Abstract
Summary
Background and objectives
Recent studies found different associations of cognitive function with albuminuria or estimated GFR (eGFR). Most studies were limited to the elderly or did not take both renal variables into account. Therefore, this study analyzed the association of cognitive function with albuminuria and eGFR in community-dwelling persons aged 35 to 82 years.
Design, setting, participants, & measurements
This was a cross-sectional study comprising 4095 participants of the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study. Cognitive function, measured with the Ruff Figural Fluency Test (RFFT), was treated as the dependent variable, and albuminuria and eGFR were treated as independent variables.
Results
The prevalence of albuminuria <10, 10 to 29, and ≥30 mg/24 h was 54%, 31%, and 15%, respectively. Mean eGFR (± SD) was 79 ± 15 ml/min per 1.73 m2. Because of interaction between albuminuria and age, analyses were performed per age tertile. After multivariate adjustment, albuminuria ≥ 30 mg/24 h, but not eGFR, was associated with lower RFFT score in the youngest tertile (B −5.3; 95% CI, −0.6 to −9.2; P = 0.05), but not in older tertiles. Moreover, subjects in the youngest tertile with increasing albuminuria (5–15 and >15 mg/24 h) before RFFT measurement had lower mean RFFT scores than subjects with stable albuminuria: mean difference −4.9 (P = 0.3) and −6.7 (P = 0.03), respectively.
Conclusions
In this community-based cohort, elevated albuminuria was associated with worse cognitive function in young but not in old persons. There was no association of eGFR with cognitive function.
Introduction
The kidneys and the brain are end organs susceptible to vascular damage. Accordingly, it has been assumed that cardiovascular risk factors can cause similar (micro)vascular injury in both organs and that there is an association between renal and cerebral markers of vascular damage (1,2). Renal vascular damage leads to chronic kidney disease (CKD), as reflected by elevated albuminuria or a diminished GFR. Vascular damage to brain tissue results in loss of cerebral function, manifesting itself as diffuse white matter lesions, focal cerebral accidents, or decreased cognitive function. Because the kidney and brain suffer from vascular damage, it seems plausible to assume that there might also be an association between cerebral (cognitive) function and either albuminuria or GFR. Previous imaging studies already showed data on diffuse cerebrovascular pathology in subjects with CKD (3–8). So far, the association of cognitive function and CKD has been investigated in only a few studies, focusing mainly on high-risk populations (3,4,9–15). Moreover, most studies analyzing the association took either albuminuria or GFR into account but omitted investigating their mutual relationship in studies on cognitive function.
Data on albuminuria and cognitive function are currently available from elderly populations only, but the data are conflicting (3,4,12,16,17). In selected populations including subjects with dementia or peripheral arterial disease, higher levels of albuminuria were associated with worse cognitive function (3,12). One study in community-dwelling elderly presented cross-sectional and longitudinal data on cognitive function, taking albuminuria and estimated GFR (eGFR) into consideration. The authors showed that, in men, albuminuria (and not GFR) was associated with greater cognitive decline. There was no association between albuminuria or eGFR with cognitive decline in women (16). Data on GFR are also mainly available for the elderly. Some authors found an association between severity of CKD and decline in cognitive function (10,14). In contrast, others did not show impaired cognitive function in elderly persons with reduced GFR (9,16). Current evidence is thus unclear, and data on younger adults are lacking. Therefore, this study aims to evaluate the cross-sectional association of albuminuria and GFR with cognitive function in a large community-based population cohort including subjects with a wide age distribution (35 to 82 years).
Study Population and Methods
This study is a cross-sectional analysis of the association of cognitive function and albuminuria with eGFR in participants of the third survey of the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study.
Setting and Participants
PREVEND is a prospective, community-based cohort study initiated to investigate the association between albuminuria and renal and cardiovascular disease. The design has been described in detail elsewhere (18,19) and can be found at www.prevend.org. In summary, 8592 persons from the general population aged 28 to 75 years completed the baseline survey (1997 to 1998) and were followed over time. The third survey was conducted from 2003 to 2006 and included assessment of cardiovascular risk factors as well as blood and urine sampling. Because 315 persons died during follow-up, and 2415 individuals refused further participation, the third survey comprised 5862 participants. Table 1 summarizes the measurements of study variables.
Table 1.
Timetable of the PREVEND study variables used in the analyses presented here
| Variables | Time of Measurement |
|
|---|---|---|
| Baseline Survey (1997 to 1998) | Third Survey (2003 to 2006) | |
| Cognitive function test | RFFT | |
| Renal | Albuminuria | Albuminuria |
| Change in albuminuriaa | ||
| eGFR | eGFR | |
| Change in eGFRb | ||
| Plasma creatinine | ||
| Demographic | Age | |
| Educational level | ||
| Cardiovascular | Diabetes mellitus | |
| Hypertension | ||
| History of cardiovascular disease | ||
| Body mass index | ||
| Alcohol use | ||
| Cholesterol | ||
| Smoking | ||
Change in albuminuria = albuminuria at third survey minus albuminuria at baseline survey. PREVEND, Prevention of Renal and Vascular End-Stage Disease; RFFT, Ruff Figural Fluency Test; eGFR, estimated GFR.
Change in eGFR = eGFR at third survey minus eGFR at baseline survey.
The PREVEND study has been approved by the local medical ethics committee and is conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Cognitive Function
Measurement of cognitive function was introduced during the third survey and was assessed by the Ruff Figural Fluency Test (RFFT) (20,21). Fluency is generally seen as an executive function that is sensitive to early cognitive dysfunction (22). The RFFT requires respondents to draw as many different designs as possible by connecting patterns of dots within a set time period (21,23). In contrast to many other neuropsychological tests, the RFFT is sensitive to changes in executive function in old and young persons (20,21,23). It has been validated in various healthy and diseased populations (20,24–27). The main outcome measure is the total number of unique designs, which varies from 0 (worst score) to 175 (best score). Two trained, independent raters scored each RFFT test according to strict scoring rules (21,23). Reassessment by a third rater was performed if the total score differed by >4 points. The mean difference in total scores (± SD) between raters was 0.1 ± 1.8, and the intraclass correlation coefficient was 1.00 (95% confidence interval [CI] 0.99 to 1.00) (28).
Albuminuria and GFR
Urinary albumin excretion (UAE) rate was calculated from the mean of two 24-hour urinary collections. Because of the skewed distribution, UAE was normalized with natural logarithmic transformation (lnUAE) when evaluating for interaction between albuminuria and other clinical terms (29,30). Because outcomes for lnUAE are difficult to interpret and classes of albuminuria are widely used in clinical practice, we incorporated albuminuria as a categorical variable in our analyses. A recent meta-analysis showed that not only the cutpoint of 20 mg/24 h for albuminuria, but also a lower cutpoint of 10 mg/24 h is associated with increased mortality and cardiovascular disease (31). We therefore classified albuminuria into low, intermediate, and elevated (defined as UAE <10, 10 to 29, and ≥30 mg/24 h, respectively). We estimated GFR using the Modification of Diet in Renal Disease formula (32,33), classifying eGFR into low, intermediate, and normal (<60, 60 to 89, and ≥90 ml/min per 1.73 m2, respectively).
Other Variables
Educational level was classified by highest achieved degree on the basis of a questionnaire and divided into four groups according to the International Standard Classification of Education (ISCED) (34). Primary school level corresponded to 0 to 8 years of education (ISCED 0 to 1), lower secondary level to 9 to 12 years (ISCED 2), higher secondary level to 13 to 15 years (ISCED 3 to 4), and university level to ≥16 years (ISCED 5). A history of vascular events was defined as a prior cardiac, cerebrovascular, or peripheral vascular event requiring hospitalization. Data on disease history were derived from a questionnaire at baseline and obtained from the Dutch national registry of hospital discharge diagnoses during follow-up. Diabetes was defined as fasting glucose ≥ 7 mmol/L or nonfasting glucose ≥ 11.1 mmol/L or the use of glucose-lowering drugs (35). Overweight status was defined as body mass index ≥ 25 kg/m2. Subject-specific information on drug use was obtained from IADB, a database comprising pharmacy-dispensing data from regional community pharmacies (www.IADB.nl) (36).
Data Analyses
Statistical analyses were performed with SPSS version 16.0 (SPSS, Inc., Chicago, IL). For normally distributed data, differences in baseline characteristics were tested with the t test or, if appropriate, ANOVA. For nonparametric data we used the Mann–Whitney U test or, if appropriate, the Kruskal–Wallis test. Outcomes on RFFT scores between different eGFR and albuminuria categories were tested by ANOVA. Adjusted RFFT scores were calculated by analysis of covariance (ANCOVA).
The independent association of albuminuria and eGFR with cognitive function was investigated in a stepwise approach. First, multivariable linear regression analysis was used controlling for age, sex, educational level, diabetes mellitus, hypertension, history of cardiovascular disease, body mass index, alcohol use, cholesterol, and smoking. Second, interaction of age and albuminuria and their association with RFFT score was considered by entering both variables and their product term in multivariate analysis: age × lnUAE, where age is in years and UAE is in milligrams per 24 hours. Finally, from all subjects longitudinal data on albuminuria and eGFR were available from baseline until the third survey (mean time span [± SD] 6.1 ± 0.7 years). Using ANCOVA, we compared RFFT scores of subjects with stable albuminuria (change −5 to 5 mg/24 h) to subjects with decreasing (change less than −15 mg/24 h, or −15 to less than −5 mg/24 h) and increasing albuminuria (change >5 to 15 mg/24 h, or >15 mg/24 h) before RFFT measurement. A similar analysis was done for eGFR using the following definitions: stable eGFR (change between −10 to 10 ml/min per 1.73 m2), decreasing eGFR (change less than −20 ml/min per 1.73 m2 or −20 to less than −10 ml/min per 1.73 m2), and increasing eGFR (change >10 to 20 ml/min per 1.73 m2, or >20 ml/min per 1.73 m2) before RFFT measurement.
Sensitivity Analyses
Various a priori-defined sensitivity analyses were performed. First, because the PREVEND cohort is enriched for individuals with elevated albuminuria, we repeated our analysis in a subsample of the PREVEND population that reflects the prevalence of elevated albuminuria in the normal population (7.5%). The representativity of this subsample for the general population has been described previously (18). Second, all analyses were repeated excluding subjects with comorbidities, which might negatively influence cognitive function. Third, we performed sensitivity analyses with other measurements of kidney function such as creatinine clearance (mean of two 24-hour urine collections), the Chronic Kidney Disease–Epidemiology Collaboration (CKD-EPI), and Cockroft–Gault formulas. Finally, to enable comparison of our data with previous studies, we repeated our analyses with albuminuria or only eGFR in subgroups of our population, creating data sets on the basis of characteristics known from earlier studies, such as elderly age (≥65 years) (3,4,9,12,37) and subjects with cardiovascular risk factors (10,13,17).
Results
Study Population
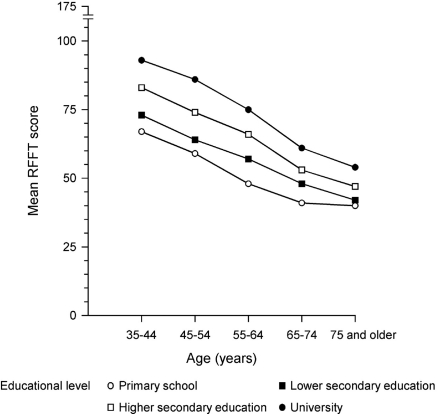
Overall, 4158 subjects completed the RFFT test. A total of 1271 subjects (21.6%) refused cognitive testing and 433 (7.4%) had incomplete RFFT data. Subjects without RFFT were slightly older (mean age [± SD] 56 ± 12 versus 55 ± 12 years [P < 0.001]) and had a lower educational level (P < 0.001). There was a small yet statistically significant difference in mean eGFR (± SD), 78 ± 15 versus 79 ± 15 ml/min per 1.73 m2, respectively (P < 0.001), but not for albuminuria (P = 0.88) or history of cardiovascular disease (P = 0.45). Of those with complete RFFT data (n = 4158), subjects lacking data on other main variables (n = 63) were excluded. The final study population thus comprised 4095 subjects (Table 2). Mean (± SD) RFFT score was 69 ± 26. Age and educational level appeared the strongest determinants of RFFT score. The association of RFFT score with age and educational level is depicted in Figure 1. Albuminuria ≥ 30 mg/24 h was present in 15% and eGFR < 60 ml/min per 1.73 m2 in 9% (Table 2). There was a clear association of increasing degrees of albuminuria and lower classes of eGFR with the presence of vascular risk factors (Table 3).
Table 2.
Characteristics of the study population dependent on tertile of age
| Tertile 1 (age 34 to 48 years) | Tertile 2 (age 49 to 59 years) | Tertile 3 (age 60 to 82 years) | All | |
|---|---|---|---|---|
| n | 1395 | 1370 | 1330 | 4095 |
| Demographics | ||||
| age (years), mean ± SD | 42 ± 4 | 54 ± 3 | 68 ± 6 | 55 ± 12 |
| men, n (%) | 679 (49) | 675 (49) | 786 (59) | 2143 (52) |
| Educational level, n (%) | ||||
| primary school | 44 (3) | 107 (8) | 248 (19) | 399 (10) |
| lower secondary | 257 (18) | 407 (30) | 548 (41) | 1212 (30) |
| higher secondary | 486 (34) | 357 (26) | 278 (21) | 1105 (27) |
| university | 626 (45) | 499 (36) | 256 (19) | 1382 (33) |
| Cardiovascular risk factors | ||||
| history of vascular events, n (%) | 25 (2) | 75 (6) | 207 (16) | 307 (8) |
| diabetes mellitus, n (%) | 13 (1) | 73 (5) | 169 (13) | 255 (6) |
| hypertension, n (%) | 133 (10) | 449 (33) | 807 (61) | 1390 (34) |
| systolic BP (mmHg), mean ± SD | 118 ± 13 | 124 ± 16 | 136 ± 19 | 126 ± 18 |
| diastolic BP (mmHg), mean ± SD | 70 ± 8 | 74 ± 9 | 75 ± 9 | 73 ± 9 |
| current smoking, n (%) | 358 (26) | 376 (27) | 238 (18) | 972 (24) |
| body mass index (kg/m2), mean ± SD | 26 ± 4 | 27 ± 5 | 28 ± 4 | 27 ± 4 |
| overweight, n (%) | 704 (51) | 868 (63) | 976 (73) | 2548 (62) |
| alcohol use, n (%) | 1118 (80) | 1076 (79) | 929 (70) | 3123 (76) |
| HDL-cholesterol (mmol/L), mean ± SD | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 |
| non-HDL cholesterol (mmol/L), mean ± SD | 5.2 ± 1.0 | 5.6 ± 1.0 | 5.4 ± 1.1 | 5.4 ± 1.1 |
| Renal variables | ||||
| eGFR MDRD (ml/min per 1.73 m2), mean ± SD | 84 ± 13 | 80 ± 13 | 72 ± 15 | 79 ± 15 |
| low (<60), n (%) | 29 (2) | 72 (5) | 243 (18) | 344 (9) |
| intermediate (60 to 89), n (%) | 955 (69) | 1025 (75) | 945 (71) | 2925 (71) |
| normal (≥90), n (%) | 411 (29) | 273 (20) | 142 (11) | 826 (20) |
| Serum creatinine (μmol/L), mean ± SD | 82 ± 14 | 83 ± 14 | 90 ± 22 | 85 ± 17 |
| UAE (mg/24 h), median [IQR] | 8.1 [6.2 to 12.3] | 9.2 [6.4 to 16.2] | 12.5 [7.2 to 31.7] | 9.4 [6.5 to 17.3] |
| low (<10), n (%) | 893 (64) | 763 (56) | 547 (41) | 2203 (54) |
| intermediate (10 to 29), n (%) | 414 (30) | 439 (32) | 443 (33) | 1296 (31) |
| elevated (≥30), n (%) | 88 (6) | 168 (12) | 340 (26) | 596 (15) |
| RFFT score, mean ± SD | 83 ± 25 | 70 ± 23 | 52 ± 20 | 69 ± 26 |
MDRD, Modification of Diet in Renal Disease; IQR, interquartile range; UAE, urinary albumin excretion.
Figure 1.
Mean Ruff Figural Fluency Test (RFFT) score and its relationship with age and educational level.
Table 3.
Vascular risk factors and renal variables dependent on clinical classes of albuminuria and eGFR
| Albuminuria (mg/24 h) |
eGFR (ml/min per 1.73 m2) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Low (<10) | Intermediate (10 to 29) | Elevated (≥30) | Pa | Low (<60) | Intermediate (60 to 89) | Normal (≥90) | Pb | |
| n | 2203 | 1296 | 596 | 344 | 2925 | 826 | ||
| Age (years), mean ± SD | 52 ± 11 | 55 ± 12 | 61 ± 12 | <0.001 | 65 ± 11 | 55 ± 11 | 50 ± 10 | <0.001 |
| Men (%) | 1010 (46) | 720 (56) | 410 (69) | <0.001 | 155 (45) | 1441 (49) | 544 (66) | <0.001 |
| Cardiovascular risk factors | ||||||||
| history of vascular events (%) | 117 (5) | 91 (7) | 100 (17) | <0.001 | 54 (16) | 213 (7) | 40 (5) | <0.001 |
| diabetes mellitus (%) | 68 (3) | 88 (7) | 99 (17) | <0.001 | 49 (14) | 162 (6) | 44 (5) | <0.001 |
| hypertension (%) | 500 (23) | 506 (39) | 383 (64) | <0.001 | 226 (66) | 946 (32) | 217 (26) | <0.001 |
| systolic BP (mmHg), mean ± SD | 121 ± 15 | 129 ± 17 | 138 ± 21 | <0.001 | 134 ± 21 | 125 ± 18 | 124 ± 16 | <0.001 |
| diastolic BP (mmHg), mean ± SD | 71 ± 8 | 75 ± 9 | 77 ± 9 | <0.001 | 75 ± 10 | 73 ± 9 | 72 ± 8 | <0.001 |
| body mass index (kg/m2), mean ± SD | 26 ± 4 | 27 ± 5 | 28 ± 5 | <0.001 | 28 ± 5 | 27 ± 4 | 26 ± 5 | <0.001 |
| overweight (%) | 1228 (56) | 874 (67) | 446 (75) | <0.001 | 259 (75) | 1838 (63) | 451 (55) | <0.001 |
| current smoking (%) | 502 (23) | 311 (24) | 159 (27) | 0.13 | 72 (21) | 632 (22) | 268 (33) | <0.001 |
| HDL cholesterol (mmol/L), mean ± SD | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.4 | <0.001 | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 | <0.001 |
| non–HDL cholesterol (mmol/L), mean ± SD | 5.4 ± 1.0 | 5.3 ± 1.0 | 5.2 ± 1.1 | 0.002 | 5.3 ± 1.2 | 5.4 ± 1.1 | 5.2 ± 1.0 | <0.001 |
| Renal variables | ||||||||
| eGFR MDRD (ml/min per 1.73 m2), mean ± SD | 79 ± 13 | 80 ± 15 | 74 ± 18 | <0.001 | 52 ± 8 | 76 ± 8 | 99 ± 7 | <0.001 |
| UAE (mg/24 h), median [IQR] | 7 [6 to 8] | 14 [12 to 20] | 64 [40 to 136] | <0.001 | 14 [7 to 47] | 9 [6 to 16] | 10 [7 to 16] | <0.001 |
P value for comparison between normal versus elevated albuminuria; P value for comparison between eGFR categories.
Cognitive Function, Albuminuria, and eGFR
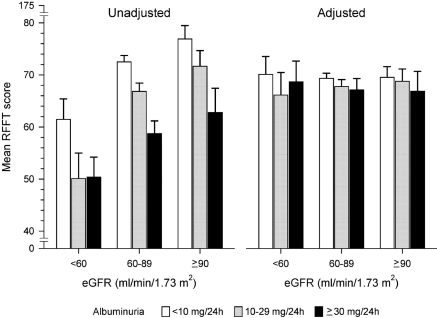
Unadjusted, subjects with albuminuria 10 to 29 mg/24 h and ≥30 mg/24 h had a lower (worse) RFFT score than subjects with albuminuria < 10 mg/24 h (Figure 2). This was found in the total study population (P < 0.001) and in each of the three eGFR categories (P < 0.001). Similarly, subjects with lower eGFR had lower unadjusted RFFT scores (Figure 2). In subjects with an eGFR < 60 ml/min per 1.73 m2, unadjusted RFFT scores were not significantly different between subjects with albuminuria 10 to 29 and ≥30 mg/24 h (P = 0.95).
Figure 2.
Mean RFFT score in subjects subdivided into clinical classes of albuminuria and estimated GFR (eGFR). Adjusted RFFT scores were calculated by analysis of covariance (ANCOVA) and adjusted for age, sex, educational level, diabetes mellitus, hypertension, history of cardiovascular disease, body mass index, alcohol use, cholesterol, and smoking. Bars represent 95% confidence intervals.
The differences in RFFT scores decreased after multivariate adjustment (Figure 2). As compared with subjects with albuminuria < 10 mg/24 h, subjects with albuminuria 10 to 29 mg/24 h scored 2 points (95% CI −3 to 0; P = 0.01) lower, and subjects with albuminuria ≥ 30 mg/24 h scored 3 points (95% CI −5 to −1; P = 0.001) lower. Considering categories of eGFR, the difference in adjusted RFFT score of subjects with albuminuria ≥ 30 mg/24 h compared with <10 mg/24 h was −0.9 points (95% CI −5.9 to 4.2; P = 0.73), −3.5 points (95% CI −5.9 to −1.1; P = 0.004), and −3.6 points (95% CI −8.2 to 1.1; P = 0.13) for eGFR <60, 60 to 89, and ≥90 ml/min per 1.73 m2, respectively. After multivariate adjustment, RFFT scores were no longer dependent on eGFR category (Figure 2).
Interaction of Albuminuria and Age
The findings depicted in Figure 2 were in line with results from linear regression analysis in the total study population, in which testing also revealed a significant interaction of lnUAE and age (B = 0.08; 95% CI 0.02 to 1.33; P = 0.008). This interaction remained significant in the fully adjusted model: for age (B = −1.0; 95% CI −1.2 to −0.9; P < 0.001); for lnUAE (B = −3.9; 95% CI −7.2 to 0.6; P = 0.02); and for lnUAE × age (B = 0.06; 95% CI 0.004 to 0.11; P = 0.04). The association with eGFR was not statistically significant in the fully adjusted model (B = 0.009; 95% CI −0.04 to 0.06; P = 0.71).
Analysis per Age Group
Because of the significant age-by-albuminuria interaction, analyses were performed in age tertiles. As shown in Table 4, the regression coefficient for RFFT score showed a stepwise decline with increasing levels of albuminuria in all age tertiles. However, the association of cognitive function with elevated albuminuria was only significant for the youngest tertile (Table 4). Young subjects with albuminuria ≥ 30 mg/24 h scored 5 points lower than age peers with albuminuria < 10 mg/24 h (B = −5.3; 95% CI −10.4 to −0.1; P = 0.048), which equals a difference in 7 years of age (as the RFFT score decreases with 0.76 points/yr; Table 4).
Table 4.
Linear regression analysis of RFFT score with albuminuria and eGFR in tertiles of age
| Tertile 1 (age 34 to 48 years) |
Tertile 2 (age 49 t o 59 years) |
Tertile 3 (age 60 to 82 years) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | P | B | 95% CI | P | B | 95% CI | P | |
| Albuminuria (mg/24 h) | |||||||||
| low (<10) | Reference | Reference | Reference | Reference | Reference | Reference | |||
| intermediate (10 to 29) | −2.09 | −4.8 to 0.6 | 0.13 | −1.68 | −4.3 to 0.8 | 0.18 | −0.20 | −2.5 to 2.1 | 0.86 |
| elevated (≥30) | −5.25 | −10.4 to −0.1 | 0.05 | −1.76 | −5.3 to 1.9 | 0.37 | −0.50 | −3.1 to 2.1 | 0.71 |
| eGFR (ml/min per 1.73 m2) | 0.04 | −0.6 to 0.1 | 0.44 | −0.03 | −0.1 to 0.1 | 0.47 | −0.03 | −0.4 to 0.1 | 0.35 |
| Age (years) | −0.76 | −1.1 to −0.4 | <0.001 | −1.17 | −1.5 to −0.8 | <0.001 | −0.79 | −1.0 to −0.6 | <0.001 |
| Gender | |||||||||
| male | Reference | Reference | Reference | Reference | Reference | Reference | |||
| female | −0.65 | −3.2 to 1.9 | 0.62 | 1.18 | −1.2 to 3.6 | 0.34 | 0.47 | −1.7 to 2.6 | 0.67 |
| Educational level | |||||||||
| primary school | Reference | Reference | Reference | Reference | Reference | Reference | |||
| lower secondary | 4.24 | −3.2 to 11.6 | 0.26 | 5.43 | 0.1 to 9.9 | 0.02 | 5.70 | −3.0 to 8.4 | <0.001 |
| higher secondary | 12.19 | 4.9 to 19.4 | 0.001 | 14.22 | 9.6 to 18.9 | <0.001 | 11.28 | 8.2 to 14.4 | <0.001 |
| university | 22.86 | 15.6 to 30.1 | <0.001 | 22.69 | 18.1 to 27.3 | <0.001 | 19.27 | 16.0 to 22.5 | <0.001 |
Model adjusted for adjusted for classes of albuminuria, eGFR, age, gender, educational level, diabetes, hypertension, history of cardiovascular disease, body mass index, alcohol use, cholesterol, and smoking. Model tertile 1: adjusted R2 = 0.15, residual SD = 23. Model tertile 2: adjusted R2 = 0.21, residual SD = 21. Model tertile 3: adjusted R2 = 0.21, residual SD = 18; CI, confidence interval.
Longitudinal Data
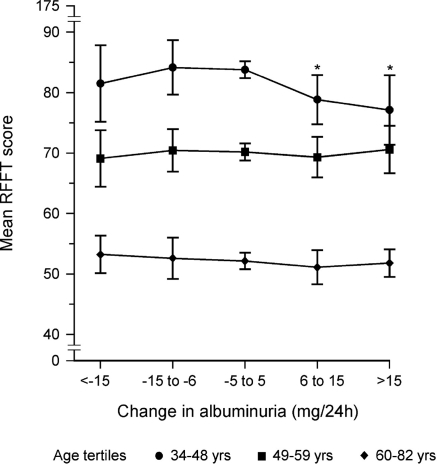
We subsequently analyzed if there also was an association between change in albuminuria or eGFR before RFFT measurement and RFFT score. In the youngest tertile, when compared with subjects with stable albuminuria before RFFT measurement, both groups with increasing albuminuria over time had significantly lower RFFT scores (Figure 3). In the older tertiles differences were not significant. A similar analysis was performed for change in eGFR before RFFT measurement. This showed no differences between subjects with a stable versus either an increasing or decreasing level of eGFR before RFFT measurement.
Figure 3.
Mean RFFT score and its relationship with the change in albuminuria (mg/24 h) between baseline (1997 to 1998) and the third survey (2000 to 2003) in subjects subdivided into tertiles of age. Adjusted RFFT scores were calculated by ANCOVA and adjusted for eGFR, age, sex, educational level, diabetes, hypertension, history of cardiovascular disease, body mass index, alcohol use, cholesterol, and smoking. Bars represent the 95% CIs of the mean RFFT score. *P < 0.05.
Sensitivity Analyses
We repeated analyses in a sample of the PREVEND cohort representative for the general population (Table 5). Results were similar to those of the total study population, showing a significant negative association of elevated albuminuria with RFFT score but not for eGFR in the youngest tertile. To determine whether the presence of comorbidities had biased the observed association, we conducted several sensitivity analyses in subgroups without specific cardiovascular risk factors. The association of albuminuria and eGFR remained similar when excluding subjects with a history of cardiovascular disease, cerebrovascular events, diabetes mellitus, or an eGFR < 60 ml/min per 1.73 m2 (Table 5). Finally, sensitivity analyses with alternative measures for kidney function (CKD-EPI, creatinine clearance, and Cockroft–Gault) were performed (Table 5).
Table 5.
Sensitivity analyses of RFFT score with albuminuria in the youngest age tertile
| Albuminuria (mg/24 h) | B | 95% CI | P |
|---|---|---|---|
| General population samplea | |||
| low (<10) | Reference | Reference | Reference |
| intermediate (10 to 29) | −1.4 | −6.2 to 3.3 | 0.55 |
| elevated (≥30) | −10.6 | −19.9 to −1.3 | 0.02 |
| Measurements of kidney function | |||
| eGFR CKD-EPI | |||
| low (<10) | Reference | Reference | Reference |
| intermediate (10 to 29) | −2.08 | −4.8 to 0.6 | 0.13 |
| elevated (≥30) | −5.23 | −10.4 to −0.04 | 0.04 |
| creatinine clearance (24-hour urine collection) | |||
| low (<10) | Reference | Reference | Reference |
| intermediate (10 to 29) | −2.26 | −4.9 to 0.4 | 0.10 |
| elevated (≥30) | −5.26 | −10.5 to −0.05 | 0.04 |
| Cockroft–Gault formula | |||
| low (<10) | Reference | Reference | Reference |
| intermediate (10 to 29) | −2.45 | −5.1 to 0.3 | 0.75 |
| elevated (≥30) | −5.27 | −10.4 to −0.09 | 0.04 |
| Exclusion of comorbidities | |||
| after exclusion of diabetes mellitus | |||
| low (<10) | Reference | Reference | Reference |
| intermediate (10 to 29) | −1.92 | −4.6 to 0.8 | 0.13 |
| elevated (≥30) | −6.04 | −11.3 to −0.7 | 0.03 |
| after exclusion of eGFR<60 ml/min per 1.73 m2 | |||
| low (<10) | Reference | Reference | Reference |
| intermediate (10 to 29) | −2.18 | −4.9 to 0.5 | 0.15 |
| elevated (≥30) | −5.77 | −11.1 to −0.4 | 0.03 |
| after exclusion of cardiovascular disease | |||
| low (<10) | Reference | Reference | Reference |
| intermediate (10 to 29) | −2.14 | −4.8 to 0.6 | 0.57 |
| elevated (≥30) | −5.62 | −10.9 to −0.3 | 0.04 |
| after exclusion of cerebrovascular disease | |||
| low (<10) | Reference | Reference | Reference |
| intermediate (10 to 29) | −2.16 | −4.9 to 0.5 | 0.13 |
| elevated (≥30) | −5.13 | −10.3 to −0.06 | 0.05 |
Model adjusted for adjusted for classes of albuminuria, eGFR, age, gender, educational level, diabetes, hypertension, history of cardiovascular disease, body mass index, alcohol use, cholesterol, and smoking. In the sensitivity analyses, no significant associations were found in the two older tertiles of age CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration.
Subsample PREVEND cohort reflecting the prevalence of elevated albuminuria in the normal population (7.5%).
Comparison with Previous Studies
Finally, we made a comparison with earlier studies by focusing on the association of RFFT scores with eGFR in elderly with cardiovascular risk factors such as diabetes mellitus, smoking, or a history of cardiovascular events. If the fully adjusted analysis was limited to persons 65 years of age or older with increased cardiovascular risk and no adjustment was made for albuminuria, we found that lower eGFR was significantly associated with lower cognitive function (B = 0.09; 95% CI 0.02 to 0.17; P = 0.04) in accordance with previous studies.
Discussion
In this community-based cohort, elevated albuminuria—but not eGFR—was associated with worse cognitive function. This association was only present in the younger cohort. Furthermore, younger subjects with stable albuminuria during 6 years of follow-up performed significantly better with respect to cognitive function testing compared with those with increasing albuminuria levels.
Our findings extend the existing knowledge on the association of albuminuria with cognitive function to younger subjects. Previous studies on this topic were predominantly limited to elderly populations (3,4,12,13,16,17). Several of these found albuminuria to be associated with worse cognitive performance (4,12,13,17). In our study such an association was not found in the elderly. This may be due to chance or a lack of power, but it may also be due to differences in study characteristics. Whereas we studied nonselected community-dwelling elderly, others studied selected subpopulations (e.g., activities-of-daily-living-dependent elderly or subjects with impaired glucose tolerance) (4,13) or found an association in parts of their population (e.g., elderly with peripheral artery disease [12,17] or dementia [3]).
The differences in the association between elevated albuminuria and cognitive function in younger versus older persons could be explained in several ways. First, albuminuria is a marker of impaired endothelial function (38–40), and elevated levels of albuminuria often precede a decrease in eGFR (41). Our data show that the association was already noticeable from the early phases of renal damage. It is plausible that this association is most evident at a young age, when the prevalence of interacting comorbid conditions that may statistically obscure this association is low. Second, ongoing vascular damage may result in distinctive pathologic processes in the kidney compared with the brain. Consequently, the direct association of albuminuria with cognitive function may become less prominent at a higher age because higher age is associated with more advanced CKD.
We found no association between eGFR and cognitive function, which is in line with data from two recent studies (4,16). However, this finding is in contrast to most other previous cross-sectional studies (10,11,42–44). Our results may differ from these studies because of various population characteristics. Our community-based population comprised a considerable number of young subjects with normal or moderately impaired eGFR, whereas others focused on elderly or high-risk populations (9–11,37) and included higher numbers of people with low eGFR (10,11,42,43). Another explanation might be that most previous studies did not take albuminuria into account as a possible confounder (37,42–44). This assumption is supported by the fact that in our study eGFR did show a significant association with cognitive function in our high-risk elderly patients, but only when not accounting for albuminuria. Analyses of the association between CKD and cognitive function should preferably include both renal variables because the presence of albuminuria has important prognostic significance. This has also been shown recently for the association between CKD and all-cause and cardiovascular mortality (31).
Some limitations of this study have to be noted. Because the PREVEND cohort is enriched for individuals with elevated albuminuria, it could be argued that selection bias influenced outcomes. However, a sensitivity analysis in a representative sample for the general population did not change results. Furthermore, the number of subjects with an eGFR < 45 ml/min per 1.73 m2 in the PREVEND cohort was limited. The link with cognitive function might be different in these persons; therefore, our conclusions cannot be extended to individuals with very low eGFR. Our results were based on a single cognitive function test, in contrast to several previous studies in which detailed neurocognitive testing was used (3,4,13,16). However, the RFFT is a sensitive measure reflecting a broad spectrum of cognitive performance (28). Finally, longitudinal data on cognitive function were lacking and the cross-sectional design does not allow determining a temporal relation. However, progressive albuminuria before RFFT testing was associated with worse cognitive function.
Our study also has several strengths. It includes a community-based population with a wide range of age, eGFR, and educational level. Furthermore, albuminuria was calculated from two 24-hour urine collections, whereas most previous studies used the albumin/creatinine ratio (3,4,12,16,17). However, elevated albumin/creatinine ratio can reflect high urinary albumin or just reflect low urinary creatinine, which is common in frail elderly. This potential misclassification is avoided by calculating albuminuria from 24-hour urine collections. Finally, contrary to cognitive tests such as the Modified Mini-Mental State Examination, the Digit Symbol Substitution Test, or the Telephone Interview for Cognitive Status as previously used (11,12,17,43), the RFFT not only distinguishes differences in cognitive function of elderly, but it is also sensitive to differences between younger adults.
In conclusion, in the younger population, elevated albuminuria was associated with worse cognitive function in this community-based cohort with relatively preserved renal function. There was no association of eGFR with cognitive function.
Disclosures
None.
Acknowledgments
This report was supported by the Dutch Kidney Foundation and the Dutch Alzheimer Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial ”To Predict Dementia, Should We Be Mindful of the Kidneys?” on pages 1232–1234.
References
- 1. Murray AM: The brain and the kidney connection: A model of accelerated vascular cognitive impairment. Neurology 73: 916–917, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Knopman DS: Albuminuria and microvascular disease of the brain: A shared pathophysiology. Am J Epidemiol 171: 287–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barzilay JI, Fitzpatrick AL, Luchsinger J, Yasar S, Bernick C, Jenny NS, Kuller LH: Albuminuria and dementia in the elderly: A community study. Am J Kidney Dis 52: 216–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiner DE, Bartolomei K, Scott T, Price LL, Griffith JL, Rosenberg I, Levey AS, Folstein MF, Sarnak MJ: Albuminuria, cognitive functioning and white matter hyperintensities in homebound elders. Am J Kidney Dis 53: 438–447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinez-Vea A, Salvadó E, Bardají A, Gutierrez C, Ramos A, García C, Compte T, Peralta C, Broch M, Pastor R, Angelet P, Marcas L, Saurí A, Oliver JA: Silent cerebral white matter lesions and their relationship with vascular risk factors in middle-aged predialysis patients with CKD. Am J Kidney Dis 47: 241–250, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Kim CD, Lee HJ, Kim DJ, Kim BS, Shin SK, Do JY, Jang MH, Park SH, Kim YS, Kim YL: High prevalence of leukoaraiosis in cerebral magnetic resonance images of patients on peritoneal dialysis. Am J Kidney Dis 50: 98–107, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Knopman DS, Mosley TH, Bailey KR, Jack CR, Schwartz GL, Turner ST: Associations of microalbuminuria with brain atrophy and white matter hyperintensities in hypertensive sibships. J Neurol Sci 271: 53–60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MMB: Kidney function is related to cerebral small vessel disease. Stroke 39: 55–61, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Slinin Y, Paudel ML, Ishani A, Taylor BC, Yaffe K, Murray AM, Fink HA, Orwoll ES, Cummings SR, Barrett-Connor E, Jassal S, Ensrud KE: Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc 56: 2082–2088, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurella M, Chertow GM, Luan J, Yaffe K: Cognitive impairment in chronic kidney disease: J Am Geriatr Soc 52: 1863–1869, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, Satterfield S, Ayonayon H, Yaffe K: Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 16: 2127–2133, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Kuo HK, Lin LY, Yu YH: Microalbuminuria is a negative correlate for cognitive function in older adults with peripheral arterial disease: Results from the U.S. National Health and Nutrition Examination Survey 1999–2002. J Intern Med 262: 562–570, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Abbatecola AM, Barbieri M, Rizzo MR, Grella R, Laieta MT, Quaranta E, Molinari AM, Cioffi M, Fioretto P, Paolisso G: Arterial stiffness and cognition in elderly persons with impaired glucose tolerance and microalbuminuria. J Gerontol A Biol Sci Med Sci 9: 991–996, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Buchman AS, Tanna D, Boyle PA, Shah RC, Leurgans SE, Bennett DA: Kidney function is associated with the rate of cognitive decline in the elderly. Neurology 73: 920–927, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruce DG, Davis WA, Casey GP, Starkstein SE, Clarnette RM, Almeida OP, Davis TM: Predictors of cognitive decline in older individuals with diabetes. Diabetes Care 31: 2103–2107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jassal SK, Kritz-Silverstein D, Barret-Connor E: A prospective study of albuminuria and cognitive function in older adults: The Rancho Bernardo study. Am J Epidemiol 171: 277–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vupputuri S, Shohan DA, Kshirsagar AV: Microalbuminuria, peripheral artery disease and cognitive function. Kidney Int 73: 341–346, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Lambers Heerspink H, Brantsma AH, de Zeeuw D, Bakker SJL, de Jong PE, Gansevoort RT; for the PREVEND study group: Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol 168: 897–905, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Mahmoodi BK, Gansevoort RT, Veeger NJGM, Matthews AG, Navis GJ, Hillege HL, van der Meer J; for the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study group: Microalbuminuria and risk of venous thromboembolism. JAMA 17: 1791–1797 2009 [DOI] [PubMed] [Google Scholar]
- 20. Ruff RM, Light R, Evans R: The Ruff Figural Fluency Test: A normative study with adults. Dev Neuropsychol 3: 37–51, 1987 [Google Scholar]
- 21. Mulder JL, Dekker PH, Dekker R: Fluency: Word Fluency Test (WFT) and Figure-Fluency Test (FFT), Leiden, The Netherlands, PITS BV, 2006 [Google Scholar]
- 22. Twamley EW, Legendre Ropacki L, Bondi MW: Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. J Int Neuropsych Soc 12: 707–735, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruff RM: Ruff Figural Fluency Test: Professional Manual, Odessa, FL, Psychological Assessment Resources, Inc., 1996 [Google Scholar]
- 24. Ruff RM, Allen CC, Farrow CE, Niemann H, Wylie T: Figural fluency, differential impairment in patients with left versus right frontal lobe lesions. Arch Clin Neuropsychol 9: 41–55, 1994 [PubMed] [Google Scholar]
- 25. Basso MR, Bornstein RA, Lang JM: Practice effects on commonly used measures of executive function across 12 months. Clin Neuropsychol 13: 283–292, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E: Verbal and design fluency in patients with frontal lobe lesions. J Int Neuropsychol Soc 5: 586–596, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Fama R, Sullivan EV, Shear PK, Cahn-Wiener DA, Yesavage JA, Tinklenberg JR, Pfefferbaum A: Fluency performance in Alzheimer's disease and Parkinson's disease. Clin Neuropsychol 12: 487–499, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Izaks GJ, Joosten H, Koerts J, Gansevoort RT, Slaets JPJ: Reference data for the Ruff Figural Fluency Test stratified by age and educational level. PLoS One 6: e17045, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gansevoort RT, Brinkman J, Bakker SJL, de Jong PE, de Zeeuw D: Evaluation of measures of urinary albumin excretion. Am J Epidemiol 164: 725–727 2006 [DOI] [PubMed] [Google Scholar]
- 30. Gansevoort RT, Lambers Heerspink H, Witte EC: Methodology of screening for albuminuria. Nephrol Dial Transpl 22: 2109–2111, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Levey AS, Greene T, Kusek J, Beck G: A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 34. United Nations Educational, Scientific and Cultural Organization: International Standard Classification of Education ISCED 1997, May 2006 Re-edition. Available at: http://www.uis.unesco.org/TEMPLATE/pdf/isced/ISCED_A.pdf Accessed October 18, 2010
- 35. World Health Organization: Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Report of a WHO Consultation, WHO/NCD/NCS/99.2. Available at: http://www.staff.ncl.ac.uk/philip.home/who_dmg.pdf Accessed December 1, 2010
- 36. Monster TBM, Janssen WMT, de Jong PE, de Jong-van den Berg LTW: Pharmacy data in epidemiological studies: An easy to obtain and reliable tool. Pharmacoepidemiol Drug Saf 11: 379–384, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Seliger SL, Siscovick DS, Stehman-Breen CO, Gillen DL, Fitzpatrick A, Bleyer A, Kuller LH: Moderate renal impairment and risk of dementia among older adults: The cardiovascular health cognition study: J Am Soc Nephrol 15: 1904–1911, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Schmieder RE, Schrader J, Zidek W, Tebbe U, Paar WD, Bramlage P, Pittrow D, Bohm M: Low-grade albuminuria and cardiovascular risk. Clin Res Cardiol 96: 247–257, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Diercks GF, van Boven AJ, Hillege JL, de Jong PE, Rouleau JL, van Gilst WH: The importance of microalbuminuria as a cardiovascular risk indicator: A review. Can J Cardiol 18: 525–535, 2002 [PubMed] [Google Scholar]
- 40. Hillege HL, Janssen WMT, Bak AAA, Diercks GFH, Grobbee DE, Crijns HJGM, van Gilst WH, de Zeeuw D, de Jong PE; for the PREVEND study group: Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 249: 519–526, 2001 [DOI] [PubMed] [Google Scholar]
- 41. de Jong PE, Curhan GC: Screening, monitoring, and treatment of albuminuria: Public health perspectives. J Am Soc Nephrol 17: 2120–2126, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA: Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant 24: 2446–2452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W: Kidney function and cognitive impairment in US adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 52: 227–234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hailpern SM, Melamed ML, Cohen HW, Hostetter TH: Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 18: 2205–2213, 2007 [DOI] [PubMed] [Google Scholar]