Abstract
Summary
Background and objectives
There are limited data on the prevalence of chronic kidney disease (CKD) and its clinical importance in the very old. We examined the prevalence of CKD in octogenarians and its association with cardiovascular disease (CVD).
Design, setting, participants, & measurements
In a cross-sectional analysis of 1028 participants from the Cardiovascular Health Study All Stars, we evaluated association of prevalent CKD with CVD using multivariable logistic regression. CKD was defined as eGFR of <60 ml/min per 1.73 m2. GFR was estimated using CKD-Epi creatinine and cystatin C equations that incorporate coefficients for age, gender, and race (eGFREPI, eGFRCYS3var) and the one-variable cystatin C equation (eGFRCYS1var). Prevalent CVD was defined as a composite of coronary heart disease, heart failure, and stroke.
Results
Mean age was 86 years, 64% were women, 86% were Caucasians, 14% had diabetes, and 39% had prevalent CVD. Mean eGFREPI, eGFRCYS3var, and eGFRCYS1var were 59, 62, and 70 ml/min per 1.73 m2, and 51%, 46%, and 33% had CKD, respectively. Associations of CKD with CVD varied by equation in adjusted analyses: CKDEPI (OR, 1.53; 95% CI, 1.15 to 2.03), CKDCYS3var (OR, 1.67; 95% CI, 1.25, 2.23), and CKDCYS1var (OR, 2.09; 95% CI, 1.55, 2.83).
Conclusions
Reduced eGFR is highly prevalent in octogenarians, and the eGFRCYS1var equation yielded the lowest prevalence of CKD but the strongest association with prevalent CVD. Because there are no validated estimating equations in the elderly, estimation of kidney function on the basis of on any one equation should be interpreted with caution.
Introduction
Chronic kidney disease (CKD) is a major public health problem disproportionately affecting the elderly. In various epidemiologic studies, approximately one-third to one-half of the individuals older than 70 years have CKD (1–3). Age was also the leading risk factor for incident CKD in the Framingham Heart Study (4). Thus, older individuals are most likely both to have CKD and to develop CKD over time. According to the United States Census Bureau, 8 million persons were 80 years or older in 2000, and by 2040 this will increase to 27 million when the “baby boomers” reach old age (5). Given the projected growth of the elderly population, understanding the prevalence and associations of CKD in octogenarians has important clinical and economic consequences.
Most prior studies that have evaluated the prevalence of CKD in the elderly have focused on individuals between the ages of 65 and 80 years (2,3). Many have utilized estimating equations on the basis of serum creatinine, but equations using serum creatinine may be particularly limited as a measure of estimated GFR (eGFR) in older adults in whom there may be a high prevalence of chronic disease associated with alterations in muscle mass and diet. Cystatin C, an alternative marker of GFR, may be less influenced by muscle mass (6,7) and has advantages as a marker of kidney function in the elderly where muscle mass is unpredictable. Conversely, cystatin C may be more affected by inflammatory status than serum creatinine (8,9).
The presence of CKD is recognized as an important, independent risk factor for all-cause mortality and cardiovascular disease (CVD) in most (10–16), but not all, longitudinal studies (17,18). Furthermore, most longitudinal studies suggest that cystatin C is more strongly associated with CVD than serum creatinine (15,19). However, few studies have evaluated the association of kidney function with CVD in octogenarians (20–22). The latter is important given the high prevalence of CKD in the elderly as well as the recognition that CKD may carry less prognostic importance as one ages (1).
We used data from Cardiovascular Health Study All Stars participants, survivors of at least 17 years from enrollment into the Cardiovascular Health Study (CHS), to assess the prevalence of CKD using different estimating equations on the basis of serum creatinine and cystatin C in octogenarians and the association of CKD with CVD in cross-sectional analyses.
Materials and Methods
Study Population
The CHS is a longitudinal study of community-dwelling adults aged 65 years and older recruited from Medicare eligibility lists in four Unites States communities (Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA) that was designed to examine subclinical and clinical CVD risk factors in older adults. Sampling and recruitment procedures have been described in detail elsewhere (23). An initial 5201 participants were recruited between 1989 and 1990, and an additional 687 black participants were added to the study between 1992 and 1993.
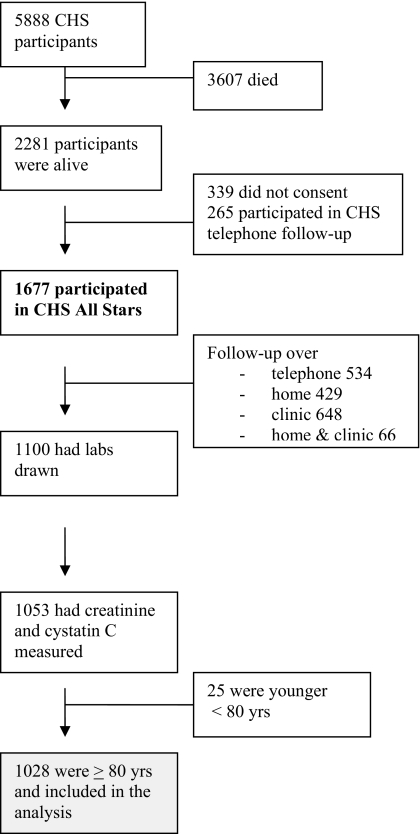
Until 1999, semiannual contact with participants alternated between clinic examinations and telephone contacts, during which time information about hospitalizations and potential CVD events were collected. Subsequently, participants were contacted twice a year by telephone to collect data, including use of various medications, and to identify all hospitalizations and potential cardiovascular events that were adjudicated by committee. During the seventeenth year of follow-up (April 2005 to May 2006), the CHS cohort was re-recruited for the CHS All Stars Study (24). The flow of patients is shown in Figure 1.
Figure 1.
Flow diagram of cardiovascular health study participants.
Exposure Variable
Serum creatinine was measured using a colorimetric method (Ektachem 700; Eastman Kodak, Rochester, NY), which was calibrated to isotope dilution mass spectrometry (IDMS). The intra-assay coefficient of variation for creatinine was 1.94%. Cystatin C was measured using a BN II nephelometer (Siemens) by a particle-enhanced immunonepholometric assay (N Latex Cystatin-C) on frozen serum samples stored at −70°C. The intra-assay coefficient of variation for cystatin C ranged from 2.0 to 2.8%. Estimated GFR was calculated using the Chronic Kidney Disease Epidemiology (CKD-Epi) equation because it may be more accurate and less biased than the Modification of Diet in Renal Disease (MDRD) Study equation at higher levels of GFR. eGFREPI = 141 × minimum (Scr/κ, 1)α × maximum(Scr/κ, 1)−1.209 × 0.993Age × 1.018 (if female) × 1.159 (if black), where κ is 0.7 for women and 0.9 for men, and α is −0.329 for women and −0.411 for men (25). eGFREPI is expressed in ml/min per 1.73 m2 of body surface area.
Most prior studies that have utilized a GFR estimate from cystatin C have chosen a single variable GFR equation (26). The cystatin C demographic equation had slightly higher accuracy and precision and lower bias when compared with the cystatin C one-variable equation in a pooled study of patients with established CKD with mean age of 52 years (8). We estimated GFR using both equations,
eGFRCYS1var = 76.7 × CysC−1.19
eGFRCYS3var = 127.7 × CysC−1.17 × age−0.13 × 1.06 (if black) × 0.91 (if female)
where eGFR is expressed as ml/min per 1.73 m2 of body surface area, and CysC is serum cystatin C expressed in mg/L (8).
For ease of interpretation, comparison with other studies, and consistency with kidney disease guidelines, CKD was defined as eGFR <60 ml/min per 1.73 m2 using any of the three equations. CKD stages 3 and stage 4 were defined as eGFR 30 to 60 ml/min per 1.73 m2 and eGFR <30 ml/min per 1.73 m2, respectively (27). We recognize that despite recent studies demonstrating an association of eGFR below 60 ml/min per 1.73 m2 (even in the absence of albuminuria) with adverse outcomes (16), there continues to be debate as to whether reduced GFR in the elderly is due to a normal decline associated with aging versus whether it meets criteria to define a disease state (28,29).
Study Outcome
The primary study outcome was prevalent clinical CVD, defined as a composite of history of coronary heart disease, heart failure, or stroke, at the time of initiation of CHS All Stars. Coronary heart disease was defined as history of angina, myocardial infarction, angioplasty, or coronary bypass surgery. Potential events were identified through contact with participants or proxies. Reports not confirmed by examination or medication use were investigated by review of medical records. All of the events were adjudicated by a committee as described previously (30).
Covariates
Covariates were chosen for multivariate analyses on the basis of prior studies or the biologic plausibility of variables that may confound the association between decreased kidney function and presence of CVD. These included: (1) demographic variables (age, gender, and race); (2) cardiovascular risk factors (body mass index calculated as weight in kilograms/height in meters squared, hypertension [defined as self-reported history or an average of three BP measurements ≥140/90 mmHg], diabetes [defined as self-reported history of diabetes, use of insulin, or oral hypoglycemic agent or fasting glucose value ≥126 mg/dl], systolic BP, diastolic BP, smoking, LDL cholesterol, and HDL cholesterol); and (3) novel cardiovascular risk factors (C-reactive protein, vitamin D25).
Statistical Analyses
Descriptive analyses were used to summarize baseline characteristics of the study participants according to stages of CKD. Continuous data are presented as means ± SD, and categorical variables are presented as proportions. ANOVA (31) and Pearson chi-squared test (32) with trend P values were used to compare the stages of CKD across continuous and categorical variables, respectively. Because the distribution of C-reactive protein is skewed, the data are presented as median and interquartile ranges, and the Kruskal-Wallis test (33) was used to compare this variable across the stages of CKD. Pearson correlation was used to assess the correlation between creatinine and cystatin C-based estimates of GFR. The overlap of CKD groups on the basis of creatinine (CKDEPI) and cystatin C three (CKDCYS3var) and cystatin C one-variable (CKDCYS1var) eGFR was evaluated, and the degree of agreement between the two was assessed using weighted kappa statistics (32). Logistic regression analyses were used to examine the association of kidney function with CVD (34). Separate models were constructed for eGFR defined either by creatinine or cystatin C. Creatinine- and cystatin C-based eGFR were initially modeled as continuous variables, and a piecewise linear model was used because of nonlinearity, where eGFR was modeled in 10 ml/min per 1.73 m2 increments for those with eGFR <60 ml/min per 1.73 m2 and ≥60 ml/min per 1.73 m2. Analyses were repeated for cystatin C and creatinine CKD stages with the reference group being those without CKD.
Initial analyses were unadjusted, and subsequent analyses were adjusted for demographic factors and CVD risk factors with the same variables in each model. Interactions were assessed a priori, between CKD and diabetes and CKD and gender, given that nephropathy in diabetes is a particularly high risk state, and there are known differences between men and women in nontraditional CVD risk factors in patients with CKD (35). To assess whether the addition of CKD defined by cystatin C added prognostic information to CKD defined by creatinine, participants were categorized into four mutually exclusive groups: eGFREPI < 60 ml/min per 1.73 m2 only, eGFRCYS3var < 60 ml/min per 1.73 m2 only, eGFREPI and eGFRCYS3var < 60 ml/min per 1.73 m2, and eGFREPI and eGFRCYS3var ≥ 60 ml/min per 1.73 m2 (reference group). A similar analysis was repeated using the cystatin C one-variable equation.
Sensitivity Analyses.
Several sensitivity analyses were performed to assess the consistency of our results. Analyses were repeated using the four-variable MDRD Study equation given that it is currently the most commonly used estimating equation (eGFR = 175 × standardized Scr−1.154 × age−0.203 × 1.212 [if black] × 0.742 [if female]), in which eGFR is expressed as ml/min per 1.73 m2 of body surface area and where Scr is serum creatinine expressed in mg/dL (36). Because prior studies have shown that kidney function may have different relationships with atherosclerotic CVD and heart failure (37), repeat analyses were performed by excluding those individuals with a history of heart failure, but with no other form of atherosclerotic CVD, from our working definition of CVD. Finally, because high GFR, a marker of low creatinine and malnutrition, has also been associated with CVD and mortality (14), we repeated the CKDEPI analyses with eGFREPI of 60 to 90 ml/min per 1.73 m2 as the reference group, rather than eGFREPI >60 ml/min per 1.73 m2. Analyses were performed using SAS software (9.2; SAS, Cary, NC). We considered two-tailed P < 0.05 as statistically significant.
Results
Characteristics of Study Participants
Of the 1053 CHS All Stars participants in whom creatinine and cystatin C was measured, 25 were excluded because they were younger than 80 years old, resulting in a sample of 1028 participants for these analyses. Participants who were alive but did not have laboratory data available were more likely to be women, to be older, and to have a higher prevalence of myocardial infarction and stroke compared with those included in the analysis.
The average age of the study sample was 86 years, and 64% were women, 86% were Caucasians, two-thirds had hypertension, and 14% had diabetes. Mean eGFREPI, eGFRCYS3var, and eGFRCYS were 59, 62, and 70 ml/min per 1.73 m2, respectively (Table 1). The Pearson correlation between eGFREPI and eGFRCYS3var was 0.75 (p = <0.01), and that between eGFREPI and eGFRCYS1var was 0.74 (p = <0.01). Compared with participants without CKD, those with CKD were more likely to be older and have a history of hypertension and diabetes, higher C-reactive protein levels, and lower HDL cholesterol and LDL cholesterol levels. Similar results were obtained when CKD was considered using cystatin C.
Table 1.
Baseline characteristics by level of kidney function using the CKD-Epi equation
| Characteristics | Total | eGFREPI (ml/min per 1.73 m2) |
Pa | ||
|---|---|---|---|---|---|
| >60 | 30 to 59 | <30 | |||
| n | 1028 | 501 | 477 | 50 | |
| Age | 85.5 (3.5) | 85.2 (3.4) | 85.7 (3.6) | 86.2 (3.7) | <0.01 |
| Women | 63.8 | 62.7 | 66.5 | 50.0 | 0.89 |
| White race | 85.9 | 83.2 | 88.5 | 88.0 | 0.03 |
| Body mass index (kg/m2) | 26.8 (4.7) | 26.6 (4.6) | 26.8 (4.7) | 27.8 (5.0) | 0.22 |
| Ever smoked | 49.2 | 50.2 | 48.2 | 48.0 | 0.54 |
| Diabetes | 14.4 | 13.1 | 13.9 | 32.0 | 0.02 |
| Hypertension | 65.6 | 60 | 69.5 | 84.0 | <0.01 |
| Systolic blood pressure (mmHg) | 132.7 (21.1) | 133.7 (20.8) | 131.4 (20.9) | 135.7 (25.9) | 0.19 |
| HDL cholesterol (mg/dl) | 54.3 (15.3) | 55.7 (15.2) | 53.7 (15.3) | 44.9 (12.3) | <0.01 |
| LDL cholesterol (mg/dl) | 101.3 (31.5) | 104.0 (32.0) | 99.0 (30.5) | 96.0 (33.7) | <0.01 |
| Vitamin D25 (ng/ml) | 25.9 (10.4) | 24.8 (9.1) | 27.2 (11.5) | 25.1 (11.3) | <0.01 |
| C-reactive protein (mg/L)b | 2.0 (1.0, 4.5) | 1.8 (0.9, 4.1) | 2.1 (1.0, 5.0) | 3.0 (1.8, 7.8) | <0.01 |
| Creatinine (mg/dl) | 1.1 (0.5) | 0.8 (0.1) | 1.2 (0.2) | 2.7 (1.4) | <0.01 |
| Cystatin C (mg/L) | 1.2 (0.5) | 1.0 (0.2) | 1.3 (0.3) | 2.4 (1.0) | <0.01 |
| MDRD eGFR | 61.8 (19.1) | 77.0 (12.9) | 49.8 (8.2) | 23.7 (7.4) | <0.01 |
| CKD-Epi eGFR | 58.8 (17.0) | 72.9 (8.5) | 47.7 (8.1) | 21.9 (7.1) | <0.01 |
| Cystatin C eGFR using one- variable equation | 69.8 (21.7) | 82.6 (17.7) | 60.3 (15.9) | 31.2 (10.6) | <0.01 |
| Cystatin C eGFR using three- variable equation | 61.9 (19.2) | 73.4 (15.9) | 53.4 (13.7) | 28.3 (9.5) | <0.01 |
The data are presented either as means (SD) or percentages. Represent P = 0.0000 as P < 0.01.
Trend P values.
Median [IQR] with Kruskal-Wallis test.
Prevalence of CKD
On the basis of eGFREPI, eGFRCYS3var, and eGFRCYS1var equations, 51.3, 46.1, and 33.0% participants, respectively, had CKD. There was moderate agreement between both the CKDEPI and CKDCYS3var stages and the CKDEPI and CKDCYS1var stages, and the weighted kappa statistic was 0.58 (95% CI, 0.53 to 0.62) and 0.51 (95% CI, 0.46 to 0.55), respectively (Table 2).
Table 2.
Agreement between CKDEPI and CKDCYS stages
| eGFREPI (ml/min per 1.73 m2) | eGFRCYS3var (ml/min per 1.73 m2) (n) (%)a |
Total patients (n) (%) | eGFRCYS1var (ml/min per 1.73 m2) (n) (%)b |
Total patients (n) (%) | ||||
|---|---|---|---|---|---|---|---|---|
| eGFR >60 | eGFR 30 to 59 | eGFR <30 | eGFR >60 | eGFR 30 to 59 | eGFR <30 | |||
| eGFR >60 | 416 (40.5) | 85 (8.3) | 0 | 501 (48.7) | 465 (45.2) | 36 (3.5) | 0 | 501 (48.7) |
| eGFR 30 to 59 | 138 (13.4) | 328 (31.9) | 11 (1.1) | 477 (46.4) | 224 (21.8) | 248 (24.1) | 5 (0.5) | 477 (46.4) |
| eGFR <30 | 0 | 20 (2.0) | 30 (2.9) | 50 (4.9) | 0 | 28 (2.7) | 22 (2.1) | 50 (4.9) |
| Total | 554 (53.9) | 433 (42.1) | 41 (4.0) | 1028 (100) | 689 (67.0) | 312 (30.4) | 27 (2.6) | 1028 (100) |
The cell percentages may not total to margin percentages because of rounding. Cells with bold type represent agreement. Cells above the cells with bold represent disagreements in which estimated GFR category was higher with the CKD-Epi equation than with the cystatin C equation; cells below the cells with bold represent disagreements in which estimated GFR category was lower with the CKD-Epi equation than with the cystatin C equation. Parentheses = percentages of total participants (n = 1028). To calculate the percentage of agreement in a particular row, divide the value in bold into the total in that row. To calculate the percentage of agreement in a particular column, divide the value in bold into the total in that column. For example, in patients with eGFR using CKDEPI of <30 ml/min per 1.73 m2, the percentage of agreement in rows = 30/50 and percentage of agreement in columns = 30/41.
Weighted kappa statistics: 0.58 (95% CI, 0.53 to 0.62).
Weighted kappa statistics: 0.51 (95% CI, 0.46 to 0.55).
Cross-sectional Association of CVD with CKD
The overall prevalence of CVD in this study sample was 38.5%, of which 30.5% had a history of coronary heart disease, 14.5% had heart failure, and 8.1% had stroke. A piecewise linear form with cut-point of 60 ml/min per 1.73 m2 was chosen for the continuous models on the basis of current definition of CKD (Table 3). In these analyses, eGFR <60ml/min per 1.73 m2 was associated with CVD with the strongest relationship in eGFRCYS1var analysis. There was no significant relationship between CVD and eGFR >60 ml/min per 1.73 m2. In both unadjusted and adjusted analyses, associations of CKD with CVD varied by the equation with increased odds of CVD in those with CKD stage 4 compared with stage 3 (Table 3). CKD when defined by cystatin C was associated with higher odds of CVD compared with CKDEPI. There was no interaction between CKD with diabetes or gender (P = 0.7 for both).
Table 3.
Association of CKD with CVD based on serum creatinine and cystatin C estimates
| eGFR (ml/min per 1.73 m2) | CKD-Epi |
Cystatin C Three-variable Equation |
Cystatin C One-variable Equation |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)a | n (%) | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)a | n (%) | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)a | |
| Piecewise linear analysis | |||||||||
| >60b | 501 (49) | 1.06 (0.89, 1.26) | 1.07 (0.88, 1.29) | 554 (54) | 0.98 (0.88, 1.11) | 0.98 (0.86, 1.11) | 689 (67) | 1.05 (0.96, 1.15) | 1.03 (0.93, 1.14) |
| <60b | 527 (51) | 1.35 (1.18, 1.55) | 1.31 (1.12, 1.53) | 474 (46) | 1.53 (1.33, 1.77) | 1.48 (1.25, 1.75) | 339 (33) | 1.55 (1.31, 1.83) | 1.49 (1.23, 1.81) |
| CKD 3 groups | |||||||||
| >60 | 501 (49) | 1.00 (ref) | 1.00 (ref) | 554 (54) | 1.00 (ref) | 1.00 (ref) | 689 (67) | 1.00 (ref) | 1.00 (ref) |
| 30 to 59 | 477 (46) | 1.45 (1.11, 1.88) | 1.40 (1.04, 1.87) | 433 (42) | 1.63 (1.26, 2.12) | 1.54 (1.15, 2.07) | 312 (30) | 2.12 (1.61, 2.78) | 2.00 (1.47, 2.72) |
| <30 | 50 (5) | 4.79 (2.55, 9.03) | 4.16 (2.03, 8.49) | 41 (4.0) | 6.60 (3.17, 13.76) | 5.68 (2.42,13.34) | 27 (3) | 5.03 (2.17, 11.67) | 4.25 (1.58, 1.46) |
| CKD 2 groups | |||||||||
| >60 | 501 (49) | 1.00 (ref) | 1.00 (ref) | 554 (54) | 1.00 (ref) | 1.00 (ref) | 689 (67) | 1.00 (ref) | 1.00 (ref) |
| <60 | 527 (51) | 1.62 (1.25, 2.08) | 1.53 (1.15, 2.03) | 474 (46) | 1.83 (1.42, 2.36) | 1.67 (1.25, 2.23) | 339 (33) | 2.26 (1.73, 2.95) | 2.09 (1.55, 2.83) |
ref, referent group.
Adjusted for age, gender, race, body mass index, smoking, diabetes, hypertension, systolic blood pressure, low density lipoprotein cholesterol, high density lipoprotein cholesterol, vitamin D, and log C-reactive protein. The sample sizes are based on the complete case data for all variables used in the multivariable models.
Values per 10 ml/min per 1.73 m2 lower eGFR.
When participants were categorized into four mutually exclusive groups using eGFREPI and eGFRCYS, CKD was associated with CVD only in those participants who were identified as having CKD by both measures when eGFRCYS3var was used (Table 4). In comparison, when CKD was defined using the eGFRCYS1var equation, there was a significant association with CVD, irrespective of CKD on the basis of eGFREPI.
Table 4.
Association of chronic kidney disease with cardiovascular disease using four mutually exclusive groups based CKD-Epi and cystatin C equations
| eGFR (ml/min per 1.73 m2) | Cystatin C Three-variable Equation |
Cystatin C One-variable Equation |
||
|---|---|---|---|---|
| n (%) | Adjusted Odds Ratio (95% CI)a | n (%) | Adjusted Odds Ratio (95% CI)a | |
| eGFRCKD-Epi and eGFRCYS >60 | 385 (41) | 1.00 (ref) | 430 (46) | 1.00 (ref) |
| eGFRCKD-Epi <60/eGFRCYS >60 | 130 (14) | 1.31 (0.84, 2.02) | 212 (23) | 1.18 (0.81, 1.70) |
| eGFRCKD-Epi >60/eGFRCYS <60 | 78 (8) | 1.59 (0.94, 2.69) | 33 (4) | 2.58 (1.22, 5.43) |
| eGFRCKD-Epi and eGFRCYS <60 | 349 (37) | 1.85 (1.33, 2.57) | 267 (28) | 2.18 (1.55, 3.06) |
ref, referent group.
Adjusted for age, gender, race, body mass index, smoking, diabetes, hypertension, systolic blood pressure, low density lipoprotein cholesterol, high density lipoprotein cholesterol, vitamin D, and log C-reactive protein.
Sensitivity Analyses
The prevalence of CKD using MDRD equation was 47.3%, which is very similar to that obtained using CKDEPI and CKDCYS3var. In multivariable analyses, the presence of CKD was also associated with similar odds of CVD (OR, 1.57; 95% CI, 1.18 to 2.10). The results were essentially unchanged when the 36 participants with heart failure and no other atherosclerotic CVD were excluded from the definition of CVD. That is, CKD was associated with a higher odds of CVD using CKDEPI (OR, 1.54; 95% CI, 1.15 to 2.06) and CKDCYS3var (OR, 1.56; 95% CI, 1.16 to 2.09). Finally, when eGFREPI 60 to 90 ml/min per 1.73 m2 was used as the reference group, the results were similar. Those with CKDEPI stage 3 (OR, 1.39; 95% CI, 1.04 to 1.87) and stage 4 (OR, 4.12; 95% CI, 2.02 to 8.43) had higher odds of CVD when compared with eGFREPI 60 to 90 ml/min per 1.73 m2.
Discussion
In this community-based cohort of octogenarians, we observed a high prevalence of CKD. Although the prevalence of CKD and strength of the association between CKD and CVD varied depending on the estimating equation used, the association was significant across all of our analyses.
There was a very high prevalence of CKD in our population, and our findings are consistent with those previously reported in the elderly (1,38,39). The prevalence of CKD was 40% among a national cohort of veterans aged 75 to 84 years (1); in the Kidney Early Evaluation Program, a high risk population, the prevalence of CKD was 60% in individuals over 80 years (38); and in the National Health and National Examination Survey, which oversamples minorities, the prevalence of CKD was 68% (38). Differences in the prevalence of CKD may be due to differences in the clinical and demographic characteristics of the study population, criteria used to define CKD, age of subjects, and measurement of serum creatinine (calibrated versus uncalibrated). Also, most studies have used serum creatinine-based estimating equations. This is one of the first studies in patients over 80 years of age where GFR was estimated using both CKD-Epi and cystatin C equations. We noted that the prevalence of CKD was significantly lower when the cystatin C one-variable equation was used to estimate kidney function. Therefore, although the prevalence of CKD remained very high in the elderly, the prevalence differs depending upon the equation used, despite a strong correlation between creatinine and cystatin C-based estimates of kidney function. Further studies in older adults with actual GFR measurements are needed to evaluate the validity of these estimating equations.
The clinical significance of CKD has been questioned in the elderly (28). Prior studies had conflicting results, with some suggesting that CKD is associated with all-cause (21) and CVD mortality (20,21), whereas others noted an attenuation of this effect with advancing age (1,40). Our results demonstrate that CKD defined using either creatinine or cystatin was independently associated with prevalent CVD in the very old. In addition, an incremental association of CKD with CVD with decreasing kidney function was observed. The reasons why CKD is independently associated with a higher prevalence of CVD are unknown. Among the possibilities are that CKD is a marker of the severity of traditional risk factors, such as hypertension, a measure of nontraditional CVD risk factors, a causal risk factor through promotion of volume retention, anemia, and abnormalities in mineral metabolism, or because of therapeutic nihilism whereby those with CKD are not treated with medications that are known to prevent CVD (41). We also noted that participants identified as having CKD using the cystatin one-variable equation had higher odds of CVD compared with those defined by eGFREPI. The cystatin one-variable equation therefore identifies a smaller but higher risk group. Follow-up of our cohort will allow assessment of the consistency of these differences in longitudinal analyses.
We were particularly interested in evaluating whether a more specific definition of CKD requiring the presence of both CKDCYS and CKDEPI would identify a higher risk group.
Our results demonstrated that when CKD was defined using the eGFRCYS3var equation, the presence of CKD by eGFREPI was also required for a significant association with CVD. In comparison, when CKD was defined using the eGFRCYS1var equation, there was a significant association with CVD, irrespective of CKD on the basis of eGFREPI. The latter is consistent with combined analyses from Multi-Ethnic Study of Atherosclerosis and CHS (participants were at baseline and therefore of a younger age than in this work), where in patients with a diagnosis of CKD using the creatinine-based CKD-Epi equation, risks for adverse outcomes (death, CVD events, heart failure, and end stage renal disease) were limited to the subset who also have CKD according to the cystatin C-based equation (42). Therefore, cystatin C could in theory be used to distinguish high and low risk individuals defined by CKDEPI.
The results of this study may have particular relevance in the context of the increasing prevalence of CKD in octogenarians. The discrepancies in GFR estimates between different creatinine and cystatin C-based equations must be taken into consideration if cystatin C-based equations become a method for assessing kidney function in the elderly. These differences in estimation of kidney function may affect clinical decision making at an individual level (such as risk/benefit decisions regarding medication dosing and assessing risk of contrast-induced nephropathy), as well as in estimating the burden of CKD in the elderly.
The strengths of our study include the use of data from a well characterized cohort of older men and women. In addition, this is one of the largest cohorts of octogenarians with detailed ascertainment of covariates and CVD. Most prior studies in octogenarians have only used estimating equations on the basis of serum creatinine to assess kidney function, whereas we used IDMS calibrated creatinine as well as cystatin C. Furthermore, we used the CKD-Epi equation, which is more accurate than the MDRD equation at higher levels of GFR, in women, and in persons of white race.
Several limitations need to be considered in interpreting our findings. The CHS All Stars Study lacks direct measurement of GFR, and therefore we cannot comment on discrepancies between creatinine and cystatin C measurements and true GFR. It is possible that some participants with reduced eGFR (e.g. 45 to 59 ml/min per 1.73 m2) may not meet the criteria for CKD, given that the equations have not been validated in this age group. Unfortunately, there are no large studies that have measured GFR or validated estimating equations in the elderly. Therefore, at the present time and into the foreseeable future, indirect estimates of GFR will be used to assess kidney function, and the limitations of this approach need to be recognized. Urinary protein was not collected in the CHS All Stars study; therefore, we were unable to adjust for the presence of albuminuria or define individuals in CKD stages 1 to 2. Furthermore, in those individuals with eGFR just below the threshold of 60 ml/min per 1.73 m2, we had no supportive data to strengthen or weaken the diagnosis of kidney disease. We may also have underestimated the prevalence of CKD, because the participants who did not provide blood samples were in general sicker. This is a cross-sectional analysis; therefore, cause or effect could not be established, and indeed recent data suggest that CVD may contribute to the progression of kidney disease (43). As opposed to the IDMS creatinine reference standard, a cystatin C reference standard is not yet available, and prevalence data may vary depending on its calibration. In addition, cystatin C may be influenced somewhat by factors other than GFR such as age, gender, body fat, smoking, and inflammation; despite adjustment for these variables, residual confounding may have remained (8,9).
In summary, there is a high prevalence of reduced eGFR in octogenarians, and the prevalence of CKD and its relationship with CVD differed depending on the estimating equation used to assess kidney function. Because there are no validated estimating equations in the elderly, estimation on the basis of any one equation should be interpreted with caution. Although octogenarians are survivors, those with CKD defined by either creatinine or cystatin C remain a high risk group. Longitudinal studies in octogenarians are needed to assess the importance of CKD, defined by either eGFREPI or eGFRCYS, for prognosis and clinical decision making.
Disclosures
None.
Acknowledgments
This manuscript was presented in abstract form at the American Society of Nephrology Annual Meeting in Denver, CO on November 18, 2010.
The research reported in this article was supported by National Institute on Aging Grant AG-023629. This material is also based upon work supported in part by Department of Veterans Affairs Grant VISN 4. The CHS was supported by Contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133 and Grant U01 HL080295 from the National Heart, Lung, and Blood Institute, with an additional contribution from the National Institute of Neurologic Disorders and Stroke. Additional support was provided through Grants R01-AG-023629, R01-AG-15928, R01 AG-20098, and AG-027058 from the National Institute on Aging; Grant R01 HL-075366 from the National Heart, Lung and Blood Institute; and University of Pittsburgh Claude. D. Pepper Older Americans Independence Center Grant P30-AG-024827. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Dr. Sarnak is supported by NIDDK Grant K24 DK078204.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. O'Hare AM, Bertenthal D, Covinsky KE, Landefeld CS, Sen S, Mehta K, Steinman MA, Borzecki A, Walter LC: Mortality risk stratification in chronic kidney disease: One size for all ages? J Am Soc Nephrol 17: 846–853, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Garg AX, Papaioannou A, Ferko N, Campbell G, Clarke JA, Ray JG: Estimating the prevalence of renal insufficiency in seniors requiring long-term care. Kidney Int 65: 649–653, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 5.United States Census Bureau. 2005. [Accessed March 8, 2011]. http://www.census.gov/prod/2006pubs/p23-209.pdf.
- 6. Fliser D, Ritz E: Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis 37: 79–83, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP: Serum cystatin C measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int 47: 312–318, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE: Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65: 1416–1421, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB: Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 41: 1364–1372, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM: Cardiovascular disease risk status in elderly persons with renal insufficiency. Kidney Int 62: 997–1004, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W: Kidney function and cognitive impairment in US adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 52: 227–234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manjunath G, Tighiouart H, Coresh J, Macleod B, Salem DN, Griffith JL, Levey AS, Sarnak MJ: Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 63: 1121–1129, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D: Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int 56: 2214–2219, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Garg AX, Clark WF, Haynes RB, House AA: Moderate renal insufficiency and the risk of cardiovascular mortality: Results from the NHANES I. Kidney Int 61: 1486–1494, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS: Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 145: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Kagiyama S, Matsumura K, Ansai T, Soh I, Takata Y, Awano S, Sonoki K, Yoshida A, Takehara T, Iida M: Chronic kidney disease increases cardiovascular mortality in 80-year-old subjects in Japan. Hypertens Res 31: 2053–2058, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Roderick PJ, Atkins RJ, Smeeth L, Mylne A, Nitsch DD, Hubbard RB, Bulpitt CJ, Fletcher AE: CKD and mortality risk in older people: a community-based population study in the United Kingdom. Am J Kidney Dis 53: 950–960, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Van den Noortgate NJ, Janssens WH, Afschrift MB, Lameire NH: Renal function in the oldest-old on an acute geriatric ward. Int Urol Nephrol 32: 531–537, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG.: The Cardiovascular Health Study: Design and rationale. Ann Epidemiol 1: 263–276, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Newman AB, Arnold AM, Sachs MC, Ives DG, Cushman M, Strotmeyer ES, Ding J, Kritchevsky SB, Chaves PH, Fried LP, Robbins J: Long-term function in an older cohort: The Cardiovascular Health Study All Stars study. J Am Geriatr Soc 57: 432–440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Glassock RJ, Winearls C: An epidemic of chronic kidney disease: Fact or fiction? Nephrol Dial Transplant 23: 1117–1121, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Glassock RJ, Winearls C: Screening for CKD with eGFR: Doubts and dangers. Clin J Am Soc Nephrol 3: 1563–1568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S: Surveillance and ascertainment of cardiovascular events: The Cardiovascular Health Study. Ann Epidemiol 5: 278–285, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Neter J, Wasserman W, Kutner MH: Applied Linear Statistical Models: Regression, Analysis of Variance, and Experimental Designs: Analysis of Variance, Homewood, IL, Richard D. Irwin, Inc., 1990 [Google Scholar]
- 32. Fleiss JL: Statistical Methods for Rates and Proportions: Kappa and Chi-square Test, New York, John Wiley & Sons, Inc., 1981 [Google Scholar]
- 33. Hollander M, Wolfe DA: Nonparametric Statistical Methods: Kruskal-Wallis Test, New York, John Wiley & Sons, Inc., 1999 [Google Scholar]
- 34. Allison PD: Logistic Regression Using the SAS System: Theory and Application, Cary NC, SAS Institute Inc., 1999 [Google Scholar]
- 35. Duncan JA, Levin A: Sex, haemoglobin and kidney disease: New perspectives. Eur J Clin Invest 35[Suppl 3]: 52–57, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG: Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med 142: 497–505, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Stevens LA, Li S, Wang C, Huang C, Becker BN, Bomback AS, Brown WW, Burrows NR, Jurkovitz CT, McFarlane SI, Norris KC, Shlipak M, Whaley-Connell AT, Chen SC, Bakris GL, McCullough PA: Prevalence of CKD and comorbid illness in elderly patients in the United States: Results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 55: S23–S33, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Christensson A, Elmstahl S: Estimation of the age-dependent decline of glomerular filtration rate from formulas based on creatinine and cystatin C in the general elderly population. Nephron Clin Pract 117: c40–c50, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Raymond NT, Zehnder D, Smith SC, Stinson JA, Lehnert H, Higgins RM: Elevated relative mortality risk with mild-to-moderate chronic kidney disease decreases with age. Nephrol Dial Transplant 22: 3214–3220, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Peralta CA, Katz R, Sarnak MJ, Ix J, Fried LF, De Boer I, Palmas W, Siscovick D, Levey AS, Shlipak MG: Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol 22: 147–155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, Sarnak MJ, Weiner DE: Cardiovascular disease and subsequent kidney disease. Arch Intern Med 167: 1130–1136, 2007 [DOI] [PubMed] [Google Scholar]