Abstract
Strains of the green alga Chlamydomonas acidophila and two chrysomonads, Ochromonas spp., isolated from each of two similar acid mining lakes (AMLs) with extremely low pH (∼2.6) were investigated to consider a possible synergistic stress effect of low pH and unfavourable temperature. We measured flagellate growth rates over a combination of four pH (2.5, 3.5, 5.0 and 7.0) and three temperatures (10, 17.5 and 25°C) in the laboratory. Our hypothesis was that, under highly acidic conditions (pH <3), an obligate acidophil species (C. acidophila) would be less sensitive to the combined stress of pH and temperature than acidotolerant species (Ochromonas spp.). We expected that the difference of the fundamental vs. realized pH niche would be greater in the latter. Another chrysomonad, Poterioochromonas malhamensis strain DS, served as a reference for a closely related neutrophil species. Surprisingly, C. acidophila did not survive temperatures >27°C. The lowest temperature tested reduced growth rates of all three chrysomonad strains significantly. Since all chrysomonads were tolerant to high temperature, growth rate of one Ochromonas spp. strain was measured exemplarily at 35°C. Only at this high temperature was the realized pH niche significantly narrowed. We also recorded significant intraspecific differences within the C. acidophila strains from the two AML, illustrating that the niche width of a species is broader than that of individual clones.
Keywords: Chlamydomonas acidophila, Ochromonas sp., acid mining lakes, pH response, temperature response
INTRODUCTION
The pH varies in freshwater ecosystems between <2 and 12 (Wetzel, 2001). Organisms living at the low and high end of this range must have developed particular physiological adaptations and life history strategies to survive and reproduce. The general reduction of species diversity with decreasing pH has been known for decades (Schindler, 1988; Baker and Christensen, 1991). However, laboratory studies on the pH effect on planktonic species relative to temperature and biotic factors are scarce (DeNicola, 2000; Gerloff-Elias et al., 2005; Weisse, 2006a; Weisse and Stadler, 2006).
Acid mining lakes (AMLs) are man-made acidic habitats with pH ranging from 2 to 5. Due to the oxidation of pyrite that is linked to brown and black coal deposits, these lakes have been acidified and contain high concentrations of iron, sulphur and heavy metals (Geller et al., 1998). Primary production is relatively low in AMLs (Nixdorf et al., 2003). Protists are the key players in the pelagic food web of these lakes (Gaedke and Kamjunke, 2006). In AMLs with pH <3, the phytoplankton is dominated by the mixotrophic flagellate species Chlamydomonas acidophila and Ochromonas spp. (Nixdorf et al., 1998). Ciliates seem to be of minor importance, relative to mesotrophic circumneutral lakes (Packroff, 2000; Gaedke and Kamjunke, 2006).
The chrysophyte genus Ochromonas is one of the most common mixotrophs in freshwater ecosystems (Boenigk et al., 2005). Many as yet undescribed species and strains of this genus are widely dispersed over a broad range of habitats and pH (Boëchat et al., 2007; Boenigk, 2008b). Mixotrophy, i.e. the ability to combine photosynthesis with the uptake of dissolved organic matter (C. acidophila) or bacteria (Ochromonas) for optimized carbon acquisition, is very common and widespread in protists (Jones, 2000; Tittel et al., 2003). In AMLs, algae have to cope with a limited supply of CO2 for photosynthesis because of the absence of a bicarbonate pool (Gross, 2000; Tittel et al., 2005). Accordingly, inorganic carbon limitation of autotrophic production has been suggested by several authors (Ohle, 1981; Lessmann et al., 1999) and experimentally demonstrated (Spijkerman, 2005) for extremely acidic lakes. As a consequence, the relative contribution of mixotrophic flagellates to the algal community tends to increase with decreasing pH and may reach 90% of the pigmented biomass in AMLs with pH <3 (Lessmann et al., 2000; Kamjunke et al., 2004).
Compared with most natural lakes, AMLs are young, disconnected water bodies, mostly located in temperate regions. Due to the buffering capacity of dissolved Fe(III), the pH is relatively constant in AMLs with pH <4 (Nixdorf et al., 1998). However, such habitats are subject to pronounced seasonal temperature variation. In the small Austrian AML investigated within this project, water temperature ranges from 0 to 30°C (M.M. and T.W., submitted for publication). Accordingly, the organisms dwelling in central European AML may temporarily suffer from combined pH and temperature stress. Recent work with C. acidophila suggests that tolerance towards temperature and pH stress may have a common denominator in the form of heat-shock proteins (Gerloff-Elias et al., 2006). The coupling of temperature and pH tolerance was also observed with four planktonic ciliate species of the genera Urotricha and Meseres (Weisse and Stadler, 2006; Weisse et al., 2007); the eurythermal species Urotricha farcta and Meseres corlissi were tolerant to wide ranges of pH, while the stenothermal ciliate Urotricha castalia responded most sensitively to pH changes.
We investigated the combined effect of pH and temperature stress on growth rates of the dominant flagellate taxa C. acidophila and Ochromonas spp. isolated from two similar AMLs located in Austria and Germany. The Poterioochromonas malhamensis strain DS originating from Lake Constance served as a reference for a closely related neutrophil species. All these flagellates reproduce asexually under favourable laboratory conditions, i.e. their population growth rates can be used as a proxy for their fitness. The primary aim of our study was to test if the flagellate species pursue different life history strategies, i.e. if we can differentiate between specialists specifically adapted to the harsh conditions (acidophil species) and generalists (acidotolerant species), which take refuge from predation or competition in these habitats. The nomenclature used in the literature to define acidophil(ic) and acidotolerant species of various taxa is somewhat ambiguous. We define an obligate acidophil strain or species as a taxon that is unable to survive at circumneutral pH (Langworthy, 1978; Gross, 2000). In a broader sense, species that show a clear fitness optimum under acidic conditions may also be termed acidophil (Weisse and Stadler, 2006). This definition is overlapping with acidotolerant species sensu stricto that grow better under (moderately) acidic conditions but are able to survive at circumneutral pH. Neutrophil species thrive best at circumneutral pH and most of them cannot survive at pH < 4–5. However, as we will demonstrate in the present work, some neutrophil species can be tolerant even to extremely low pH. Accordingly, the precision of the definition for a given taxon depends on its pH niche width.
We expected the acidophil species to be better adapted to the extremely low pH than the acidotolerant species and thus to be more resistant to an additional stressor. Our hypothesis was that, under highly acidic conditions (pH <3), obligate acidophil species would be less sensitive to the combined stress of pH and temperature than acidotolerant species. If this assumption holds, the difference of the fundamental vs. realized pH niche should be greater in the latter. Secondly, we wanted to test for intraspecific differences among the flagellate strains isolated from the two distant, but similar AML. Recent studies from circumneutral water bodies have revealed that large intraspecific ecophysiological and smaller genetic differences are rather the rule than the exception for freshwater protists (Gächter and Weisse, 2006; Weisse and Rammer, 2006; Weisse et al., 2008; Logares et al., 2009). Similar observations were made with marine protists (Lowe et al., 2005, 2010). The current work extends this previous research to an extreme aquatic biotope.
METHOD
Study sites
We investigated two AMLs located at Langau (Lower Austria, 48°50′N, 15°43′E) and in Lusatia (East Germany, 51°29′N, 13°38′E). These man-made lakes are similar in their hydrochemical composition, pH and origin but differ in their geographic location; they are ∼330 km apart from each other (Weithoff et al., 2010). Both lakes arose more than 40 years ago as a consequence of open cast mining. Due to the oxidation of pyrite and marcasite, they have been acidified and now have pH ranging from 2.3 to 3.0. Total acidity (base capacity KB 4.3) ranges from 8 to 12 mmol L−1 (Nixdorf et al., 1998 and own unpublished data). Another consequence of this oxidation is the high concentrations of iron (110–430 mg L−1) and sulphate (1100–1600 mg L−1). Most physico-chemical and biotic environmental parameters are less variable in AML compared with circumneutral lakes (Nixdorf et al., 1998).
Study organisms
All flagellate species were kept as non-clonal, non-axenic batch cultures. The flagellates used in this study, C. acidophila Negoro and Ochromonas spp. Vysotskii, were isolated both from the AML at Langau and from Lake ML 111 in the Lusatian mining area. For simplicity, all strains originating from Langau will be denoted LG, those from Lusatia ML 111 in the following. Isolation was achieved via enrichment cultures or dilution of the lake water and pipetting of several cells each. The flagellate strain P. malhamensis strain DS, which served as a reference for a neutrophil species, had been isolated from Lake Constance (47°35′N, 9°28′E) by Doris Springmann in the late 1980s and has been kept since then in our laboratory.
Species identity of C. acidophila was verified by sequencing of the 18S rDNA and consecutive BLASTn search. Sequences of both strains were identical and matched the uncultured eukaryote of the highly acidic (pH ∼2) Spanish River Rio Tinto (GenBank accession number AY082979; Amaral Zettler et al., 2002). Sequence identity of this strain with another C. acidophila isolate from Lake ML 111 used earlier by Gerloff-Elias et al. is 99% (1674/1675 bp); accession number of this strain is AJ852427; Gerloff-Elias et al., 2005). Sequences of the 18S rDNA gene of all chrysomonad strains used in this study are also available in GenBank. Accession numbers are FN429125 (Ochromonas sp. LG), EF165126 (Ochromonas sp. ML 111) and AM981258.1 (P. malhamensis). Pairwise sequence similarity was 95% between the two Ochromonas strains and 94% between Ochromonas spp. and P. malhamensis. Although species circumscription is uncertain in many nanoflagellates (Boenigk, 2008a), the three chrysomonads used in this study represent, most likely, different species.
Flagellate stock cultures were maintained in modified Woods Hole Medium (MWC) at a continuous light intensity of 90–100 μmol m−2 s−1 and 17.5°C. The chrysomonad cultures were supplied with a wheat grain to stimulate bacterial growth. In the flagellate stock cultures originating from the AML, pH ranged from 2.8 to 3.0. Poterioochromonas malhamensis was kept at pH 7.0.
Experimental design
Experiments were carried out in 50 mL tissue culture flasks. The experimental volume in each flask was 40 mL. We first assessed the response to pH (pH reaction norm) of each strain at 17.5°C and 7 pH ranging from 2.5 to 7. We then measured flagellate growth rates over a combination of four pH (2.5, 3.5, 5.0 and 7.0) and three temperatures (10, 17.5 and 25°C). To study pH tolerance at extreme temperature conditions, the thermotolerant Ochromonas strain LG was additionally incubated at 35°C at the four different pH levels. However, both C. acidophila strains could not be tested at this temperature as they did not even tolerate an experimental temperature of 27.5°C for more than a few days.
We first acclimated the cultures to the different pH levels at a temperature of 17.5°C, i.e. at the temperature of the flagellate stock cultures. The pH was adjusted with small amounts of 0.1 or 1 mol L−1 NaOH or HCl, respectively. When the target pH levels were reached, the cultures were acclimated to the temperatures of 10 and 25°C. The temperature was manipulated and controlled in incubators (KB 53 and KB 115, WTB Binder) during the entire acclimation procedure and all growth experiments. The incubators kept the temperature constant within ±0.5°C of the target temperature. The various steps of the acclimation procedure are summarized in Table I. The cultures remained for 1 day at each step. Cultures used for the growth experiments had been acclimated to the final experimental pH and temperature conditions for at least 3–4 days. The acclimation procedure and all experiments were conducted at a continuous light intensity of 90–100 μmol m−2 s−1.
Table I:
Stepwise acclimation procedure to the experimental conditions for the flagellate strains and species investigated (T, temperature)
| Species/Strain | pH of stock culture | Adapted to pH extremes | Acclimation steps (pH units day−1) | T of stock culture (°C) | Adapted to | Acclimation steps (°C day−1) |
|---|---|---|---|---|---|---|
| Chlamydomonas acidophila strain LG | 3.0 | 2.5 and 7.0 | 0.5 | 17.5 | 10 and 25°C | 2 |
| Chlamydomonas acidophila strain ML 111 | 3.0 | 2.5 and 7.0 | 0.5 | 17.5 | 10 and 25°C | 2 |
| Ochromonas sp. strain LG | 3.0 | 2.5 and 7.0 | 0.5 | 17.5 | 10 and 25°C | 2 |
| Ochromonas sp. strain ML 111 | 3.0 | 2.5 and 7.0 | 0.5 | 17.5 | 10 and 25°C | 2 |
| Poterioochromonas malhamensis | 7.0 | 2.5 | 0.5 | 17.5 | 10 and 25°C | 2 |
We aimed to compare the fitness of the organisms on the basis of their maximum growth rates. Accordingly, we measured their growth rates during the exponential growth phase. Preliminary experiments with C. acidophila (Spijkerman, 2005; Spijkerman, Univ. of Potsdam, personal communication) and Ochromonas spp. (own unpublished results) had shown that these flagellates begin to grow exponentially at abundances of 10 000 cells mL−1 almost immediately after inoculation. The growth curve usually levels off at abundances of ∼100 000 cells mL−1. Therefore, the acclimated flagellate cultures were diluted with MWC medium to yield initial abundances of 10 000–20 000 cells mL−1. The dilution was done 3–4 h prior to the beginning of each experiment to minimize the risk of an initial lag-phase, potentially causing a reduction in the calculated growth rate. The experiments lasted for 24 h; pH was measured at 0, 6, 12 and 24 h after the beginning of the experiments. The pH measurements were conducted with a pH meter (Mettler Toledo, Seven Easy pH Meter S20) to the nearest 0.01 unit. The pH sensor was three-point calibrated with standard buffer solutions of pH = 4.01, pH = 7.00 and pH = 9.21 prior to each measurement. If the pH differed by more than 0.2 units from the target value, it was adjusted by the addition of small amounts of 0.1 or 1 mol L−1 NaOH or HCl, respectively. Each experiment was run in triplicate. Results reported are mean values ±1 standard deviation (SD).
Measurement of flagellate abundance and calculation of experimental results
Flagellate cell concentrations were measured in each container at the beginning and end of each experiment in formalin-fixed samples. Measurements were performed with a flow cytometer (FacsCalibur, Becton Dickinson), using the software programmes “Cellquest” and “Attractors” (Becton Dickinson). The abundance of C. acidophila was also measured in unfixed samples with an electronic particle counter (CASY 1-Model TTC Schärfe System). We could not use the electronic particle counting for Ochromonas spp., because these cells were too small and overlapped in size with their aggregated food bacteria. To check for the precision of the flow cytometric and electronic cell counts, some samples were also measured by inverted microscopy using a 3 mL settling chamber. Growth rates of the flagellates (μ, day−1) were calculated from their initial (N0) and final (N24) cell numbers assuming exponential growth over the experimental period according to μ = ln(Nt/N0)/t.
Two-way ANOVA and Tukey's post hoc tests were performed to test for the effect of pH and strain on flagellate growth rates and to identify which treatments were statistically different. Two three-way ANOVAs (followed by Tukey's post hoc tests) were used to test for the combined effects of pH, temperature and taxon on flagellate growth rates and all their possible interactions. The first compared strains of C. acidophila (i.e. two levels of the taxon factor) and the second compared the two species of Ochromonas spp. and the closely related P. malahamensis (i.e. three levels of the taxon factor). Results were considered significant if P < 0.05. All statistical analyses were performed with Sigma Stat 2.03 (SPSS Inc.).
RESULTS
pH tolerance
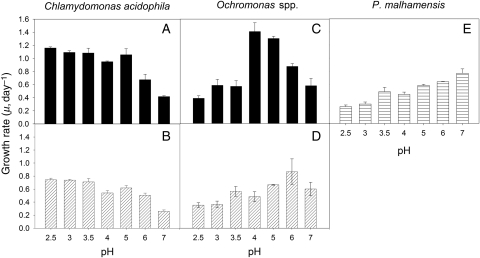
Growth rates (μ, day−1) of C. acidophila were significantly affected by pH in the range of pH 2.5–7 (two-way ANOVA and Tukey's test). In both strains, μ changed only little between 2.5 and 5.0. Only at pH 4.0 were growth rates significantly reduced, relative to the lower pH (Fig. 1A and B). Although μ of both strains appeared to be lower at pH 4 than at pH 5, this difference was not significant (P = 0.091 for strain LG, P = 0.127 for strain ML 111). Growth rates declined significantly from pH 6 to 7. The lack of a statistically significant interaction between pH and strain (P = 0.960) indicated that the pH effect was independent of the strain effect. The overall response to pH of the two C. acidophila strains was thus similar, but averaged over the entire pH range, μ of strain ML 111 was significantly lower (0.57 ± 0.18 day−1) than that of strain LG (0.92 ± 0.27 day−1; P = 0.014). The maximum growth rate, μmax = 1.16 day−1, observed for strain LG at pH 2.5 corresponded to a generation time of 14.3 h, i.e. C. acidophila was able to double almost twice per day at 17.5°C.
Fig. 1.
Growth rates of C. acidophila (A and B), Ochromonas sp. (C and D) and P. malhamensis from Lake Constance (E) at a temperature of 17.5°C. For Chlamydomonas (A) and Ochromonas (C), the top panels represent the strains from Langau, Austria; the bottom panels (B and D) show the respective strains isolated from lake ML 111 in Lusatia, Germany. All bars indicate mean values of triplicates; the error bars denote 1 SD.
Growth rates of the two Ochromonas spp. isolates from Langau and ML111 showed species-specific differences in response to pH. The pH response of P. malhamensis from Lake Constance was also different to both AML isolates (Fig. 1C–E). The effects of species, pH and their interaction on the three chrysomonads were all statistically significant (two-way ANOVA, Tukey's test). Averaged over the entire pH range, μ was highest in Ochromonas sp. strain LG (0.80 ± 0.37 day−1) and lowest in P. malhamensis (0.47 ± 0.19 day−1). Maximal growth rates were shifted from pH 4 to 5 in Ochromonas sp. strain LG (Fig. 1C; μmax = 1.41 ± 0.13 day−1) to pH 5–7 in Ochromonas sp. strain ML 111 (Fig. 1D; μmax = 0.86 ± 0.20 day−1) and pH 7 in P. malhamensis (Fig. 1E; μmax = 0.73 ± 0.09 day−1). Growth rates of strain LG measured at pH 4 and 5 (Fig. 1C) were not different (t-test, P = 0.219).
Combined pH and temperature effects
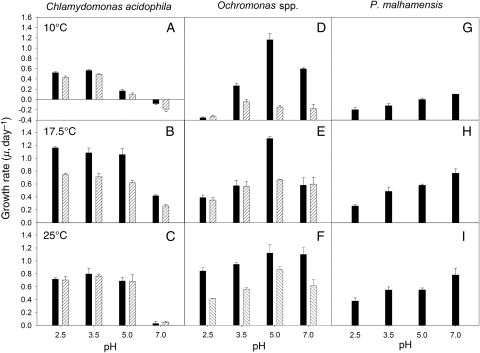
Temperature significantly modified the pH response of both C. acidophila strains, i.e. μ of strain LG was significantly lower both at lower and at higher temperatures than at 17.5°C (Fig. 2A–C). Growth rates of strain ML 111 recorded under neutral conditions were significantly different at the three temperatures. At all other pH, μ was lower at 10°C than at the two higher temperatures. At the highest pH (7.0) and lowest temperature (10°C) tested, μ was negative in both strains (Fig. 2A). At pH 5.0, μ was significantly reduced relative to μ measured at pH 2.5 and 3.5 at the same temperature (10°C). At the highest pH (7.0) and temperature (25°C) investigated, μ was close to zero in both strains (Fig. 2C). There was no temperature effect for Ochromonas sp. strain LG at pH 5.0, its pH optimum (μ = 1.12–1.30 day−1). At the other three pH tested, the temperature effect on μ was significant (Fig. 2D–F). In Ochromonas sp. strain ML 111 and P. malhamensis, μ was significantly lower at 10°C than at the two higher temperatures at which μ was not different. Growth rates of P. malhamensis increased with pH at each temperature (Fig. 2G–I).
Fig. 2.
Growth rates of C. acidophila (left), Ochromonas sp. (centre) and P. malhamensis from Lake Constance (right) at a temperature of 10°C (top panels), 17.5°C (central panels) and 25°C (bottom panels). The black bars within Chlamydomonas and Ochromonas represent the strains isolated from Langau, Austria; the dashed bars indicate the strains isolated from lake ML 111 in Lusatia, Germany. All bars indicate mean values of triplicates; the error bars denote 1 SD.
For C. acidophila, temperature, pH, strain and the interactions of temperature × pH, temperature × strain and temperature × pH × strain were all significant (three-way ANOVA, Table II). Only the interaction of pH × strain was not significant (P = 0.065), thus confirming the results reported above for the experiments measured at seven pH and the standard temperature of 17.5°C. Within Ochromonas spp. and P. malhamensis, all these interactions were significant (three-way ANOVA, Table III); only the interaction of temperature × pH was less clear (P = 0.051).
Table II:
ANOVA results for the effects of temperature, pH and strain on growth rates of C. acidophila
| Source of variation | DF | SS | MS | F | P-value |
|---|---|---|---|---|---|
| Temperature | 2 | 3.104 | 1.552 | 637.249 | <0.001 |
| pH | 3 | 4.889 | 1.630 | 669.211 | <0.001 |
| Strain | 1 | 0.395 | 0.395 | 162.166 | <0.001 |
| Temperature × pH | 2 | 0.358 | 0.0596 | 24.468 | <0.001 |
| Temperature × strain | 6 | 0.354 | 0.177 | 72.601 | <0.001 |
| pH × strain | 3 | 0.0188 | 0.00625 | 2.567 | 0.065 |
| Temp. × pH × Strain | 6 | 0.0549 | 0.00915 | 3.757 | 0.004 |
| Residual | 48 | 0.117 | 0.00244 | ||
| Total | 71 | 9.290 | 0.131 |
Table III:
ANOVA results for the effects of temperature, pH and species on growth rates of Ochromonas spp./P. malhamensis
| Source of variation | DF | SS | MS | F | P-value |
|---|---|---|---|---|---|
| Temperature | 2 | 2.069 | 1.035 | 91.023 | <0.001 |
| pH | 3 | 2.608 | 0.869 | 76.484 | <0.001 |
| Species | 2 | 2.625 | 1.313 | 115.474 | <0.001 |
| Temperature × pH | 6 | 0.151 | 0.0252 | 2.213 | 0.051 |
| Temperature × species | 4 | 0.555 | 0.139 | 12.208 | <0.001 |
| pH × species | 6 | 1.071 | 0.178 | 15.698 | <0.001 |
| Temp. × pH × species | 12 | 0.903 | 0.0752 | 6.619 | <0.001 |
| Residual | 72 | 0.818 | 0.0114 | ||
| Total | 107 | 10.801 | 0.101 |
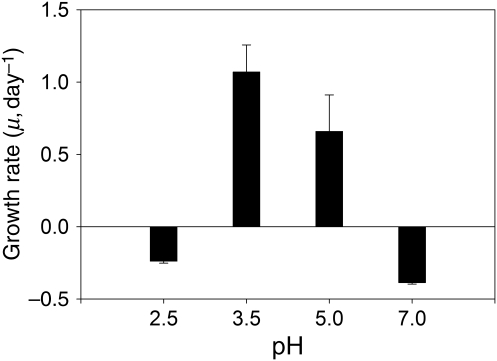
Since we did not observe negative growth rates in Ochromonas spp. and P. malhamensis at temperatures ranging from 10 to 25°C, the chrysomonad strains appeared less sensitive to temperature than C. acidophila. However, the temperature effect on the former became more obvious when Ochromonas sp. strain LG was exposed to 35°C (Fig. 3). At this exceptionally high temperature, the flagellate population declined both at the lowest and highest pH. Our attempts to gradually acclimate our C. acidophila cultures to higher temperatures of 30 and 35°C failed; both strains did not even tolerate an experimental temperature of 27.5°C for more than a few days (own unpublished results).
Fig. 3.
Growth rates of Ochromonas sp. strain LG at a temperature of 35°C. The bars show mean values of triplicates; the error bars indicate 1 SD.
The mean cell volume of both Chlamydomonas strains (strain LG: 157 ± 38 μm3; strain ML 111: 137 ± 38 μm3) was insensitive to pH and temperature changes over the ranges investigated (data not shown).
DISCUSSION
Coupling of tolerance towards pH and temperature stress does not depend on life history strategy
To our knowledge, this is the first study that has investigated the combined effect of pH and temperature stress for several planktonic flagellate species. We demonstrated that the realized niche is distinctly narrower than the fundamental niche if only two abiotic factors, i.e. pH and temperature, are combined. According to the results obtained, we define the two C. acidophila strains as acidophil because they showed a clear fitness optimum under extremely acidic conditions. The two Ochromonas isolates from the AMLs were acidotolerant because they grew best under moderately acidic conditions. Poterioochromonas malhamensis strain DS is neutrophil because its highest growth was measured at neutral pH.
Our results confirmed the earlier conjecture that the resistance to both stressors is coupled (Gerloff-Elias et al., 2006; Weisse and Stadler, 2006; Weisse et al., 2007). Accordingly, the relative effect of temperature was lowest at the pH optimum of each strain and species observed. Close to the lower and upper threshold of the pH tolerance, the temperature effect was more important, narrowing the width of the pH niche of the flagellates if temperature became unfavourable. However, we must reject our hypothesis that, under highly acidic conditions, acidotolerant species are generally more sensitive to the combined stress effect than acidophil species. The three (Poterio-)Ochromonas isolates from the three different lakes were all less sensitive to temperature changes than the two C. acidophila isolates. Even the neutrophil P. malhamensis from Lake Constance achieved positive growth rates at pH 2.5 over a wide temperature range. We conclude that competition by its superior acidotolerant congeners is more likely for its exclusion or reduction to low numbers in AML than physiological constraints, which agrees with recent experimental evidence (M.M. and T.W., submitted for publication). Obviously, the realized niche width of all three chrysomonads was broad with respect to pH and temperature, confirming earlier results obtained with Spumella-like chrysomonad flagellates from various freshwater habitats (Boenigk et al., 2006; Boenigk, 2008b). Niche width was narrower in the acidophil species C. acidophila, which could not sustain neutral conditions both at moderately low and high temperatures. Maximum growth rates that we measured for C. acidophila strain LG at pH 2.5 (µmax = 1.16 day−1) are at the high end of maximum growth rates reported for a large number of unicellular green algae under similar continuous light (200 μmol m−2 s−1) and temperature (17°C) conditions but higher, unspecified pH (Nielsen, 2006). The green algae investigated by this author comprised 11 other Chlamydomonas species. We conclude that C. acidophila reaches a fitness in AML that is comparable to that of similar sized (cell length ∼8 µm) green algae in circumneutral lakes. However, its specialist life history strategy leads to a reduced competitive ability in most natural aquatic environments. This conclusion is in accordance with contemporary evolutionary theory that specialists evolved in homogeneous environments and are, relative to generalists, characterized by a narrow ecological niche width (Kassen, 2002). However, as we will point out in the following section, classification as specialists or generalists may be sensitive to the number of strains investigated. The overall niche width of a flagellate species may be considerably broader than that of individual strains (clones).
Results reported in this study are consistent with in situ observations. In the AML at Langau, cell numbers of C. acidophila are 2-fold higher than those of Ochromonas sp. (Weithoff et al., 2010). Since pH is relatively stable in this AML, ranging from 2.5 to 3.5, and temperature rarely exceeds 27°C in the course of the year (M.M. and T.W., submitted for publication), these conditions should favour C. acidophila, which reached higher growth rates than Ochromonas sp. at pH 2.5 and temperature <25°C in our experiments (Fig. 2). If pH rises in the course of natural or man-made neutralization, we would expect a shift to Ochromonas sp., which reaches higher growth rates than the green algae at pH ≥5.0, irrespective of temperature (Fig. 2). More importantly, various other algae that thrive at pH >3 may become superior competitors in the course of neutralization (Rönicke et al., 2010).
Intraspecific differences within C. acidophila affect the niche width of the species
Our findings clearly demonstrate the existence of clonal differences among C. acidophila. Averaged over the entire pH range, growth rates of strain LG were almost twice as high as those of strain ML 111 under identical laboratory conditions at a temperature of 17.5°C. Our results revealed that both strains are able to tolerate a wide pH range from 2.5 to 7.0. In the field, C. acidophila can withstand pH even as low as 1.0 for short time, but its pH limit for sustained growth seems to be a somewhat higher. Growth rates obtained by Gerloff-Elias et al. (Gerloff-Elias et al., 2005) and Spijkerman (Spijkerman, 2005), also working with a C. acidophila strain from ML 111, are similar to ours (Table IV). These authors also measured reduced growth rates at pH 4, which they explained by extremely stressful conditions caused by a reversal of the proton flux across the plasmalemma of the cells. The reversal point lies at pH ∼4, and as a result, the proton efflux decreases. Most nutrient transport is coupled to the H+ co-transport. Since nutrient acquisition around the reversal point is most likely hampered, this might explain the reduced growth at pH 4 (Gerloff-Elias et al., 2005). In contrast to these findings, Visviki and Palladino (Visviki and Palladino, 2001) reported that several axenic C. acidophila strains obtained from the University of Toronto Culture Collection grew best in media with pH ranging from 6.4 to 8.4 (Table IV). Although these authors concluded that their results indicate strain-specific differences among various, locally adapted C. acidophila populations, their short acclimation time (5 days) to the acidic experimental conditions may have caused biased results. Physiological stress caused by the rapidly changing culture conditions may also explain why Visviki and Palladino (Visviki and Palladino, 2001), in contrast to our findings, observed an increase in cell volume with decreasing pH. An increase in cell volume at the lower threshold of the pH tolerance was also observed with other flagellates (Weisse and Stadler, 2006).
Table IV:
Range of pH tolerance and pH of maximum growth rates (pHopt) of C. acidophila reported from different studies (n.d., not determined)
| pH tolerance | pHopt | Reference |
|---|---|---|
| 1.5–6.0 | 4.0–6.0 | Satake and Saijo (1974) |
| 2.0–6.9 | 3.0–6.0 | Cassin (1974) |
| 1.5–n.d. | 3.5–4.5 | Pollio et al. (2005) |
| 1.5–7.0 | 3.0–5.0 | Gerloff-Elias et al. (2005) |
| 2.0–7.0 | 2.0–6.0 | Spijkerman (2005) |
| 2.0–7.0 | 2.5–5.0 | This study |
| 3.4–8.4 | 6.4–8.4 | Visviki and Palladino (2001) |
Similar to the pH optimum and tolerance, there is contrasting evidence concerning the temperature optimum of C. acidophila. The two strains used in our study showed relatively high growth rates at 25°C, but could not be acclimated to 27.5°C. Above 30°C, cell numbers of both strains rapidly declined. In contrast to these findings, closely related species of the genus Chlamydomonas have been reported from hot springs with temperatures of up to 50°C, but laboratory experiments suggest that the generic tolerable temperature maximum is at ∼42°C (Pollio et al., 2005). Positive growth of C. acidophila strains from different localities was supported up to 34°C, with a temperature optimum of 26°C (Pollio et al., 2005).
While the conflicting results reported in the literature provide at best indirect evidence that intraspecific differences may be pronounced in this species, our findings clearly demonstrate the existence of clonal differences among C. acidophila. Although our isolates were originally non-clonal, it appears likely that, after many generations under optimized growth condition in culture, each strain was represented by its fastest growing clone. Our isolates did not differ in their SSU rDNA sequences (D. Barth, Leipzig, unpublished data). The SSU rDNA gene can be used to differentiate among several closely related Chlamydomonas species. Five C. acidophila isolates from Argentina, Czech Republic and Germany were identical in their SSU rDNA sequences but differed from other Chlamydomonas species (Gerloff-Elias et al., 2005). Strains with identical SSU rDNA sequences may differ in more variable genes. Several clonal lineages of the C. acidophila isolates from LG and ML 111 differed by 0.4–1.7% in the nucleotide sequence of their non-coding internal transcribed spacer (ITS1) region, indicating some genetic clonal variation (Dorow, 2009). Genomic fingerprinting methods, which have not yet been applied to C. acidophila, may reveal a higher genetic intraspecific variability.
The results reported in the present study agree with conclusions derived from the recent work with planktonic ciliates (Weisse et al., 2008). These authors reported that intraspecific variation was low at common genetic markers (SSU rDNA, ITS: 0–4%), moderate at the morphological level (5–15%) and high at the ecophysiological level (10–100%). This high, strain-specific ecophysiological variability cautions against generalizations at the species level if only a single strain or clone was investigated. Lowe et al. (Lowe et al., 2005) recently came to a similar conclusion for marine protists.
Within Ochromonas spp., we also recorded large differences in their ecophysiological performance. Sequencing the 18s rDNA of the two Ochromonas sp. strains from Langau and Lusatia revealed a 5% difference between these two strains, i.e. we probably were dealing with different species. In several treatments, growth rate of Ochromonas sp. strain LG was 3-fold higher than that of strain ML 111 and the maximum fitness was shifted from pH 4–5 in strain LG to pH 5–7 in strain ML 111 at 17.5°C. Due to the fact that AMLs are disconnected from other surface water bodies, they can be regarded as islands in a circumneutral environment. Relative to natural lakes, rates of dispersal are thus reduced in AML. This is also because of the absence of waterfowl, which are an important vector for dispersal of microorganisms in most neutral lakes (Weisse, 2006b). The reduced rates of dispersal result in a restricted gene flow, which is a prerequisite for the evolution of local adaptation (Kawecki and Ebert, 2004). The different ecophysiological performance of the flagellates of different origin suggests that both flagellate species are locally adapted to their habitat.
Irrespective of the species uncertainty in Ochromonas, the pH reaction norm of the two C. acidophila and the two Ochromonas spp. strains, respectively, from the AML investigated in this study was similar enough to each other to warrant a general conclusion: The dominant flagellate flora in the AML is composed of a mixture of acidophil specialists (C. acidophila) and acidotolerant generalists (Ochromonas spp.).
FUNDING
This work was supported by the Austrian Science Fund (FWF Grant P20118-B17).
ACKNOWLEDGEMENTS
We thank G. Weithoff, Univ. of Potsdam, for providing the flagellate isolates from Lake ML 111 and D. Barth and T. Berendonk, Univ. of Leipzig, for performing the molecular analyses. We also acknowledge technical assistance in the laboratory by P. Stadler and U. Scheffel, ILIM Mondsee. We appreciate constructive comments offered by two anonymous reviewers.
REFERENCES
- Amaral Zettler L. A., Gómez F., Zettler E., et al. Microbiology: eukaryotic diversity in Spain's River of Fire. Nature. 2002;417:137. doi: 10.1038/417137a. doi:10.1038/417137a. [DOI] [PubMed] [Google Scholar]
- Baker J. P., Christensen S. W. Effects of acidification on biological communities in aquatic ecosystems. In: Charles D. F., editor. Acidic Deposition and Aquatic Ecosystems: Regional Case Studies. New York: Springer; 1991. pp. 83–106. [Google Scholar]
- Boëchat I. G., Weithoff G., Krüger A., et al. A biochemical explanation for the success of mixotrophy in the flagellate Ochromonas sp. Limnol. Oceanogr. 2007;52:1624–1632. doi:10.4319/lo.2007.52.4.1624. [Google Scholar]
- Boenigk J. The past and present classification problem with nanoflagellates exemplified by the genus Monas. Protist. 2008a;159:319–337. doi: 10.1016/j.protis.2008.01.001. doi:10.1016/j.protis.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Boenigk J. Nanoflagellates: functional groups and intraspecific variation. Denisia. 2008b;23:331–335. [Google Scholar]
- Boenigk J., Pfandl K., Garstecki T., et al. Evidence for geographic isolation and signs of endemism within a protistan morphospecies. Appl. Environ. Microbiol. 2006;72:5159–5164. doi: 10.1128/AEM.00601-06. doi:10.1128/AEM.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boenigk J., Pfandl K., Stadler P., et al. High diversity of the ‘Spumella-like' flagellates: an investigation based on the SSU rRNA gene sequences of isolates from habitats located in six different geographic regions. Environ. Microbiol. 2005;7:685–697. doi: 10.1111/j.1462-2920.2005.00743.x. doi:10.1111/j.1462-2920.2005.00743.x. [DOI] [PubMed] [Google Scholar]
- Cassin P. E. Isolation, growth, and physiology of acidophilic chlamydomonads. J. Phycol. 1974;10:439–447. [Google Scholar]
- DeNicola D. M. A review of diatoms found in highly acidic environments. Hydrobiologia. 2000;433:111–122. doi:10.1023/A:1004066620172. [Google Scholar]
- Dorow J. Leipzig: Faculty of Biosciences, Pharmacy and Psychology, University of Leipzig; 2009. Genetische Diversität im Süßwasser: Regenerations-Potential der Büschelmücke Chaoborus cristallinus und genetische Variabilität im Plankton in versauerten Tagebauseen; p. 114. Diploma thesis. [Google Scholar]
- Gächter E., Weisse T. Local adaptation among geographically distant clones of the cosmopolitan freshwater ciliate Meseres corlissi. I. Temperature response. Aquat. Microb. Ecol. 2006;45:291–300. doi:10.3354/ame045291. [Google Scholar]
- Gaedke U., Kamjunke N. Structural and functional properties of low- and high-diversity planktonic food webs. J. Plankton Res. 2006;28:707–718. doi:10.1093/plankt/fbl003. [Google Scholar]
- Geller W., Klapper H., Schultze M. Natural and anthropogenic sulfuric acidification of lakes. In: Geller W., Klapper H., Salomons W., editors. Acidic Mining Lakes: Acid Mine Drainage, Limnology and Reclamation. Berlin: Springer; 1998. pp. 3–14. [Google Scholar]
- Gerloff-Elias A., Barua D., Mölich A., et al. Temperature- and pH-dependent accumulation of heat-shock proteins in the acidophilic green alga Chlamydomonas acidophila. FEMS Microbiol. Ecol. 2006;56:345–354. doi: 10.1111/j.1574-6941.2006.00078.x. doi:10.1111/j.1574-6941.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- Gerloff-Elias A., Spijkerman E., Pröschold T. Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acid lake (pH 2.6) Plant Cell Environ. 2005;28:1218–1229. doi:10.1111/j.1365-3040.2005.01357.x. [Google Scholar]
- Gross W. Ecophysiology of algae living in highly acidic environments. Hydrobiologia. 2000;433:31–37. doi:10.1023/A:1004054317446. [Google Scholar]
- Jones R. I. Mixotrophy in planktonic protists: an overview. Freshw. Biol. 2000;45:219–226. doi:10.1046/j.1365-2427.2000.00672.x. [Google Scholar]
- Kamjunke N., Gaedke U., Tittel J., et al. Strong vertical differences in the plankton composition of an extremely acidic lake. Arch. Hydrobiol. 2004;161:289–306. doi:10.1127/0003-9136/2004/0161-0289. [Google Scholar]
- Kassen R. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 2002;15:173–190. doi:10.1046/j.1420-9101.2002.00377.x. [Google Scholar]
- Kawecki T. J., Ebert D. Conceptual issues in local adaptation. Ecol. Lett. 2004;7:1225–1241. doi:10.1111/j.1461-0248.2004.00684.x. [Google Scholar]
- Langworthy T. A. Microbial life at extreme pH values. In: Kushner D. J., editor. Microbial Life in Extreme Environments. New York: Academic Press; 1978. pp. 279–315. [Google Scholar]
- Lessmann D., Deneke R., Ender R., et al. Lake Plessa 107 (Lusatia, Germany)—an extremely acidic shallow mining lake. Hydrobiologia. 1999;408/409:293–299. doi:10.1023/A:1017038616513. [Google Scholar]
- Lessmann D., Fyson A., Nixdorf B. Phytoplankon of the extremely acidic mining lakes of Lusatia (Germany) with pH <3. Hydrobiologia. 2000;, 433:, 123–128. [Google Scholar]
- Logares R., Boltovskoy A., Bensch S., et al. Genetic diversity patterns in five protist species occurring in lakes. Protist. 2009;160:301–317. doi: 10.1016/j.protis.2008.10.004. doi:10.1016/j.protis.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Lowe C. D., Day A., Kemp S. J., et al. There are high levels of functional and genetic diversity in Oxyrrhis marina. J. Eukaryot. Microbiol. 2005;52:250–257. doi: 10.1111/j.1550-7408.2005.00034.x. doi:10.1111/j.1550-7408.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Lowe C. D., Montagnes D. J. S., Martin L. E., et al. Patterns of genetic diversity in the marine heterotrophic flagellate Oxyrrhis marina (Alveolata: Dinophyceae) Protist. 2010;161:212–221. doi: 10.1016/j.protis.2009.11.003. doi:10.1016/j.protis.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Nielsen S. L. Size-dependent growth rates in eukaryotic and prokaryotic algae exemplified by green algae and cyanobacteria: comparisons between unicells and colonial growth forms. J. Plankton Res. 2006;28:489–498. doi:10.1093/plankt/fbi134. [Google Scholar]
- Nixdorf B., Krumbeck H., Jander J., et al. Comparison of bacterial and phytoplankton productivity in extremely acidic mining lakes and eutrophic hard water lakes. Acta. Oecol. 2003;24(Suppl. 1):281–288. doi:10.1016/S1146-609X(03)00031-6. [Google Scholar]
- Nixdorf B., Mischke U., Leßmann D. Chrysophytes and chlamydomonads: pioneer colonists in extremely acidic ming lakes (pH >3) in Lusatia (Germany) Hydrobiologia. 1998;369/370:315–327. doi:10.1023/A:1017010229136. [Google Scholar]
- Ohle W. Photosynthesis and chemistry of an extremely acidic bathing pond in Germany. Verh. Int. Verein. Limnol. 1981;21:1172–1177. [Google Scholar]
- Packroff G. Protozooplankton in acidic mining lakes with special respect to ciliates. Hydrobiologia. 2000;433:157–166. doi:10.1023/A:1004095426532. [Google Scholar]
- Pollio A., Cennamo P., Ciniglia C., et al. Chlamydomonas pitschmannii Ettl, a little known species from thermoacidic environments. Protist. 2005;156:287–302. doi: 10.1016/j.protis.2005.04.004. doi:10.1016/j.protis.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Rönicke H., Schultze M., Neumann V., et al. Changes of the plankton community composition during chemical neutralisation of the Bockwitz pit lake. Limnologica. 2010;40:191–198. [Google Scholar]
- Satake K., Saijo Y. Carbon dioxide content and metabolic activity of microorganisms in some acid lakes in Japan. Limnol. Oceanogr. 1974;19:331–338. doi:10.4319/lo.1974.19.2.0331. [Google Scholar]
- Schindler D. W. Effects of acid rain on freshwater ecosystems. Science. 1988;239:149–157. doi: 10.1126/science.239.4836.149. doi:10.1126/science.239.4836.149. [DOI] [PubMed] [Google Scholar]
- Spijkerman E. Inorganic carbon acquisition by Chlamydomonas acidophila across a pH range. Can. J. Bot. 2005;83:872–878. doi:10.1139/b05-073. [Google Scholar]
- Tittel J., Bissinger V., Gaedke U., et al. Inorganic carbon limitation and mixotrophic growth in Chlamydomonas from an acidic mining lake. Protist. 2005;156:63–75. doi: 10.1016/j.protis.2004.09.001. doi:10.1016/j.protis.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Tittel J., Bissinger V., Zippel B., et al. Mixotrophs combine resource use to outcompete specialists: Implications for aquatic food webs. Proc. Natl Acad. Sci. USA. 2003;100:12776–12781. doi: 10.1073/pnas.2130696100. doi:10.1073/pnas.2130696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visviki I., Palladino J. Growth and cytology of Chlamydomonas acidophila under acidic stress. Bull. Environ. Contam. Toxicol. 2001;66:623–630. doi: 10.1007/s001280054. [DOI] [PubMed] [Google Scholar]
- Weisse T. Freshwater ciliates as ecophysiological model organisms—lessons from Daphnia, major achievements, and future perspectives. Arch. Hydrobiol. 2006a;167:371–402. doi:10.1127/0003-9136/2006/0167-0371. [Google Scholar]
- Weisse T. Biodiversity of freshwater microorganisms—achievements, problems, and perspectives. Pol. J. Ecol. 2006b;54:633–652. [Google Scholar]
- Weisse T., Rammer S. Pronounced ecophysiological clonal differences of two common freshwater ciliates, Coleps spetai (Prostomatida) and Rimostrombidium lacustris (Oligotrichida), challenge the morphospecies concept. J. Plankton Res. 2006;27:55–63. [Google Scholar]
- Weisse T., Scheffel U., Stadler P., et al. Local adaptation among geographically distant clones of the cosmopolitan freshwater ciliate Meseres corlissi. II. Response to pH. Aquat. Microb. Ecol. 2007;47:289–297. doi:10.3354/ame047289. [Google Scholar]
- Weisse T., Stadler P. Effect of pH on growth, cell volume, and production of freshwater ciliates, and implications for their distribution. Limnol. Oceanogr. 2006;51:1708–1715. doi:10.4319/lo.2006.51.4.1708. [Google Scholar]
- Weisse T., Strüder-Kypke M. C., Berger H., et al. Genetic, morphological, and ecological diversity of spatially separated clones of Meseres corlissi Petz and Foissner, 1992 (Ciliophora, Spirotrichea) J. Eukaryot. Microb. 2008;55:257–270. doi: 10.1111/j.1550-7408.2008.00330.x. doi:10.1111/j.1550-7408.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- Weithoff G., Moser M., Kamjunke N, et al. Lake morphometry strongly shapes the plankton community structure in acidic mining lakes. Limnologica. 2010;40:161–166. doi: 10.1016/j.limno.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel R. G. Limnology—Lake and River Ecosystems. San Diego: Academic Press; 2001. p. 1006. [Google Scholar]