Abstract
Summary
The case of a 12-year-old with a hybrid CFH/CFHL1 gene and atypical hemolytic uremic syndrome (aHUS) that had previously developed native kidney and then renal allograft loss is reported. This case illustrates the relatively common occurrence of renal loss from the late presentation of aHUS. Also presented is a protocol for the pre-emptive use of eculizumab and plasmapheresis as part of a renal transplant plan for the treatment of aHUS in patients deemed at high risk for recurrent disease. This protocol was a result of a multidisciplinary approach including adult and pediatric nephrology, transplant surgery, transfusion medicine, and infectious disease specialists. This protocol and the justifications and components of it can function as a guideline for the treatment of a group of children that have waited in limbo for the first U.S. transplant to open the door to this type of definitive care for this devastating disease.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a disease of the microvasculature classically characterized by the triad of hemolytic anemia, thrombocytopenia, and acute kidney injury (1–3). It disproportionately affects children, with more than half progressing to ESRD (4). A mortality rate of 10% to 15% has been reported during acute episodes. The likelihood of recurrence of aHUS after renal transplantation can make the decision to transplant particularly difficult, especially in aHUS patients who suffer multisystem dysfunction during renal exacerbations or at the time of native or transplant kidney loss (5–10). For many aHUS patients, lifelong dialysis has been prescribed as the treatment of choice for ESRD.
aHUS is caused by uncontrolled activation of the alternative pathway (AP) of the complement system, with more than 120 reported loss-of-function mutations in the AP regulatory proteins complement factor H (CFH), complement factor I (CFI), and membrane-cofactor protein (4,9–13). Gain-of-function mutations in complement factor B (CFB) and complement component 3 (C3) genes have also been reported, as have mutations in THBD, encoding thrombomodulin, a membrane-bound glycoprotein with anticoagulant properties (14–18). In addition to these point mutations, genomic rearrangements of the CFH and the complement factor H-related (CFHR) gene region have been reported in aHUS patients. Some of these rearrangements lead to novel fusion proteins, whereas others, such as homozygosity for deletion of CFHR3 and CFHR1, lead to the development of autoantibodies to CFH (19,20).
Case
Our patient initially presented at 8 years of age with marked hypertension, anuric renal failure (blood urea nitrogen [BUN] 196 mg/dl [70 mmol/L], creatinine [Cr] 13.7 mg/dl [1211 μmol/L]), and severe anemia (hemoglobin 5.5 g/dl [3.4 pmol/L]) (Figure 1). Hemolysis parameters were not assessed. Complement component 3 (C3) and 4 (C4) levels were normal at 98 and 31 mg/dl, respectively. Dialysis was initiated at the time of presentation and no return of renal function was noted. No cause of the renal dysfunction was discovered. The patient had an uncomplicated 8-month course on dialysis and then underwent a living-related renal transplant. Native kidney biopsy at the time of transplantation showed severe chronic interstitial inflammation (Figure 2A). The etiology for the loss of native renal function remained unknown.
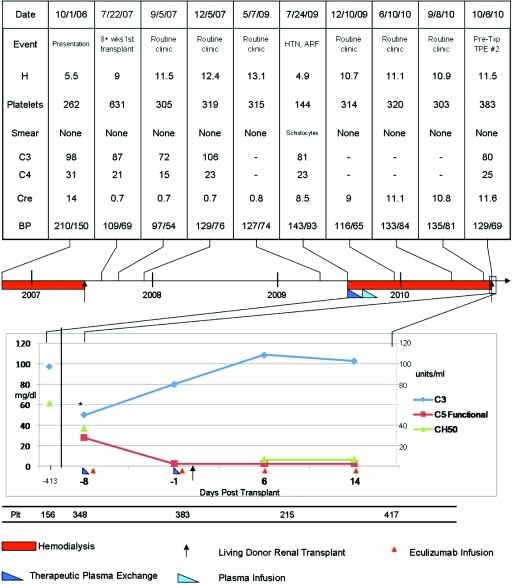
Figure 1.
Laboratory values at key time points in the course of the patient's aHUS episodes. (Upper) Laboratory and clinical parameters that would have played a role in establishing a clinical diagnosis of aHUS. Of note, the complement levels and platelet count were normal at the time of presentation of native kidney loss (October 1, 2006). The hemoglobin was the only assessed indication of possible hemolysis. The hemoglobin was low at the time of graft loss and this time was associated with a low platelet count (July 24, 2009). (Lower) The C3, C5, total complement pathway hemolytic assay, and platelet count at the time of the second transplant and perieculizumab use. The patient's baseline remission laboratory results are noted on the left of the figure for reference. HTN, hypertension; ARF, acute renal failure; TPE, therapeutic plasma exchange; h, hemoglobin (g/dl).
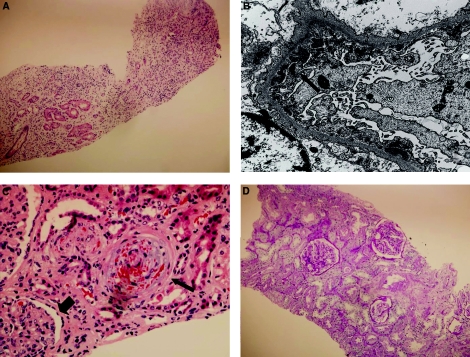
Figure 2.
Sequential renal biopsy micrographs in patient with aHUS. (A) Native kidney at the time of renal transplantation (8 months after the start of dialysis). The predominant finding is that of scarred renal parenchyma with no viable glomeruli and few tubules with marked interstitial inflammation. H&E 100X. (B) On the electron microscopy (native kidney), there are moderate amounts of subepithelial electron-dense deposits in an interrupted pattern in one glomerulus (thin arrow). Also present on this biopsy were moderate amounts of mesangial electron-dense deposits and mild amounts of subendothelial deposits associated with glomerular basement membrane double contours (not shown). EM 15000X. (C) One glomerulus (first transplant) with endothelial swelling (arrowhead) and an arteriole showing severe TMA with intramural fragmented erythrocytes (thin arrow). H&E 400X. (D) The posteculizumab transplant biopsy demonstrates normocellular glomeruli and normal capillary loops. There is no interstitial fibrosis, inflammation, or tubular atrophy, although there is patchy dilation of tubules. The arteries and arterioles are unremarkable. PAS 100X.
Immediately after her first renal transplantation, the patient had one episode of thrombocytopenia during which her platelet count decreased by half (postoperative day 0) but then recovered to normal by 1 month posttransplant. No other concerning laboratory or clinical parameters to suggest systemic disease were noted. During routine surveillance over a 2-year period, renal function was stable with no episodes of rejection.
Twenty-five months after renal transplantation, the patient developed higher-than-usual BP as noted by home monitoring. In a nonroutine clinic follow-up for evaluation of hypertension and vague systemic complaints, her laboratory evaluation documented renal failure, with a BUN of 109 mg/dl (38.9 mmol/L) and a Cr of 8.5 mg/dl (751.4 μmol/L). Ten weeks earlier, the BUN and Cr had been 12 mg/dl (4.28 mmol/L) and 0.8 mg/dl (70.7 μmol/L), respectively. Her hemoglobin decreased from 13.1 to 4.5 g/dl, and her platelet count fell from 315 to 144 mm3 during this same time period. At presentation, her C3 was mildly depressed at 81 mg/dl (normal 90 to 180 mg/dl) and the C4 was normal. Workup for her anemia suggested a hemolytic process. A transplant renal biopsy was remarkable for severe thrombotic microangiopathy (TMA), moderate amounts of subepithelial electron-dense deposits, and no evidence of tubulitis (Figure 2, B and C).
Thrice-weekly hemodialysis and a regimen of daily plasma exchange (PE) was initiated. Over the subsequent 19 days, PE elicited a remission of hemolysis as reflected by a normalization of her lactic acid dehydrogenase, the disappearance of schistocytes, and the platelet count rose from 144,000 to 337,000 mm3. Renal function did not return and chronic hemodialysis ensued. Over the next several weeks, the patient was weaned off PE without signs or symptoms of aHUS. She received a total of 28 plasma therapy interventions during this time period.
Serologic and Genetic Workup
The patient underwent an extensive serologic workup once TMA was identified in her transplant kidney. CFH, CFB, and CFI levels were normal, and AP hemolytic assays and total complement hemolytic assays were normal. C3 was mildly depressed at 81 mg/dl (normal 90 to 180 mg/dl), but C4 was normal (Figure 1). Antiphospholipid antibodies were normal and antinuclear antibodies were negative. Her HIV test was negative. Her ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin Motifs) 13 level was normal.
Genetic workup completed by the University of Iowa Molecular Otolaryngology and Renal Research Laboratory (http://www.healthcare.uiowa.edu/labs/morl/) did not identify mutations in CFH, CFI, CFHR5, MCP (gene for membrane-cofactor protein), CFB, C3, or THBD. Copy number variation of the CFHR region was assessed by multiplex ligation-dependent probe amplification and identified a hybrid CFH/CFHR1 gene that encodes a fusion protein comprised of the first 18 short consensus repeats of CFH and the last 2 short consensus repeats of CFHR1 (21). This hybrid CFH/CFHR1 gene encodes a protein product identical to a functionally significant CFH mutant (c.3572C>T, S1191L and c.3590T>C, V1197A) that has been previously described in association with aHUS.
Pretransplant Risk-Benefit Considerations
The patient's clinical history and her genotype results suggested a substantial risk for recurrence of her native disease should a kidney transplant be attempted. The patient's life was not immediately in danger; however, she had a significant long-term risk for cardiac disease and a shortened life span if she remained on dialysis. The possibility of a combined deceased-donor liver-kidney transplant was considered, but given the significant morbidity and mortality reported with the procedure when first attempted for factor H–associated aHUS, this was not pursued further. It was felt that the long-term risk of dialysis and the emerging evidence of the effectiveness of eculizumab represented an opportunity to utilize an alternative approach for this patient.
With a transplant there were several potential risks: aHUS recurrence, infection due to complement blockade (along with usual transplant immune suppression), the development of antibodies to the biologic agent eculizumab, and the long-term social and financial burden of being maintained indefinitely on a frequently delivered intravenous medication. The decision to proceed with kidney transplant was made only after the family had been instructed on each of these risks and had decided along with their medical team that transplantation was the preferred option.
Preparation for Transplantation
A living nonrelated renal donor was identified after 15 months of chronic hemodialysis. Insurance approval for eculizumab preconditioning and subsequent therapy was obtained, and members of the Rare Renal Disease Clinic approved the patient for transplantation. She was immunized against meningococcus 6 months before transplant; however, no meningococcal vaccine response was noted with titer assessment. Before transplantation, the patient was re-immunized and placed on ciprofloxacin for meningococcal prophylaxis. All routine childhood immunizations were current per the American Academy of Pediatrics recommendations.
Transplantation and Subsequent Course
The patient received her usual thrice-weekly dialysis before renal transplantation. She underwent her second renal transplant 1 month before her 13th birthday using the protocol outlined in Table 1. One week before transplantation, PE with 1.5 volumes of albumin was performed and one dose of eculizumab (900 mg) was given immediately thereafter. The day before transplant, before hemodialysis, the patient received a second PE (1.5 volumes of fresh frozen plasma) and was admitted in preparation for surgery. A second dose of eculizumab (900 mg) was given the evening before surgery.
Table 1.
Protocol for the prophylactic use of eculizumab for renal transplant in aHUS
| Pretransplant evaluation |
| Donor evaluation for pathogenic aHUS mutations. |
| Immunize against meningococcus (as well as hemophilus and pneumococci if not current). |
| Verify titer if on dialysis or if immune suppressed at the time of vaccination—consider re-immunization and antibacterial prophylaxis as necessary. |
| Zero minus 1 week (before transplant) |
| Measure C3, C4, AH50, CH50, C5 functional level, CBC, LDH, haptoglobin, platelet count, and if appropriate C3Nef, and/or CFI levels. (Laboratory test samples must be drawn before plasma therapy and before eculizumab.) |
| PE or infusion—first dose (1.5 volumes of albumin). |
| Administer 900 mg eculizumaba—first dose. |
| Zero minus 24 to 48 h before transplant |
| Measure C3, C4, AH50, CH50, C5 functional level, CBC, LDH, haptoglobin, platelet count, and if appropriate C3Nef, and/or CFI levels. (Laboratory test samples must be drawn before plasma therapy and before eculizumab.) |
| PE or infusion—second dose (1.5 volumes of FFP). |
| Zero hour 0 to 24 h before transplant |
| Administer 900 mg eculizumab—second dose. |
| Posttransplant |
| Measure C3, C4, AH50, CH50, C5 functional level, CBC, LDH, haptoglobin, platelet count, and if appropriate C3Nef, and/or CFI levels. (Laboratory test samples must be drawn before plasma therapy and before eculizumab.) |
| Administer 900 mg eculixumab every 7 days times two (third and fourth dose). |
| Administer 900 mg eculizumab every 14 days. |
AH50, alternate complement pathway hemolytic assay; CH50, total complement pathway hemolytic assay; CBC, complete blood count; C3Nef, complement component C3 nephritic factor; aHUS, atypical hemolytic uremic syndrome; LDH, lactate dehydrogenase; CFI, complement factor I; PE, plasma exchange.
Dose adjustment based on standard adult dosing as recommended by manufacturer or by weight in children.
Induction therapy included thymoglobulin (50 mg), methylprednisolone (350 mg), tacrolimus (0.1 mg/kg per dose), and mycophenolate mofetil (300 mg/m2 per dose), with a prednisone taper. It is our standard of care to use a steroid protocol when there is a high risk for recurrence, when steroids have been used to treat native renal disease, and/or if patients have had prior renal transplants. The third and fourth doses of eculizumab were given 1 and 2 weeks after transplantation, and then dosing was transitioned to every other week, with laboratory studies immediately before dosing. This is a similar regimen as has been followed to block complement in paroxysmal nocturnal hemoglobinuria (PNH) patients (22,23).
Within hours of her transplantation, urine output was well established; the Cr level fell from 11.7 mg/dl (1034 μmol/L) pretransplant to 1.5 mg/dl (133 μmol/L) by 7 days posttransplant. Because the Cr did not immediately decrease to the level expected (0.8 mg/dl, or 71 μmol/L), a renal biopsy was performed on day 8 (Figure 2D). No evidence of TMA was noted.
Laboratory studies collected during the protocol were as follows: serum C3 complement levels, which had been normal at baseline for this patient (95 mg/dl or 0.95 g/L), were 50 mg/dl (0.5 g/L) after her first PE, recovered to 80 mg/dl (0.8 g/L) before second PE, and recovered to normal by 2 weeks posttransplant as evidenced by her laboratory results immediately before her third dose of eculizumab. Her total complement pathway hemolysis assay, which was normal at baseline (>60 U/ml), was low at 37 U/ml after PE and <13 U/ml 1 week after her second PE. Her complement component 5 (C5) functional assay was 28 U/ml (normal 29 to 53 U/ml) before her first eculizumab dose (and after her first PE). It has been <5 U/ml with each check drawn immediately before subsequent eculizumab doses (Figure 2).
The patient is now more than 4 months past her second renal transplant with a Cr level that continues to decline. The most recent check was 0.9 mg/dl (80 μmol/L). All hemolytic laboratory results are within the normal range. Her total complement and terminal complement assays remain low. Her BP was normal at 112/78 mmHg at her last clinic visit and she has resumed the routine of an active seventh grader.
Discussion
Until recently, renal transplantation alone has not been an option for many aHUS patients because the procedure carried an unacceptably high morbidity/mortality because of disease recurrence. However, at least in children, chronic hemodialysis is a poor alternative because the expected remaining lifetime for children 0 to 14 years of age on dialysis is only 18.3 years, whereas the prevalent transplant population of the same age has an expected remaining lifetime of 50 years (24). Therefore, there is an impetus to re-establish renal function by transplantation in aHUS patients.
On the basis of the pathophysiology of aHUS, several combined liver-kidney transplants have been done with the goal to have the transplanted liver supply normal complement regulatory proteins in those aHUS patients with mutations in liver-derived factors (CFH, CFI, CFB, and C3) (25–30). However, this approach does not address the problem in aHUS patients with CFH autoantibodies and carries a significant added risk when considering the possibility of increased complement activation and the increased complexity of a dual organ transplant.
The identification of an anticomplement therapy has permitted a safer approach, allowing transplantation of a kidney alone in aHUS patients by pharmacologically controlling AP dysregulation with eculizumab. This humanized anti-C5 monoclonal antibody blocks activation of the terminal complement cascade. The U.S. Food and Drug Administration has approved eculizumab for PNH. In Europe, it has been used successfully in patients with native renal disease secondary to aHUS and in those in which the disease has occurred in the postrenal-transplant setting (9,22,31–38). It is currently not approved for aHUS in the United States, although given the PNH experience showing it to be relatively safe, the case reports of success in native aHUS, and the not infrequent need to use unapproved drugs in the pediatric population, the stage has been set for this to be a useful tool to treat aHUS in the pediatric population (22,23).
No standard of care exists for renal transplantation in aHUS patients, reflecting the myriad of issues that must be addressed. An evolving approach for the treatment of native renal aHUS appears to be eculizumab, and the results of a trial in adults and adolescents with aHUS is now pending. A pediatric trial is beginning. One significant issue to contend with when deciding to use eculizumab for native aHUS remains the fact that it is unclear whether scheduled maintenance dosing long term is absolutely required as opposed to an option that would allow for discontinuation of eculizumab with the use of a “rescue” protocol when an aHUS episode develops. One point in favor of long-term dosing is that published reports suggest that eculizumab when used late in established disease does not always reverse renal injury. The identification of robust biomarkers that predict an imminent clinically significant recurrence is essential before contemplating a rescue protocol. Although an increase in LDH and a drop in platelets reflect increased activity of the terminal complement pathway, these are secondary changes and coupled with interlaboratory variation and a broad range of normal parameters lack the sensitivity to detect disease activity in a timely fashion.
The protocol for renal transplantation used for our aHUS patient represents an empiric approach with the respect to (1) the timing of dosing of eculizumab (partially on the basis of what we were aware of regarding the pharmocokinetics of the drug), (2) whether and when to use PE (done because of the presumed presence of an abnormal protein and to limit potential graft incompatibility issues), (3) what laboratory parameters to monitor based on (the assumption that monitoring the terminal compliment pathway should be a useful biomarker), and (4) the length of therapy (which rests on risk of recurrence of disease and the fact that there is currently no established acute predictive biomarker). As in native aHUS, the final consideration is paramount because it is a well documented fact that successive renal insults risk total loss of renal function. We believe this protocol offers a current best approach to patients in this complex situation.
There are, of course, no randomized control protocols and there will likely be none based on the rarity of this disease and because of each of the many components of this protocol that could be manipulated. Suffice to say we feel that the justifications for meningococcal vaccination before therapy with eculizumab are well supported. The lack of need for PE after transplant appears to be supported by case reports and by the inhibition of complement that is noted biochemically with eculizumab alone. It remains to be seen if PE could be avoided pretransplant. Which hemolysis and/or complement activation laboratory results to monitor posttransplant remains an important question, as does the timing of such laboratory tests. These are key questions that need to be answered before considering a withdrawal/rescue protocol. Finally, there is currently no evidence that allows a clear definition of the length of therapy that is required. Until there is better evidence and improved early biomarkers, we have elected to provide indefinite therapy to our patient.
We are cautiously optimistic about the promise of a terminal complement assay such as the C5 functional assay, to monitor eculizumab therapy. In another patient we are treating with native kidney aHUS, two significant events occurred without an aHUS flare: a concurrent Clostridium difficile infection and a transition through a dose-for-weight cutoff point. In each case, we noted only a slight decrease in platelet count, although the C5 functional assay normalized. With eculizumab, the assay should remain low. With treatment of the C. difficile infection in the first instance and when the dose of eculizumab was increased to account for the patient's weight increase in the second instance, the C5 functional assay returned to the low range. This finding suggests that assessing the terminal complement pathway may allow earlier recognition of disease activity or flare risk than is offered by the traditional hemolysis markers of LDH, platelet count, and haptoglobin.
With respect to long-term follow-up for our transplant patient, one paramount concern will be that of the increased risk for infection. Neisseria meningitides is the organism of primary concern because the natural immunologic defense against this organism requires terminal complement pathway activation, hence the recommendation in this protocol for meningococcal vaccination before receiving the drug. As reported by the manufacturer of the drug in the package insert, the incidence of meningococcal meningitis with eculizumab is <0.1% (23). In our patient's case, she will remain on meningococcal prophylaxis in the form of daily ciprofloxacin, and she and her family have been educated on the associated symptoms.
A further concern that may be raised for this patient long term is that of the immunogenicity of eculizumab. Again utilizing PNH data and as reported in the package insert, low-titer antibodies to eculizumab have been reported in placebo and treated individuals (2.1%) (22). There was no apparent correlation of antibody development to clinical response to the drug. We have no specific plan to look for antibodies in this patient, but we will seek to do so if we note any indication of change in drug effectiveness.
Finally, the social and economic burden of delivering an expensive pharmaceutical on a biweekly basis for an extended period of time must be considered. It may be true in some patients that the reduction in relapse of aHUS with subsequent intensive care interventions, including plasma therapy and dialysis (at our institution $3500/intermittent episode and $2000/episode, respectively), and avoidance of associated comorbidities will justify the expense. However, when orphan diseases are considered, the cost of treatment is often difficult to reconcile purely economically. Similarly, there is precedent for the need for frequent medical appointments to receive therapy. Chronic dialytic therapy provides a prototypical model for the frequent, repeated need to access maintenance medical care. In the end, the choice to proceed with chronic pharmaceutical therapy must be based on each patient's specific circumstance in close consultation with the medical caregivers, the patient, and the patient's family.
In summary, we present the use of eculizumab and plasmapheresis pre-emptively as part of a renal transplant protocol for the treatment of aHUS in a patient with a known genetic defect deemed at high risk for recurrent disease. Although our follow-up is short (4 months), we suggest that this protocol offers the promise of kidney transplantation for aHUS patients in the United States.
Disclosures
Dr. Nester is a member of Alexion Pharmaceutical's Atypical Hemolytic Uremic Syndrome International Advisory Board.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Zimmerhackl LB, Besbas N, Jungraithmayr T, Van de Kar N, Karch H, Karpman D, Landau D, Loirat C, Proesmans W, Prüfer F, Rizzoni G, Taylor MC; European Study Group for Haemolytic Uraemic and Related Disorders: Epidemiology, clinical presentation, and pathophysiology of atypical and recurrent hemolytic uremic syndrome. Semin Thromb Haemost 32: 113–120, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, Rizzoni G, Taylor CM, Van de Kar N, Zimmerhackl LB: A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int 70: 423–431, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Ariceta G, Besbas N, Johnson S, Karpman D, Landau D, Licht C, Loirat C, Pecoraro C, Taylor C, Van de Kar N, Vandewalle J, Zimmerhackl L: Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol 24: 687–696, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Loirat C, Noris M, Fremeaux-Bacchi V: Complement and the atypical hemolytic uremic syndrome in children. Pediatr Nephrol 23: 1957–1972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bresin E, Daina E, Noris M, Castelletti F, Stefanov R, Hill P, Goodship TH, Remuzzi G; International Registry of Recurrent and Familial HUS/TTP: Outcome of renal transplantation in patients with non-Shiga toxin-associated hemolytic uremic syndrome: Prognostic significance of genetic background. Clin J Am Soc Nephrol 1: 88–99, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Olie KH, Florquin S, Groothoff JW, Verlaak R, Strain L, Goodship TH, Weening JJ, Davin JC: Atypical relapse of hemolytic uremic syndrome after transplantation. Pediatr Nephrol 19: 1173–1176, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Chan MR, Thomas CP, Torrealba JR, Djamali A, Fernandez LA, Nishimura CJ, Smith RJ, Samaniego MD: Recurrent atypical hemolytic uremic syndrome associated with factor I mutation in a living related renal transplant recipient. Am J Kid Dis 53: 321–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donne RL, Abbs I, Barany P, Elinder CG, Little M, Conlon P, Goodship TH: Recurrence of hemolytic uremic syndrome after live related renal transplantation associated with subsequent de novo disease in the donor. Am J Kid Dis 40: E22–E22, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Kavanagh D, Goodship T: Genetics and complement in atypical HUS. Pediatr Nephrol 25: 2431–2442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, Daina E, Fenili C, Castelletti F, Sorosina A, Piras R, Donadelli R, Maranta R, van der Meer I, Conway EM, Zipfel PF, Goodship TH, Remuzzi G: Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 5: 1844–1859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waters AM, Pappworth I, Marchbank K, Bockenhauer D, Tullus K, Pickering MC, Strain L, Sebire N, Shroff R, Marks SD, Goodship TH, Rees L: Successful renal transplantation in factor H autoantibody associated HUS with CFHR1 and 3 deficiency and CFH variant G2850T. Am J Transplant 10: 168–172, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJH: Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat 31: E1445–E1460, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi G; International Registry of Recurrent and Familial HUS/TTP: Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108: 1267–1279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tawadrous H, Maga T, Sharma J, Kupferman J, Smith R, Schoeneman M: A novel mutation in the complement factor B gene (CFB) and atypical hemolytic uremic syndrome. Pediatr Nephrol 25: 947–951, 2010 [DOI] [PubMed] [Google Scholar]
- 15. de Jorge EG, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, Garrido CA, López-Trascasa M, Sánchez-Corral P, Morgan BP de Córdoba SR: Gain-of-function mutations in complement factory B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A 104: 240–245, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lhotta K, Janecke AR, Scheiring J, Petzlberger B, Giner T, Fally V, Wurzner R, Zimmerhackl LB, Mayer G, Fremeaux-Bacchi V: A large family with gain-of-function mutation of complement C3 predisposing to atypical hemolytic uremic syndrome, microhematuria, hypertension and chronic renal failure. Clin J Am Soc Nephrol 4: 1356–1362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fremeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, de Ligny BH, Fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJC, Goodship TH, Atkinson JP: Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112: 4948–4952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delvaeye M, Noris M, De Vriese A, Esmon CT, Esmon NL, Ferrell G, Del-Favero J, Plaisance S, Claes B, Lambrechts D, Zoja C, Remuzzi G, Conway EM: Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med 361: 345–357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore I, Strain L, Pappworth I, Kavanagh D, Barlow PN, Herbert AP, Schmidt CQ, Staniforth SJ, Holmes LV, Ward R, Morgan L, Goodship TH, Marchbank KJ: Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood 115: 379–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skerka C, Józsi M, Zipfel PF, Dragon-Durey MA, Fremeaux-Bacchi V: Autoantibodies in haemolytic uraemic syndrome (HUS). Thromb Haemost 101: 227–232, 2009 [PubMed] [Google Scholar]
- 21. Venables JP, Strain L, Routledge D, Bourn D, Powell HM, Warwicker P, Diaz-Torres ML, Sampson A, Mead P, Webb M, Pirson Y, Jackson MS, Hughes A, Wood KM, Goodship JA, Goodship TH: Atypical haemolytic uraemic syndrome associated with a hybrid complement gene. PLoS Med 3: e431, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, Gaya A, Coyle L, de Castro C, Fu CL, Maciejewski JP, Bessler M, Kroon HA, Rother RP, Hillmen P: Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood 111: 1840–1847, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Hillmen P, Young NS, Schubert J, Brodsky RA, Socié G, Muus P, Röth A, Szer J, Elebute MO, Nakamura R, Browne P, Risitano AM, Hill A, Schrezenmeier H, Fu CL, Maciejewski J, Rollins SA, Mojcik CF, Rother RP, Luzzatto L: The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 355: 1233–1243, 2006 [DOI] [PubMed] [Google Scholar]
- 24. 2005 USRDS Annual Data Report, Minneapolis, MN, U.S. Renal Data System, 2005 [Google Scholar]
- 25. Remuzzi G, Ruggenenti P, Codazzi D, Noris M, Caprioli J, Locatelli G, Gridelli B: Combined kidney and liver transplantation for familial haemolytic uraemic syndrome. Lancet 359: 1671–1672, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Remuzzi G, Ruggenenti P, Colledan M, Gridelli B, Bertani A, Bettinaglio P, Bucchioni S, Sonzogni A, Bonanomi E, Sonzogni V, Platt JL, Perico N, Noris M: Hemolytic uremic syndrome: A fatal outcome after kidney and liver transplantation performed to correct factor h gene mutation. Am J Transplant 5: 1146–1150, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Saland JM, Ruggenenti P, Remuzzi J; Consensus Study Group: Liver-kidney transplantation to cure atypical hemolytic uremic syndrome. J Am Soc Nephrol 20: 940–949, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Saland JM, Emre SH, Shneider BL, Benchimol C, Ames S, Bromberg JS, Remuzzi G, Strain L, Goodship TH: Favorable long-term outcome after liver-kidney transplant for recurrent hemolytic uremic syndrome associated with a factor H mutation. Am J Transplant 6: 1948–1952, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Saland JM, Shneider BL, Bromberg JS, Shi PA, Ward SC, Magid MS, Benchimol C, Seikaly MG, Emre SH, Bresin E, Remuzzi G: Successful split liver-kidney transplant for factor H associated hemolytic uremic syndrome. Clin J Am Soc Nephrol 4: 201–206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jalanko H, Peltonen S, Koskinen A, Puntila J, Isoniemi H, Holmberg C, Pinomäki A, Armstrong E, Koivusalo A, Tukiainen E, Mäkisalo H, Saland J, Remuzzi G., De Cordoba S, Lassila R, Meri S, Jokiranta TS: Successful liver-kidney transplantation in two children with aHUS caused by a mutation in complement factor H. Am J Transplant 8: 216–221, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Waters A, Licht C: aHUS caused by complement dysregulation: New therapies on the horizon. Pediatr Nephrol 26: 41–57, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zimmerhackl LB, Hofer J, Cortina G, Mark W, Würzner R, Jungraithmayr TC, Khursigara G, Kliche KO, Radauer W: Prophylactic eculizumab after renal transplantation in atypical hemolytic-uremic syndrome. N Engl J Med 362: 1746–1748, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Larrea CF, Cofan F, Oppenheimer F, Campistol JM, Escolar G, Lozano M: Efficacy of eculizumab in the treatment of recurrent atypical hemolytic-uremic syndrome after renal transplantation. Transplantation 15: 903–904, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Davin JC, Gracchi V, Bouts A, Groothoff J, Strain L, Goodship T: Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation. Am J Kid Dis 55: 708–711, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Nürnberger J, Philipp T, Witzke O, Opazo Saez A, Vester U, Baba HA, Kribben A, Zimmerhackl LB, Janecke AR, Nagel M, Kirschfink M: Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med 360: 542–544, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Gruppo RA, Rother RP: Eculizumab for congenital atypical hemolytic-uremic syndrome. N Engl J Med 360: 544–546, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Mache CJ, Acham-Roschitz B, Fremeaux-Bacchi V, Kirschfink M, Zipfel PF, Roedl S, Vester U, Ring E: Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 4: 1312–1316, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chatelet V, Frémeaux-Bacchi V, Lobbedez T, Ficheux M, Hurault de Ligny B: Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome. Am J Transplant 9: 2644–2645, 2009 [DOI] [PubMed] [Google Scholar]