Abstract
A number of physiological changes occur during pregnancy and amongst them, audiological and nasal changes are quite significant. These are mainly due to the changing levels of sex hormones and return to normal once the pregnancy is over. This study was conducted to document these changes. Forty (pregnant 40 and non-pregnant 40) consenting subjects in age group of 20–35 years were assigned to test and control groups. They underwent complete ENT and Obstetric examination. In test group Pure Tone Audiometry was performed in all trimesters of pregnancy and within 3 months of delivery. The subjects in the control group underwent pure tone audiometry only once. The nasal patency was measured by Gertner’s plate method. Results from each trimester and postpartum period were compared. A highly significant difference in pure tone thresholds was observed at frequencies ranging from 125 to 1000 Hz (P < 0.001). However frequencies higher than 1000 Hz demonstrated no significant correlation. Nasal patency as measured by mean area of vapour condensation in all trimesters and control groups was highly significant (P < 0.001). The results of this study confirm that these changes occur in the first trimester and gradually improve during the subsequent trimesters returning to normal in post partum period. However number of pregnancies bear no relationship with these changes
Keywords: Pregnancy , Hearing loss, Nasal symptoms, Pure tone audiometry, Longitudinal study
Introduction
The hormonal system of women is unique because of the cyclical changes observed during pregnancy, menstrual cycle and menopause. During these periods there are physiological changes in the body due to changing levels of estrogen and progesterone hormones. Sex hormones exert regulatory influences on central nervous system [1]. The production rates for these hormones in non-pregnant women (oestrogen 0.02–0.1 mg/24 h and progesterone 0.1–40 mg/24 h, respectively) show considerable increase in near term pregnant women (oestrogen 50–100 mg/24 h and progesterone 250–600 mg/24 h). While creating optimal conditions for pregnancy, these hormonal changes cause an increase of 6.5 l in extra-cellular and 1.25 l intra-cellular fluids. As a result of these osmotic changes in body, water and sodium retention takes place. As it is evident from previous studies that circulating sex hormones affect the Sensorineural hearing system, one may expect changes in hearing levels with so much fluid retention during pregnancy [2]. Uchide reported a case of Meniere’s disease in which symptoms worsened during pregnancy. The patient experienced ten times more attack during early pregnancy. He correlated the increase in vertigo attacks with the decline in serum osmolality during pregnancy. Therefore, changes in fluid osmolality may affect inner ear during pregnancy [3].
The pregnancy is associated with a low frequency sensorineural hearing loss and tolerance problem mimicking cochlear pathology. There is a decrease in hearing level for 125, 250 and 500 Hz, beginning in the first trimester and increasing over second and third trimesters. For frequencies of 1000 Hz and above, there occurs no significant difference in between the trimesters and the postpartum period. The speech audiometric findings are normal during pregnancy. However, this low frequency hearing loss never reaches the pathologic levels and returns to normal in the postpartum period [4].
Nasal obstruction during pregnancy or rhinitis during pregnancy has been accepted as distinct and very common pathological entity. It occur in 5–32% of pregnant women and most commonly noted during the end of first trimester, and may persist up to the time of delivery or a few weeks afterward, associated with clear rhinorrhea and edematous nasal mucosa [5].
Materials and Methods
The study was carried out in the ENT and Obstetrics & Gynecology Department of Medical College Amritsar. Eighty healthy individuals were evaluated. The pregnant group consisted of 40 females diagnosed and followed in the department of Obstetrics and Gynecology. Their age ranged between 20 and 29 years (mean 24.5 years). The control group consisted of 40 non-pregnant/non-lactating females, whose age ranged between 21 and 32 years (mean 25.1 years) and who were not using any oral contraceptives. The subjects were selected according to following criteria: (1) No systemic disease; (2) Normal ENT examination; (3) No ear/nasal complaint before pregnancy; (4) No use of medication during pregnancy; (5) No toxaemia during pregnancy; (6) Non diabetic and non hypertensive After complete ENT examination, all subject underwent pure tone audiometry and Nasal obstruction was assessed by using plate method of Gertner’s. The pregnant subjects were investigated periodically in each of the four stages according to the classification.
Group I, 1–14 weeks (First trimester);
Group II, 15–28 weeks (Second trimester);
Group III, 29–42 weeks (Third trimester);
Group IV, post partum period up to 3 months after delivery;
The Control group subjects were categorized as Group V.
Clinical Protocol
Audiometric Assessment
Hearing acuity was assessed by pure tone audiometry and hearing threshold were measured in all subjects between 125 and 8000 Hz frequencies for air conduction and between 250 and 4000 Hz frequencies for bone conduction. Thresholds were measured using Elkon Model EPA3N3plus Audiometer, in all subjects. Tuning fork tests (Rinne’s, Weber and Absolute Bone Conduction) were performed using 128, 256, 512 and 1024 Hz tuning fork. Speech testing was performed using spondee words for speech reception threshold and phonetically balanced mono syllable words for speech discrimination. Short Increment Sensitivity Index (SISI) and Tone Decay using Pure Tone Audiometry was performed in subjects who develop sensorineural deafness during the course of study. Uncomfortable loudness levels were also evaluated. All tests were conducted with subjects comfortably seated in an IAC sound isolated room where the background sound level was below the accepted level (<35 dB SPL for 125 Hz).
Nasal Assessment
The nose was examined for any changes in the nasal mucosa, nasal patency. Nasal patency was measured by Gertner’s plate method. First, the test of homogeneity of variance was applied to all data and comparisons of baseline measurements between groups were performed using one-way analysis of variance (ANOVA). Paired t and unpaired t was used for comparison between the groups.
Results
The pure tone thresholds are shown in Table 1. As there is no air bone gap, only air conduction threshold were given. There is a gradual decrease in hearing acuity at low frequencies (125, 250, 500 and 1000 Hz) from first trimester to third trimester of pregnancy. The hearing threshold get stabilized in the third trimester and return to normal in post partum period. Furthermore it was observed that there was a highly statistically significant difference of threshold during pregnancy (Group I, II and III) from the post partum (Group IV) indicating that these metabolic/hormonal changes which result in increased threshold are reversible. During this double blind study it has been observed that the threshold for low frequencies revealed a statistically significant difference in control group (V) in relation to pregnant subjects (P > 0.05).
Table 1.
Mean pure tone threshold (dBHL) in each trimester and control group (air conduction)
| Frequency (Hz) | Group I | Group II | Group III | Group IV | Group V | F value | P value |
|---|---|---|---|---|---|---|---|
| 125 | 28.25 ± 2.16 | 28.63 ± 5.40 | 26.63 ± 3.99 | 16.63 ± 5.14 | 9.63 ± 2.60 | 86.09 | <0.001 |
| 250 | 25.88 ± 2.95 | 26.75 ± 4.94 | 24.25 ± 3.89 | 16.13 ± 4.40 | 12.13 ± 2.60 | 57.02 | <0.001 |
| 500 | 24.38 ± 4.57 | 25.38 ± 3.65 | 23.38 ± 3.56 | 15.25 ± 4.79 | 11.00 ± 3.58 | 49.23 | <0.001 |
| 1000 | 20.13 ± 3.92 | 19.37 ± 3.12 | 16.62 ± 4.31 | 11.88 ± 4.35 | 9.88 ± 3.92 | 26.32 | <0.001 |
| 2000 | 16.38 ± 5.03 | 15.75 ± 6.28 | 14.38 ± 6.58 | 11.00 ± 5.98 | 8.75 ± 2.36 | 5.43 | <0.051 |
| 4000 | 12.75 ± 7.94 | 11.13 ± 5.64 | 10.38 ± 7.40 | 7.00 ± 6.71 | 5.63 ± 1.37 | 4.46 | 0.052 |
| 8000 | 9.00 ± 10.04 | 9.88 ± 13.21 | 10.75 ± 13.52 | 7.25 ± 10.66 | 4.50 ± 2.08 | 1.06 | 0.379 |
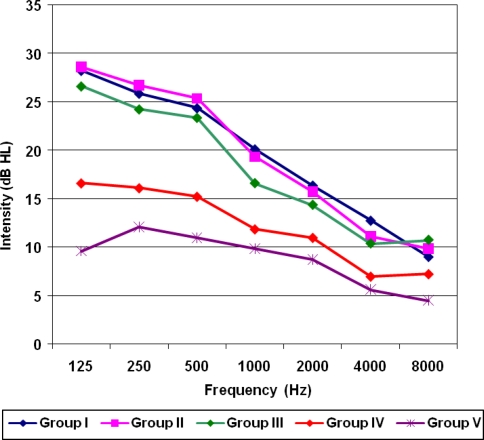
For 125 to 1000 Hz, post partum (group IV) and control group (group V) showed a highly statically significant difference from first, second and third trimesters (group I, II and III respectively) (P < 0.05). For 2000 Hz and higher frequencies, there were no significant difference between the trimesters, post partum and control groups (P > 0.05) (Fig. 1).
Fig. 1.
Showing mean pure tone thresholds for pregnant and non pregnant subjects
During study two subjects revealed bilateral mild to moderate sensorineural hearing loss which was mild in low and moderate in higher frequencies. However there was no significant changes in threshold during pregnancy (Group I, II & III) as well as during postpartum period.
Nasal patency (one of the feature of pregnancy rhinitis) is affected in the first trimester but it improves during the subsequent trimester and post partum period. A highly statistically significant difference is seen in mean area of vapour condensation (cm2) in all trimesters and control groups (P < 0.05). Out of 40 pregnant subjects 14 women when questioned about any nasal problem complaint of nasal obstruction which was associated with clear rhinorrhea and physical examination showed edematous nasal mucosa. These subjects were made aware of these physiological changes commonly seen during pregnancy and however were prescribed isotonic nasal spray 0.9% w/v of sodium chloride in purified water only. Four subjects were prescribed topical nasal decongestant along with systemic oral antihistaminic for 5 days only. Only two subjects complained of hearing loss along with nasal symptoms and 26 subjects were asymptomatic. As it is evidence from Fig. 2 there was a decrease in mean area of vapour condensation in all trimester (Group I, II and III). However mean area of vapour condensation improve significantly after delivery (Group IV) and was almost consistent with the control group (Group V).
Fig. 2.
Showing mean area of vapour condensation cm2 (nasal patency)
The audiological and nasal changes that occur during pregnancy are not affected by the number of pregnancies in women. Statistical values are not significant (P > 0.05) between gravida I and gravida II or more. Pregnancy does not affect the hearing acuity at high frequencies.
Discussion
Audiological Changes
One of the main outcome of this study is that while pregnancy does not affect the hearing acuity at high frequencies, there is a gradual decrease in hearing acuity at low frequencies (125, 250, 500 and 1000 Hz) from first trimester to third trimester (P < 0.05) and returned to normal in the post partum period. Although there is a statistically significant difference with respect to mean pure tone thresholds between the different groups, this cannot be regarded as a hearing loss according to American National Standard Institute (ANSI) [6]. The result also demonstrate that there is no change in the hearing sensitivity between 2000 and 8000 Hz across different groups (P > 0.05) (Fig. 1).
At frequencies 125 to 1000 Hz there is statistically significant difference amongst the first, second and third trimester groups (Group I, II and III respectively) from group IV and group V (P < 0.05). For frequencies 125, 250 and 500 Hz the difference between group V and group IV is significant (P < 0.05), but at higher frequencies (2000 to 8000 Hz) no significant difference is measurable (P > 0.05). Another key finding of this study is that there is no significant difference amongst primigravida and multi gravida in all trimesters (P > 0.05).
Our study compares well with Gonca and Erol [4] who also reported a gradual decrease in pure tone averages at 125, 250, 500 Hz from first trimester to third trimester. This decrease returned to normal in the post-partum period. The result of this study also demonstrated that there is no change in the hearing sensitivity between 1000 and 8000 Hz. Thus they opined that pregnancy does not effect the high frequency hearing level (P > 0.05). The study suggested that the low tone decrease in acuity level may be due to excessive water and salt retention during pregnancy which increases as pregnancy progress and water retention at term is 6.5 l where as plasma volume is also increased to 1.25 l Although hearing loss pattern resembles that of Meniere’s disease, yet none of the pregnant women however had any of the characteristics of Meniere’s disease. The findings are always within physiological limits.
In this study two subject developed sensorineural hearing loss which affected the higher frequencies. This hearing loss persisted not only in the first trimester itself but also in second, third and post partum groups. A study conducted by Lavy [7] also reported sudden onset of sensorineural deafness in two cases associated with pregnancy. Kandays and Oleszczok [8] also reported a case of sudden sensorineural hearing loss in 23-years-old healthy nulliparous women during an uncomplicated pregnancy.
Tsunoda et al. [9] investigated ear problems in a group of pregnant women and found that pure tone audiometry and impedance audiometry showed normal hearing in all cases. However, they did not mention the finding in different frequencies and also did not take into account the different stages of pregnancy. Therefore it is difficult to compare our results with their study.
Although in present study subject with prior history suggestive of meniere’s disease were not included, none of the subject recruited in this study presented with meniere’s like symptoms during course of pregnancy or in the post-partam period. However Uchide et al. [3] reported a case of meniere’s disease in which symptoms worsened during pregnancy and correlated the increase in vertigo attack with the decline in serum osmolality during pregnancy.
Nasal Changes
Pregnancy rhinitis can really impact upon the quality of life and is actually one of the most common discomforts associated with morning sickness, sneezing and running nose. As a result nasal mucosa becomes inflamed, irritated and congested. It can occur at anytime throughout pregnancy, commonly occurring in the first trimester and disappearing completely within 2 weeks after delivery [5].
In this study nasal patency measured by Gertner’s [10] plate method shows highly significant difference in group I, group II and group III (P < 0.05) and in between groups (Group I and group V, Group II and Group V, Group III and group V, Group I and Group II, Group II and Group III, Group III and IV (P < 0.05). But there is no significant difference in group V and group IV (P > 0.05).
Our study showed 14 (35%) subject with decreased nasal flow which is minimum in group I subjects (mean nasal patency 12.92 cm2) but in second (MNP 15.73 cm2) and third trimester (MNP 19.41 cm2) some improvement occurs. No significant difference is seen between primigravida and multigravida in all trimester (P > 0.05).
Our study also compares with Ellegard et al. [11] who describe physiological variations of nasal obstruction during pregnancy. Subjective scores and nasal as well as oral peak expiratory flow values were recorded daily in 23 pregnancies until 1 month after delivery. Scores were high during early and late pregnancy than in the month after delivery. Objectively registered blockage increased during pregnancy in eight women only. Unexpectedly nine women showed declining blockage. Incaudo [12] also showed that 18–30% of patients reported substantial symptoms of rhinitis and sinusitis. Our study also shows that 35% subjects presented with rhinitis.
Philpott et al. [13] also studied the nasal physiological changes during pregnancy. This study examined all the variables of the nasal airway simultaneously for the first time. Measurements of the nasal airway included anterior rhinoscopy, peak inspiratory nasal flow, acoustic rhinometry, anterior rhinomanometry and the saccharin test with rhinitis questionnaire scores providing a symptomatic measurement. All the tests showed a trend consistent with decreasing nasal patency when expressed as an average for the group as a whole, although only anterior rhinoscopy, anterior rhinomanometry, mucociliary clearance time and rhinitis questionnaire scores were statistically significant (P < 0.05). Thus they concluded the effect of pregnancy on the nasal mucosa and coincide with the rise in the serum concentration of the female sex hormones with gestational age, returning to normal in postpartum.
Ellegard and Karisson [14] studied nasal mucociliary transport in pregnancy and concluded that pregnancy rhinitis affects at least 20% of pregnancies. The mucociliary transport speed was higher in the group of women with pregnancy rhinitis, and was reduced during pregnancy in the group of women without that condition. They found no significant correlation between mucociliary transport speed and objectively registered nasal peak expiratory flow index.
Key Message
To conclude we have come to inference that the pregnancy related changes that occur in ear, nose and throat have been a subject of investigation. A number of studies are available in literatures that address the subject from a number of angles. The audio-rhinological changes observed appear to be transient in nature and are most marked in first trimester of pregnancy. As the pregnancy progress these changes tend to disappear with complete resolution in post partum period in most cases, indicating that all subjective complaints of these symptoms during pregnancy are physiological and not pathological. The number of pregnancies a female undergoes does not appear to affect these symptoms. Thus a clear-cut understanding of these changes, both the otolaryngologist and obstetrician can team up to improve the quality of life of pregnant subject by avoiding use of drugs for their symptoms during pregnancy especially in first trimester.
References
- 1.Wharton JA, Church GT. Influence of menopause on the auditory brain stem response. Audiology. 1990;29:196–201. doi: 10.3109/00206099009072850. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald PC, Leveno KJ, Gant NF, Gilstrap LC. Williams’ obstetrics. New York: Appleton and Lange; 1993. [Google Scholar]
- 3.Uchide K, Suzuki N, Takiguchi T, Terada T, Inoue M. The possible effect of pregnancy on Meniere’s disease. ORL. 1997;59:292–295. doi: 10.1159/000276956. [DOI] [PubMed] [Google Scholar]
- 4.Gonca S, Erol B. Audiological findings in pregnancy. J Laryngol Otol. 2001;115:617–621. doi: 10.1258/0022215011908603. [DOI] [PubMed] [Google Scholar]
- 5.Mabry RL. Rhinitis of pregnancy. South Med J. 1986;79:965–971. doi: 10.1097/00007611-198608000-00012. [DOI] [PubMed] [Google Scholar]
- 6.American national standard specification for audiometers. New York: American National Standards Institute; 1969. [Google Scholar]
- 7.Lavy JA. Sudden onset deafness: two cases associated with pregnancy. Int J Clin Pract. 1998;52(2):129–130. [PubMed] [Google Scholar]
- 8.Kanadys WM, Oleszezuk J. Sudden sensorineural hearing loss during pregnancy. Ginekol Pol. 2005;76(3):225–227. [PubMed] [Google Scholar]
- 9.Tsunoda K, Takahshi S, Takanosawa M, Shimoji Y. The influence of pregnancy on sensation of ear problems. Ear problems associated with healthy pregnancy. J Laryngol Otol. 1999;113:318–320. doi: 10.1017/S0022215100143877. [DOI] [PubMed] [Google Scholar]
- 10.Gertner R, Podoshin L, Fradis M. A simple method of measuring the nasal airway in clinical work. J Laryngol Otol. 1984;98:351–355. doi: 10.1017/S0022215100146729. [DOI] [PubMed] [Google Scholar]
- 11.Ellegard E, et al. Nasal congestion during pregnancy. Clin Otolaryngol Allied Sci. 1999;24:307. doi: 10.1046/j.1365-2273.1999.00264.x. [DOI] [PubMed] [Google Scholar]
- 12.Incouda GA. Diagnosis and treatment of allergic rhinitis and sinusitis during pregnancy and location. Clin Rev Allergy Immunol. 2004;27(2):159–177. doi: 10.1385/CRIAI:27:2:159. [DOI] [PubMed] [Google Scholar]
- 13.Philipott, et al. Physiological nasal changes during pregnancy. Clinical Otolayngol. 2004;29:343–351. doi: 10.1111/j.1365-2273.2004.00815.x. [DOI] [PubMed] [Google Scholar]
- 14.Ellegard EK, Karisson NG. Nasal mucocillary transport in pregnancy. Am J Rhinol. 2000;14(6):375–378. doi: 10.2500/105065800779954356. [DOI] [PubMed] [Google Scholar]