Abstract
Summary
Background and objectives
Whether chronic kidney disease (CKD) should also be considered a coronary disease equivalent like diabetes is not clear.
Design, setting, participants, & methods
Veterans with and without diabetes and with and without CKD were prospectively recruited. A competing Cox regression model was used to describe the risk of myocardial infarction (MI) in the two groups (CKD and diabetes) over a decade of follow-up.
Results
The incidence rate of MI in those without CKD was 0.047/yr and in those with CKD was 0.206/yr. Multivariate adjustment revealed the incident rate ratio for MI in CKD as 3.5 and for diabetes mellitus as 2.5. The cumulative incidence for MI was influenced by CKD and diabetes. CKD was associated with a subhazard ratio for MI of 3.74; in contrast, diabetes was associated with a subhazard ratio for MI of 2.6. For the outcome of all-cause mortality, after multivariate adjustment, CKD was associated with a hazard ratio (HR) of 1.86, which was similar to the HR of 2.27 for prevalent coronary artery disease. The HR for diabetes was NS at 1.35.
Conclusions
CKD is associated with a risk of death similar to that of established coronary artery disease and higher than that of diabetes mellitus. CKD is associated with a risk of MI that is at least as much as that from diabetes mellitus. Among veterans, CKD appears to be a coronary disease equivalent.
Introduction
Compared with the general population, patients with chronic kidney disease (CKD) have an increased burden of cardiovascular disease, cardiovascular events, and all-cause mortality (1–3). Patients with mild CKD, moderate CKD, and those with ESRD on dialysis are all at high risk of cardiovascular events (1,4,5); however, CKD is not considered a coronary disease equivalent. In contrast, patients with diabetes mellitus have an increased burden of cardiovascular disease, cardiovascular events, and all-cause mortality. Diabetes mellitus is not considered simply a cardiovascular risk factor but also a cardiovascular disease equivalent (6). In other words, the presence of type II diabetes mellitus is considered equivalent to having pre-existing atherosclerotic cardiovascular disease. This dichotomy in consideration of these cardiovascular risks of CKD and diabetes mellitus may be because of lack of head-to-head comparisons of these two conditions.
The Veterans Administration (VA) hospitals serve predominantly an older male population with a high prevalence of diabetes mellitus and CKD. These patients often have a high prevalence of cardiovascular risk factors and cardiovascular disease. These patients can thus provide an estimate of risk posed by diabetes mellitus and CKD on the future risk of myocardial infarction (MI). Accordingly, the purpose of this study was to describe the rate of MI, determine the risk factors associated with this risk, and to ascertain the cumulative incidence of MI in the context of competing risk of death. We used a factorial design with CKD and diabetes as risk factors to determine the risk of future MI. A long duration of follow-up that spanned a decade allowed us to capture many events to provide head-to-head information on the comparative hazards of diabetes and CKD on the future risk of MI.
Materials and Methods
Study Cohort
This was a prospective cohort study of patients recruited from the renal clinic and a general medicine clinic of the Richard L. Roudebush Veterans Affairs Medical Center in Indianapolis. Patients were excluded for a body mass index (BMI) > 40 kg/m2, acute renal failure, receiving renal replacement therapy, atrial fibrillation, or a change in their antihypertensive drugs within 2 weeks of study enrollment. These exclusion criteria allowed for accurate BP measurement. CKD was defined as the presence of proteinuria on a spot urine specimen when the protein/creatinine ratio was ≥0.22 g/g or estimated GFR was <60 ml/min per 1.73 m2 by the four-component Modification of Diet in Renal Disease formula: 186 × creatinine−0.1.54 × age−0.203 × 0.74 if female and × 1.21 if black (7). A urine protein/creatinine ratio of >0.22 g/g correlates with a urine protein excretion of >300 mg/d, the standard definition of clinical proteinuria (8). Accordingly, we selected this threshold of urine protein/creatinine ratio to reflect CKD.
The Institutional Review Board of Indiana University and the Research and Development Committee of the Veterans Affairs Medical Center approved this study, and all patients gave their written, informed consent.
A medical history was obtained and physical examination performed on each participant. Coronary artery disease was defined as previous evidence of MI, coronary artery bypass surgery, or percutaneous coronary intervention after consideration of the individual's history and evaluation of his medical records. Diabetes mellitus was defined as treatment with insulin or oral hypoglycemic agents. Actual medications being taken by the subjects were recorded.
A urine creatinine assay was performed with a modification of the Jaffe reaction (Boehringer Mannheim no. 450019) and a urine protein assay was performed with a turbidometric method using benzethonium chloride read at 550 nm using a Hitachi 911 analyzer (Boehringer Mannheim, Roche Diagnostics Corporation, Indianapolis, IN). A single random urine specimen was used from each participant.
Patients received follow-up care, generally three to four annual visits, by their respective physicians at the Veterans Affairs Medical Center. No interventions occurred as a result of participation in the study. We only ascertained the outcomes of MI or mortality at follow-up.
Ascertainment of MI and Mortality
Each patient's electronic medical record was manually examined for notation of MI. Specifically, the diagnosis of MI was not based on an International Classification of Diseases code. The diagnosis of MI required anginal pain or the equivalent (e.g., shortness of breath) and changes on electrocardiogram consistent with a MI or time-concordant elevation of cardiac enzymes (9). The ascertainment of death was established using the computerized VA electronic medical record system. The last date of visit to any VA facility was used to determine the last date of follow-up. In patients who were not seen at a VA facility in the prior 6 months, the social security death index was checked for mortality.
Statistical Analyses
Using a 2 × 2 factorial design with diabetes (present or absent) and CKD (present or absent) as factors, we compared the baseline characteristics for continuous variables using a two-way ANOVA and for categorical variables using the Cochran–Mantel Haenszel test. The incident rate of MI and the incident rate ratio for CKD and diabetes mellitus were calculated using Poisson regression. This rate was further adjusted for age, sex, and race. Multivariate adjustments were then made by using demographic factors, coronary risk factors, pre-existing coronary disease, and coronary risk equivalents. In a model that included indicator variables for CKD and diabetes mellitus, the following variables were used for multivariate adjustment: age, sex, race, prevalent coronary artery disease, ambulatory systolic BP, use of antihypertensive medication, current smoking, angiotensin converting enzyme (ACE) inhibitor (ACE) or angiotensin II receptor blocker (ARB) use, and the use of aspirin or statin.
Estimates of mortality were made by the Kaplan–Meier method. Estimates of time to multiple MIs were made by a Cox proportional hazards model. Proportionality assumption of the model was tested by analyzing the statistical significance of the Schoenfeld residuals. Given the limited number of multiple MIs, more than two MIs were coded as 2. Multivariable adjustments followed the same strategy as above.
A competing risk Cox regression model was used to analyze the independent risk of MI after allowing for the competing risk for death. Competing risks were evaluated using standard procedures with death as the competing outcome for MI using stcrreg in Stata 11.0. All analyses were performed using Stata 11.0 (Stata Corporation, College Station, TX). P values are two-sided and significance was set at 0.05.
Results
The baseline characteristics of the sample are shown in Table 1. Because we have analyzed the risk of MI by CKD and diabetes, the baseline characteristics are broken into these strata. Patients with CKD had the following characteristics that were statistically significant: older, more men, more blacks, fewer nonsmokers, more prevalent coronary artery disease (especially coronary artery bypass grafts), greater numbers of antihypertensive medications (particularly the use of loop diuretics, dihydropyridine calcium channel blockers, β-blockers, α-blockers, ACE inhibitors, or ARBs), greater use of statins and aspirin, higher systolic BP, lower heart rate, lower hemoglobin, and lower cholesterol. Patients with diabetes mellitus had the following characteristics that were statistically significant: greater BMI, more prevalent coronary artery disease (especially MIs and coronary artery bypass grafts), greater number of antihypertensive medications (particularly the use of loop diuretics, centrally acting agents, ACE inhibitors, or ARBs), greater use of statins and aspirin, and lower cholesterol. The presence of diabetes mellitus and CKD (interaction effect) had the following characteristics that were statistically significant: fewer nonsmokers, more loop diuretic use, less ACE inhibitor use, higher ambulatory systolic BP, lower estimated GFR, greater proteinuria, and higher cholesterol.
Table 1.
Baseline characteristics of the study participants by CKD and diabetes mellitus
| Clinical Characteristic | No CKD |
CKD |
Total | P–CKD | P–DM | P–CKD × DM | ||
|---|---|---|---|---|---|---|---|---|
| No DM | DM | No DM | DM | |||||
| n | 126 (30%) | 29 (7%) | 168 (40%) | 100 (24%) | 423 (100%) | |||
| Age (years) | 54.8 ± 13.2 | 57.8 ± 11.7 | 62.8 ± 12.8 | 63.4 ± 10.4 | 60.2 ± 12.8 | <0.0001 | 0.2 | 0.4 |
| Gender | 116 (92%) | 26 (90%) | 161 (96%) | 99 (99%) | 402 (95%) | 0.03 | 0.5 | 0.2 |
| Race | <0.0001 | 0.4 | 0.9 | |||||
| White | 112 (89%) | 26 (90%) | 108 (64%) | 69 (69%) | 315 (74%) | |||
| Black | 14 (11%) | 3 (10%) | 60 (36%) | 31 (31%) | 108 (26%) | |||
| Weight (kg) | 93.6 ± 23.6 | 104.1 ± 28.2 | 90.4 ± 21.5 | 97.4 ± 20.6 | 93.9 ± 22.7 | 0.2 | 0.02 | 0.5 |
| Height (in.) | 69.8 ± 3.0 | 70.0 ± 3.4 | 69.0 ± 3.2 | 69.2 ± 2.7 | 69.3 ± 3.1 | 0.02 | 0.8 | 0.9 |
| BMI (kg/m2) | 29.7 ± 6.7 | 32.9 ± 8.6 | 29.2 ± 6.0 | 31.5 ± 5.9 | 30.1 ± 6.5 | 0.6 | 0.01 | 0.5 |
| History of smoking | <0.01 | 0.7 | 0.02 | |||||
| never | 32 (25%) | 12 (41%) | 32 (19%) | 11 (11%) | 87 (21%) | |||
| past | 47 (37%) | 14 (48%) | 94 (56%) | 63 (63%) | 218 (52%) | |||
| current | 46 (37%) | 3 (10%) | 40 (24%) | 26 (26%) | 115 (27%) | |||
| Prevalence of | ||||||||
| MI | 16 (13%) | 7 (24%) | 28 (17%) | 29 (29%) | 80 (19%) | 0.3 | <0.01 | 0.9 |
| coronary artery bypass graft | 8 (6%) | 2 (7%) | 19 (11%) | 21 (21%) | 50 (12%) | 0.03 | 0.04 | 0.5 |
| percutaneous coronary intervention | 7 (6%) | 4 (14%) | 21 (13%) | 14 (14%) | 46 (11%) | 0.09 | 0.3 | 0.3 |
| coronary artery disease | 21 (17%) | 8 (28%) | 42 (25%) | 37 (37%) | 108 (26%) | 0.05 | 0.01 | 0.9 |
| BP medication use | 77 (61%) | 25 (86%) | 151 (90%) | 99 (99%) | 352 (83%) | <0.0001 | <0.001 | 0.4 |
| BP medications (number) | 1.2 ± 1.3 | 2.1 ± 1.4 | 2.4 ± 1.5 | 3.4 ± 1.3 | 2.3 ± 1.6 | <0.0001 | <0.01 | 0.9 |
| thiazide diuretic use | 26 (21%) | 11 (38%) | 44 (26%) | 26 (26%) | 107 (25%) | 0.8 | 0.3 | 0.1 |
| loop diuretic use | 13 (10%) | 4 (14%) | 51 (30%) | 70 (70%) | 138 (33%) | <0.0001 | <0.0001 | 0.04 |
| dihydropyridine use | 13 (10%) | 3 (10%) | 60 (36%) | 36 (36%) | 112 (26%) | <0.0001 | 1 | 1 |
| nondihydropyridine calcium channel blocker use | 9 (7%) | 5 (17%) | 14 (8%) | 10 (10%) | 38 (9%) | 0.8 | 0.2 | 0.3 |
| β-blocker use | 32 (25%) | 7 (24%) | 72 (43%) | 53 (53%) | 164 (39%) | <0.0001 | 0.2 | 0.4 |
| α-blocker use | 18 (14%) | 5 (17%) | 51 (30%) | 30 (30%) | 104 (25%) | <0.001 | 0.9 | 0.7 |
| centrally acting agent use | 4 (3%) | 1 (3%) | 12 (7%) | 15 (15%) | 32 (8%) | 0.03 | 0.05 | 0.5 |
| vasodilator use | 1 (1%) | 0 (0%) | 10 (6%) | 2 (2%) | 13 (3%) | 0.02 | 0.1 | |
| ACE inhibitor use | 33 (26%) | 22 (76%) | 72 (43%) | 68 (68%) | 195 (46%) | 0.03 | <0.0001 | 0.04 |
| ARB use | 5 (4%) | 3 (10%) | 22 (13%) | 26 (26%) | 56 (13%) | <0.01 | <0.01 | 0.8 |
| statin use | 37 (29%) | 9 (31%) | 84 (50%) | 74 (74%) | 204 (48%) | <0.0001 | <0.001 | 0.07 |
| ASA use | 38 (30%) | 14 (48%) | 81 (48%) | 63 (63%) | 196 (46%) | <0.001 | <0.01 | 0.7 |
| ACE and/or ARB use | 38 (30%) | 24 (83%) | 90 (54%) | 86 (86%) | 238 (56%) | <0.001 | <0.0001 | 0.2 |
| Clinic systolic BP (mmHg) | 137.6 ± 18.5 | 139.8 ± 22.0 | 144.8 ± 20.6 | 153.6 ± 23.7 | 144.4 ± 21.7 | <0.01 | 0.6 | 0.2 |
| Clinic diastolic BP (mmHg) | 83.3 ± 10.6 | 81.0 ± 14.2 | 83.7 ± 12.6 | 78.8 ± 12.1 | 82.2 ± 12.2 | 0.8 | 0.3 | 0.4 |
| Clinic heart rate (bpm) | 73.8 ± 11.9 | 77.9 ± 12.6 | 70.0 ± 12.3 | 70.4 ± 12.5 | 71.8 ± 12.4 | 0.01 | 0.1 | 0.2 |
| Ambulatory systolic BP (mmHg) | 127.8 ± 12.5 | 127.9 ± 13.5 | 131.8 ± 15.2 | 140.4 ± 18.3 | 132.5 ± 15.9 | 0.05 | 1 | 0.05 |
| Ambulatory diastolic BP (mmHg) | 74.9 ± 8.2 | 70.8 ± 8.2 | 75.2 ± 10.6 | 71.5 ± 11.1 | 74.0 ± 10.1 | 0.8 | 0.1 | 0.9 |
| Ambulatory heart rate (bpm) | 74.3 ± 11.2 | 71.8 ± 12.0 | 69.4 ± 10.9 | 70.6 ± 11.4 | 71.3 ± 11.3 | <0.01 | 0.4 | 0.3 |
| Estimated GFR (ml/min per 1.73 m2) | 81.2 ± 14.6 | 82.8 ± 16.9 | 39.3 ± 16.8 | 31.1 ± 14.9 | 52.1 ± 26.9 | <0.0001 | 0.6 | 0.01 |
| CKD stage | <0.001 | |||||||
| 1 | 2 (1%) | 0 (0%) | 2 (1%) | |||||
| 2 | 4 (2%) | 5 (5%) | 9 (3%) | |||||
| 3A | 56 (33%) | 15 (15%) | 71 (26%) | |||||
| 3B | 47 (28%) | 20 (20%) | 67 (25%) | |||||
| 4 | 50 (30%) | 52 (52%) | 102 (38%) | |||||
| 5 | 9 (5%) | 8 (8%) | 17 (6%) | |||||
| Urine protein/creatinine ratio (g/g) (median, IQR) | 0.078 (0.062, 0.106) | 0.096 (0.068, 0.126) | 0.134 (0.068, 0.480) | 0.604 (0.174, 2.44) | 0.118 (0.068, 0.469) | <0.01 | 1 | 0.01 |
| Albumin (g/dl) | 3.8 ± 0.4 | 3.7 ± 0.3 | 3.8 ± 0.4 | 3.7 ± 0.3 | 3.8 ± 0.4 | 0.9 | 0.5 | 0.5 |
| Hemoglobin (g/dl) | 14.6 ± 1.5 | 14.1 ± 1.3 | 13.5 ± 1.7 | 12.4 ± 1.8 | 13.5 ± 1.8 | <0.0001 | 0.1 | 0.2 |
| Cholesterol (mg/dl) | 196.6 ± 37.5 | 177.0 ± 42.8 | 181.0 ± 35.3 | 183.2 ± 39.2 | 185.4 ± 37.9 | <0.01 | 0.02 | 0.02 |
DM, diabetes mellitus; ASA, acetylsalicylic acid; IQR, interquartile range.
Among those with CKD, more than half of the patients had stage 3 CKD and 38% had stage 4 CKD. Patients with diabetes mellitus also had more advanced kidney disease.
Incidence Rate of MI in CKD and Diabetes
Sixty MIs were documented over a decade. Of these, 45 patients experienced single events, 4 patients had two MIs, and 1 each had three and four MIs. Most of these events were in the CKD group; the non-CKD group had only six patients experiencing one MI and one patient having two MIs.
In the non-CKD group, the unadjusted incident rate of MI was 0.047/yr (95% confidence interval [CI] 0.014 to 0.079). In the CKD group, the unadjusted incident rate of MI was 0.206/yr (95% CI 0.150 to 0.262). The incident rate ratio for MI in CKD was 4.4 (95% CI 2.1 to 9.3, P < 0.001). Adjusting for age, sex, and race reduced the incident rate ratio for MI in CKD to 4.3 (95% CI 2.0 to 9.4, P < 0.001).
In the nondiabetic group, the unadjusted incident rate of MI was 0.094/yr (95% CI 0.060 to 0.128). In the diabetic group, the unadjusted incident rate of MI was 0.273/yr (95% CI 0.177 to 0.369). The incident rate ratio for MI in diabetes was 2.9 (95% CI 1.8 to 4.8, P < 0.001). Adjusting for age, sex, and race reduced the incident rate ratio for MI in diabetes to 2.8 (95% CI 1.7 to 4.6, P < 0.001).
In a model containing indicator variables for CKD and diabetes mellitus, multivariate adjustment revealed the incident rate ratio for MI in CKD as 3.5 (95% CI 1.4 to 8.5, P = 0.005) and for diabetes mellitus as 2.5 (95% CI 1.4 to 4.7, P = 0.003). None of the other risk factors besides CKD and diabetes mellitus were significant at the 5% level.
Competing Risks of MI and Mortality
Table 2 shows the subhazard ratios of the competing risk model. The subhazards for MI were increased among patients with CKD and those with diabetes mellitus. The risk of MI was nearly fourfold elevated in preference to all-cause mortality in those with CKD. The competing risk for MI was more than twofold elevated in those with diabetes mellitus. The remaining risk factors did not achieve statistical significance.
Table 2.
Subhazard ratios for MI with competing risk of all-cause mortality
| Clinical Characteristic | HR | 95% CI | P |
|---|---|---|---|
| CKD | 3.74 | 1.02 to 13.76 | 0.05 |
| Prevalent coronary artery disease | 0.52 | 0.21 to 1.29 | 0.16 |
| Age (years) | 1.02 | 0.98 to 1.06 | >0.2 |
| Male gender | 0.25 | 0.05 to 1.21 | 0.08 |
| Black race | 0.7 | 0.28 to 1.76 | >0.2 |
| Ambulatory systolic BP (mmHg) | 1 | 0.98 to 1.02 | >0.2 |
| ACE and/or ARB use | 0.64 | 0.29 to 1.4 | >0.2 |
| DM | 2.6 | 1.25 to 5.39 | 0.01 |
| Aspirin use | 1.14 | 0.54 to 2.42 | >0.2 |
| Statin use | 1.04 | 0.5 to 2.15 | >0.2 |
| BP medication use | 4.51 | 0.49 to 41.84 | 0.19 |
| Current smoker | 1.31 | 0.53 to 3.24 | >0.2 |
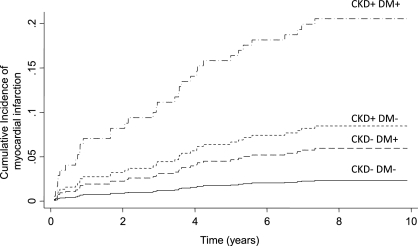
Figure 1 shows the cumulative incidence of MI as a function of CKD and diabetes after accounting for multivariable risks. Compared with patients without CKD and without diabetes mellitus, those with diabetes mellitus had a greater cumulative incidence of MI. However, compared with patients without CKD but with diabetes mellitus, those with CKD but without diabetes had a higher cumulative incidence for MI. It is clear that those with CKD and diabetes mellitus had the greatest subhazard for incident MI of approximately 20% at 10 years.
Figure 1.
Cumulative incidence rates for MI as a function of diabetes and CKD. Cumulative incidence rates assume the covariates shown in Table 2 to be at their means. DM, diabetes mellitus.
Mortality and Risk of Mortality Due to MI
We observed 184 deaths (40 in the non-CKD group, 144 in the CKD group) over a cumulative follow-up of 1112 patient-years in the non-CKD group and 1663 patient-years in the CKD group. This yielded a crude mortality rate of 3.59 per 100 patient-years in the non-CKD group and 8.66 per 100 patient-years in the CKD group (P < 0.001 by log-rank test).
Of the 184 deaths, 108 occurred among nondiabetics and 76 among diabetics over a cumulative follow-up of 2036 patient-years in the nondiabetic group and 739 patient-years in the diabetic group. This yielded a crude mortality rate of 5.30 per 100 patient-years in the nondiabetic group and 10.28 per 100 patient-years in the diabetic group (P < 0.001 by log-rank test).
Table 3 shows the risk for all-cause mortality for CKD and MIs. As expected, CKD increased the all-cause mortality rate 1.86-fold. Prevalent coronary artery disease more than doubled this risk, whereas incident MI more than tripled this risk. Having multiple MIs had a risk of death that was similar to having one MI. Diabetes mellitus was not associated with a statistically significant elevation in all-cause mortality.
Table 3.
HRs for all-cause mortality
| Clinical Characteristic | HR | 95% CI | P |
|---|---|---|---|
| CKD | 1.86 | 1.15 to 3.01 | 0.01 |
| Number of myocardial infarctions | |||
| 1 | 3.92 | 2.43 to 6.31 | <0.0001 |
| ≥2 | 3.17 | 0.98 to 10.25 | 0.06 |
| Prevalent coronary artery disease | 2.27 | 1.5 to 3.42 | <0.0001 |
| Age (years) | 1.03 | 1.01 to 1.05 | 0.002 |
| Male gender | 1.02 | 0.31 to 3.35 | >0.2 |
| Black race | 0.59 | 0.36 to 0.96 | 0.03 |
| Ambulatory systolic BP (mmHg) | 1.02 | 1 to 1.03 | 0.005 |
| ACE and/or ARB use | 1.05 | 0.7 to 1.59 | >0.2 |
| DM | 1.35 | 0.91 to 1.99 | >0.2 |
| Aspirin use | 0.88 | 0.61 to 1.28 | >0.2 |
| Statin use | 0.95 | 0.64 to 1.39 | >0.2 |
| BP medication use | 0.91 | 0.43 to 1.94 | >0.2 |
| Current smoker | 1.15 | 0.74 to 1.79 | >0.2 |
Discussion
The major findings of our study are as follows. (1) The incidence rate of MI in those with diabetes is approximately 2.5 times, but in those with CKD the hazard ratio (HR) at 3.5 is even higher. (2) The cumulative incidence for MI over a decade of follow-up is undoubtedly increased with diabetes. However, whereas diabetes increases this risk by approximately 2.4, CKD elevates this risk 4.3-fold. Thus, the cumulative incidence of MI is higher among people with CKD than those with diabetes without CKD (see Figure 1). When CKD and diabetes are present, the cumulative incidence of MI approaches 20% over 10 years. These effects of CKD and diabetes on mortality and MI are seen despite a large percent of patients using antihypertensive medications, statins, and aspirin. (3) The HR for all-cause mortality is twofold elevated among those with prevalent coronary artery disease. In comparison, the HR for all-cause mortality among those with CKD is 1.8-fold, but that of diabetes is only 1.3-fold elevated. Thus, the mortality hazard for CKD is similar to that of prevalent coronary artery disease.
Among patients with type 2 diabetes mellitus, it has been argued that the “clock starts ticking” years before the onset of hyperglycemia (6,10). The preceding period of metabolic derangements that accompanies insulin resistance and that is characterized by the metabolic syndrome (e.g., hypertension, abdominal obesity, impaired fasting glucose, low HDL cholesterol, and high triglycerides) is thought to accelerate the atherosclerotic process (11). Thus, in the management of cardiovascular risk factors, the presence of type 2 diabetes mellitus has been given the same importance as established cardiovascular disease (12,13). As in patients with type 2 diabetes mellitus, the diagnosis of CKD occurs at a late stage of kidney disease. A large mass of nephrons is lost before serum creatinine rises and estimated GFR falls (14). The risk factors for nephron loss such as diabetes mellitus and hypertension are also the same that cause accelerated atherosclerosis. In addition, novel risk factors such as cardiovascular calcification, anemia and accumulation of uremic toxins may aggravate the atherosclerotic process (3). Accordingly, as in patients with diabetes mellitus, proatherosclerotic risk factors accumulate long before CKD is diagnosed. The “silent attack” on nephrons and the vasculature may be even more occult among patients with CKD. We found that compared with those with diabetes mellitus, the incidence of MI and all-cause mortality was similar or increased among those with CKD. This congruent increase in cardiovascular morbidity and all-cause mortality suggests that CKD is a marker of established cardiovascular disease. This risk was seen in a cohort of veterans seen at one medical center, which presumably reduces heterogeneity in access to or in the delivery of care and makes the results even more striking.
Although a previous study pooling several community-based cohorts has found CKD to be an independent risk factor for a composite outcome of stroke, MI, fatal coronary heart disease, and death, the HR of CKD was much smaller than seen in our study (HR 1.19, 95% CI 1.07 to 1.32) (2). These differences may be due to differences in study design. For example, we used proteinuria to define CKD and measured 24-hour ambulatory BP, covariates that were not accounted for in the above study. Second, by using a competing risk model, we separated the hazards of MI from the competing event of death. Third, our population was older and sicker. As examples, in contrast to the mortality rate of 9.2% in the above study, the mortality rate in our study was 43%; in contrast to the cardiac event rate seen of 5.7%, we uncovered MI in 12.1% of the participants. Fourth, our patients had a lower estimated GFR, which is a strong risk factor for the risk of death and cardiovascular events (1). These differences may have magnified the HR of CKD on the risk of MI and death. Although our study is smaller, it extends the observations seen in other cohorts and suggests that the cardiovascular risk of CKD may be even more profound, especially among older and sicker patients. That CKD can increase the risk for MI, stroke, or cardiovascular death among those with diabetes is well recognized (15–17).
Our study has several strengths and limitations. First, we ascertained mortality and MI in each patient who was in the cohort. We did so not via a computerized database query (commonly done via International Classification of Disease codes), but by searching each patient's record for MI. Second, we prospectively collected information on proteinuria, systolic ambulatory BP, medication use, and prevalent coronary artery disease, which are all important risk factors for the competing endpoints and provide meaningful analyses to be conducted. These risk factors are often missing from competing risk analyses (18,19). Third, we extended studies that have analyzed the relationship between cardiovascular events and CKD as the time to first cardiovascular event. We found few studies that have examined the risk for MI in an individual over time. The analysis of repeated MI is clinically relevant for patients with CKD because if the first MI was fatal, then repeated MIs cannot occur. If this were the case, then secondary prevention for MI would not be possible.
Nonetheless, there are several limitations of our data. First, our study is limited to predominantly male veterans and may not apply to younger people and women. Thus, additional studies are needed to validate our findings in other cohorts. Although our sample size was small, the many events and follow-up extending up to a decade provided adequate power to perform these analyses. In fact, recruitment of patients from a single center may have reduced heterogeneity introduced because of access to and delivery of care. Second, we did not collect follow-up BP, medication, and proteinuria information; thus, we cannot comment on the time dependence of the outcomes on these risk factors. Third, deaths were not adjudicated but were extracted from administrative records. It is possible that some of the all-cause mortalities could in fact have represented acute coronary events, especially outside of the hospital. Finally, we did not analyze novel risk factors such as asymmetrical dimethylarginine, which may be important in a competing risk model (20).
The implications of this research affect the design of clinical trials, policy, and clinical care. Because patients with diabetes mellitus and CKD have an increased risk of MI, clinical trials may need to recruit fewer patients to see a relative risk reduction for interventions that can truly reduce future risk of MI. Increased absolute risk for MI requires fewer numbers needed to treat to prevent an outcome for a therapy that is effective. Therefore, from public health policy perspective, the risk factor reduction may be more aggressively targeted to reduce costs associated with MI. Thus, like diabetes mellitus, CKD may be considered a cardiovascular disease equivalent. The implication of this research for clinical care is more complicated. For example, recent studies have indicated that the use of statins in dialysis patients does not protect from cardiovascular events (21,22). However, these trials were not adequately powered. The well powered Study of Heart and Renal Protection trial (23) has now informed us that statins truly improve major atherosclerotic cardiovascular events without changing mortality in this group of patients. Therefore, it appears prudent to aggressively target known cardiovascular risk factors (e.g., by reduction of BP and use of aspirin, statins, and ACE inhibitors) among those with CKD and diabetes mellitus. Our data suggest that patients with CKD have a risk for MI similar to pre-existing coronary disease. If so, interventions to reduce MIs may reduce to a greater extent coronary morbidity and costs among those with CKD than they do among those with diabetes mellitus (24).
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Sarnak MJ, Levey AS: Cardiovascular disease and chronic renal disease: A new paradigm. Am J Kidney Dis 35: S117–S131, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Herzog CA, Ma JZ, Collins AJ: Poor long-term survival after acute myocardial infarction among patients on long-term dialysis [see comments]. N Engl J Med 339: 799–805, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction [see comments]. N Engl J Med 339: 229–234, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Manjunath G, Sarnak MJ, Levey AS: Prediction equations to estimate glomerular filtration rate: An update. Curr Opin Nephrol Hypertens 10: 785–792, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Thygesen K, Alpert JS, White HD: Universal definition of myocardial infarction. J Am Coll Cardiol 50: 2173–2195, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR: Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation 100: 1134–1146, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA: Clinical management of metabolic syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation 109: 551–556, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ: Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110: 227–239, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ: Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Levey AS: Measurement of renal function in chronic renal disease [clinical conference]. Kidney Int 38: 167–184, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Gerstein HC, Mann JF, Pogue J, Dinneen SF, Halle JP, Hoogwerf B, Joyce C, Rashkow A, Young J, Zinman B, Yusuf S: Prevalence and determinants of microalbuminuria in high-risk diabetic and nondiabetic patients in the Heart Outcomes Prevention Evaluation Study. The HOPE Study Investigators. Diabetes Care 23[Suppl 2]: B35–B39, 2000 [PubMed] [Google Scholar]
- 16. Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S: Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med 134: 629–636, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Eriksen BO, Ingebretsen OC: The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int 69: 375–382, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Johnson ES, Thorp ML, Yang X, Charansonney OL, Smith DH: Predicting renal replacement therapy and mortality in CKD. Am J Kidney Dis 50: 559–565, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C: Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: A competing risks modeling approach. J Am Soc Nephrol 16: 2449–2455, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F: Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Baigent C, Landry M: Study of Heart and Renal Protection (SHARP). Kidney Int Suppl S207–S210, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Bakris G, Vassalotti J, Ritz E, Wanner C, Stergiou G, Molitch M, Nesto R, Kaysen GA, Sowers JR: National Kidney Foundation consensus conference on cardiovascular and kidney diseases and diabetes risk: An integrated therapeutic approach to reduce events. Kidney Int 78: 726–736, 2010 [DOI] [PubMed] [Google Scholar]