Abstract
The aim of this study was to investigate and compare histopathological and computerized tomographic (CT) findings of experimental acute sinusitis in an animal model. The noses of five healthy rabbits were inoculated with a gelatin sponge impregnated with a solution containing Staphylococcus aureus, and one healthy rabbit acted as the control. The animals were sacrificed on the tenth day, following the acquisition of paranasal CT scans. Specimens were obtained from the lateral nasal walls, and the ethmoid and maxillary sinuses of the animals for histopathological examination. Histopathological and CT findings were compared. Various degrees of epithelial disorganization, foci of ruptured epithelial cells, and inflammatory cell infiltration in the lamina propria were seen in the histopathological examinations of the five study rabbits, and mucosal thickening and soft tissue density were noted in their CTs. There was no correlation between the histopathological and CT findings. It was shown that CT did not reflect the acute changes in the sinus mucosa. Patients with chronic sinusitis must be evaluated for a chronic process. Computerized tomographic scans should not be obtained in acute sinusitis cases. In this way, both unnecessary radiation exposure and economic waste can be avoided.
Keywords: Experimental, Rabbits, Sinusitis, Tomography, Histology
Introduction
Experimental sinusitis is frequently induced in rabbits in experimental sinusitis studies. Various methods have been used to create sinusitis in animals. Many recent studies have used the rhinogenic method, in which tampons soaked in bacterial solutions are placed into the nasal cavity [1].
Computerized tomography (CT) has been rarely employed in experimental sinusitis studies. Kerschner et al. [2] compared CT and magnetic resonance imaging (MRI) for the imaging of normal paranasal sinuses and sinusitis in rabbits. To the best of our knowledge the literature does not contain any studies that have investigated the correlation between histopathological and radiological findings in experimental rabbit sinusitis.
Materials and Method
The study included six healthy adult albino rabbits; five in the study group and one as the control. The rabbits weighed between 1000 and 1600 g and were between 6 and 8 months old. The Hacettepe University Ethics Committee approved the study protocol. The animals were fed and followed-up for adaptation 2 weeks before the onset of the study.
All five rabbits in the study group were administered intramuscular ketamine (50 mg/kg) and xylazine (5 mg/kg) anesthesia. Gelatin sponges were soaked in 0.05 ml of a solution containing 2 × 107 CFU Staphylococcus aureus per ml and were then inserted into both nasal cavities of the rabbits. The animals were fed and monitored for systemic signs and symptoms for 10 days. Then, a paranasal sinus CT scan was obtained following anesthesia as described above. The animals were then sacrificed with a lethal dose of thiopental sodium.
The rabbits were decapitated. The mucosa of the lateral nasal walls, maxillary sinuses, and ethmoid were removed from both sides. The specimens were fixed in phosphate-buffered 2.5% glutaraldehyde. They were then placed into paraffin blocks. They were stained with hematoxylin and eosin (H & E) and examined under a light microscope.
Inflammatory CT findings were scored as follows: 1: mild; 2: moderate; 3: severe; 4: very severe. Absence of any inflammation was scored as 0. Radiological evaluation was performed by an experienced radiologist blinded to the histopathological findings.
Histopathological findings, including epithelial disorganization, foci of ruptured epithelial cells, and inflammatory cell infiltration in the lamina propria were scored as follows: 1: mild; 2: moderate; 3: severe; 4: very severe. Absence of any inflammation was scored as 0.
The correlation between CT and histopathological findings was statistically investigated using Spearman’s rho test and SPSS v.13.0.
Results
Anatomy
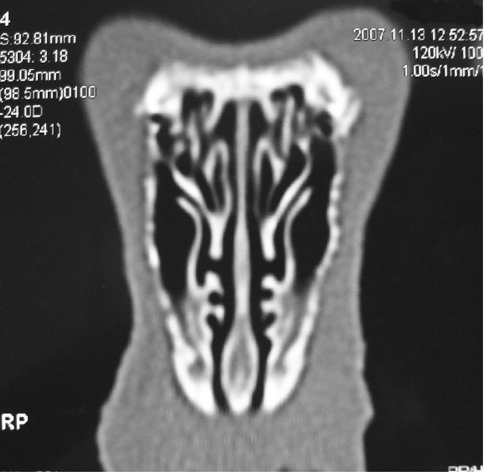
Similarities exist between the rabbit and human nasal and paranasal anatomy. There are three turbinate-like structures in the lateral nasal wall of rabbits. The first turbinate-like structure, which contains indentations and processes, is referred to as the maxilloturbinal and is suggested to have a role in heating and humidifying inspired air. The second turbinate-like structure is the nasoturbinal. The third turbinate-like structure, the endoturbinal, is located posterior and superior to the nasoturbinal, and medial to the maxillary sinus ostium (Fig. 1). Maxillary sinuses are large and suitable for experimental studies. They have interconnected anterior and posterior sections. The maxillary sinus opens laterally to the endoturbinal through an oval ostium. Ethmoid sinuses in rabbits are less developed when compared to humans and are located medial to the endoturbinal [3, 4].
Fig. 1.
Normal rabbit paranasal sinuses: The maxillary sinus ostium is seen medial to the endoturbinal
Radiological Findings
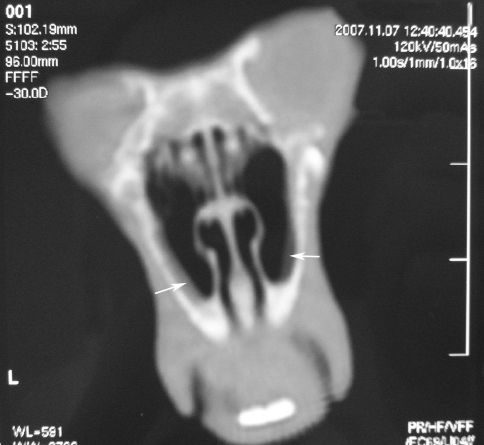
The anatomical structures of the rabbits were similar in CT scans. The control rabbit did not have any mucosal thickening or soft tissue density in its paranasal sinus CT. The maxilloturbinal was seen as a large structure with many indentations. The dimensions of the two-compartment maxillary sinus were similar in all the rabbits. The ethmoid sinuses were small sinuses located posteriorly. Various degrees of mucosal thickening were observed (Fig. 2). Inflammatory findings were scored between 0 and 4 on both sides. The results are presented in Table 1.
Fig. 2.
Mucosal thickening (white arrow) in both maxillary sinuses
Table 1.
Radiological sinusitis scores of the rabbits
| Radiological score | |
|---|---|
| Rabbit 1, right | 1 |
| Rabbit 1, left | 2 |
| Rabbit 2, right | 2 |
| Rabbit 2, left | 1 |
| Rabbit 3, right | 0 |
| Rabbit 3, left | 2 |
| Rabbit 4, right | 1 |
| Rabbit 4, left | 0 |
| Rabbit 5, right | 1 |
| Rabbit 5, left | 1 |
| Control rabbit, right | 0 |
| Control rabbit, left | 0 |
Histopathological Findings
The control rabbit had a pseudostratified prismatic ciliated epithelium and a continuous basal lamina. There were serous and mucous glands, and fibroblasts in the subepithelial lamina propria. In the mucosa of the lateral nasal wall, maxillary sinus, and ethmoid sinus, the five rabbits in the study group had various degrees of epithelial disorganization, foci of ruptured epithelial cells, increased lymphocytes throughout the epithelium, and polymorphonuclear cell and lymphocyte infiltration in the lamina propria (Figs. 3, 4). The findings were graded between 0 and 4 according to the severity of the inflammation. The results are shown in Table 2.
Fig. 3.
Epithelial disorganization and vacuolization in the epithelium (black arrow), and infiltration of inflammatory cells in the lamina propria (white arrow)
Fig. 4.
Epithelial rupture (black arrow) and infiltration of inflammatory cells in the lamina propria
Table 2.
Histopathological sinusitis scores of the rabbits
| Histopathological score | |
|---|---|
| Rabbit 1, right | 1 |
| Rabbit 1, left | 1 |
| Rabbit 2, right | 2 |
| Rabbit 2, left | 1 |
| Rabbit 3, right | 2 |
| Rabbit 3, left | 4 |
| Rabbit 4, right | 4 |
| Rabbit 4, left | 2 |
| Rabbit 5, right | 3 |
| Rabbit 5, left | 2 |
| Control rabbit, right | 0 |
| Control rabbit, left | 0 |
Comparison of Radiological and Histopathological Findings
The radiological and histopathological findings were statistically analyzed. Spearman’s rho test did not reveal a statistically significant correlation between radiological and histopathological findings (P = 0.940, correlation coefficient = 0.028).
Discussion
Rabbits have been increasingly used in experimental sinusitis studies since the 1940s because of their large maxillary sinuses and the similarities between human and rabbit paranasal sinuses [5].
Many routes have been used to induce sinusitis in animals. The anterior wall of the maxillary sinus is surgically opened, the maxillary sinus ostium is blocked, and then bacteria are injected into the sinus via the sinogenic route; this route was the preferred route in the first experimental sinusitis studies [1]. The rhinogenic route, however, has become the most frequently used method in recent studies. In this method different tampons are placed into the nasal cavity to induce experimental sinusitis and surgical trauma is avoided. The rhinogenic route is considered the most physiological way to induce sinusitis. Nasal tampons are usually soaked in a solution containing bacteria. Some authors suggest that adding bacteria to the nasal tampons just accelerates the development of sinusitis [5, 6].
We used the rhinogenic route to induce sinusitis in the present study. Gelatin sponges were impregnated with a solution containing bacteria and then were placed in the nose near the region of the endoturbinal.
Streptococcus pneumoniae, Staphylococcus aureus, and Bacteroides fragilis are the most frequently used bacteria to induce sinusitis [7]. In the present study Staphylococcus aureus was used to induce sinusitis.
The literature contains many experimental sinusitis studies that have investigated the histopathological features of sinusitis and the development of nasal polyps, as well as adhesion molecules, free oxygen radicals, inflammatory mediators, and blood flow to the sinuses [7–9]. Yet, imaging modalities were rarely employed in those studies. The anatomical features of normal rabbit paranasal sinuses and their similarities to those of humans were investigated, and the maxillary sinuses were reported to be suitable for experiments because they are large [3, 4]. One study compared CT and MRI for imaging sinusitis in rabbits and concluded that CT was more useful [2].
The present study investigated the correlation between the CT and histopathological findings in experimental rabbit sinusitis.
Patients with acute sinusitis constitute a significant percentage of patients that present to otolaryngology outpatient clinics. In the US, 31 million cases of acute sinusitis are reported each year [10]. Previously, conventional sinus X-rays were frequently used for the diagnosis of sinusitis; however, their use has recently declined. The use of paranasal CT has increased simultaneously with the increased use of endoscopic sinus surgery [11]. Computerized tomography is a very useful tool for the diagnosis of chronic sinusitis, as well as for determining the necessity for surgery. Computerized tomography also serves as a guide during sinus surgery. Computerized tomography may be used in acute sinusitis.
In the present study histopathological and CT findings in experimental rabbit sinusitis were not correlated. We think that CT did not effectively image the acute changes in the sinus mucosa.
In conclusion, CT should not be performed in acute sinusitis cases. Medical history must be carefully taken and nasal endoscopy must be performed. Medical therapy must be administered in adequate doses and duration. Computerized tomography scans should be obtained in nasal polyposis cases and when chronic sinusitis is suspected. Obtaining CT scans in acute sinusitis cases may result in unnecessary radiation exposure and economic waste.
References
- 1.Kara CO, Demirkan N. A review on experimental sinusitis models in rabbits. Kulak Burun Bogaz Ihtis Derg. 2003;10(3):122–130. [PubMed] [Google Scholar]
- 2.Kerschner JE, Cruz MJ, Beste DJ, Danahue KM, Kehl KS. Computed tomography vs. magnetic resonance imaging of acute bacterial sinusitis: a rabbit model. Am J Otolaryngol. 2000;21(5):298–305. doi: 10.1053/ajot.2000.9874. [DOI] [PubMed] [Google Scholar]
- 3.Koybasıoglu A, Ileri F, Beder L, Inal E. Anatomy of the rabbit maxillary sinus. KBB ve Bas Boyun Cerrahisi Dergisi. 1997;5:41–44. [Google Scholar]
- 4.Ozuer MZ, Bayramoglu I, Kara CO, Akkemik B, Callı N. Anatomicalş and radiological observation on rabbit paranasal sinuses. Pamukkale Universitesi Tip Fakultesi Dergisi. 1998;4:13–16. [Google Scholar]
- 5.Marks SC. Acute sinusitis in the rabbit: a new rhinogenic model. Laryngoscope. 1997;107(12):1579–1585. doi: 10.1097/00005537-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Marks SC. Acute sinusitis in the rabbit model: histologic analysis. Laryngoscope. 1998;108(3):320–325. doi: 10.1097/00005537-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Norlander T, Fukami M, Westrin PS, Carlsoo B. Formation of mucosal polyps in the nasal and maxillary cavities by infection. Otolaryngol Head Neck Surg. 1993;109:522–529. doi: 10.1177/019459989310900322. [DOI] [PubMed] [Google Scholar]
- 8.Kumlien JA, Schiratzki H, Drettner B. Blood flow in the rabbit maxillary sinus mucosa. Acta Otolaryngol. 1985;99:144–153. doi: 10.3109/00016488509119157. [DOI] [PubMed] [Google Scholar]
- 9.Doner F, Delibas N, Dogru H, Sari I, Yorgancigil B. Malondialdehyde levels and superoxide dismutase activity in experimental maxillary sinusitis. Auris Nasus Larynx. 1999;26:287–291. doi: 10.1016/S0385-8146(98)00078-9. [DOI] [PubMed] [Google Scholar]
- 10.Thaler ER. Management of acute rhinosinusitis. In: Kennedy DW, Bolger WE, Zinreich SJ, editors. Disease of the sinuses. 1. London: BC Decker Inc; 2001. pp. 149–155. [Google Scholar]
- 11.Yousem DM. Imaging in sinus disease. In: Kennedy DW, Bolger WE, Zinreich SJ, editors. Disease of the sinuses. 1. London: BC Decker Inc; 2001. pp. 129–149. [Google Scholar]