Abstract
The aim of this study was to evaluate whether sampling of cerebrospinal fluid (CSF) via the cisterna magna and of blood via the heart affects brain water content in a rat subarachnoid hemorrhage (SAH) model. Twenty-nine animals were divided into four groups: sham-operated group with sampling of CSF and blood (Sham S+), sham-operated group without sampling of CSF and blood (Sham S−), SAH group with sampling of CSF and blood (SAH S+), and SAH without sampling of CSF and blood (SAH S−). SAH was induced via endovascular perforation of the left internal carotid artery bifurcation. Cerebrospinal fluid via the cisterna magna and blood via cardiac puncture was collected in the Sham S+ and SAH S+ groups before killing the animals for brain water content measurements. Left hemisphere brain water content was significantly higher in the SAH S− group compared with the Sham S− group (p< 0.05) and in Sham S+ group compared with the Sham S− group (p<0.05). There was no significant difference in brain water content of the left hemisphere between the SAH S+ and Sham S+ groups (p=NS). There was no significant difference in brain water content in other parts of brains. Sampling of CSF and blood affected brain water content in Sham animals and therefore it is not accurate to use these values from Sham animals for comparison with SAH animals.
Keywords: SAH, Rat, Brain water content, Blood, CSF
Introduction
Subarachnoid hemorrhage (SAH) is a devastating event with an incidence of seven per 100,000 each year [1, 2]. Nearly 50% of patients die within 1 month and approximately 60% of surviving patients have a relevant neurological deficit [1, 2]. SAH research often involves brain edema monitoring as well as cerebrospinal fluid (CSF) and blood collection [3–5]. In experimental animal SAH models, brain water content and CSF/blood sampling are often conducted in the same animal to reduce cost [6–8]. Similarly, brain water content and CSF/blood sampling are conducted in the same animal in experimental intracerebral hemorrhage [9] and brain ischemia [10] animal models.
However, we are not aware of evidence suggesting whether sampling of CSF and blood affects brain water content in these animal models. The aim of this study was to evaluate whether sampling of CSF via the cisterna magna and blood via the heart affects the brain water content in a rat SAH model.
Materials and Methods
General Information and Experimental Design
All experiments were approved by the Institutional Animal Care and Use Committee of Loma Linda University. Adult male Sprague–Dawley rats were purchased from Harlan Laboratories (Indianapolis, Indiana). In total, 29 animals were operated in this study, 12 in Sham groups, 17 in SAH groups from which five animals died due to massive subarachnoid hemorrhage. The animals were divided into the following groups: sham-operated group with sampling of CSF and blood (Sham S+, n=6), sham-operated group without sampling of CSF and blood (Sham S−, n= 6), SAH group with sampling of CSF and blood (SAH S+, n=6), and SAH without sampling of CSF and blood (SAH S−, n=6).
Body weight was measured before surgery and before killing. SAH was induced via endovascular perforation of the left internal carotid artery bifurcation. Sham-operated animals underwent the introduction of the suture into internal carotid artery, but no perforation was performed. Neurological score was evaluated at 23 h after surgery. CSF via cisterna magna and blood via heart was collected in the Sham S+ and SAH S+ groups immediately before killing. Animals were killed 24 h after surgery, brains were collected, quickly photographed for SAH grading evaluation, and then the brains were processed for brain water content evaluation.
Surgery
The endovascular perforation model of SAH in rats was used for this study as previously described [11]. Briefly, rats were anesthetized with 3% isoflurane in 60/40% medical air/oxygen. The animals were intubated and kept on artificial ventilation with 3% isoflurane in 60/40% medical air/oxygen during surgery. Body temperature was monitored by rectal probe and normothermia was maintained by heating lamp during surgery. Left common carotid artery, external carotid artery, and internal carotid artery were isolated. The external carotid artery was ligated, coagulated, cut, and shaped into a 3-mm stump. A sharpened 4–0 monofilament nylon suture was introduced into the internal carotid artery from the external carotid artery stump until resistance was felt (approximately 18 mm from the common carotid bifurcation). The suture was then pushed further to perforate the bifurcation of the anterior and middle cerebral arteries until resistance was overcome. Sham-operated animals underwent the identical procedures, but the suture was not inserted over skull base. The incision was closed, and rats were individually housed in heated cages until recovery from anesthesia.
Neurological Evaluation
Neurological scores were evaluated 23 h after surgery in a blinded fashion with a modification of the scoring system described by Garcia [12]. An 18-point scoring system was used to evaluate the neurological deficits. In S+ groups, neurological testing was performed before collecting samples.
CSF and Blood Collection
In S+ groups, CSF was collected during terminal anesthesia and blood was collected immediately after completion of terminal anesthesia just before decapitation. CSF was collected as previously described [13]. The animals were terminally anesthetized with 5% isoflurane in 60/40% medical air/oxygen by face mask. The 27 G needle attached on tuberculin syringe was inserted into the cisterna magna trough the occipital membrane and 50 μl of CSF was collected. The syringe was guided through a holder to prevent damage of the brain. Terminal anesthesia was maintained for 5 min while CSF collection procedure was performed. Directly after CSF collection 3 ml of blood was sampled by cardiac puncture as previously described [14]. Animals were decapitated immediately after blood collection and the brains were collected. Only Sham S+ and SAH S+ animals were subjected to CSF and blood collection. Sham S− and SAH S− animals were anesthetized by 5% isoflurane for the same duration and then decapitated.
SAH Grade
After decapitation, brains were photographed and SAH grade was evaluated according to blood clot occurrence (maximum of 18 points) as previously described [15].
Brain Water Content Evaluation
Brain water content was evaluated as previously described [16]. Brains were removed 24 h after surgery and separated into four parts (left hemisphere, right hemisphere, cerebellum, and brain stem). Each part was weighed immediately after removal (wet weight) and after drying in 100°C for 72 h (dry weight). The percentage of water content was calculated as [(wet weight − dry weight)/wet weight]× 100%.
Statistical Analysis
Data are expressed as a mean and standard error of mean. Brain water content data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test. Mortality data was analyzed by Fischer exact test. Body weight data were analyzed using two-way ANOVA followed by Bonferroni test. SAH grading data were evaluated by two-tail t test. A p value of <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 5.02 for Windows.
Results
Mortality
The mortality rate in the SAH groups was as follows: 25% (two of eight animals) in the SAH (S+) group and 33% (three of nine animals) in the SAH (S−) group. There was no statistical difference in mortality between SAH groups (Fischer’s exact test, p=NS). None of the Sham animals died.
Body Weight Before Surgery and Before Killing
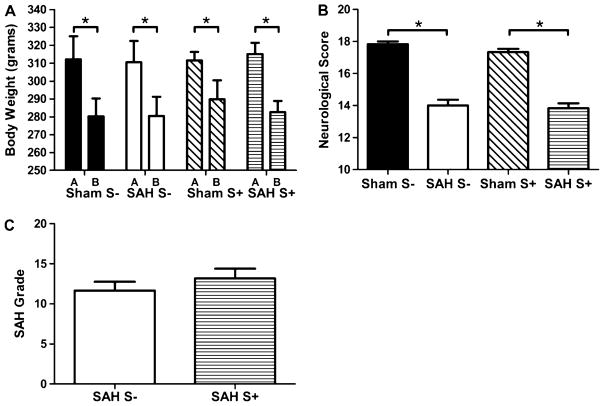
There was no significant difference in body weight between groups before surgery (p=NS), or 24 h after surgery (p=NS). Body weight was significantly decreased in all groups 24 h after surgery compared with body weight before surgery (p< 0.05). Body weight loss after surgery was between 7% and 10% of body weight before surgery (Fig. 1a, Table 1).
Fig. 1.
Body weight (a), neurological score (b), and SAH grade (c). *P<0.05. a There was a statistically significant body weight loss after surgery (b) compared with the body weight before surgery (a) in all groups (p<0.05). There was no significant difference between groups in body weight, before surgery, nor in the body weight loss after surgery (p=NS). b There was a significant neurological deficit in SAH animals compared with Sham animals (p<0.05). The differences between Sham and SAH animals were comparable between S+ and S− groups. c There was no significant difference in SAH grade between SAH S+ and SAH S− animals (p=NS)
Table 1.
Parameters and results of experiment
| Sham S− | SAH S− | Sham S+ | SAH S+ | |
|---|---|---|---|---|
| Numbers of operated animals | 6 | 9 | 6 | 8 |
| Survived/Dead animals (mortality %) | 6/0 (0%) | 6/3 (33%) | 6/0 (0%) | 6/2 (25%) |
| Average body weight (g) and SEM before surgery | 312 (5.25) | 311 (4.83) | 312 (1.98) | 315 (2.55) |
| Average body weight (g) and SEM after surgery | 280 (4.06) | 281 (4.39) | 290 (4.36) | 283 (2.55) |
| Average body weight loss (%) after surgery | 10.3 | 9.6 | 7.1 | 10.2 |
| Average neurological score and SEM after surgery | 17.83 (0.17) | 14.00 (0.37) | 17.33 (0.21) | 13.83 (0.31) |
| Average SAH grade and SEM | 0 | 11.67 (1.12) | 0 | 13.17 (1.22) |
| BWC in left hemisphere (%) and SEM | 78.88 (0.11) | 79.52 (0.13) | 79.49 (0.15) | 79.42 (0.17) |
| BWC in right hemisphere (%) and SEM | 78.93 (0.07) | 79.43 (0.25) | 79.34 (0.11) | 79.34 (0.17) |
| BWC in cerebellum (%) and SEM | 78.26 (0.11) | 78.95 (0.52) | 79.19 (0.19) | 78.95 (0.32) |
| BWC in brain stem (%) and SEM | 73.62 (0.17) | 74.33 (0.79) | 74.47 (0.26) | 73.83 (0.25) |
Sham S− sham-operated group without sampling of cerebrospinal fluid (CSF) and blood, SAH S− subarachnoid hemorrhage-operated group without sampling of CSF and blood, Sham S+ sham-operated group with sampling of CSF and blood, SAH S+ subarachnoid hemorrhage-operated group with sampling of CSF and blood, Mortality (number of dead animals/number of operated animals)×100 (%), SEM standard error of mean, BWC brain water content
Neurological Score
Neurological score was significantly lower in the SAH groups compared with the Sham groups (p<0.05). There was no significant difference between the SAH+ group and SAH S− group (p=NS). There was no significant difference between the Sham S+ group and Sham S− group (p=NS) (Fig. 1b, Table 1).
SAH Grade
There was no significant difference in SAH grade between the SAH S+ and SAH S− groups (p=NS) (Fig. 1c, Table 1).
Brain Water Content
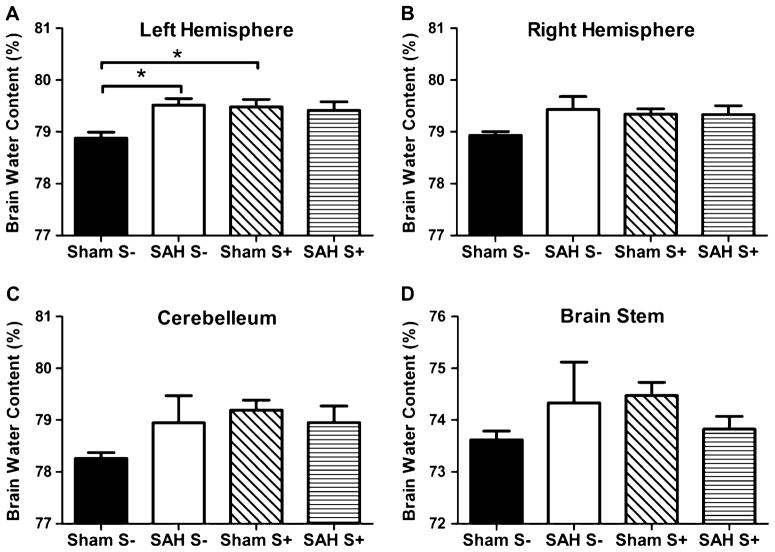
Brain water content of the left hemisphere was significantly higher in the SAH S− group compared with Sham S− group (p<0.05). There was no significant difference in brain water content of the left hemisphere between the SAH S+ and Sham S+ groups (p=NS). Brain water content was significantly higher in the Sham S+ group compared with Sham S− group (p<0.05) (Fig. 2, Table 1).
Fig. 2.
Brain water content in the left hemisphere (a), right hemisphere (b), cerebellum (c), and brain stem (d). *P<0.05. a In the left hemisphere, there was a significant difference in BWC between the Sham S− and SAH S− groups (p<0.05). There was a significant difference in BWC between the Sham S− and Sham S+ groups (p<0.05). There was no difference between Sham S+ and SAH S+ (p=NS). b In the right hemisphere, there was no statistically significant difference (p=NS); however, there was a similar tendency compared with the left hemisphere. c In the cerebellum, there was no statistically significant difference (p=NS); however, there was a similar tendency compared with the left hemisphere. d In the brain stem, there was no statistically significant difference (p=NS)
There was no significant difference between groups either in the right hemisphere, cerebellum nor brain stem (p=NS for all). The right hemisphere showed a similar tendency compared with the left hemisphere, however there was no statistical significance seen.
Discussion
The aim of this study was to evaluate whether collection of CSF the via cisterna magna and of blood via the heart could affect brain water content in a rat SAH model. We used CSF collection via the cistern magna and blood collection via the heart, because these techniques have been commonly used in rat experiments [17, 18].
All parameters except brain water content were comparable between the SAH S− and SAH S+ groups, same as between the Sham S− and Sham S+ animals. There was 0% mortality in the sham groups, 33% mortality in the SAH S− group and 25% mortality in the SAH S+ group. There is high variation in the mortality of rat SAH perforation model between different researchers from 18% [8] to 46% [19]. Body weight before surgery was similar between all groups, same as the body weight before killing. Body weight loss after surgery was between 7% and 10% of body weight before surgery, which is similar to that previously described [8]. No significant difference in body weight loss between the Sham and SAH animals implies that body weight decreases more by the surgical procedure than by SAH. The neurological deficits were comparable between the SAH Sand SAH S+ groups, same as the neurological score between the Sham S− and Sham S+ groups. We used a neurologic scoring system proposed by Garcia [12] and modified in our laboratory [11]. There was no significant difference in SAH grade between the SAH S− an SAH S+ groups. We used SAH scoring system described by Sugawara [15] which is well established in our laboratory.
Brain water content was significantly increased ipsilateraly in SAH S− group compared with Sham S− animals, while the increases of brain water content in the contralateral hemisphere, cerebellum and brain stem were not significant, which is in line with previously published data. In the literature there are references regarding an increase of brain water content in rat perforation model of SAH in the ipsilateral hemisphere only [6], both in ipsilateral and contralateral hemisphere [8] and some authors report an increase of brain water content in hemispheres, cerebellum and brain stem [7]. In S+ groups there was no significant difference in brain water content between Sham and SAH animals in the left hemisphere or in any other part of the brain. The brain water content of the left hemisphere in SAH S+ group was comparable with SAH S− group, while the brain water content in Sham S+ animals was significantly higher than in the Sham S− group. Thus brain water content was affected by simultaneous collection of CSF and blood selectively in Sham animals, which suggests that preexisting edema (SAH animals) was not affected by simultaneous collection of CSF and blood while this procedure caused a rise in brain water content in animals without preexisting edema (Sham animals).
The mechanism of changes in brain water content in the Sham animals after CSF and blood sampling is not clear. Animals were decapitated shortly after sampling (approximately 2 min after CSF collection and seconds after blood collection) and this time is likely not long enough for added brain edema to form. Therefore a possible mechanism could be increased cerebral blood volume (CBV), due to vascular engorgement. Both CSF and blood collection procedures can theoretically increase CBV. CSF sampling can cause dilation predominantly in the venous system [20], while blood collection can cause dilation in the arterial system [21].
CSF collection could affect CBV mainly via the venous system. Decreased CSF pressure without decrease in mean arterial pressure (MAP) leads to venodistension and accumulation of blood in the brain, because veins, with a thin muscle layer, cannot resist pressure differences across their walls [20]. In SAH animals, preformed brain edema could attenuate the venodistension. Decreased CSF pressure could be compensated more by brain tissue expansion than by blood compartment expansion. Additionally, the accumulation of blood in the brain can be reduced by arterial vasoconstriction. CSF collection could also result in decreased ICP, which would lead to an increased cerebral perfusion pressure (CPP). An increased CPP results in autoregulatory vasoconstriction of cerebral arteries reducing CPP back to normal [21]. Vasoconstrictor mechanisms are augmented after SAH [22] thus lowering blood volume in cerebral arteries in SAH animals compared with Sham animals, regardless of CSF collection. Therefore in summary, CSF collection could have a larger effect on brain water content in Sham animals, because of higher venodistention and a lower level of vasoconstriction, compared with SAH animals.
While there are complex mechanisms following CSF collection that can possibly affect brain water content, the effect of blood collection could be more straightforward. Hypovolemia caused by blood sampling, leads to decreased MAP and CPP which results in compensatory vasodilation of cerebral arteries to bring the CPP back up [21]. After SAH vasodilation is impaired [22], so vascular engorgement and resulting effects on brain water content could be more pronounced in Sham animals than in SAH.
Comparing Sham S− animals to Sham S+ animals, brain water content could be higher in Sham S+ because of vascular engorgement resulting from venodistension and vasodilation due to collection of CSF and blood. However, in SAH S+ animals the increase in CBV after sample collection can possibly be reduced by the presence of preformed brain edema, attenuating the degree of venodistension. Additionally, impairment in vasodilation associated with SAH can reduce CBV. Therefore theoretically the brain water content of SAH S+ may not be higher than in SAH S− animals.
Our study was focused on brain water content and simultaneous collection of CSF and blood. Direct comparison of CSF and blood parameters is important from a clinical point of view and the relationship of these parameters to brain edema could provide interesting information. We realize that from a pathophysiological point of view our study is limited and to further clarify which of these procedures mainly affected brain water content, additional experiments would be needed using separate groups collecting only CSF and blood.
Our results suggest that simultaneous collection CSF via the cisterna magna and of blood via the heart affected brain water content in Sham animals and for that reason it is not suitable to simultaneously collect the CSF and blood in Sham animals and then measure brain water content for comparison with SAH animals.
Acknowledgments
This study was partially supported by grant NS053407 from the National Institutes of Health to J.H.Z.
Contributor Information
Kamil Duris, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA, USA.
Anatol Manaenko, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA, USA.
Hidenori Suzuki, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA, USA.
William Rolland, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA, USA.
Jiping Tang, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA, USA.
John H. Zhang, Email: johnzhang3910@yahoo.com, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA, USA. Department of Neurosurgery, Loma Linda University School of Medicine, Loma Linda, CA 92354, USA
References
- 1.Van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306–18. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–36. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- 3.Lad SP, Hegen H, Gupta G, Deisenhammer F, Steinberg GK. Proteomic biomarker discovery in cerebrospinal fluid for cerebral vasospasm following subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2010 doi: 10.1016/j.jstrokecerebrovasdis.2010.04.004. (in press) [DOI] [PubMed] [Google Scholar]
- 4.Kleindienst A, Schmidt C, Parsch H, Emtmann I, Xu Y, Buchfelder M. The passage of S100B from brain to blood is not specifically related to the blood–brain barrier integrity. Cardiovasc Psychiatry Neurol. 2010;2010:801295. doi: 10.1155/2010/801295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarrafzadeh A, Schlenk F, Gericke C, Vajkoczy P. Relevance of cerebral interleukin-6 after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2010 doi: 10.1007/s12028-010-9432-4. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Thal SC, Sporer S, Klopotowski M, Thal SE, Woitzik J, Schmid-Elsaesser R, et al. Brain edema formation and neurological impairment after subarachnoid hemorrhage in rats. Laboratory investigation. J Neurosurg. 2009;111(5):988–94. doi: 10.3171/2009.3.JNS08412. [DOI] [PubMed] [Google Scholar]
- 7.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25(5):554–71. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Ayer R, Sugawara T, Chen W, Sozen T, Hasegawa Y, et al. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2010;38(2):612–8. doi: 10.1097/CCM.0b013e3181c027ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu B, Ma Q, Khatibi N, Chen W, Sozen T, Cheng O, et al. Ac-YVAD-CMK decreases blood–brain barrier degradation by inhibiting caspase-1 activation of Interleukin-1beta in intracerebral hemorrhage mouse model. Transl Stroke Res. 2010;1(1):57–64. doi: 10.1007/s12975-009-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cipolla MJ, Godfrey JA. Effect of hyperglycemia on brain penetrating arterioles and cerebral blood flow before and after ischemia/reperfusion. Transl Stroke Res. 2010;1(2):127–34. doi: 10.1007/s12975-010-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24(8):916–25. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26(4):627–34. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- 13.Nirogi R, Kandikere V, Mudigonda K, Bhyrapuneni G, Muddana N, Saralaya R, et al. A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system. J Neurosci Methods. 2009;178(1):116–9. doi: 10.1016/j.jneumeth.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Beeton C, Garcia A, Chandy KG. Drawing blood from rats through the saphenous vein and by cardiac puncture. J Vis Exp. 2007;7:266. doi: 10.3791/266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167(2):327–34. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after sub-arachnoid hemorrhage. Stroke. 2004;35(10):2412–7. doi: 10.1161/01.STR.0000141162.29864.e9. [DOI] [PubMed] [Google Scholar]
- 17.Vargas C, Tannhauser M, Barros HM. Dissimilar effects of lithium and valproic acid on GABA and glutamine concentrations in rat cerebrospinal fluid. Gen Pharmacol. 1998;30(4):601–4. doi: 10.1016/s0306-3623(97)00328-5. [DOI] [PubMed] [Google Scholar]
- 18.Clark SR, McMahon CJ, Gueorguieva I, Rowland M, Scarth S, Georgiou R, et al. Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J Cereb Blood Flow Metab. 2008;28(2):387–94. doi: 10.1038/sj.jcbfm.9600537. [DOI] [PubMed] [Google Scholar]
- 19.Prunell GF, Svendgaard NA, Alkass K, Mathiesen T. Inflammation in the brain after experimental subarachnoid hemorrhage. Neurosurgery. 2005;56(5):1082–92. [PubMed] [Google Scholar]
- 20.Levine DN, Rapalino O. The pathophysiology of lumbar puncture headache. J Neurol Sci. 2001;192(1–2):1–8. doi: 10.1016/s0022-510x(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 21.Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234(4):H371–83. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- 22.Sobey CG, Faraci FM. Subarachnoid haemorrhage: what happens to the cerebral arteries? Clin Exp Pharmacol Physiol. 1998;25 (11):867–76. doi: 10.1111/j.1440-1681.1998.tb02337.x. [DOI] [PubMed] [Google Scholar]