Abstract
Summary
Background and objectives
Low bone mineral density and coronary artery calcification (CAC) are highly prevalent among chronic kidney disease (CKD) patients, and both conditions are strongly associated with higher mortality. The study presented here aimed to investigate whether reduced vertebral bone density (VBD) was associated with the presence of CAC in the earlier stages of CKD.
Design, setting, participants, & measurements
Seventy-two nondialyzed CKD patients (age 52 ± 11.7 years, 70% male, 42% diabetics, creatinine clearance 40.4 ± 18.2 ml/min per 1.73 m2) were studied. VBD and CAC were quantified by computed tomography.
Results
CAC > 10 Agatston units (AU) was observed in 50% of the patients (median 120 AU [interquartile range 32 to 584 AU]), and a calcification score ≥ 400 AU was found in 19% (736 [527 to 1012] AU). VBD (190 ± 52 Hounsfield units) correlated inversely with age (r = −0.41, P < 0.001) and calcium score (r = −0.31, P = 0.01), and no correlation was found with gender, creatinine clearance, proteinuria, lipid profile, mineral parameters, body mass index, and diabetes. Patients in the lowest tertile of VBD had expressively increased calcium score in comparison to the middle and highest tertile groups. In the multiple logistic regression analysis adjusting for confounding variables, low VBD was independently associated with the presence of CAC.
Conclusions
Low VBD was associated with CAC in nondialyzed CKD patients. The authors suggest that low VBD might constitute another nontraditional risk factor for cardiovascular disease in CKD.
Introduction
Low bone mineral density (BMD) is a common feature among chronic kidney disease (CKD) patients (1). Abnormal remodeling, cell differentiation, hormonal and growth factor abnormalities, and disorders in mineral content are likely to alter bone architecture, resulting in bone mass loss in these patients (2,3). Reduced BMD has been associated not only with increased incidence of fractures (1) but also with higher cardiovascular disease (CVD) and subsequent higher mortality in dialysis populations (2,3).
In the general population, the inverse relationship between BMD and coronary artery calcification (CAC) has been demonstrated (4,5). More importantly, loss of BMD has been implicated with the progression of CAC over time (6,7). In dialysis patients, the few studies using computed tomography (CT) have found an association of vertebral bone density (VBD) with the extent of CAC (8,9). CAC is a highly prevalent condition among CKD patients, and it has been described as a strong predictor of worse outcome in the dialysis population (10,11). Recently, we demonstrated that the presence of CAC was associated with future cardiovascular events, hospitalization, and mortality in nondialyzed patients as well (12). In the investigation presented here, we aimed to evaluate the relationship between VBD and CAC, both assessed by CT, in nondialyzed CKD patients. Our hypothesis is that the high prevalence of CAC may be associated with low VBD, and that this linkage starts in the earlier stages of CKD.
Materials and Methods
Patients
A total of 72 asymptomatic nondialyzed CKD patients (stages 2 to 4) followed by a nephrologist for at least 3 months and older than 18 years of age were recruited from the outpatient clinic of the Federal University of São Paulo, Brazil. Exclusion criteria were evidence of inflammation; neoplasic or infectious diseases; and use of phosphate binder, calcium, vitamin D analogs, or corticosteroids.
The study was reviewed and approved by the Ethics Advisory Committee of the Federal University of São Paulo, and each patient signed the informed consent form.
Study Protocol
In this cross-sectional study all selected patients underwent clinical and physical evaluation. Weight and height were used to calculate body mass index (BMI). Previous CVD was characterized by the presence of myocardial infarction, angina pectoris, coronary artery revascularization, ischemic stroke, or a positive diagnostic procedure (stress test, coronary angiography, and radionuclide image). Laboratory tests and multislice vertebral and coronary CT were performed within a period of 30 days after patients' selection.
Laboratory Tests
Blood samples were drawn in a fasting state for the following laboratory tests: hemoglobin, glucose, total cholesterol, HDL and LDL cholesterol, triglycerides, creatinine, ionized calcium, phosphorus, alkaline phosphatase, bicarbonate, intact parathyroid hormone (iPTH; Immulite assay, DPC, Los Angeles, CA; reference range 10 to 65 pg/ml), and 25-hydroxyvitamin D (25(OH)D; radio immunoassay, DiaSorin, Stillwater, MN; reference range 18 to 62 ng/ml). The creatinine clearance and proteinuria were measured by obtaining 24-hour urine samples. Creatinine clearance was corrected for body surface area. Abnormal proteinuria was defined as urinary protein excretion >150 mg/24 h. According to the Kidney Disease Improving Global Outcomes Guideline on Mineral and Bone Disorder of Chronic Kidney Disease (13), hyperphosphatemia, hypercalcemia, and hyperparathyroidism were considered when serum phosphorus, ionized calcium, and iPTH levels were higher than the upper limit of normal range (>4.6 mg/dl, >1.40 mmol/L, and >65 pg/ml, respectively). Vitamin D deficiency and insufficiency were diagnosed when 25(OH)D values were <15 ng/ml and between 15 and 30 ng/ml, respectively.
VBD
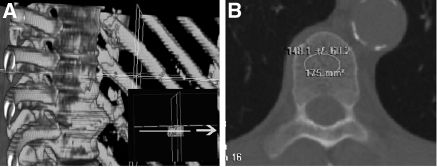
Stored vertebral images were obtained in a 16-detector-row scanner (Somatron Volume Zoom Siemens AG, Erlhagen, Germany). A thoracic vertebra was used for VBD analysis because the patients of this study were participating in a protocol that required the performance of a chest CT scan for measurement of coronary calcification. For the assessment of VBD by CT, the mean density within a region of interest placed at the midvertebral body was measured including only medullar (trabecular) bone (Figure 1). The vertebral body chosen was the thoracic vertebra located at the level of aortic root on the axial images. The mean density obtained from this region of interest was considered as a surrogate measure of VBD and was expressed in Hounsfield units (HU). All analyses were performed in a Vitrea 2 workstation (Vital Images, Inc., Plymouth, MN) by two independent observers blind to the clinical data, and the mean values were considered for statistical analyses.
Figure 1.
Quantitative computed tomography (CT) images of the thoracic vertebral body. The white circle in panel B depicts the tissue attenuation data measured by the computer software in the region of interest (trabecular bone) chosen for analysis.
CAC
Patients underwent coronary calcium quantification by a multislice CT scanner (Somatrom Volum Zoom Siemens AG, Erlhagen, Germany) using a gantry rotation of 0.4 seconds, a collimation of 2.5 mm (slice thickness), and a reconstruction time of 6 frames/s. A calcium threshold of ≥130 HU was used. The images were scored by a single radiologist blinded to all clinical and biochemical aspects of the patient. As described by Agatston (14), the calcium score was determined by multiplying the area of each calcified lesion by a weighting factor corresponding to the peak pixel intensity for each lesion. The sum of each lesion of all coronary arteries was used for analysis. Presence of CAC was defined as calcium score >10 Agatston units (AU), and severe CAC as calcium score ≥400 AU.
Statistical Analyses
Mean and SD, median and interquartile range, or frequencies were calculated for all variables. Comparisons for continuous variables among the VBD tertiles were done by ANOVA or Kruskal–Wallis test for normal and skewed data, respectively. Comparisons of proportions were done by χ2 analysis or by Fischer's exact test, as appropriate. The correlation between calcium score or VBD and other variables was assessed using Pearson's or Spearman's correlation coefficients according to the distribution of the variables. For graphical representation, the calcium score was logarithimic transformed (ln[lnCaSc + 1], where CaSc is the calcium score). Multiple logistic regression analysis was applied to evaluate the independent association between VBD and CAC. Statistical analyses were performed using SPSS for Windows (SPSS, Inc., Chicago, IL), and P < 0.05 was considered statistically significant.
Results
The characteristics of the studied patients are shown in Table 1. As can be seen, patients were predominantly middle-aged men and had been followed by a nephrologist for a median of 22 months (interquartile range 10 to 51 months). Hypertension and diabetes were the main causes of CKD. Overweight status (BMI 25 to 29.9 kg/m2) and obesity (BMI ≥ 30 kg/m2) were found in 40.3% and 22.2% of the patients, respectively. On the basis of the CKD classification proposed by the National Kidney Foundation (Kidney Disease Outcomes Quality Initiative) (15), most patients (85%) were in stages 3 and 4. Proteinuria was found in 52.8% of patients. Regarding bone metabolism parameters, hyperphosphatemia, hypercalcemia, and hyperparathyroidism were observed in 7%, 3%, and 67% of the patients, respectively. Elevated levels of alkaline phosphatase were seen in 43% of the patients. Vitamin D deficiency and insufficiency were observed in 9.5% and 28.5% of the patients, respectively.
Table 1.
Characteristics of the study participants
| All (n = 72) | Tertile of VBD |
P | |||
|---|---|---|---|---|---|
| VBD (77.6 to 162 HU) (n = 24) | VBD (168.5 to 210.1 HU) (n = 24) | VBD (212.2 to 292.8 HU) (n = 24) | |||
| Age (years) | 52 ± 11.7 | 55.7 ± 10.8 | 54.9 ± 8.9 | 45.9 ± 12.9 | 0.005 |
| Male (%) | 70 | 62.5 | 79.2 | 58.3 | 0.27 |
| CKD etiology (%) | |||||
| hypertension | 40 | 37.5 | 25 | 29 | |
| diabetes | 30 | 25 | 54.2 | 20.8 | |
| others | 30 | 37.5 | 20.8 | 50.2 | |
| Diabetes (%) | 42 | 41.7 | 41.7 | 41.7 | 1.00 |
| BMI (kg/m2) | 25.3 ± 4.5 | 26.4 ± 4.5 | 27 ± 5.2 | 25.7 ± 3.8 | 0.66 |
| Creatinine clearance (ml/min per 1.73 m2) | 40.4 ± 18.2 | 38.4 ± 18 | 40.7 ± 17 | 42.2 ± 20.2 | 0.78 |
| Proteinuria (g/24 h) | 0.22 (0 to 1.7) | 0.31 (0 to 1.03) | 0.14 (0 to 1.22) | 0.17 (0 to 1.18) | 0.85 |
| Hemoglobin (g/dl) | 13.1 ± 2.1 | 12.8 ± 1.9 | 13.1 ± 2.5 | 13.5 ± 1.8 | 0.51 |
| Glucose (mg/dl) | 100 (92 to 153.7) | 107 (96 to 164.7) | 102.5 (90.5 to 178.5) | 99.5 (84.5 to 133.5) | 0.68 |
| Total cholesterol (mg/dl) | 203 ± 44 | 204 ± 42 | 207 ± 44 | 199 ± 48 | 0.82 |
| HDL (mg/dl) | 49 ± 11.7 | 51 ± 12.8 | 50 ± 12.5 | 47.6 ± 9.7 | 0.58 |
| LDL (mg/dl) | 111 (93 to 134) | 106.5 (81 to 135.5) | 122 (96 to 135) | 111 (92 to 133.7) | 0.70 |
| Triglycerides (mg/dl) | 145 (101 to 202) | 161.5 (98 to 243) | 139.5 (110.7 to 230) | 124 (94 to 176.7) | 0.51 |
| CRP (mg/dl) | 0.41 (0.13 to 0.83) | 0.33 (0.13 to 0.54) | 0.46 (0.18 to 1.73) | 0.43 (0.1 to 0.69) | 0.79 |
| Bicarbonate (mEq/L) | 24 (21 to 26) | 24 (21.8 to 26) | 23.9 (22.4 to 27) | 24 (21.5 to 26) | 0.90 |
| Ionized calcium (mmol/L) | 1.30 ± 0.59 | 1.3 ± 0.05 | 1.31 ± 0.60 | 1.31 ± 0.66 | 0.70 |
| Phosphorus (mg/dl) | 3.7 ± 0.66 | 3.65 ± 0.72 | 3.7 ± 0.56 | 3.7 ± 0.66 | 0.92 |
| Alkaline phosphatase (U/L) | 118.5 (75 to 179.7) | 116 (95 to 171) | 120 (71 to 168.7) | 121.5 (67.5 to 211.7) | 0.85 |
| iPTH (pg/ml) | 103 (26 to 1028) | 111.5 (75 to 224.5) | 99.5 (65 to 132) | 97.5 (49.5 to 174.7) | 1.00 |
| 25(OH)D (ng/dl)a | 33.6 ± 1.66 | 33.6 ± 8 | 30.6 ± 14.8 | 36.7 ± 14.9 | 0.32 |
| Calcium score (AU) | 10.5 (0 to 120) | 85.7 (2.8 to 517.7) | 3.7 (0 to 78.1) | 0.55 (0 to 31.8) | 0.03 |
Results are presented as mean ± SD, median and interquartile range, or proportions. CRP, C-reactive protein; CKD, chronic kidney disease; HU, Hounsfield unit; VBD, vertebral bone density; BMI, body mass index; iPTH, intact parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D; AU, Agatston unit.
n = 63.
CAC was observed in 50% of the patients; median calcium score in these patients was 120 AU (32 to 584 AU). Severe CAC was found in 19% of the patients, and the median calcium score was 736 AU (527 to 1012 AU). The calcium score was higher among diabetic patients (32.1 [0.3 to 453.3] versus 3.2 AU [0 to 58.1]; P = 0.04). Calcium score correlated with age (r = 0.47; P < 0.001), urinary protein (r = 0.24; P = 0.04), and serum glucose (r = 0.24; P = 0.04). There was no correlation between CAC and BMI, creatinine clearance, C-reactive protein, lipids, 25(OH)D, iPTH, or other mineral parameters.
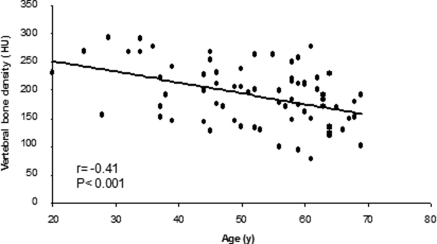
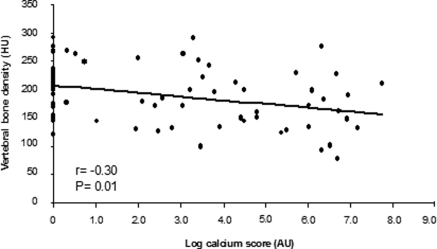
The mean VBD was 190 ± 52 HU. VBD correlated inversely with age (r = −0.41; P < 0.001; Figure 2) and calcium score (r = −0.30; P = 0.01; Figure 3), and no correlation was found with BMI, creatinine clearance, proteinuria, C-reactive protein, lipids, 25(OH)D, iPTH, or other mineral parameters. In addition, there were no significant differences in VBD among patients in CKD stages 2, 3, and 4 (200 ± 61, 194 ± 53 and 180 ± 46 HU [mean ± SD], respectively; P = 0.49) as well as when comparing men and women (187 ± 49 versus 196 ± 58 HU; P = 0.51), patients with and without diabetes (187 ± 52 versus 192 ± 53 HU; P = 0.70), and patients with BMI ≥25 and <25 kg/m2 (193 ± 56 versus 188 ± 49 HU; P = 0.70) or iPTH >65 and ≤65 pg/ml (185 ± 54 versus 201 ± 46 HU; P = 0.20).
Figure 2.
Correlation between vertebral bone density (VBD) and age. HU, Hounsfield unit.
Figure 3.
Correlation between VBD and calcium score. AU, Agatston unit.
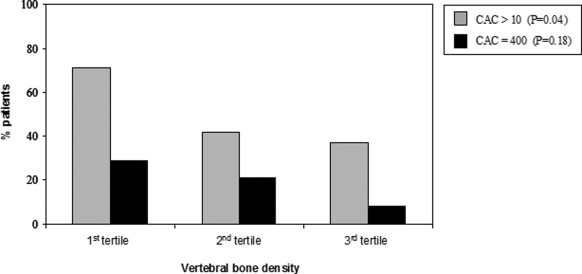
Patients were divided into groups according to tertiles of VBD (Table 1). Not unexpectedly, patients with lower VBD were older. Patients in the lowest tertile of VBD had expressively increased calcium score in comparison to the middle and highest VBD groups. Figure 4 depicts the prevalence of CAC and severe CAC according to the tertiles of VBD. As lower was the VBD higher was the proportion of patients with CAC >10 AU (P = 0.042). Although this trend was observed for the presence of CAC ≥ 400 AU, statistical significance was not reached (P = 0.18). No other differences were observed among the VBD tertile groups.
Figure 4.
Proportion of patients with coronary artery calcification (CAC) according to the tertiles of VBD.
Adjusting for confounding variables in the multiple logistic regression analysis, the lowest tertile of VBD was independently associated with the presence of CAC (Table 2).
Table 2.
Factors associated with the presence of CAC in nondialyzed CKD patients (n = 72)
| Factor | Coefficient | SEM | P | 95% Confidence Interval |
|---|---|---|---|---|
| Constant | −7.611 | 2.421 | 0.002 | |
| Female | 0.149 | 0.663 | 0.82 | 0.317 to 4.253 |
| Serum glucose | −0.005 | 0.004 | 0.24 | 0.987 to 1.003 |
| Creatinine clearance | 0.035 | 0.019 | 0.07 | 0.997 to 1.075 |
| Smoking | −0.282 | 0.802 | 0.73 | 0.157 to 3.632 |
| BMI (kg/m2) | 0.062 | 0.063 | 0.33 | 0.940 to 1.203 |
| Urinary protein | 0.593 | 0.319 | 0.06 | 0.968 to 3.382 |
| Age | 0.084 | 0.032 | 0.008 | 1.022 to 1.158 |
| Low VBD (first tertile) | 1.432 | 0.646 | 0.03 | 1.181 to 14.852 |
Multiple logistic regression analysis.
Discussion
The study presented here demonstrated that reduced VBD was associated with the presence of CAC in nondialyzed CKD patients. To the best of our knowledge, this is the first study that evaluated this relationship by measuring VBD by CT in this population.
It is well known that low BMD is highly prevalent even in the early stages of CKD (16–18). Increasing age, diabetes, smoking, glucocorticoid therapy, and mineral metabolism have been described as risk factors for bone loss (19,20). The prevalence of CAC is also elevated among nondialyzed CKD patients and it has been associated with future cardiovascular events, hospitalization, and mortality (12). The available literature identifies age, hypertension, diabetes, dyslipidemia, smoking, and disturbances of bone mineral metabolism as the main risk factors for CAC in CKD patients (21,22). In the study presented here, advanced age was the most important factor common for low VBD and presence of CAC. Accordingly, although bone mineralization processes peak at age 25 to 35 years and thereafter, bone mineral content decreases gradually (23). CAC is thought to initiate around 25 to 35 years old and progress until death (24). Therefore, aging seems to be a key determinant for both conditions.
In the last few years, some evidence of an inverse relationship between BMD, analyzed by dual-energy x-ray absorptiometry, and vascular calcification have emerged in CKD groups (25). However, the findings are somewhat controversial (26), possibly because of the limitation of the dual-energy x-ray absorptiometry technique in the assessment of BMD (27–29). In the study presented here, VBD was assessed by CT, a method that allows distinction between cortical and trabecular bone and avoids miscalculation of the VBD due to the presence of aortic calcification. In dialysis patients, the few studies using CT have found an association between low trabecular VBD and the extent of CAC (8,9). Herein, we demonstrated that such association starts early in the course of CKD.
It has been suggested that bone loss and CVD share some similar etiopathogenic mechanisms. However, no data in the literature have established a clear physiopathological mechanism to explain the shift of calcium from bone to the vessels. Some potential mechanisms mutual to both complications have been advocated based on animal models. For instance, factors such as PTH, vitamin D, estrogen, and cytokines play a role in both processes, independently or through the mobilization of calcium from the skeleton to the vessels. Actually, evidence of the relationship between bone loss and vascular calcification has come from knockout mice. Animals deficient in matrix gla protein have impaired cartilage growth plate mineralization, leading to osteopenia and severe medial vascular calcification (30). In an interesting study by Price and colleagues, vascular calcification was induced in rats by administration of excessive vitamin D (cholecalciferol), and they were able to inhibit calcification by adopting therapies that inhibited the bone resorption (31–33). The evidences linking bone disease to vascular calcification in CKD patients have been based on bone turnover derangements and not on bone density. In studies with bone biopsy, an association between CAC and low bone turnover has been observed in CKD patients (17,34,35). It seems that the decreased bone formation rate leads to an inability of bone to buffer the calcium overload, predisposing to metastatic calcification (36). Although low-turnover bone disease is frequent among nondialyzed CKD patients (16,17), it remains unclear whether loss of bone mass is mandatory in the course of low-turnover bone disease. In a study with hemodialysis patients, Barreto et al. (35) showed that lower trabecular bone volume was associated with the development of CAC, and the improvement of bone turnover was associated with lower CAC progression. These data provide evidence that disturbances in bone metabolism may lead to vascular calcification.
In the study presented here, the cause-and-effect relationship between low VBD and CAC cannot be established because of the cross-sectional design. Another limitation of this study is the relatively small number of nondialyzed CKD patients included. However, it must be emphasized that the sample was representative in terms of age, sex, prevalence of diabetes, and CKD stage distribution. In addition, considering the high burden of risk factors associated with bone mineral disturbances that predispose CKD patients to cardiovascular injuries and the lack of conception on this issue particularly among nondialyzed CKD patients, we believe that the result of this study is important.
In conclusion, low VBD was associated with the presence of CAC in nondialyzed CKD patients. We suggest that low VBD might constitute another nontraditional risk factor for CVD in CKD patients. Interventional studies are warranted in the earlier stages of the disease to investigate whether the treatment of low bone density could ameliorate the vascular calcification in this population.
Disclosures
None.
Acknowledgments
We thank Maria Ayako Kamimura for valuable input during the preparation of this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C: Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58: 396–399, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Taal MW, Masud T, Green D, Cassidy MJ: Risk factors for reduced bone density in haemodialysis patients. Nephrol Dial Transplant 14: 1922–1928, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Moe SM: Vascular calcification and renal osteodystrophy relationship in chronic kidney disease Eur J Clin Invest 36[Suppl 2]: 51–62, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC: Progression of aortic calcification is associated with metacarpal bone loss during menopause: A population-based longitudinal study. Arterioscler Thromb Vasc Biol 20: 1926–1931, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Hydera JA, Allison MA, Barrett-Connor E, Detranoc R, Wong ND, Sirlin C, Gapsturf SM, Ouyangg P, Carr JJ, Criqui MH: Bone mineral density and atherosclerosis: The Multi-Ethnic Study of Atherosclerosis, Abdominal Aortic Calcium Study. Atherosclerosis 209: 283–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frost M, Grella R, Millasseau S, Jiang B, Hampson G, Fogelman I, Chowienczyk P: Relationship of calcification of atherosclerotic plaque and arterial stiffness to bone mineral density and osteoprotegerin in postmenopausal women referred for osteoporosis screening. Calcif Tissue Int 83: 112–120, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Hsia J, Klouj A, Prasad A, Burt J, Adams-Campbell L, Howard B: Progression of coronary calcification in healthy postmenopausal women. BMC Cardiovasc Disord 4: 21, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC: Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 27: 394–401, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Raggi P, James G, Burke SK, Bommer J, Chasan-Taber S, Holzer H, Braun J, Chertow GM: Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res 20: 764–772, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary artery calcification in young adults with end stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 11. London GM, Guerin AP, Marchais SJ: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Watanabe R, Lemos MM, Manfredi SR, Draibe SA, Canziani MEF: Impact of cardiovascular calcification in nondialyzed patients after 24 months of follow-up. Clin J Am Soc Nephrol 5: 189–194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int 76[Suppl 113]: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 15. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 16. Lobão R, Carvalho AB, Cuppari L, Ventura R, Lazaretti-Castro M, Jorgetti V, Vieira JG, Cendoroglo M, Draibe AS: High prevalence of low bone mineral density in pre-dialisis chronic kidney disease: Bone histomophometric analysis. Clin Nephrol 62: 432–439, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Tomiyama C, Carvalho AB, Higa A, Jorgetti V, Draibe SA, Canziani ME: Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J Bone Miner Res 25: 499–504, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Mehrotra R, Kermah D, Budoff M, Salusky IB, Mao SS, Gao YL, Takasu J, Adler S, Norris K: Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol 3: 1144–1151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Binici D, Gunes N: Risk factors leading to reduced bone mineral density in hemodialysis patients with metabolic syndrome. Renal Failure 32: 469–474, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Huang GS, Chu TS, Lou MF, Hwang SL, Yang RS: Factors associated with low bone mass in the hemodialysis patients—A cross-sectional correlation study. BMC Musculoskelet Disord 10: 60, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Couri CE, da Silva GA, Martinez JA, Pereira F de A, de Paula FJ: Mönckeberg's sclerosis—Is the artery the only target of calcification? BMC Cardiov Disord 12: 34, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giachelli CM: Vascular calcification: In vitro evidence for the role of inorganic phosphate. J Am Soc Nephrol 14[Suppl 4]: S300–S304, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Garraway WM, Stauffer RN, Kurland LT, O'Fallon WM: Limb fractures in a defined population. I. Frequency and distribution. Mayo Clin Proc 54: 701–707, 1979 [PubMed] [Google Scholar]
- 24. Wexler L, Brundage B, Crouse J, Detrano R, Fuster V, Maddahi J, Rumberger J, Stanford W, White R, Taubert K: Coronary artery calcification: Pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation 94: 1175–1192, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Raggi P, Kleerekoper M: Contribution of bone and mineral abnormalities to cardiovascular disease in patients with chronic kidney disease. Clin J Am Soc Nephrol 3: 836–843, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG: Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 23: 586–593, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Genant HK, Engelke K, Fuerst T, Glüer CC, Grampp S, Harris ST, Jergas M, Lang T, Lu Y, Majumdar S, Mathur A, Takada M: Noninvasive assessment of bone mineral and structure: State of the art. J Bone Miner Res 11: 707–730, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Guglielmi G, Grimston SK, Fischer KC, Pacifici R: Osteoporosis: Diagnosis with lateral and posteroanterior dual x-ray absorptiometry compared with quantitative CT. Radiology 192: 845–850, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Orwoll ES, Oviatt SK, Mann T: The impact of osteophytic and vascular calcifications on vertebral mineral density measurements in men. J Clin Endocrinol Metab 70: 1202–1207, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G: Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386: 78–81, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Price PA, Buckley JR, Williamson MK: The amino bisphosphonate ibandronate prevents vitamin D toxicity and inhibits vitamin D-induced calcification of arteries, cartilage, lungs and kidneys in rats. J Nutr 131: 2910–2915, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Price PA, June HH, Buckley JR, Williamson MK: Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol 21: 1610–1616, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Price PA, Omid N, Than TN, Williamson MK: The amino bisphosphonate ibandronate prevents calciphylaxis in the rat at doses that inhibit bone resorption. Calcif Tissue Int 71: 356–363, 2002 [DOI] [PubMed] [Google Scholar]
- 34. London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul M-C: Arterial calcifications and bone histomorphometry in endstage renal disease. J Am Soc Nephrol 15: 1943–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Barreto DV, Barreto FC, Carvalho AB, Cuppari L, Draibe SA, Dalboni MA, Moyses RM, Neves KR, Jorgetti V, Miname M, Santos RD, Canziani ME: Association of changes in bone remodeling and coronary calcification in hemodialysis patients: A prospective study. Am J Kidney Dis 52: 1139–1150, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Kurz P, Monier-Faugere MC, Bognar B, Werner E, Roth P, Vlachojannis J, Malluche HH: Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int 46: 855–861, 1994 [DOI] [PubMed] [Google Scholar]