Abstract
Summary
Background and objectives
Pre-existing hepatitis B virus (HBV) infection has been associated in inferior renal transplant outcomes. We examined outcomes of HBV+ renal recipients in a more recent era with availability of oral anti-viral agents.
Design, setting, participants, & measurements
Using the Organ Procurement Transplant Network/United Network for Organ Sharing database, we selected adult primary kidney recipients transplanted in the United States (2001 to 2007). The cohort was divided into HBV+ (surface antigen positive, n = 1346) and HBV− patients (surface antigen negative; n = 74,335). Five-year graft survival, patient survival, hepatic failure incidence, and associated adjusted risks were compared.
Results
HBV+ recipients were more frequently Asian, had a lower body mass index, and glomerulonephritis was more prevalent as the etiology of ESRD. HBV+ recipients had less pretransplant diabetes and cardiovascular disease, were less likely a living donor recipient, and were less likely to receive steroids at discharge. Five-year patient survival was 85.3% and 85.6% and graft survival was 74.9% and 75.1% for HBV+ and HBV−, respectively. HBV infection was not a risk factor for death or kidney failure, although 5-year cumulative incidence of hepatic failure was higher in HBV+ recipients (1.3% versus 0.2%; P < 0.001), and HBV+ was associated with 5.5- and 5.2-fold increased risk for hepatic failure in living and deceased donors, respectively, compared with HBV−.
Conclusions
In a recent era (2001 to 2007), HBV-infected renal recipients were not at higher risk for kidney failure or death; however, they remain at higher risk of liver failure compared with HBV− recipients.
Introduction
Pre-existing hepatitis B virus (HBV) infection has been considered a relative contraindication to kidney transplant. The use of immunosuppressive drugs enhances viral replication, leading to acceleration of liver injury and progression to hepatocellular failure (1). Moreover, HBV-associated GN may recur or develop de novo in the graft, reducing function or even ultimately inducing graft failure (2,3).
Earlier studies had reported an adverse impact of HBV on patient and graft survivals (4–7). However, in the last decade, introduction of effective oral anti-viral therapies for HBV with judicious use of immunosuppressive drugs in recipients with viral hepatitis have altered management of HBV-infected recipients (8). Using the Organ Procurement Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database, we examined outcomes of HBV-infected kidney recipients in a recent era (2001 to 2007) compared with noninfected HBV recipients.
Materials and Methods
Study Population
A retrospective cohort study was conducted using data from OPTN/UNOS as of May 2009. Adults (>18 years old) who were primary renal graft recipients between 2001 and 2007 and had at least one follow-up visit reported to OPTN/UNOS were included. Recipients of multiorgan transplant and those with pretransplant hepatitis C virus (HCV) infection (defined as positive antibody to HCV) were excluded. A recipient was characterized as HBV infected (HBV+) when seropositivity for hepatitis B surface antigen (HbsAg+ve) was recorded on the transplant registration form. A total of 75,681 recipients were included in the study population: 1346 were HBV+ and 74,335 were HBV−.
Statistical Analysis
Donor, recipient, and transplant characteristics, including immunosuppressive regimens at discharge, were compared. The Kruskal-Wallis test was used to detect significant differences in continuous variables. The χ2 test was used to compare categorical variables. Post-transplant complications including primary nonfunction, delayed graft function (DGF1, need dialysis in the first week after transplant; DGF2, combination of DGF1 with cases of creatinine decline <25% or urine output <40 ml in the first 24 hours after transplantation), 1-year cumulative acute rejection rates, and occurrence of glomerular disease in the graft were examined. Univariate comparisons of hepatic failure after kidney transplant, and graft and patient survival were performed using the Kaplan-Meier product limits method, with differences compared by log-rank test. Overall kidney graft survival was determined from date of transplantation until recipient death, kidney retransplantation, or return to dialysis. Hepatic failure was defined as either listing for or receipt of a liver transplant after prior kidney transplant or hepatic failure as the reported cause of death (primary, secondary, or tertiary). Patients were censored at the end of the study period (60 months). Potential confounding covariates examined on univariate analysis included donor (living/deceased, expanded criteria donor), recipient (age, gender, race, diabetes, hypertension, cardiovascular disease, cerebrovascular disease, body mass index, dialysis duration, and peak panel reactive antibody), and transplant (cold ischemia time, HLA mismatch, and immunosupression type) factors. Covariates with P < 0.1 were included in multivariate analyses. Multivariate estimates of hazards of hepatic failure, overall graft failure, death-censored graft failure, and patient mortality were calculated using stepwise Cox proportional hazards model. The results were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs) and associated P value. All reported P values were two-tailed, and P < 0.05 was considered significant. All analyses were conducted using STATA Statistical Software, Release 10 (StateCorp LP, College Station, TX).
Comparing Transplant Outcomes in Different Eras
To evaluate the impact of HBV infection on long-term graft survival in different transplant eras, we also compared outcomes of recipients transplanted in three consecutive periods: 1987 to 1994, 1995 to 2000, and 2001 to 2007 (1987 to 1994: HbsAg+ve, n = 585, HBsAg−ve, n = 50,192; 1995 to 2000: HBsAg+ve, n = 698, HBsAg−ve, n = 51,344; 2001 to 2007: HBsAg+ve, n = 1346, HBsAg−ve, n = 74,335). The impact of recipient HBsAg on overall kidney graft survival, patient survival, and liver-failure-free survival, as defined in the main analysis, was examined.
Results
Baseline Characteristics
The study population included 75,681 adults primary kidney recipients transplanted between 2001 and 2007, of whom 1346 were HBV+ and 74,335 were HBV−. The median follow-up was 1098 days. Recipient, donor, and transplant baseline characters are shown in Table 1. The HBV+ group had a significantly higher proportion of younger (<40 years) and Asian recipients, donors with seropositivity for HBV core antibody (HBcAb), recipients with a longer pretransplant dialysis period, recipients with increased HLA mismatch, and recipients discharged more frequently on rapamycin for immune suppression compared with the HBV− group. The HBV− group had a higher percentage of white recipients. Overall, the HBV− group had higher body mass index, more recipients with diabetes as the cause of ESRD, an increased frequency of associated comorbidities (notably hypertension and cerebrovascular disease) received more organs from living donors, and were more frequently discharged on steroids. There were no significant differences in recipient gender or transplant factors, including peak panel reactive antibody >10%; extended criteria donor; and cold ischemia time.
Table 1.
Baseline characteristics of the study groups
| HBV+(n = 1346) | HBV−(n = 74,335) | P | |
|---|---|---|---|
| Recipient factors | |||
| male (%) | 59.2 | 59.8 | 0.68 |
| age: 18 to 40 years (%) | 30.2 | 24.7 | <0.01 |
| age: 40 to 60 years (%) | 46.7 | 49.4 | |
| age: ≥60 years (%) | 23.1 | 25.9 | |
| body mass index (kg/m2): <25 (%) | 40.8 | 36.3 | <0.01 |
| body mass index (kg/m2): 25 to 30 (%) | 30.5 | 33.4 | |
| body mass index (kg/m2): ≥30 (%) | 28.7 | 30.3 | |
| race: white (%) | 40.9 | 56.4 | <0.01 |
| race: AA (%) | 27.5 | 23.3 | |
| race: Hispanic (%) | 11.4 | 13.4 | |
| race: Asian (%) | 16.9 | 4.7 | |
| race: others (%) | 3.3 | 2.2 | |
| pretransplant DM (%) | 28.6 | 31.0 | <0.01 |
| ESRD: DM (%) | 22.3 | 25.0 | <0.01 |
| ESRD: GN (%) | 24.2 | 20.0 | |
| ESRD: hypertension | 24.7 | 23.4 | |
| ESRD: polycystic kidney | 7.9 | 10.3 | |
| ESRD: others | 20.9 | 21.3 | |
| previous hypertension (%) | 74.3 | 80.3 | <0.01 |
| previous CVD (%) | 7.1 | 9.6 | <0.01 |
| pre-TX dialysis (%): no | 14.3 | 19.2 | <0.01 |
| pre-TX dialysis (%): <1 years | 14.5 | 17.9 | |
| pre-TX dialysis (%): 1 to 3 years | 30.0 | 30.6 | |
| pre-TX dialysis (%): ≥3 years | 41.2 | 32.4 | |
| PPRA ≥ 10% (%) | 18.3 | 18.1 | 0.9 |
| Donor factors | |||
| living donor (%) | 37.4 | 42.5 | <0.01 |
| HBcAb+ve (%) | 12.2 | 3.4 | <0.01 |
| ECD (%) | 12.9 | 12.1 | 0.4 |
| HLA-DR mismatch: 0 (%) | 21.3 | 25.2 | <0.01 |
| 1 (%) | 43.9 | 46.4 | |
| 2 (%) | 34.8 | 28.4 | |
| CIT: <24 hours (%) | 83.4 | 83.1 | 0.9 |
| Immunosuppressants | |||
| induction (%) | 71.6 | 72.0 | 0.7 |
| Tac at discharge (%) | 72.0 | 69.8 | 0.08 |
| CsA at discharge (%) | 18.9 | 21.6 | 0.02 |
| MMF/EC-MFS at discharge (%) | 84.0 | 84.4 | 0.7 |
| mTOR at discharge(%) | 13.4 | 11.6 | 0.04 |
| steroid at discharge (%) | 91.8 | 94.2 | <0.01 |
AA, African American; CVD, cerebrovascular disease; PPRA, peak panel of reactive antibodies; HLA, human leukocyte antigens; ECD, expanded criteria donor; Tac, tacrolimus; CsA, cyclosporine A; MMF, mycophenolate mofetil; EC-MPS, enteric-coated mycophenolate sodium; MTOR, mammalian target of rapamycin inhibitor; HBV, hepatitis B virus; DM, diabetes mellitus; CIT = cold ischemia time; TX, transplantation; HBV, HBcAB+ve, HBV core antibody; EC-MPS, enteric-coated mycophenolate sodium.
Impact of Recipient HBV Infection on Outcomes in 2001 to 2007
Post transplant complications.
The median length of hospitalization was 6 (4 to 8) and 6 (4 to 9) days (P = 0.82); incidence of primary nonfunction was 1.0 and 1.2% (P = 0.49); rate of DGF1 was 24.8% and 29.3% (P < 0.001) in deceased donors; rate of DGF2 was 47.4% and 49.9% (P < 0.14) in deceased donors; 1-year cumulative rejection rate was 12.2% and 10.3% (P = 0.08); and recurrence of graft glomerular disease was 0.24% versus 0% (P = 0.07) for HBV− and HBV+ recipients, respectively.
Hepatic failure after kidney transplant.
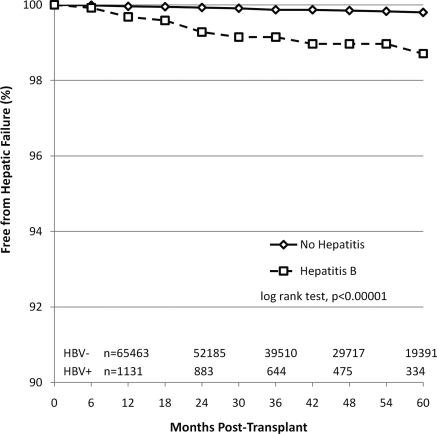
The proportion of recipients who developed hepatic failure is shown in Figure 1. The HBV+ group had a higher percentage of recipients with hepatic failure after kidney transplant during the follow-up period compared with the HBV− group (11 in 1346, 0.82% versus 113 in 74,335, 0.15%, P < 0.01). Of the recipients with liver failure, 9 (0.67%) HBV+ and 61 (0.08%) HBV− (P < 0.01) were listed for liver transplant. Five (0.37%) HBV+ and 36 (0.05%) HBV− (P < 0.01) received a liver transplant, and 3 (0.22%) HBV+ and 49 (0.07%) HBV− (P = 0.03) died of liver failure. The median time to development of liver failure was 629 days (231 to 850 days) and 857 days (309 to 1381 days) in HBV+ and HBV−, respectively. The unadjusted cumulative incidence of hepatic failure after kidney transplant was significantly higher in the HBV+ group compared with the HBV− group (at 12, 36, and 60 months: 0.32%, 0.85%, and 1.29% for HBV+ and 0.04%, 0.13%, and 0.20% for HBV−; P < 0.0001). The unadjusted and adjusted HRs for hepatic failure after kidney transplant are shown in Table 2. By multivariate Cox regression analysis, pretransplant HBV infection was associated with an increased risk for hepatic failure after kidney transplant in both living (adjusted HR = 6.5, P < 0.01) and deceased (adjusted HR = 6.21, P < 0.01) donor recipients. Among the 120 recipients with liver failure, HBV was implicated in 7 (63.6%) of the 11 HBV+ recipients compared with 6 (5.3%) of the 113 HBV− recipients (P < 0.01).
Figure 1.
Proportion of kidney transplant recipients free from hepatic failure. Transplants were performed between 2000 and 2007. Hepatic failure was defined as either listing for or receipt of a liver transplant after prior kidney transplant or hepatic failure as the cause of death.
Table 2.
HR (95% CI) for hepatic failure after kidney transplant in Cox regression analyses
| Living Donor Transplant |
Deceased Donor Transplant |
|||
|---|---|---|---|---|
| Unadjusted HR | Adjusted HR | Unadjusted HR | Adjusted HR | |
| Recipient HBV infection | ||||
| +ve versus −ve | 5.96 (2.14 to 16.6)a | 6.5 (1.96 to 21.3)a | 6.02 (2.76 to 13.1)a | 6.21 (2.67 to 14.4)a |
| Donor HBcAb | ||||
| +ve versus −ve | 0.89 (0.12 to 6.56) | 0.77 (0.10 to 5.58) | 0.98 (0.31 to 3.11) | 0.66 (0.16 to 2.70) |
| Recipient age | ||||
| 40 to 60 versus <40 years | — | — | 3.36 (1.33 to 8.50)b | 5.05 (1.56 to 16.4)a |
| >60 versus <40 years | — | — | 4.08 (1.57 to 10.6)a | 6.20 (1.86 to 30.6)a |
| Pretransplant diabetes | 0.48 (0.22 to 1.08)c | 0.48 (0.19 to 1.25) | — | — |
| yes versus no | ||||
| cold ischemia time | — | — | 1.93 (1.17 to 3.18)a | 1.89 (1.14 to 3.13)b |
| >24 versus <24 hours | ||||
| Steroid at discharge | — | — | ||
| yes versus no | 0.39 (0.18 to 0.85)b | 0.38 (0.17 to 0.83)b | ||
Potential risk factors in the univariate analysis with a P < 0.1 were selected to be included in the multivariate analysis. Other analyzed covariates: recipient gender, body mass index, race, comorbidity (diabetes, hypertension, cerebrovascular disease), dialysis duration, induction therapy, and immunosuppressants at discharge (tacrolimus, mycophenolate mofetil and enteric coated mycophenolate sodium, mammalian target of rapamycin inhibitors). Recipient age and cold ischemia time were not included in the multivariate model for living donor recipients because the P value in univariate analysis was >0.1. Expanded criteria donor was not included in the multivariate model for deceased donor recipients because the P value in univariate analysis was >0.1. HR, hazard ratio; CI, confidence interval.
P < 0.01.
P < 0.05.
P < 0.1.
Kidney graft survival.
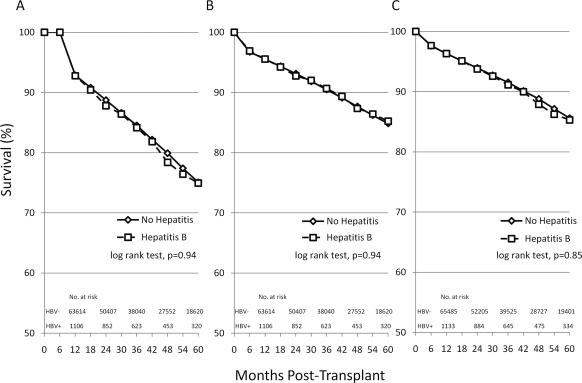
Kaplan-Meier survival curves for overall kidney graft survival according to the presence or absence of recipient HBV infection are shown in Figure 2A. There was no significant difference in overall graft survival between recipients with and without HBV infection. Kidney graft survival in HBV+ recipients at 12, 36, and 60 months was 92.8%, 84.2%, and 74.9% and in HBV− recipients was 92.9%, 84.5%, and 75.1%, respectively (log-rank test, P = 0.94). Multivariate Cox regression analysis confirmed that HBV infection was not associated with graft failure in living donor transplant (HR = 0.90, P = 0.57) or in deceased donor transplant (HR = 1.10, P = 0.52), as seen in Table 3.
Figure 2.
Kaplan-Meier curves for graft survival (A), death-censored graft survival (B), and patient survival (C) in kidney transplant recipients with and without HBV acquired during the pretransplant period. HBV was defined by serum positivity for HbsAg+ve. Data are representative of transplants performed during the years 2000 to 2007.
Table 3.
Effect of HBV infection on overall graft survival, death-censored graft survival, and mortality in Cox regression analysis
| Living Donor Transplant |
Deceased Donor Transplant |
|||
|---|---|---|---|---|
| Unadjusted HR | Adjusted HR | Unadjusted HR | Adjusted HR | |
| Overall graft failure | 0.91 (0.71 to 1.15) | 0.90 (0.62 to 1.30) | 1.01 (0.88 to 1.17) | 1.10 (0.82 to 1.47) |
| Death-censored graft failure | 0.95 (0.70 to 1.28) | 0.74 (0.45 to 1.24) | 0.98 (0.81 to 1.19) | 1.06 (0.85 to 1.33) |
| Mortality | 0.88 (0.62 to 1.24) | 0.98 (0.59 to 1.63) | 0.99 (0.82 to 1.20) | 1.09 (0.88 to 1.36) |
Potential risk factors in the univariate analysis (P < 0.1) were selected into the multivariate analysis. Other analyzed covariates: recipient age, gender, body mass index, race, comorbid (diabetes, hypertension, cerebrovascular disease), dialysis duration, donor HBcAb, expanded criteria donor, HLA DR mismatch, cold ischemia time (in deceased donor), induction therapy, and immunosuppressants at discharge (tacrolimus, mycophenolate mofetil and enteric coated mycophenolate sodium, mammalian target of rapamycin inhibitors, or steroids).
Kaplan-Meier survival curves for death-censored graft survival are shown in Figure 2B. There was no significant difference in death-censored graft survival between recipients with and without HBV infection (at 12, 36, and 60 months: 95.6%, 90.7%, and 85.2% for HBV+ and 95.6%, 90.5%, and 84.9% for HBV−; log-rank test, P = 0.94). By multivariate Cox regression analysis, recipient HBV infection was not significantly associated with death-censored graft failure in living donor transplant (HR = 0.74, P = 0.26) or deceased donor transplant (HR = 1.06, P = 0.61; Table 3).
Patient mortality.
The Kaplan-Meier survival curves for patient survival according to the presence or absence of recipient HBV infection are shown in Figure 2C. There was no significant difference in patient survival between recipients with and without HBV infection (at 12, 36, and 60 months: 96.3%, 91.1%, and 85.3% for HBV+ and 96.3%, 91.6%, and 85.6% for HBV−; log-rank test, P = 0.85). By multivariate Cox regression analysis, recipient HBV infection was not significantly associated with patient mortality in living (HR = 0.98, P = 0.93) and deceased (HR = 1.09, P = 0.42) donor transplant (Table 3).
Impact of HBV infection on transplant outcomes in earlier transplant eras.
Table 4 shows outcomes as patient and graft survival and proportion of recipients that did not develop liver failure, according to recipient HBsAg status in three consecutive eras (1987 to 1994, 1995 to 2000, and 2001 to 2007). Graft and recipient survivals in HBsAg+ve recipients were inferior to those of HBsAg−ve recipients in 1987 to 1994. However, in more recent eras, 1995−2000 and 2001−2007, no difference in kidney graft and patient survival occurred in HBV-infected recipients. Conversely, a small but significant difference in the proportion of recipients without hepatic failure between HBV+ and HBV− recipients persisted in the more recent eras of 1995 to 2000 and 2001 to 2007. Fewer HBsAg+ve recipients were without hepatic failure compared with HBsAg−ve recipients.
Table 4.
Survival outcomes of HBsAg+ and HBsAg− kidney recipients in three different eras (1987 to 1994, 1995 to 2000, and 2001 to 2007)
| 1987 to 1994 (n = 50,777) |
1995 to 2000 (n = 52,042) |
2001 to 2007 (n = 75,681) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HBV+ (1.2%) | HBV− (98.8%) | P | HBV+ (1.3%) | HBV− (98.7%) | P | HBV+ (1.8%) | HBV− (98.2%) | P | ||
| Overall graft survival | 1 year | 85.4% | 86.9% | <0.01 | 90.4% | 90.9% | 0.51 | 92.8% | 92.9% | 0.94 |
| 3 years | 71.5% | 77.4% | 80.3% | 82.4% | 84.2% | 84.5% | ||||
| 5 years | 60.8% | 67.3% | 72.8% | 72.6% | 74.9% | 75.1% | ||||
| Patient survival | 1 year | 92.5% | 94.5% | <0.01 | 94.3% | 95.6% | 0.22 | 96.3% | 96.3% | 0.85 |
| 3 years | 83.5% | 89.5% | 89.5% | 91.0% | 91.1% | 91.6% | ||||
| 5 years | 78.1% | 83.5% | 85.0% | 85.4% | 85.3% | 85.6% | ||||
| Free from liver failure | 1 year | 99.8% | 100% | <0.01 | 99.2% | 100% | <0.01 | 99.7% | 100% | <0.01 |
| 3 years | 99.6% | 99.9% | 98.4% | 99.9% | 99.2% | 99.9% | ||||
| 5 years | 99.4% | 99.9% | 98.0% | 99.8% | 98.7% | 99.8% | ||||
Cohort analyzed included only HBsAg+ and HBsAg− recipients. Recipients with HBsAg unknown and with anti-HCV+ were excluded. P value was determined by log-rank test. HbsAg, hepatitis B surface antigen.
Discussion
There were two important findings in our study. First, in the most recent period (2001 to 2007), patient and kidney graft survival in HBV+ recipients was comparable to HBV− recipients, and after adjusting for confounders, the risks for death and graft loss were not different between HBV+ and HBV− recipients. This is in contrast to earlier eras in transplant, 1984 to 1997, when HBV infection was implicated in inferior patient and graft survivals. Second, the incidence of severe liver disease was higher in HBV+ recipients, and after adjustment, risk for developing severe liver disease associated with HBV+ was 5.5- and 5.2-fold higher in HBV+ living and deceased donors recipients, respectively, compared with HBV− recipients.
In HBV infected organ transplant recipients the course of liver disease may be accelerated by therapeutic immunosuppression. Previous reports described an increase in HBV viral load after renal transplantation with progression of liver disease to overt hepatocellular failure (4–6). Consistent with prior reports, we found an increased risk of developing liver disease in HBV+ recipients, although overall patient survival was not inferior to that of recipients without HBV infection. The advent of lamivudine in the 1990s expanded treatment options for HBV-infected renal transplant recipients in whom IFN is contraindicated because of concerns about graft loss. Fabrizi et al. (10), in a meta-analysis of clinical trials of lamivudine for the treatment of HBV-related liver disease in renal transplantation, observed that lamivudine reduced viremia (91% clearance of HBV DNA) and normalized alanine aminotransferase in 81% of recipients and may slow progression of liver disease after renal transplant. However, viral resistance to lamivudine is frequent and limits its long-term efficiency. Adevofir was subsequently approved by the FDA in September 2002 for the treatment of chronic HBV in adults, with evidence of active viral replication including those with lamivudine resistance. Newer drugs, entecavir and tenofovir, more potent anti-viral agents than lamivudine and adefovir, are now licensed to treat HBV infection (9). Thus anti-viral therapy for HBV has become increasingly effective and potentially may prevent HBV-related hepatocellular failure in HBV-infected renal transplant recipients.
Fabrizi et al. (10), in a meta-analysis of kidney transplant outcomes in HBV+ recipients, concluded that these recipients are at increased risk for mortality and kidney graft failure compared with HBV− recipients. Data were based on results of six older observational studies: three from Europe (11–13), two from Asia (14,15), and one from South America (16). In contrast, using a North American database of renal transplants from a more recent era, we found that patient and kidney survival was not diminished in HBV-infected recipients.
A potential explanation for the differences in our findings from the meta-analysis of Fabrizi et al. (10) is that the former included recipients transplanted between 1972 and 1999 compared with our recipients from 2001 to 2007. Lamivudine licensed for therapy of HBV in 1996 and subsequent anti-viral agents have contributed to better outcomes in HBV-infected recipients. Two reports have appeared on lamivudine therapy and survival outcomes in kidney transplant recipients. Chan et al. (14) described 12 HBsAg+ recipients who received lamivudine preemptively, i.e., when a increase in HBV DNA levels with or without elevation in aminotransferase levels were detected, had similar renal allograft recipient survival compared with HBSAg− controls in contrast to their HBV-infected recipients transplanted before lamivudine was available. Ahn et al. (17) included transplants performed between 1979 and 2004 and found 10-year patient, graft, and death-censored graft survival were inferior in HBsAg+ve (n = 66) compared with HBsAg−ve (n = 1988) recipients. However, in 27 HBsAg+ve recipients who received lamivudine, 10-year patient overall and kidney death censored survivals were comparable with HBsAg−ve recipients. We also confirmed that outcomes of HBV+ recipients have improved significantly in the last decade. We found that overall kidney and patient survival in HBV+ was superior in the years 1995 to 2007 compared with 1987 to 1994. No difference was seen between 1995 to 2000 and 2001 to 2007 (Table 4). This finding is similar to a report from Kim et al. (18) in a liver transplant cohort. Using data from the UNOS, Kim et al. divided liver transplant HBV+ recipients according to three eras: era 1 (1987 to 1991), era 2 (1992 to 1996), and era 3 (1997 to 2002) and showed that the survival of HBV+ recipients was significantly better for era 2 than in era 1 and for era 3 than in era 2; also, there were no difference in survival between patients with HBV+ and other diagnoses for era 3. These similar findings in kidney and liver transplants suggest an overall improvement in HBV+ organ transplant recipient outcomes in the United States.
Demographic changes may also have contributed to the improvement in survival outcomes in renal transplant recipients. In the OPTN/UNOS database, the percentage of Asian kidney recipients has increased in recent eras (3.2% in 1987 to 1994, 3.8% in 1995 to 2000, 4.9% in 2001 to 2007; P < 0.001). In our study, in kidney transplants from 2000 to 2007, 16.9% of HBV+ were of Asian origin compared with 4.7% in the HBV− group. Asian recipients fair better than other ethnicities after kidney transplant (19,20). Asian renal transplant recipients have a reduced prevalence of hypertension and diabetes; they are also shorter and have smaller weight, which implies a better proportionality between kidney allograft mass and body mass, which may provide a greater kidney allograft function and functional reserve.
Another possibility for death reduction in HBV+ recipients in different eras is access to liver transplant after the kidney transplant. Of the 11 HBV+ recipients who developed severe liver failure in our study, 9 were listed for liver transplant, 5 of whom (55.5%) underwent the procedure. Only 3 of 11 died of liver failure.
Death-censored graft survival in the HBV+ group was not different from the HBV− group. In HBV+, kidney allograft dysfunction can be caused by recurrence of hepatitis B–related graft nephropathy in the graft or by the de novo occurrence of nephropathy. In our data, we did not identify an increased incidence of GN in HBV+ recipients. Use of anti-viral drugs may have reduced the incidence of renal allograft nephropathy related to HBV infection.
Limitations of our study included those related to the nature of retrospective analyses. However, it is clear that the advent of potent anti-viral agents permits excellent graft and patient survival in HBV-infected recipients, although there is still an excess of liver failure compared with uninfected patients. Future prospective data are need to determine whether earlier introduction of anti-viral therapy or exclusion of potential renal transplant recipients with more histologically advanced liver disease from isolated renal transplant avoid progression to overt liver failure after renal transplant.
Disclosures
P.M. has been a consultant, speaker, and investigator for Gilead and Bristol-Meyers-Squibb. The other authors of this manuscript have no conflicts of interest to disclose.
Acknowledgments
This work was supported in part by Health Resources and Services Administration Contract 234-2005-370011C. The content is responsibility of the authors alone and does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. P.N.R. was supported in part by NIH Training Grant T32-DK-07789. M.S.S.'s participation in this work has been made possible through an ISN-funded fellowship. An abstract related to this manuscript was presented as a poster at the American Transplant Congress (San Diego, CA, 2010).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Fabrizi F, Martin P, Ponticelli C: Hepatitis B virus and renal transplantation. Nephron 90: 241–251, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Choy BY, Chan TM, Lai KN: Recurrent glomerulonephritis after kidney transplantation. Am J Transplant 6: 2535–2542, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Ivanyi B: A primer on recurrent and de novo glomerulonephritis in renal allografts. Nat Clin Pract Nephrol 4: 446–457, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Degos F, Lugassy C, Degott C, Debure A, Carnot F, Theirs V, Tiollais P, Kreis H, Brechot C: Hepatitis B virus and hepatitis B-related viral infection in renal transplant recipients. A prospective study of 90 patients. Gastroenterology 94: 151–156, 1988 [DOI] [PubMed] [Google Scholar]
- 5. Mathurin P, Mouquet C, Poynard T, Sylla C, Benalia H, Fretz C, Thibault V, Cadranel JF, Bernard B, Opolon P, Coriat P, Bitker MO: Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology 29: 257–263, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Parfrey PS, Forbes RD, Hutchinson TA, Beaudoin JG, Dauphinee WD, Hollomby DJ, Guttmann RD: The clinical and pathological course of hepatitis B liver disease in renal transplant recipients. Transplantation 37: 461–466, 1984 [DOI] [PubMed] [Google Scholar]
- 7. Pirson Y, Alexandre GP, Ypersele C: Long-term effect of hbs antigenemia on patient survival after renal transplantation. N Engl J Med 296: 194–196, 1977 [DOI] [PubMed] [Google Scholar]
- 8. Perrillo RP: Hepatitis B and renal transplantation: Securing the sword of damocles. Hepatology 36: 1041–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Carey I, Harrison PM: Monotherapy versus combination therapy for the treatment of chronic hepatitis B. Expert Opin Investig Drugs 18: 1655–1666, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Fabrizi F, Martin P, Dixit V, Kanwal F, Dulai G: HBsAg seropositive status and survival after renal transplantation: Meta-analysis of observational studies. Am J Transplant 5: 2913–2921, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Aroldi A, Lampertico P, Montagnino G, Passerini P, Villa M, Campise MR, Lunghi G, Tarantino A, Cesana BM, Messa P, Ponticelli C: Natural history of hepatitis B and C in renal allograft recipients. Transplantation 79: 1132–1136, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Breitenfeldt MK, Rasenack J, Berthold H, Olschewski M, Schroff J, Strey C, Grotz WH: Impact of hepatitis B and C on graft loss and mortality of patients after kidney transplantation. Clin Transplant 16: 130–136, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Morales JM, Dominguez-Gil B, Sanz-Guajardo D, Fernandez J, Escuin F: The influence of hepatitis B and hepatitis C virus infection in the recipient on late renal allograft failure. Nephrol Dial Transplant 19[Suppl 3]: 72–76, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Chan TM, Fang GX, Tang CS, Cheng IK, Lai KN, Ho SK: Preemptive lamivudine therapy based on HBV DNA level in HBsAg-positive kidney allograft recipients. Hepatology 36: 1246–1252, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Lee WC, Shu KH, Cheng CH, Wu MJ, Chen CH, Lian JC: Long-term impact of hepatitis B, C virus infection on renal transplantation. Am J Nephrol 21: 300–306, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Ridruejo E, Brunet Mdel R, Cusumano A, Diaz C, Michel MD, Jost L, Jost L, Jr, Mando OG, Vilches A: HBsAg as predictor of outcome in renal transplant patients. Medicina (B Aires) 64: 429–432, 2004 [PubMed] [Google Scholar]
- 17. Ahn HJ, Kim MS, Kim YS, Kim SI, Huh KH, Ju MK, Ahn SH, Han KH: Clinical outcome of renal transplantation in patients with positive pre-transplant hepatitis B surface antigen. J Med Virol 79: 1655–1663, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kim WR, Poterucha JJ, Kremers WK, Ishitani MB, Dickson ER: Outcome of liver transplantation for hepatitis B in the United States. Liver Transpl 10: 968–974, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Katznelson S, Cecka JM: The great success of Asian kidney transplant recipients. Transplantation 64: 1850–1852, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Katznelson S, Gjertson DW, Cecka JM: The effect of race and ethnicity on kidney allograft outcome. Clin Transpl 1995: 379–394, 1995 [PubMed] [Google Scholar]