Changes in laboratory values and medication dosages were analyzed using one-way repeated-measures ANOVA and the Friedman test, respectively. P < 0.05 was considered statistically significant. All analyses were performed using Dr. SPSS II for Windows, version 11.01J (SPSS Japan, Tokyo, Japan).
Cost-Effectiveness Analysis
Model structure.
The model-based cost-effectiveness analysis was conducted from the healthcare system perspective in Japan. A patient-level state transition model was constructed in TreeAge Pro 2009 (TreeAge Software, Williamstown, MA) to predict lifetime costs and effects (quality-adjusted life years [QALYs]) associated with additive LC treatment compared with conventional treatment alone. The starting age is 60 years, based on the mean age of the trial participants. The incremental cost-effectiveness ratio (ICER) was calculated using the following formula: ICER = (Costadditive LC − Costconventional treatment)/(QALYadditive LC − QALYconventional treatment).
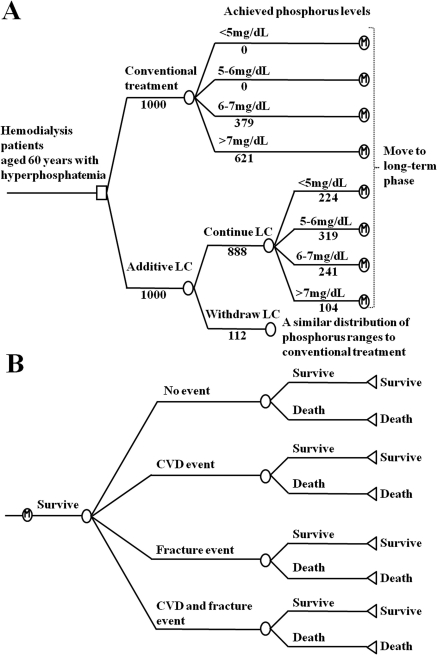
The state transition model has two components: a short-term phase (Figure 1A) and a long-term phase (Figure 1B). In the short-term phase, a transition to four different phosphorus ranges occurs (<5, 5 to <6, 6 to <7, and ≥7 mg/dl), depending on the treatment received. In the additive LC arm, the distribution of patients in each phosphorus range was derived from the clinical trial data, using the phosphorus levels after 12 to 16 weeks of LC treatment (Table 1). The distribution of patients in the conventional treatment arm was derived from the clinical trial data at baseline. Patients who withdraw from LC treatment are assumed to switch back to conventional treatment, and the distribution of these patients in each phosphorus range is assumed to be similar to that of the conventional treatment arms. The proportion of withdrawal was derived from the clinical trial (Table 2). The costs and effects of patients who withdraw from LC treatment are included in the additive LC arm. In the long-term phase, patients are vulnerable to various risks such as CVD, bone fracture, and death. During each cycle of the model, patients maintain their achieved phosphorus levels and experience one of the following clinical events: “no event,” “CVD event,” “fracture event,” and “CVD and fracture event.” The cycle length of the long-term phase was set to 1 year.
Figure 1.
Structure of state transition model. (A) Short-term phase representing 16 weeks of initial therapy. Patients receive either additional LC or conventional treatment and are distributed in each phosphorus range (<5, 5 to <6, 6 to <7, and ≥7 mg/dl). Patient numbers are shown for the base case. (B) Long-term phase representing clinical course after initial therapy. Each patient experiences one of the following clinical events: “no event,” “CVD event,” “fracture event,” and “CVD and fracture event.” Risks for CVD, fracture, and death are estimated from their age, achieved phosphorus levels, and history of experienced events. Patients who survived repeat the cycle until their death.
Table 1.
Initial transition probability for phosphorus ranges
| Phosphorus Ranges |
Plausible Range | Distributions | Source | ||||
|---|---|---|---|---|---|---|---|
| <5 mg/dl | 5 to 6 mg/dl | 6 to 7 mg/dl | ≥7 mg/dl | ||||
| Conventional treatment arm | 0.000 | 0.000 | 0.379 | 0.621 | − | Beta | Original data |
| Additive LC arm | 0.252 | 0.359 | 0.272 | 0.117 | − | Dirichlet | Original data |
LC, lanthanum carbonate.
Table 2.
Transition probabilities
| Base Case Value | Plausible Range | Distributions | Source | |
|---|---|---|---|---|
| Withdrawal of LC due to adverse events | 0.112 | 0.05 to 0.2 | Beta | Original data |
| Probabilities for CVD or fracture event | ||||
| CVD event | 0.055 | ±20% | − | (15, 16) |
| fracture event | 0.012 | ±20% | − | (15, 16) |
| CVD and fracture event | 0.001 | ±20% | − | (15, 16) |
| Annual mortality rate | ||||
| CVD | 0.021 | ±20% | − | (15, 16) |
| non-CVD | 0.030 | ±20% | − | (14) |
CVD, cardiovascular disease.
Transition probabilities.
A transition probability (P) of an event occurring over a time interval (t) was calculated using a rate (r) according to the following formula: P = 1 − exp(−rt) (13). The incidence rates of CVD, fracture, and death were obtained from a nationwide registry data of dialysis patients in Japan (14) and the Q-cohort study, a large-scale, prospective observational study conducted in Kyushu area in Japan (15,16) (Table 2). Because the direct effects of LC treatment on the risk of CVD and mortality have not been examined in randomized controlled trials, we estimated CVD rates and age-dependent mortality rates based on phosphorus ranges transited, using observational data from Japanese studies (14,17,18) (Tables 3 and 4). With regard to bone disease, there is no sufficient evidence that links better phosphorus management to decreased fracture risks in hemodialysis patients (4,19–21); therefore, we estimated fracture rates based on each patient's age, using data from large observational studies (19) (Table 4).
Table 3.
RR for clinical events according to phosphorus ranges
| Phosphorus Ranges |
Plausible Range | Distributions | Source | ||||
|---|---|---|---|---|---|---|---|
| <5 mg/dl | 5 to 6 mg/dl | 6 to 7 mg/dl | ≥7 mg/dl | ||||
| RR for mortality | |||||||
| Japan | 0.880 | 0.934 | 0.991 | 1.370 | −80 to 0% | − | (l) |
| US | 0.842 | 0.896 | 1.047 | 1.341 | − | − | (4) |
| RR for CVD | |||||||
| Japan | 0.673 | 0.971 | 1.145 | 1.204 | −80 to 0% | − | (17) |
| US | 0.894 | 0.983 | 1.028 | 1.165 | − | − | (4) |
RR, relative risk.
Table 4.
RR for clinical events according to age
| Base Case Value | Plausible Range | Distributions | Source | |
|---|---|---|---|---|
| RR for mortality according to age (per year) | 1.057 | — | Log-normal | (18) |
| RR for fracture according to age ranges | ||||
| 18 to 54 years old | 0.302 | — | — | (19) |
| 55 to 64 years old | 0.653 | — | — | (19) |
| 65 to 74 years old | 1.151 | — | — | (19) |
| >75 years old | 2.096 | — | — | (19) |
RR, relative risk.
Costs.
Drug costs were based on the price set by the Ministry of Health, Labor, and Welfare in Japan in 2010 (22) (Table 5). Drug dosage in the additive LC arm was derived from the clinical trial data at 12 to 16 weeks of LC treatment, whereas that of the conventional treatment arm was derived from the trial data at baseline. We did not include the cost of dialysis in base-case analysis; instead, the effect of its inclusion was examined in the sensitivity analysis. Costs for CVD and fracture events and dialysis treatment were derived from Japanese data (15,16,23–25). All of the costs were calculated in Japanese Yen and converted to US dollars ($1 = ¥100).
Table 5.
Unit cost
| Variable | Base Case Value ($) | Plausible Range | Distributions | Source |
|---|---|---|---|---|
| Annual drug cost | ||||
| additive LC arm | 5,055 | — | Gamma | (22) |
| conventional treatment arm | 2,900 | — | Gamma | (22) |
| Drug prices | ||||
| LC (250 mg) | 1.941 | — | — | (22) |
| sevelamer hydrochloride (250 mg) | 0.301 | — | — | (22) |
| calcium carbonate (500 mg) | 0.063 | — | — | (22) |
| calcium lactate (1 g) | 0.037 | — | — | (22) |
| oral vitamin D sterols | ||||
| calcitriol (0.25 μg) | 0.375 | — | — | (22) |
| alfacalcidol (0.25 μg) | 0.255 | — | — | (22) |
| falecalcitriol (0.15 μg) | 3.520 | — | — | (22) |
| intravenous vitamin D sterols | ||||
| calcitriol (0.5 μg) | 11.470 | — | — | (22) |
| maxacalcitol (2.5 μg) | 12.730 | — | — | (22) |
| cinacalcet hydrochloride (25 mg) | 5.351 | — | — | (22) |
| CVD event | 15,062 | ±20% | Dirichlet | (15, 16, 23) |
| Fracture event | 22,079 | ±20% | Gamma | (24) |
| Annual dialysis cost | 0 | Alternate value: 45,178 | Gamma | (25) |
LC, lanthanum carbonate; CVD, cardiovascular disease.
Utilities.
Health benefits were expressed as QALYs, a measure of life expectancy weighted by the health-state utility, where complete health and death have a score of 1 and 0, respectively. The utility values for event-free hemodialysis patients were calculated based on the Short Form-36 among Japanese hemodialysis patients (26–28) (Table 6). The utilities for patients who experienced other clinical events were calculated by multiplying the event-free utility values by the utility associated with various clinical events. The utilities for various clinical events were obtained from the literature (15,16,29–32). An annual discount rate of 3% for all costs and health benefits was applied.
Table 6.
Utility values used in the model
| Variable | Base Case Value | Plausible Range | Distributions | Source |
|---|---|---|---|---|
| Event free | 0.680 | ±20% | Beta | (26–28) |
| CVD | ×0.704 | ±20% | Conditional beta | (15, 16, 29, 30) |
| Fracture event | ×0.759 | ±20% | Conditional beta | (32) |
| CVD and fracture events | ×0.534 | ±20% | Conditional beta | (15, 16, 29, 30, 32) |
| History of CVD | ×0.787 | ±20% | Conditional beta | (15, 16, 31) |
| History of fracture | ×0.855 | ±20% | Conditional beta | (32) |
| History of CVD and fracture | ×0.673 | ±20% | Conditional beta | (15, 16, 31, 32) |
CVD, cardiovascular disease.
Sensitivity analysis.
One-way sensitivity analysis was conducted to assess the effects of changes in key parameters on base-case result. Assessed key parameters are shown in Tables 1 to 6. We also conducted one-way sensitivity analysis by using data derived from a US study (4) for the effect of serum phosphorus on the risk of CVD and mortality. A probabilistic sensitivity analysis was performed to examine parameter uncertainty. Each set of random input values was obtained from their distributions for 1000 patients, and the results were iterated 1000 times. We constructed a cost-effectiveness acceptability curve and determined the proportion of simulations in which additive LC would be preferred, assuming a willingness-to-pay of $50,000 per QALY gained.
Results
Clinical Trial
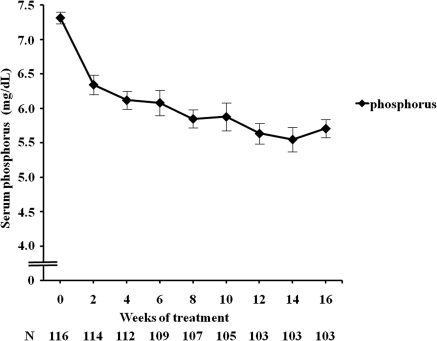
A total of 136 patients were enrolled in the study, of whom 20 were excluded from the study because pretreatment serum phosphorus was decreased to <6 mg/dl. Baseline characteristics of the patients (n = 116) are shown in Table 7. After 16 weeks of LC treatment, serum phosphorus decreased significantly from 7.30 ± 0.90 to 5.71 ± 1.32 mg/dl (Figure 2). There were no significant changes in serum calcium and intact PTH levels (Supplemental Figure 1). At the end of the study, the mean dose of LC was 988 ± 447 mg/d. The doses of sevelamer hydrochloride and calcium-based phosphate binders were progressively decreased, and the doses of vitamin D sterols and cinacalcet hydrochloride remained unchanged (Supplemental Table 1). Adverse events were reported in 16 patients (Supplemental Table 2). Nine patients withdrew because of adverse events. Two patients withdrew because they could not chew the LC tablet. Two patients withdrew because of refusal to take LC.
Table 7.
Baseline characteristics of the study participants (n = 116)
| All patients (n = 116) | |
|---|---|
| Age (years) | 58.4 ± 14.4 |
| Males (%) | 61 |
| Duration of hemodialysis (months) | 123 (60 to 190) |
| Cause of CKD (%) | |
| GN | 73 |
| diabetes | 12 |
| cystic kidney disease | 3 |
| hypertension | 3 |
| others | 9 |
| Corrected calcium (mg/dl) | 9.35 ± 0.72 |
| Phosphorus (mg/dl) | 7.31 ± 0.90 |
| 6 to <7 mg/dl (%) | 38 |
| ≥7 mg/dl (%) | 62 |
| Intact PTH (pg/ml) | 217 (142 to 323) |
| Medication (%) | |
| sevelamer hydrochloride | 62 |
| calcium carbonate | 66 |
| calcium lactate | 3 |
| oral vitamin D sterols | |
| calcitriol | 9 |
| alfacalcidol | 22 |
| falecalcitriol | 2 |
| intravenous vitamin D sterols | |
| calcitriol | 5 |
| maxacalcitol | 44 |
| cinacalcet hydrochloride | 29 |
Continuous variables are presented as mean ±SD or median (interquartile range), as appropriate. Categorical variables are presented as a percentage.
Figure 2.
Mean (±SE) serum phosphorus during the study.
Cost-Effectiveness Analysis
Base-case results for the incremental cost-effectiveness of additive LC are shown in Table 8. The total cost per patient for conventional treatment and additive LC was $38,781 and $60,835, respectively, and the mean QALYs were 5.771 and 6.403, respectively. Compared with conventional treatment, additive LC resulted in an increase in cost and QALYs by $22,054 and 0.632, respectively. This result provided an ICER of $34,896 per QALY gained.
Table 8.
Results of the base-case analysis
| Cost ($) | QALYs | Incremental Costs ($) | Incremental QALYs | ICER ($/QALY) | |
|---|---|---|---|---|---|
| Conventional treatment | 38,781 | 5.771 | — | — | — |
| Additive LC | 60,835 | 6.403 | 22,054 | 0.632 | 34,896 |
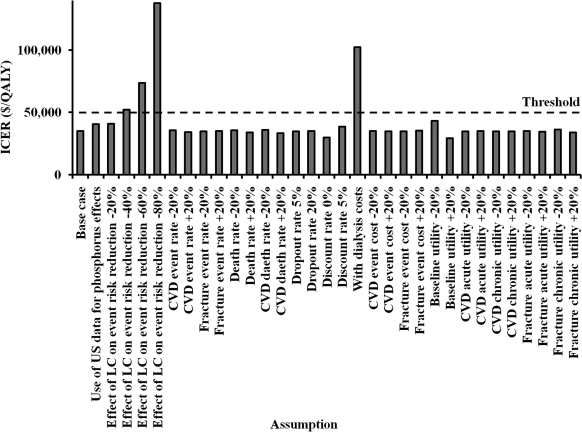
One-way sensitivity analysis showed that the ICER was not sensitive to changes in various key parameters (Figure 3). When relative risks of mortality and CVD according to each phosphorus range were derived from a US study (4), the ICER still remained below $50,000 per QALY gained. When the effect of improved phosphorus management on the incidence of CVD and mortality is assumed to be reduced by 40%, the ICER was increased to approximately $50,000 per QALY gained. When the dialysis cost was included in the analysis, however, the ICER dramatically increased to $102,523 per QALY gained.
Figure 3.
One-way sensitivity analysis of ICER for additive LC.
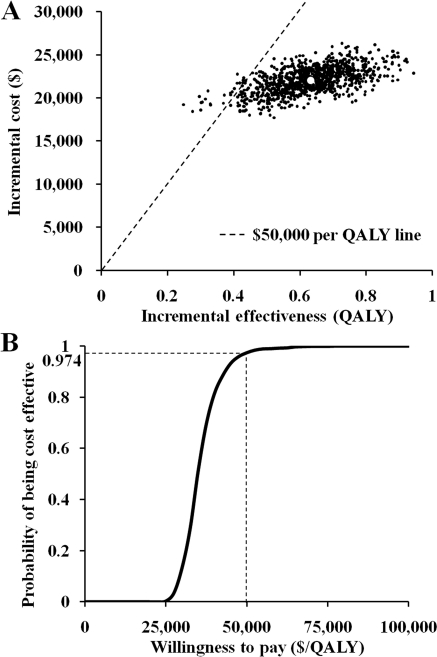
The results of the probabilistic sensitivity analysis are shown as a scatterplot (Figure 4A) and cost-effectiveness acceptability curve (Figure 4B). Based on a willingness to pay of $50,000 per QALY gained, additive LC showed a 97.4% probability of being cost-effective compared with conventional treatment.
Figure 4.
(A) Simulation output (1000 trials) for cost-effectiveness of additive LC. (B) Cost-effectiveness acceptability curve showing the probability that additive LC is cost-effective at various levels of willingness to pay.
Discussion
Our clinical trial showed that LC is effective and well tolerated as second-line therapy for uncontrolled hyperphosphatemia in hemodialysis patients. In addition, by using these data in the simulation analysis, we showed that additive LC treatment is cost-effective compared with conventional phosphorus-lowering treatment in Japan.
The cost-effectiveness of LC as second-line therapy has also been examined by Brennan et al. (10). In their economic analysis, however, the investigators used data derived from a clinical trial of LC as first-line therapy and did not include sevelamer hydrochloride in the analysis. In contrast, in this study, we first conducted a clinical trial to survey the costs and effects of LC as second-line therapy, as well as its effects on other medications related to CKD-MBD, for the subsequent cost-effectiveness analysis. Therefore, we believe that our findings are clinically more relevant to the actual healthcare settings compared with the results of previous studies.
The most important limitation to this study is that the long-term effect of LC on clinical outcomes is estimated from changes in serum phosphorus. It should be noted that the possible benefits expected from the effect of phosphate binders on surrogate markers are not always consistent with the real benefits. For example, the first pharmacoeconomic analysis of sevelamer hydrochloride (33) used surrogate endpoints (vascular calcification scores and progression) from the Treat to Goal study (34) and showed that sevelamer hydrochloride was cost-effective compared with calcium-based binders. However, this result was not confirmed by subsequent pharmacoeconomic analyses (35,36) based on the Dialysis Clinical Outcomes Revisited trial (37,38), which examined the effect of sevelamer hydrochloride on mortality, CVD morbidity, and CVD hospitalization compared with calcium-based binders. Thus, using surrogate endpoints to model the probability of hard endpoints is a major assumption, and the results should be interpreted with caution. However, several observational studies have shown an association between high serum phosphorus and increased risk of CVD and death (4–6,39–41), and two observational studies have shown an association between use of any phosphate binder and improved survival in CKD patients (42,43). Despite the fact that these studies do not prove a causal relationship between lowering serum phosphorus with phosphate binders and improved survival, until a clinical trial proves otherwise, it seems reasonable to use serum phosphorus as a surrogate maker to estimate the potential effect of LC on patient outcomes.
In this study, we also assessed whether the uncertainty of the efficacy of LC influences the results through sensitivity analysis. We found that the use of US data (4) for the effect of serum phosphorus on the risk of CVD and mortality did not alter the results of base-case analysis. Furthermore, even if we assumed that the reduction in the risk of clinical events among patients treated with LC is reduced by 40%, the ICER was still approximately $50,000 per QALY gained.
Changes in adherence to phosphate binders over time may also affect study results. However, we did not examine this point in the sensitivity analysis, because of difficulties in predicting the effect on either costs or clinical outcomes. Thus, although long-term clinical trials have shown that LC maintains reduction in serum phosphorus over time (44), further studies are needed to examine the effect of adherence to LC on phosphorus management and its resultant clinical outcomes.
Our cost-effectiveness analysis was performed from the healthcare system perspective in Japan. Thus, we mainly used data derived from Japanese studies, some of which are different between Japan and other countries. However, we evaluated parameter uncertainty through sensitivity analyses by varying model inputs for some key parameters that may vary from country to country to assess impact on the ICER. These results seems worthy of consideration with regard to the application of our results to other populations. First, it is well known that one of the features of hemodialysis patients in Japan is longevity (45); however, changes in mortality rates had little impact on the ICER as shown in Figure 3. Differences in underlying primary kidney disease may also affect the incidences of CVD, fracture, and death, but changes in these incidence rates had little impact on the ICER in our analysis. Because the average doses of phosphate binders used in the US are 1.5 to 2.5 times greater than those in Japan (34,46–49), we performed additional cost-effectiveness analysis in which the doses of phosphate binders were doubled and their costs were derived from the US data (Table 9) (50–52). In this simulation, the ICERs based on the US drug prices in 2008, 2009, and 2010 were $47,240, $57,591, and $68,441 per QALY gained, respectively. Although healthcare decision makers in the US generally agree that interventions that cost less than $50,000 per QALY gained are reasonably efficient, it should be noted that the choice of an efficient cost-effectiveness threshold is dependent on several factors, and several cost-effectiveness analyses considered $50,000 to $100,000 per QALY gained to be cost-effective (53–55). Thus, the results of our additional analysis suggest that LC may also be cost-effective for uncontrolled hyperphosphatemia patients receiving hemodialysis in the US, unless the dose or cost of LC increases further. We believe that our analytical approach will provide useful information for the analysis of the cost-effectiveness of LC in other countries.
Table 9.
Prices of phosphate binders in Japan and the USa
| Cost ($) | Source | ||
|---|---|---|---|
| Lanthanum carbonate (per 500 mg) | Japan, 2010 | 3.88 | (22) |
| US, 2008 | 2.59 | (50) | |
| US, 2009 | 3.21 | (51) | |
| US, 2010 | 3.75 | (52) | |
| Sevelamer hydrochloride (per 800 mg) | Japan, 2010 | 0.96 | (22) |
| US, 2008 | 2.05 | (50) | |
| US, 2009 | 2.56 | (51) | |
| US, 2010 | 2.56 | (52) | |
| Calcium carbonate (per 500 mg) | Japan, 2010 | 0.06 | (22) |
| US, 2008 | 0.04 | (50) | |
| US, 2009 | 0.04 | (51) | |
| US, 2010 | 0.04 | (52) |
Drug costs in the US were derived from the average wholesale price.
Finally, it should be noted that we did not include the cost of dialysis therapy in the base-case analysis. Whether cost-effectiveness analyses of dialysis patients should include the cost of dialysis is a matter of discussion. Because the cost of dialysis is very high, inclusion of its cost in the analysis could result in refusing acceptance of interventions that are relatively inexpensive but improve patient survival (56). Thus, although it still remains controversial, exclusion of the cost of dialysis in economic analysis is generally considered appropriate.
In conclusion, our study suggests that the use of LC as second-line therapy is effective and cost-effective for hemodialysis patients in Japan. Further studies are needed to determine the validity of our simulation model and to ascertain more definite evidence for the benefits and costs of LC therapy for patients with uncontrolled hyperphosphatemia.
Disclosures
M.F. has received consultant fees and research grants from Bayer Yakuhin, Japan. I.K. has received consultant fees from Bayer Schering Pharma AG. The other authors have no conflicts of interests.
Acknowledgments
We thank the following investigators who participated in the study: Dr. Masahiko Sugiki, Dr. Shohei Nakanishi, and Mr. Yasushi Shimizu. Preliminary results of this study were presented in part at the XLVII ERA-EDTA Congress in Munich, Germany, on June 28, 2010.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org
References
- 1. U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2009 [Google Scholar]
- 2. Fukuhara S, Yamazaki C, Hayashino Y, Higashi T, Eichleay MA, Akiba T, Akizawa T, Saito A, Port FK, Kurokawa K: The organization and financing of end-stage renal disease treatment in Japan. Int J Health Care Finance Econ 7: 217–231, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Komaba H, Moriwaki K, Kamae I, Fukagawa M: Towards cost-effective strategies for treatment of chronic kidney disease-mineral and bone disorder in Japan. Ther Apher Dial 13[Suppl 1]: S28–S35, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Nakai S, Masakane I, Shigematsu T, Hamano T, Yamagata K, Watanabe Y, Itami N, Ogata S, Kimata N, Shinoda T, Syouji T, Suzuki K, Taniguchi M, Tsuchida K, Nakamoto H, Nishi S, Nishi H, Hashimoto S, Hasegawa T, Hanafusa N, Fujii N, Marubayashi S, Morita O, Wakai K, Wada A, Iseki K, Tsubakihara Y: An overview of regular dialysis treatment in Japan (as of 31 December 2007). Ther Apher Dial 13: 457–504, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Hutchison AJ: Oral phosphate binders. Kidney Int 75: 906–914, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Tonelli M, Pannu N, Manns B: Oral phosphate binders in patients with kidney failure. N Engl J Med 362: 1312–1324, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Goto S, Komaba H, Moriwaki K, Kamae I, Fukagawa M: The cost-effectiveness for first-line lanthanum carbonate therapy of Japanese dialysis patients with hyperphosphatemia [Abstract]. J Am Soc Nephrol 20: 538A, 2009 [Google Scholar]
- 10. Brennan A, Akehurst R, Davis S, Sakai H, Abbott V: The cost-effectiveness of lanthanum carbonate in the treatment of hyperphosphatemia in patients with end-stage renal disease. Value Health 10: 32–41, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Guideline working group, Japanese Society for Dialysis Therapy: Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Aphr Dial 12: 511–522, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Payne RB, Little AJ, Williams RB, Milner JR: Interpretation of serum calcium levels in patients with abnormal serum proteins. BMJ 4: 643–646, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drummond MF, McGuire A: Economic Evaluation in Health Care: Merging Theory With Practice, New York, Oxford University Press, 2001 [Google Scholar]
- 14. Nakai S, Akiba T, Kazama J, Yokoyama K, Fukagawa M, Tominaga Y, Iseki K, Tsubakihara Y: Patient Registration Committee of the Japanese Society for Dialysis Therapy, Tokyo, Japan: Effects of serum calcium, phosphorous, and intact parathyroid hormone levels on survival in chronic hemodialysis patients in Japan. Ther Apher Dial 12: 49–54, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Yamada S, Taniguchi M, Fujisaki K, Tsuruya K, Hirakata H, Iida M: Incidence and risk factors for bone fractures among hemodialysis patients in the Q-Cohort Study: A prospective multicenter cohort study in Japan [Abstract]. J Jpn Soc Dial Ther 41[Suppl 1]: S597, 2008. (in Japanese) [Google Scholar]
- 16. Taniguchi M, Yamada S, Tsuruya K, Iida M, Hirakata H: Association between cardiovascular events and bone fractures among hemodialysis patients in the Q-Cohort Study: A prospective multicenter cohort study in Japan [Abstract]. Kidney Metab Bone Dis 21: 352, 2008. (in Japanese) [Google Scholar]
- 17. Kimata N, Albert JM, Akiba T, Yamazaki S, Kawaguchi T, Fukuhara S, Akizawa T, Saito A, Asano Y, Kurokawa K, Pisoni RL, Port FK: Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: The Japan dialysis outcomes and practice patterns study. Hemodial Int 11: 340–348, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Nakai S, Shinzato T, Sanaka T, Kikuchi K, Kitaoka T, Shinoda T, Yamazaki C, Sakai R, Omori H, Morita O, Iseki K, Kubo K, Tabei K, Masakane I, Fushimi K, Wada A, Miwa N, Akiba T: The current state of chronic dialysis treatment in Japan. J Jpn Soc Dial Ther 35: 1155–1184, 2002 [Google Scholar]
- 19. Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL: Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Stehman-Breen CO, Sherrard DJ, Alem AM, Gillen DL, Heckbert SR, Wong CS, Ball A, Weiss NS: Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 58: 2200–2205, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM: PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 47: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Jihou: National Health Insurance Price List, Tokyo, Jihou Press, 2010. (in Japanese) [Google Scholar]
- 23. Saito I, Kobayashi M, Matsushita Y, Mori A, Kawasugi K, Saruta T: Cost-utility analysis of antihypertensive combination therapy in Japan by a Monte Carlo simulation model. Hypertens Res 31: 1373–1383, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Kondo A, Zierler BK, Isokawa Y, Hagino H, Ito Y: Comparison of outcomes and costs after hip fracture surgery in three hospitals that have different care systems in Japan. Health Policy 91: 204–210, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Sugisaki H, Ohta Y, Ohira S, Onoyama O, Kuma H, Suzuki M, Yamakawa T, Yoshida T, Suzuki M, Ono T, Tozawa S, Miyamoto T, Yamazaki C: Cost of dialysis treatment in Japan: Eleventh annual report of the Japanese Association of Dialysis Physicians, 2007. J Jpn Ass Dial Physicians 23: 49–94, 2008. (in Japanese) [Google Scholar]
- 26. Fukuhara S, Lopes AA, Bragg-Gresham JL, Kurokawa K, Mapes DL, Akizawa T, Bommer J, Canaud BJ, Port FK, Held PJ: Worldwide Dialysis Outcomes and Practice Patterns Study: Health-related quality of life among dialysis patients on three continents: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 64: 1903–1910, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Inada O, Nishimura S, Matsushima M, Seino Y, Tsuda K: The direct medical costs and the quality of life in patients with artificial dialysis and type 2 diabetes. J Jpn Diab Soc 50: 1–8, 2007. (in Japanese) [Google Scholar]
- 28. Ara R, Brazier J: Predicting the short form-6D preference-based index using the eight mean short form-36 health dimension scores: Estimating preference-based health-related utilities when patient level data are not available. Value Health 12: 346–353, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Mahoney EM, Jurkovitz CT, Chu H, Becker ER, Culler S, Kosinski AS, Robertson DH, Alexander C, Nag S, Cook JR, Demopoulos LA, DiBattiste PM, Cannon CP, Weintraub WS: TACTICS-TIMI 18 Investigators: Treat angina with aggrastat and determine cost of therapy with an invasive or conservative strategy-thrombolysis in myocardial infarction. Cost and cost-effectiveness of an early invasive vs conservative strategy for the treatment of unstable angina and non-ST-segment elevation myocardial infarction. JAMA 16: 1851–1858, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Pickard AS, Johnson JA, Feeny DH: Responsiveness of generic health-related quality of life measures in stroke. Qual Life Res 14: 207–219, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Hisashige T, Katayama T, Ishibashi T, Imai M, Iwamoto Y, Ohishi M, Ohsawa I, Kawaguchi T, Kishikami H, Yoshikawa R, Kobayashi T, Bando H, Hotta N: Estimation of the quality of the life in patients with diabetes. In: Economic Estimation of Healthcare Service by Disease Management, Report on Research on Health Services 2000, edited by Hisashige T, Tokyo, Ministry of Health, Labour and Welfare of Japan, 2000, pp 91–98 (in Japanese) [Google Scholar]
- 32. Hagino H, Nakamura T, Fujiwara S, Oeki M, Okano T, Teshima R: Sequential change in quality of life for patients with incident clinical fractures: A prospective study. Osteoporos Int 20: 695–702, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Huybrechts KF, Caro JJ, Wilson DA, O'Brien JA: Health and economic consequences of sevelamer use for hyperphosphatemia in patients on hemodialysis. Value Health 8: 549–561, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Chertow GM, Burke SK, Raggi P: Treat to Goal Working Group: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Manns B, Klarenbach S, Lee H, Culleton B, Shrive F, Tonelli M: Economic evaluation of sevelamer in patients with end-stage renal disease. Nephrol Dial Transplant 22: 2867–2878, 2007 [DOI] [PubMed] [Google Scholar]
- 36. St Peter WL, Fan Q, Weinhandl E, Liu J: Economic evaluation of sevelamer versus calcium-based phosphate binders in hemodialysis patients: a secondary analysis using centers for Medicare & Medicaid services data. Clin J Am Soc Nephrol 4: 1954–1961, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK: Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 72: 1130–1137, 2007 [DOI] [PubMed] [Google Scholar]
- 38. St Peter WL, Liu J, Weinhandl E, Fan Q: A comparison of sevelamer and calcium-based phosphate binders on mortality, hospitalization, and morbidity in hemodialysis: A secondary analysis of the Dialysis Clinical Outcomes Revisited (DCOR) randomized trial using claims data. Am J Kidney Dis 51: 445–454, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT: Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group: The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: Association with mortality in dialysis patients. Am J Kidney Dis 46: 925–932, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Isakova T, Gutiérrez OM, Chang Y, Shah A, Tamez H, Smith K, Thadhani R, Wolf M: Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 20: 388–396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kovesdy CP, Kuchmak O, Lu JL, Kalantar-Zadeh K: Outcomes associated with phosphorus binders in men with non-dialysis-dependent CKD. Am J Kidney Dis 56: 842–851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hutchison AJ, Barnett ME, Krause R, Kwan JT, Siami GA: SPD405–309 Lanthanum Study Group: Long-term efficacy and safety profile of lanthanum carbonate: Results for up to 6 years of treatment. Nephron Clin Pract 110: c15–c23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, Held PJ, Young EW: Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 14: 3270–3277, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Finn WF, Joy MS, Hladik G: Lanthanum Study Group: Efficacy and safety of lanthanum carbonate for reduction of serum phosphorus in patients with chronic renal failure receiving hemodialysis. Clin Nephrol 62: 193–201, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Joy MS, Finn WF: LAM-302 Study Group: Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: a new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis 42: 96–107, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Shigematsu T: Lanthanum Carbonate Research Group: Lanthanum carbonate effectively controls serum phosphate without affecting serum calcium levels in patients undergoing hemodialysis. Ther Apher Dial 12: 55–61, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Iwasaki Y, Takami H, Tani M, Yamaguchi Y, Goto H, Goto Y, Goto Y, Shigematsu T: Efficacy of combined sevelamer and calcium carbonate therapy for hyperphosphatemia in Japanese hemodialysis patients. Ther Apher Dial 9: 347–351, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Côté C: Red Book Pharmacy's Fundamental Reference 2008 edition, Montvale, NJ, PDR Network, 2008 [Google Scholar]
- 51. Côté C: Red Book Pharmacy's Fundamental Reference 2009 edition, Montvale, NJ, PDR Network, 2009 [Google Scholar]
- 52. Côté C: Red Book Pharmacy's Fundamental Reference 2010 edition, Montvale, NJ, PDR Network, 2010 [Google Scholar]
- 53. Tonelli M, Winkelmayer WC, Jindal KK, Owen WF, Manns BJ: The cost-effectiveness of maintaining higher hemoglobin targets with erythropoietin in hemodialysis patients. Kidney Int 64: 295–304, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Ito K, Elkin EB, Girotra M, Morris MJ: Cost-effectiveness of fracture prevention in men who receive androgen deprivation therapy for localized prostate cancer. Ann Intern Med 152: 621–6219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sanders GD, Bayoumi AM, Holodniy M, Owens DK: Cost-effectiveness of HIV screening in patients older than 55 years of age. Ann Intern Med 148: 889–903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Manns B, Meltzer D, Taub K, Donaldson C: Illustrating the impact of including future costs in economic evaluations: An application to end-stage renal disease care. Health Econ 12: 949–958, 2003 [DOI] [PubMed] [Google Scholar]