Abstract
Nasal polyposis are common presentations in patients of chronic rhinosinusitis and are considered to be associated with more severe forms of disease with poor treatment outcome. The presentation and treatment outcome after endoscopic sinus surgery in patients of chronic rhinosinusitis and nasal polyposis have been analysed in this study. A prospective analysis of 90 patients of chronic rhinosinusitis who were classified into two groups depending on presence and absence of nasal polyps was performed in the study. The two groups were evaluated using subjective (patient complaints) and objective (computed tomography scan and endoscopy scores) criteria. Preoperative data were compared with data obtained 12 months post endoscopic sinus surgery. The study included 38 patients of chronic rhinosinusitis and 52 patients of nasal polyps. The patients of nasal polyp group presented with increased severity of symptoms of nasal blockage, nasal discharge and reduced sense of smell as compared to the chronic rhinosinusitis group who had significantly higher presentation of headache and facial pain. The preoperative CT scan revealed significantly higher bilateral disease with increased involvement of multiple sinuses in nasal polyp group. Post endoscopic sinus surgery both the groups showed significant improvement in their symptoms with the nasal polyp group demonstrating reduction in improvement on 1 year follow up. In our study we have found the patients with chronic rhinosinusitis and nasal polyp have varied severity of symptoms with the nasal polyp group having higher nasal symptoms and increased severity as compared to chronic rhinosinusitis group. Though the universal rationale of management by adequate drainage and ventilation of sinus is similar in both groups, there is a reduction in both objective and subjective scores during 1 year follow up in the nasal polyp group.
Keywords: Chronic rhinosinusitis, Nasal polyps, Endoscopic sinus surgery
Introduction
Chronic rhinosinusitis (CRS) is a common health problem which leads to frequent visits to primary care physicians and ear, nose, throat specialists. In spite of the recent advances, the etiology, pathogenesis and treatment of CRS is a matter of debate [1]. Nasal polyposis (NP) is considered as a subgroup of CRS with an incidence of 4% in general population [2] and 25–30% in patients suffering from CRS [3]. Though studies in literature suggest that patients of NP as a distinct entity but the present investigation and treatment modality do not distinguish CRS from NP [4].
In this study we have analysed the demographics, clinical features and treatment outcomes in a group of patients with CRS and NP.
Materials and Methods
The study design was a prospective analysis performed at a zonal and tertiary care referral hospital. The study group included 90 adult patients who were classified into two groups depending on the presence of polyps; CRS (without polyps) and NP (with polyps). The two groups of patient underwent ESS after failing medical treatment.
Inclusion criteria for the study were: (i) Established diagnostic criteria of CRS as per Task Force criteria 1997 [5]; (ii) patients were diagnosed as NP based on presence of polyps on endoscopic examination; (iii) age > 16 years; (iv) confirmatory radiological diagnosis of CRS and (v) previous nasal or paranasal sinus surgery was not a criteria for exclusion.
Exclusion criteria were: (i) Patients with chronic diseases like cystic fibrosis, primary ciliary dyskinesia, immune deficiencies etc; (ii) pregnancy; (iii) not complying to study protocol; (iv) tumours and (v) medical or surgical treatments influencing the study.
Patients who were clinically diagnosed as per major and minor criteria for CRS underwent detailed clinical and physical examination followed by diagnostic nasal endoscopy using rigid nasal endoscope. The study group were initially treated with maximal medical therapy with antibiotics, antihistaminics, saline nasal irrigation, topical and oral steroid where indicated. Patients found refractory to medical management were advised ESS.
The patients demographic data was obtained after which each patient completed a questionnaire which catalogued the severity of disease in a five point scale of 0–IV (no symptoms, mild, moderate, moderately severe and severe symptoms). The two groups of patients were compared by the following subjective and objective scores: (i) Preoperative Lund Mackay CT scan score [5] (postoperative CT scan cannot be used ethically for follow up and can be done only in patients of symptoms or recurrence); (ii) pre and postoperative Lund Kennedy nasal endoscopy scores [6]; (iii) change in patient symptoms (worse, no change, better). Worse and no change in symptoms were considered as failures and better as success. The symptoms were evaluated at 1, 6 and 12 months postoperatively.
ESS was carried out as per standard techniques under general anesthesia. The extent of surgery was determined by the disease and included a minimum of uncinectomy and middle meatal antrostomy. Anterior or posterior ethmoid sinus, frontal sinus and sphenoid were explored in cases of involvement. Following surgery all patients of allergy were given topical nasal steroids for 6 months.
Statistical analysis was done using statistical software SPSS. The χ2 test was used to analyse the two groups. The preoperative and postoperative results were analysed as per student t test. The level of significance was P < 0.05.
Results
The study included 90 patients of the age group 16–71 years with a mean age of 34.8 years. CRS consisted of 38 patients with a mean age of 31.2 years and male female ratio of 9:10. NP included 52 patients with a mean age of 37.5 years and male female ratio of 9:17.
Six patients of CRS and 39 patients of NP gave history and symptoms of allergy. Three and 16 patients of CRS and NP were on treatment of asthma. Three patients of CRS had undergone previous surgery (1 Caldwell Luc, 2 ESS) whereas seven patients of NP had previous surgery (1 Caldwell Luc, 6 ESS).
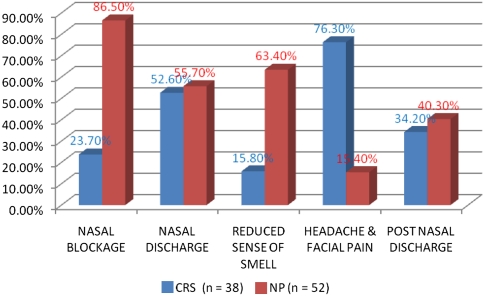
Figure 1 illustrates the distribution of symptoms preoperatively among the patients. It was observed that except the symptom of headache and facial pain, patients of NP had more symptoms than CRS. Nasal blockage and reduced sense of smell in NP and headache in CRS was found to be significantly higher (P < 0.05). On analysis of the severity of symptoms it was observed that the patients of NP presented with increased severity of symptoms of nasal blockage, nasal discharge and reduced sense of smell. Similar findings were observed in CRS with symptom of headache and facial pain (Table 1).
Fig. 1.
Patients with symptoms in CRS and NP group
Table 1.
Severity of symptoms in CRS and NP group
| Symptoms | CRS (n = 38) | NP (n = 52) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No (%) | Mild (%) | Moderate (%) | Moderately severe (%) | Severe (%) | No (%) | Mild (%) | Moderate (%) | Moderately severe (%) | Severe (%) | |
| Nasal blockage | 76.30 | 10.50 | 7.90 | 5.20 | 0 | 13.50 | 17.30 | 25 | 19.20 | 25 |
| Nasal discharge | 47.30 | 13.10 | 15.80 | 13.10 | 10.50 | 44.20 | 5.70 | 11.50 | 17.30 | 21.10 |
| Reduced sense of smell | 84.20 | 13.10 | 2.60 | 0 | 0 | 36.50 | 5.70 | 13.50 | 17.30 | 26.90 |
| Headache and facial pain | 23.70 | 7.90 | 18.40 | 28.90 | 21.10 | 84.60 | 9.60 | 5.70 | 0 | 0 |
| Post nasal discharge | 65.80 | 18.40 | 2.60 | 7.90 | 5.20 | 59.60 | 5.70 | 5.70 | 11.50 | 17.30 |
Preoperative CT scan analysis showed higher bilateral disease with involvement of multiple sinuses in NP. There was a significant difference in the mean Lund Mackay score of CRS (8.7) as compared to NP (14.2) (P < 0.05).
Post ESS patients of both groups showed significant improvement except for improvement in smell perception among the NP patients. CRS patients showed a higher percentage of post ESS improvement in symptoms as compared to NP. Nasal blockage was found to have a higher improvement in NP (Table 2).
Table 2.
Symptoms post surgery after 1 year in CRS and NP group
| Symptoms post surgery | CRS | NP | ||||||
|---|---|---|---|---|---|---|---|---|
| Failure | Success | Failure | Success | |||||
| Worse | No change | Better | Success % | Worse | No change | Better | Success % | |
| Nasal blockage | 0 | 1 | 8 | 89 | 1 | 2 | 42 | 93 |
| Nasal discharge | 0 | 3 | 17 | 85.00 | 4 | 6 | 19 | 65.50 |
| Reduced sense of smell | 0 | 1 | 5 | 91.70 | 7 | 10 | 16 | 48.50 |
| Headache and facial pain | 2 | 5 | 22 | 75.80 | 0 | 2 | 6 | 75.00 |
| Post nasal discharge | 0 | 2 | 11 | 84.60 | 4 | 2 | 15 | 71.40 |
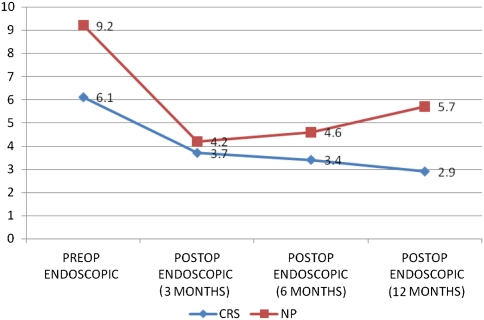
The endoscopic scores according to Lund Kennedy scoring showed significantly higher preoperative scores in NP as compared CRS. Both the groups had significant improvement in endoscopy scores after ESS. Patients of NP had an increase in endoscopic scores in the postoperative review period of 1 year (Fig. 2). One patient of CRS underwent revision during the period of review as compared to 04 patients of NP (Fig. 3).
Fig. 2.
Endoscopic scores in CRS and NP group
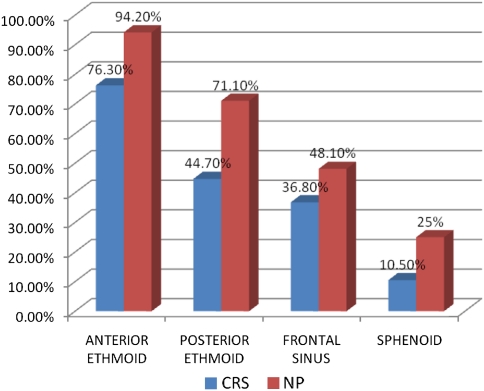
Fig. 3.
Surgery performed in CRS and NP group
10(11.2%) patients had minor complications like synechiae formation 6(6.7%) and hemorrhage 4(4.5%). Two patients of NP group had breach of periorbita during surgery with ecchymosis which was managed conservatively.
Discussion
Chronic rhinosinusitis (CRS) is a common condition affecting the quality of life of millions of involved patients [7]. It is a multifactorial disease which has been extensively studied but its variable presentations, aetiology and exact pathogenesis is still a matter of debate. CRS has been related to bacterial infection, allergy, biofilms and recently superantigens [8]. The symptom manifestation of CRS is varied, hence clinical evaluation by major and minor criteria, assisted by nasal endoscopy and CT scan are the usual methods of diagnosis and management. The universal rationale of treatment is adequate drainage and aeration of the sinus.
Nasal polyps (NP) are mucosal sacs containing oedema, fibrous tissue, vessels, inflammatory cells and glands. Zuckerkandl had described them as arising from lateral wall of nose [9] and Stammberger in his study had shown that 80% of polyps arise from middle meatal mucosa, uncinate process and infundibulum [10]. NP usually are seen in adults in the age group of 20–60 years and presence in children is usually associated with cystic fibrosis [11]. The incidence of NP is 4% in general population, 7–15% in asthmatics and up to 36–60% in patients of Samters triad [2, 3, 12].
NP are common presentations seen in patients of CRS and are considered to be associated with more severe forms of disease with poor post treatment outcome. The presence of ballooning of mucosa into polyps and also recurrence of polyps after surgery when aeration is improved has led to the thought that NP is a separate entity. Also presence of markers like eosinophils and expression of IL 5 in NP has given credence to the fact that NP has a different pathology as compared to CRS. Though EPOS document considers NP as a subgroup of CRS, [13] there are other studies who fail to differentiate among them due to similar prognosis observed after treatment [14].
The top three symptoms of CRS are postnasal drip, nasal obstruction, and facial congestion in terms of prevalence and severity [15]. Various studies in literature have reiterated the findings of increased nasal symptoms (nasal blockage, anosmia) in patients of NP and facial symptoms (headache and facial pain) in CRS [16–18]. The distribution of symptoms in CRS and NP in our study is in consonance with published reports.
The severity of symptoms in our study was observed to have a substantial negative impact on the daily routine of the patients especially in the NP group. Other authors on analysis of the severity of symptoms with objective scores like SNOT 16, SNOT 20, CSS and RDSI symptom scores have found varied results among the CRS and NP patients. Deal et al. has demonstrated worse results of QOL in patients of NP and hypothesized it due to mass effect of polyps causing blockage, reduced mucociliary clearance, retained secretions, increased mucosal oedema and inflammation and reduced sense of smell [19]. Toros et al. in their study of symptoms have observed higher symptom scores and worse objective findings in patients of NP [20]. Contrary to the above studies Smith et al. in a study of preoperative factors that predict surgical outcome and Poetker et al. on the outcomes of ESS on chronic rhinosinusitis with NP have found better preoperative CSS scores in patients with NP resulting in a positive impact on the QOL [21, 22].
The objective evaluations of patients of CRS are based on nasal endoscopy and CT scan paranasal sinus. Various authors have observed significantly higher scores in patients of NP as compared to CRS [20]. The endoscopic scoring of Lund Kennedy is based on presence of oedema and polyps and CT scan scores of Lund Mackay on presence of mucosal oedema and opacification of sinus. Hence NP group would principally have higher scores in endoscopy and CT scan which was also observed in this study.
ESS has been found to have a beneficial impact on the sinonasal outcome in patients of chronic rhinosinusitis with 85–93% of patients reporting relief from symptoms and improved QOL [23, 24]. In spite of the success in CRS and the use of ESS commonly for NP, the results of long term improvement in QOL of patients of NP has been variable with studies indicating equivocal, better or worse results. Dunlop et al. in their analysis of 50 asthmatic patients with CRS and NP recorded no major differences in outcome after ESS in both the groups [17]. Bugten et al. in a prospective trial indicated the two groups to be different entities with similar response to ESS [23]. Bhattacharya et al. in their study of RSI scores revealed similar clinically significant symptomatic improvement after ESS in both the groups [14].
Other authors have described NP to have a significant negative impact on patients with more severe symptoms and less improvement after operative interventions. In a retrospective study in 212 patients, Deal and Kountakis have described a statistically higher improvement among CRS patients as compared to NP patients [19]. Darsum et al. have observed a lower success rate in NP group (54.3%) as compared to CRS group (93.7%) after ESS [24]. They found the NP patients to have refractory disease with a tendency to recur even after prolonged medication and surgical intervention. Contrary to the above Smith et al. in their study of 119 patients of CRS found NP to be a positive effect on RDSI scores after ESS [21]. Poetker et al. also have described worse objective scores in patients of NP but have registered a significantly better QOL after ESS as compared to CRS group [22].
In this study there was a statistically significant improvement in both subjective and objective parameters in the patients of CRS and NP. Although initial improvement in the NP group was substantial, the positive effects in symptoms and endoscopic scores were found to reduce on 1 year followup. In patients of CRS these effects were found to be sustained and also showed improvement during the follow up period. These effects may be due to the fact that many patients of NP have the tendency to recur and are found refractory to treatment after successful surgery [25]. Though the exact mechanism of recurrence in these patients of NP are not well understood, certain factors like allergy, asthma, hereditary and fungus have been implicated [26]. In this study the NP group had a significantly higher percentage of patients with asthma, allergy, recurrence and revision surgery.
ESS is a safe procedure with very low incidence of major complications [27, 28]. Hopkins et al. in their prospective analysis of 3,128 patients undergoing sinonasal surgery have observed a 7% risk of minor complications like synechiae and mild bleeding [29]. The patients with higher risk of complications are usually those having higher subjective and objective disease preoperatively [29]. In our study there were no major complications with 11.2% of patients with minor complications. Synechiae formation was the commonest complication seen and was managed in the postoperative follow up with excision and diligent care. Four patients had bleeding which was found to be bothersome during surgery and no patient required postoperative re-intervention, ligation of vessels or blood transfusion. Except for two cases of breach in periorbita seen in NP group there was no significant increase in other complications in both the groups.
In our study we have found the patients with CRS and NP have varied severity of symptoms with the NP group having higher nasal symptoms and increased severity as compared to CRS group. The endoscopy and CT scores were also found to be significantly higher in the NP group. Post ESS, CRS and NP groups showed significant improvement in symptoms with the NP group having a reduction in both objective and subjective scores during 1 year follow up.
An acknowledged weakness of this study is that we have not used specific quality of life indices in assessing patient’s symptoms.
Conclusion
On review of literature there is varied data on the symptomatology, severity of disease, effects on QOL and post improvement of patients of CRS and NP. Though the universal rationale of management by adequate drainage and ventilation of sinus is similar in both groups, there exists a growing perception among otolaryngologists regarding the differences between the two entities.
In our study both the groups showed significant improvement after ESS with the symptomatic improvement of NP group reducing on long follow up. Further studies are necessary to determine effective management of NP patients with sustained improvement of symptoms.
References
- 1.Benson V, Marano MA (1995) Current estimates from the National Health Interview survey, 1995. Hyattsville, MD: National Center for Health Statistics; 1995. Data from vital and health statistics, series 10: data from the National Health Survey, no. 199: 1–428 [PubMed]
- 2.Hedman J, Kaprio J, Poussa T, Mieminem MM. Prevalence of asthma, aspirin intolerance, nasal poliposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28:717–722. doi: 10.1093/ije/28.4.717. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya N. Clinical and symptom criteria for the accurate diagnosis of chronic rhinosinusitis. Laryngoscope. 2006;116(Pt 2 Suppl 110):1–22. doi: 10.1097/01.mlg.0000224508.59725.19. [DOI] [PubMed] [Google Scholar]
- 4.Głowacki R, Strek P, Zagórska-Swiezy K, Składzień J, Oleś K, Hydzik-Sobocińska K, Miodoński A. Biofilm from patients with chronic rhinosinusitis, morphological SEM studies. Otolaryngol Pol. 2008;62(3):305–310. doi: 10.1016/S0030-6657(08)70260-9. [DOI] [PubMed] [Google Scholar]
- 5.Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol. Head Neck Surg. 1997;117:S1–S7. doi: 10.1016/S0194-5998(97)70001-9. [DOI] [PubMed] [Google Scholar]
- 6.Lund VJ, Kennedy DW. Quantification for staging sinusitis. International conference on sinus disease: terminology, staging, therapy. Ann Otol Rhinol Laryngol. 1995;104(suppl):17–21. [PubMed] [Google Scholar]
- 7.Bhattacharyya N. The economic burden and symptom manifestations of chronic rhinosinusitis. Am J Rhinol. 2003;17:27–32. [PubMed] [Google Scholar]
- 8.Wang M, Shi P, Yue Z, Chen B, Zhang H, Zhang D, Wang H. Superantigens and the expression of T-cell receptor repertoire in chronic rhinosinusitis with nasal polyps. Acta Otolaryngol. 2008;128(8):901–908. doi: 10.1080/00016480701760122. [DOI] [PubMed] [Google Scholar]
- 9.Zuckerkandl E (1882) Normale und Pathologische anatomie der Nasenhohle und Ihrer Pneumatischen Anhange. Wien, W. Braumuller
- 10.Stammberger H. Functional endoscopic sinus surgery. The Messerklinger technique. Toronto: BC Decker; 1991. [DOI] [PubMed] [Google Scholar]
- 11.Lurie H. Cystic fibrosis of pancreas and nasal mucosa. Ann Otol Rhinol Laryngol. 1959;68:478–482. doi: 10.1177/000348945906800215. [DOI] [PubMed] [Google Scholar]
- 12.Larsen K. The clinical relationship of nasal polyps and asthma. Allergy asthma Proc. 1996;17:243–249. doi: 10.2500/108854196778662255. [DOI] [PubMed] [Google Scholar]
- 13.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps group. EP3OS 2007: European position paper on rhinosinusitis and nasal polyps 2007. A summary for otorhinolaryngologists. Rhinology. 2007;45(2):97–101. [PubMed] [Google Scholar]
- 14.Bhattacharyya N. Influence of polyps on outcomes after endoscopic sinus surgery. Laryngoscope. 2007;117(10):1834–1838. doi: 10.1097/MLG.0b013e3180caa19d. [DOI] [PubMed] [Google Scholar]
- 15.Ling FT, Kountakis SE. Important clinical symptoms in patients undergoing functional endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2007;117(6):1090–1093. doi: 10.1097/MLG.0b013e31804b1a90. [DOI] [PubMed] [Google Scholar]
- 16.Banerji A, Piccirillo JF, Thawley SE, Levitt RG, Schechtman KB, Kramper MA, Hamilos DL. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol. 2007;21:19–26. doi: 10.2500/ajr.2007.21.2979. [DOI] [PubMed] [Google Scholar]
- 17.Dunlop G, Scadding GK, Lund VJ. The effect of endoscopic sinus surgery on asthma: management of patients with chronic rhinosinusitis, nasal polyposis, and asthma. Am J Rhinol. 1999;13(4):261–265. doi: 10.2500/105065899782102809. [DOI] [PubMed] [Google Scholar]
- 18.Turecka L, Scierski W, Namysłowski G, Misiołek M, Lisowska G, Czecior E, Polok A, Orecka B, Misiołek H. Surgical treatment of patients suffering from allergic chronic sinusitis with nasal polyps. Otolaryngol Pol. 2007;61(5):811–813. doi: 10.1016/S0030-6657(07)70532-2. [DOI] [PubMed] [Google Scholar]
- 19.Deal RT, Kountakis SE. Significance of nasal polyps in chronic rhinosinusitis: symptoms and surgical outcomes. Laryngoscope. 2004;114:1932–1935. doi: 10.1097/01.mlg.0000147922.12228.1f. [DOI] [PubMed] [Google Scholar]
- 20.Toros SZ, Bölükbasi S, Naiboğlu B, Er B, Akkaynak C, Noshari H, Egeli E. Comparative outcomes of endoscopic sinus surgery in patients with chronic sinusitis and nasal polyps. Eur Arch Otorhinolaryngol. 2007;264(9):1003–1008. doi: 10.1007/s00405-007-0301-5. [DOI] [PubMed] [Google Scholar]
- 21.Smith TL, Mendolia-Loffredo S, Loehrl T, et al. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2005;115:2199–2205. doi: 10.1097/01.mlg.0000182825.82910.80. [DOI] [PubMed] [Google Scholar]
- 22.Poetker DM, Mendolia-Lofredo S, Smith TL. Outcomes of endoscopic sinus surgery for chronic rhinosinusitis associated with sinonasal poliposis. Am J Rhinol. 2007;21:84–88. doi: 10.2500/ajr.2007.21.2978. [DOI] [PubMed] [Google Scholar]
- 23.Bugten V, Nordgård S, Romundstad P, Steinsvåg S. Chronic rhinosinusitis and nasal polyposis; indicia of heterogeneity. Rhinology. 2008;46(1):40–44. [PubMed] [Google Scholar]
- 24.Dursun E, Korkmaz H, Eryilmaz A, Bayiz U, Sertkaya D, Samim E. Clinical predictors of long-term success after endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2003;129:526–531. doi: 10.1016/S0194-5998(03)01576-6. [DOI] [PubMed] [Google Scholar]
- 25.Bonfils P, Nores JM, Halimi P, Avan P. Corticosteroid treatment in nasal polyps with a three year follow up period. Laryngoscope. 2003;113:683–687. doi: 10.1097/00005537-200304000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Nores JM, Avan P, Bonfils P. Medical management of nasal polyps: a study in a series of 152 patients. Rhinology. 2003;41:97–102. [PubMed] [Google Scholar]
- 27.Mehrzad H, Irvine M, Kundu S, Bleach N. A 5-year audit of rhinology procedures carried out in a district general hospital. Ann R Coll Surg Engl. 2007;89(8):804–807. doi: 10.1308/003588407X209275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharp HR, Crutchfield L, Rowe-Jones JM, Mitchel D. Major complications and consent prior to endoscopic sinus surgery. Clin Otolaryngol. 2001;26:33–38. doi: 10.1046/j.1365-2273.2001.00394.x. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins C, Browne JP, Slack R, Lund VJ, Topham J, Reeves BC, Copley LP, Brown P. Complications of surgery for nasal polyposis and chronic rhinosinusitis: the results of national audit in England and Wales. Laryngoscope. 2006;116:1494–1499. doi: 10.1097/01.mlg.0000230399.24306.50. [DOI] [PubMed] [Google Scholar]