Abstract
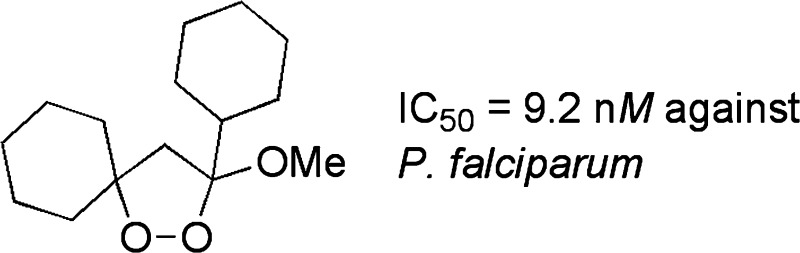
A number of 3-alkoxy-1,2-dioxolanes exhibit promising levels of antimalarial activity against Plasmodium falciparum. A new route to the 1,2-dioxolane core is reported based on tandem peroxidation/cyclization of enones.
Keywords: Malaria; peroxide; 3-alkoxy-1,2-dioxolane; dioxolane
Malaria is a global health epidemic affecting approximately 250 million people and causing over 800000 deaths annually.1 The peroxide artemesinin, along with closely related artesunate and arteether, are a critical part of therapies against drug-resistant strains of P. falciparum, the most virulent form of the disease.2 Strong antimalarial activity has also been observed with a number of artemisinin analogues.3−7 However, reports of delayed parasite clearance in patients receiving artemisinin combination therapy (ACT) have led to renewed interest in structurally distinct classes of peroxides.8 Promising levels of antimalarial activity have been observed from a number of relatively simple peroxide skeletons.9,10−105 Struck by the high antimalarial activity of 1,2,4-trioxolanes (ozonides), one of which is currently in phase III trials,105,11 we became interested in nearly isosteric 3-alkoxy-1,2-dioxolanes. We now report the synthesis of a number of alkoxydioxolanes along with in vitro antimalarial data.
The antimalarial activity of artemisinin is believed to derive from Fe(II)-mediated fragmentation of the peroxide to generate intermediate alkoxy radicals, which undergo rapid β-fragmentation or 1,5-hydrogen abstraction to generate reactive carbon radicals.12,13 The ozonides discussed above have also been established to undergo Fe(II)-mediated cleavage to generate carbon radicals.14 1,2-Dioxolanes, which are nearly isosteric with ozonides, would appear to offer a promising platform for development of new peroxide antimalarials.15 However, to date, few 1,2-dioxolanes have demonstrated significant antimalarial activity.16 One reason may be the lack of an α-oxygen, resulting in a reduced propensity for β-scission in the derived alkoxy radicals.17 We postulated that suitably substituted 3-alkoxy-1,2-dioxolanes might be excellent antimalarial candidates, providing a stable framework that would undergo activation by Fe(II) to furnish α-oxygenated alkoxy radicals predisposed toward β-scission (Scheme 1).
Scheme 1. Competing Modes of Alkoxydioxolane Activation.
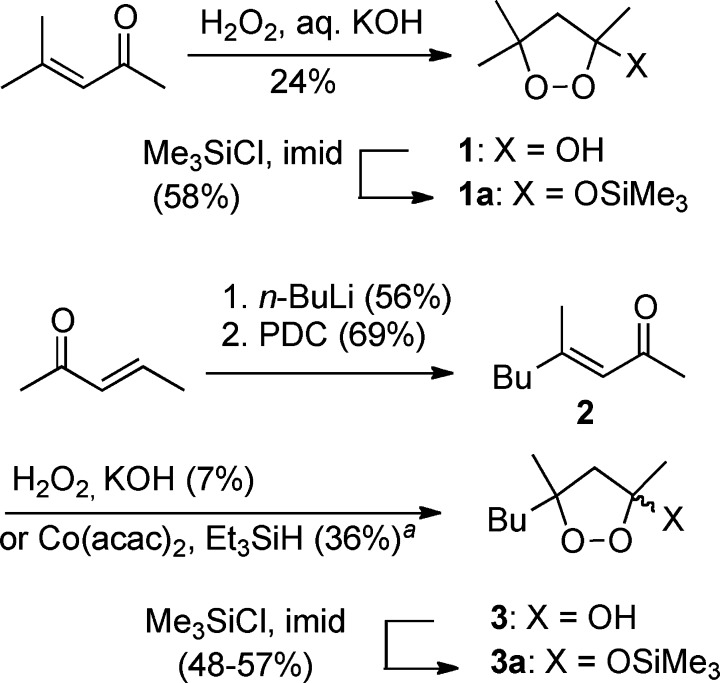
3-Alkoxy-1,2-dioxolanes are available from the corresponding 1,2-dioxolan-3-ols via acid- or base-promoted etherification.18,19 We initially pursued the 3,5,5-trimethyl-1,2-dioxolan-3-ol (1) and 5-butyl-3,5-dimethyl-1,2-alkoxydioxolan-3-ol (3) core structures (Scheme 2). A number of methods have been reported for the synthesis of 1,2-dioxolanols, including base-mediated addition of hydrogen peroxide to α,β-unsaturated ketones,20,21 radical oxygenation of cyclopropanols,22 thermolysis of aza-hydroperoxides,23 or addition of singlet oxygen to methylallyl aldehydes.24 However, none of these methods have been generally applied. Peroxide 1 was readily prepared through base-mediated addition of hydrogen peroxide to mesityl oxide.21 However, as feared, application of similar conditions to the more hydrophobic 4-methyl-3-octen-2-one (2) mainly furnished the product of nucleophilic epoxidation.18 Fortunately, cobalt-mediated oxygenation of 2,25 originally envisaged as a route to an intermediate 3-trialkylsilylperoxy alkanone, directly afforded the desired 1,2-dioxolan-3-ol 3. Acid-catalyzed tranestherification to form the alkoxydioxolanes could be conducted on the 1,2-dioxolanol-3-ols (not shown)18 but proceeded more cleanly on the corresponding trimethylsilyl ethers (1a and 3a; Table 1). Three of the alkoxydioxolanes (5, 7, and 8) demonstrated significant in vitro activity against P. falciparum with dioxolane 8 displaying an IC50 less than 100 nM.26,101 On the basis of these results, we became interested in surveying influences of the following structural elements: a spirocyclic constraint at C5/C5, the size of the C3 alkoxide, and the nature of the putative radical leaving group at C3.
Scheme 2. Synthesis of Dioxolanol Core Structures.
On the basis recovered starting material.
Table 1. Initial Alkoxydioxolane Synthesis and Bioassay.

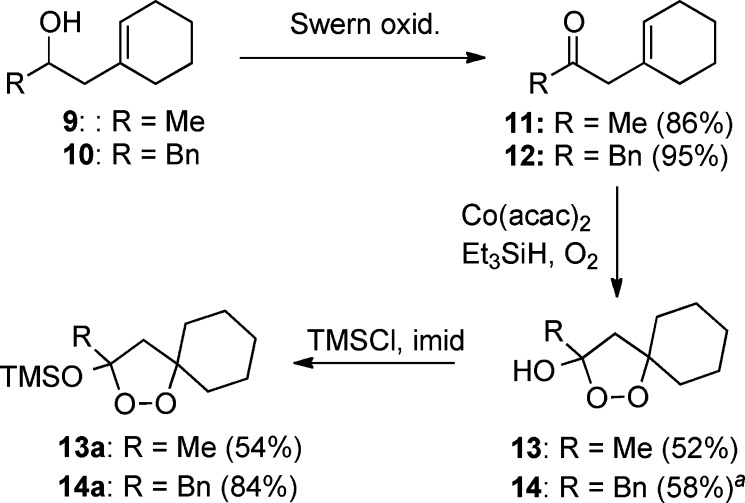
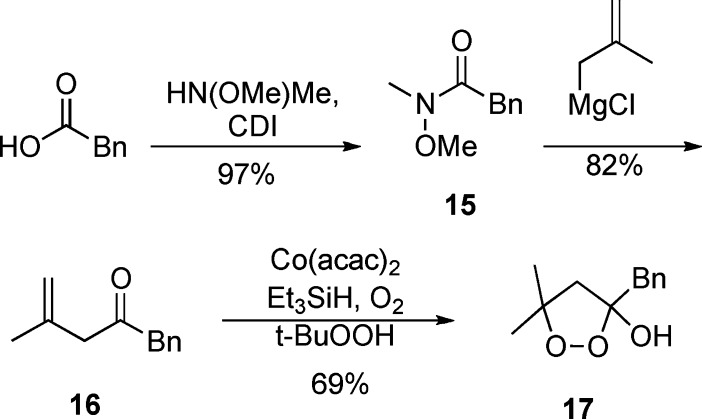
The synthesis of 1,2-dioxolanes incorporating a spirocyclic constraint is illustrated in Scheme 3. Prins reaction of methylenecyclohexane with acetaldehyde or phenylacetaldehyde furnished homoallyl alcohols 9 and 10,27 which underwent Swern oxidation to furnish β,γ-unsaturated ketones 11 and 12. Co-mediated dioxygenation (O2, Et3SiH) by a modification of Isayama's protocol directly furnished 1,2-dioxolan-3-ols 13 and 14.28,100 Although the dioxolanols could be carried on directly, purification was more easily conducted on the derived trimethylsilyl ethers, 13a and 14a. Dioxolanol 17, which incorporates a better radical leaving group (benzyl) at C3, was prepared via a benzyl/methallyl ketone, as illustrated in Scheme 4.
Scheme 3. Synthesis of 5,5′-spiro-1,2-Dioxolan-3-ols.
Added t-BuOOH (stoichometric).
Scheme 4. Synthesis of a 3-Benzyl-1,2-dioxolan-3-ol.
We were unsuccessful in applying a similar approach to a more hindered 1,2-dioxolan-3-ol (20) incorporating both a C5/C5′ spirocycle and a branched side chain at C3 (not shown). However, this target could be prepared via dioxygenation of a cyclopropanol.22 As illustrated in Scheme 5, conversion of dicyclohexylketone to the corresponding trimethylsilyl enol ether, followed by Simmons−Smith cyclopropanation, furnished trimethylsilyloxycyclopropane 18, accompanied by varying amounts of 19, presumably reflecting during work up. Desilylation with TBAF furnished a tetrasubstituted cyclopropanol (19), which could be converted to 20 by stirring in benzene under an atmosphere of oxygen.
Scheme 5. Dioxolanol via Oxidative Ring Expansion.
Cy = cyclohexyl.
Includes ∼10% of 19.
Acid-catalyzed SN1 transetherification of the dioxolanols or their trimethylsilyl ethers with primary alcohols furnished 3-alkoxydioxolanes 21−25 (Table 2). The alkoxydioxolanes proved to possess remarkable chemical stability, failing to react with either PPh3 (≥24 h, room temperature) or i-Bu2AlH (≥4 h, room temperature).
Table 2. Additional Alkoxydioxolanes.

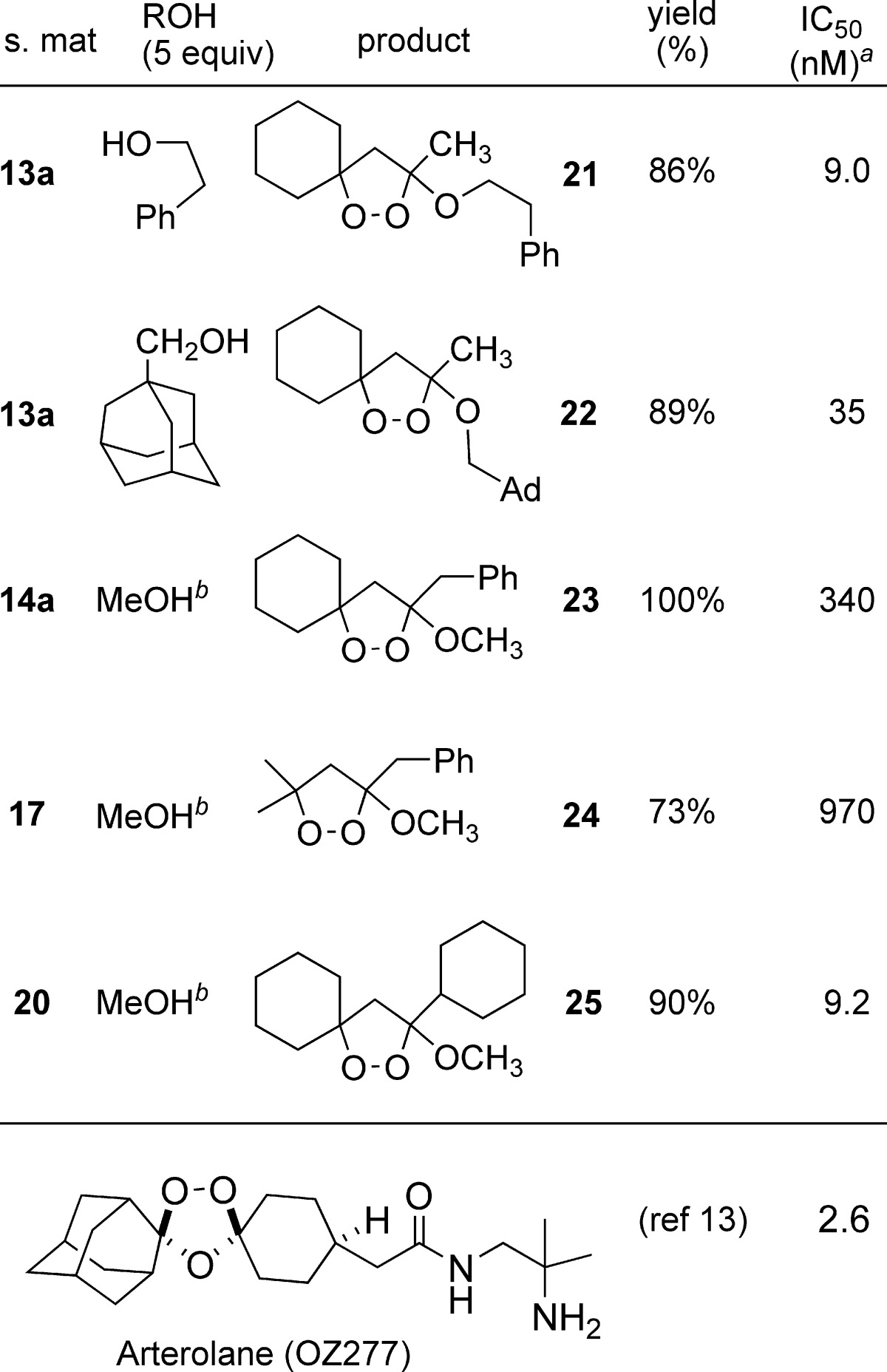 |
The results of in vitro testing for compounds 21−25 (Table 2), when taken together with the results described in Table 1, suggest clear trends in terms of antimalarial structure−activity relationships. Activity is enhanced by a spirocyclohexyl constraint at C5/C5′ and by the presence of steric bulk at C3. Dioxolanes 21 and 25, which combine a spirocyclohexyl unit with either a bulky C3 alkyl substituent or a large C3 alkoxide displayed IC50 values below 10 nM.
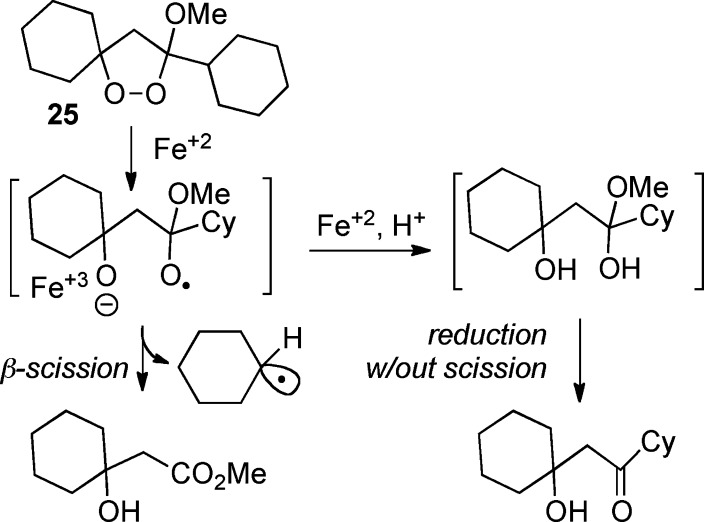
Finally, we were interested in investigating the Fe(II) reactivity of the alkoxydioxolanes, and, in particular, the extent of cleavage of the derived alkoxy radicals (see Scheme 1). Alkoxydioxolane 25, one of our most active of our initial leads, underwent reaction with FeBr2 to furnish the corresponding 3-hydroxyester in 80% yield (reaction illustrated in Scheme 1), supporting the postulated formation and fragmentation of an alkoxy-substituted alkoxy radical.12,13 Similar results have been reported for a monocyclic alkoxydioxolane.20
In conclusion, we have developed new and expanded routes to 1,2-dioxolan-3-ols and 3-alkoxy-1,2-dioxolanes. The latter are demonstrated to provide a highly promising platform for development of a new family of antimalarial peroxides.
Supporting Information Available
Complete experimental procedures and characterization data; 1H and 13C NMR spectra for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by the Medicines for Malaria Venture and the Nebraska Research Initiative and conducted in facilities remodeled with support from the NIH (RR0116544-01). NMR spectra were acquired, in part, on spectrometers purchased with NSF support (MRI 0079750 and CHE 0091975). We thank Dr. Xuejun Liu for initial preparation of several dioxolanes, Tony Lloyd for technical assistance, and Prof. Jonathan Vennerstrom (University of Nebraska Medical Center) for helpful discussions.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- World Health Organization Malaria Report 2008.
- White N. J. Qinghaosu (Artemisinin): The Price of Success. Science 2008, 320, 330–334. [DOI] [PubMed] [Google Scholar]
- Moon D. K.; Singhal V.; Kumar N.; Shapiro T. A.; Posner G. H. Antimalarial preclinical drug development: A single oral dose of a 5-carbon-linked trioxane dimer plus mefloquine cures malaria-infected mice. Drug Dev. Res. 2010, 71, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. J.; Coughlan D.; Heneghan N.; Gaynor C.; Bell A. A novel artemisinin-quinine hybrid with potent antimalarial activity. Bioorg. Med. Chem. Lett. 2007, 17, 3599–3602. [DOI] [PubMed] [Google Scholar]
- Li Y.; Zhu Y.-M.; Jiang H.-J.; Pan J.-P.; Wu G.-S.; Wu J.-M.; Shi Y.-L.; Yang J.-D.; Wu B.-A. Synthesis and antimalarial activity of artemisinin derivatives containing an amino group. J. Med. Chem. 2000, 43, 1635–1640. [DOI] [PubMed] [Google Scholar]
- O'Neill P. M.; Pugh M.; Stachulski A. V.; Ward S. A.; Davies J.; Park B. K. Optimisation of the allylsilane approach to C-10 deoxo carba analogues of dihydroartemisinin: synthesis and in vitro antimalarial activity of new, metabolically stable C-10 analogues. J. Chem. Soc., Perkin Trans. 1 2001, 2682–2689. [Google Scholar]
- Lin A. J.; Li L. Q.; Andersen S. L.; Klayman D. L. Antimalarial activity of new dihydroartemisinin derivatives. J. Med. Chem. 1992, 35, 1639–1642. [DOI] [PubMed] [Google Scholar]
- Noedl H.; Se Y.; Schaecher K.; Smith B. L.; Socheat D.; Fukuda M. M. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620. [DOI] [PubMed] [Google Scholar]
- For an overview, see Tang Y.; Dong Y.; Vennerstrom J. L. Synthetic peroxides as antimalarials. Med. Res. Rev. 2004, 24, 425–448. [DOI] [PubMed] [Google Scholar]
- 1,2,4,5-Tetraoxanes: O'Neill P.; Amewu R.; Nixon G. L; El Garah F. B; Mungthin M.; Chadwick J.; Shone A. E.; Vivas L.; Lander H.; Barton V.; Muangnoicharoen S.; Bray P. G.; Davies J.; Park B. K.; Wittlin S.; Brun R.; Preschel M.; Zhang K.; Ward S. A. Identification of a 1,2,4,5-Tetraoxane Antimalarial Drug-Development Candidate (RKA 182) with Superior Properties to the Semisynthetic Artemisinins. Angew. Chem., Int. Ed. 2010, 49, 5693–5697. [DOI] [PubMed] [Google Scholar]
- Spiroperoxyketals: Ghorai P.; Dussault P. H.; Hu C. H. Synthesis of Spiro-bisperoxy ketals. Org. Lett. 2008, 10, 2401–2404. [DOI] [PubMed] [Google Scholar]
- 1,2,4-Trioxanes: Posner G. H; Oh C. H.; Wang D.; Gerena L.; Milhous W. K.; Meshnick S. R.; Asawamadhasadka W. Mechanism-Based Design, Synthesis, and in vitro Antimalarial Testing of New 4-Methylated Trioxanes Structurally Related to Artemisinin. J. Med. Chem. 1994, 37, 1256–1258. [DOI] [PubMed] [Google Scholar]
- 1,2,4-Trioxolanes: Dong Y.; Wittlin S.; Sriraghavan K.; Chollet J.; Charman S. A.; Charman W. N.; Scheurer C.; Urwyler H.; Santo Tomas J.; Snyder C.; Creek D. J.; Morizzi J.; Koltun M.; Matile H.; Wang X.; Padmanilayam M.; Tang Y.; Dorn A.; Brun R.; Vennerstrom J. L. The Structure−Activity Relationship of the Antimalarial Ozonide Arterolane (OZ277). J. Med. Chem. 2010, 53, 481–91. [DOI] [PubMed] [Google Scholar]
- Fugi M. A.; Wittlin S.; Dong Y.; Vennerstrom J. L. Probing the antimalarial mechanism of artemisinin and OZ277 (arterolane) with nonperoxidic isosteres and nitroxyl radicals. Antimicrob. Agents Chemother. 2010, 54, 1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner G. H.; O'Neill P. M. Knowledge of the Proposed Chemical Mechanism of Action and Cytochrome P450 Metabolism of Antimalarial Trioxanes Like Artemisinin Allows Rational Design of New Antimalarial Peroxides. Acc. Chem. Res. 2004, 37, 397–404. [DOI] [PubMed] [Google Scholar]
- Several alternatives to Fe(II)-mediated formation of reactive radicals have been proposed as the basis for the biological activity of peroxide antimalarials. For an excellent overview of this discussion, see O'Neill P. M.; Barton V. E.; Ward S. A. The Molecular Mechanism of Action of Artemisinin—The Debate Continues. Molecules 2010, 15, 1705–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.; Dong Y.; Wang X.; Sriraghavan K.; Wood J. K.; Vennerstrom J. L. Dispiro-1,2,4-trioxane Analogues of a Prototype Dispiro-1,2,4-trioxolane. J. Org. Chem. 2005, 70, 5103–5110. [DOI] [PubMed] [Google Scholar]
- Dai P.; Trullinger T. K.; Liu X.; Dussault P. H. Asymmetric Synthesis of 1,2-Dioxolane-3-acetic Acids. J. Org. Chem. 2006, 71, 2283–2292. [DOI] [PubMed] [Google Scholar]
- Martyn D. C.; Beletsky G.; Cortese J. F.; Tyndall E.; Liu H.; Fitzgerald M. M.; O'Shea T. J.; Liang B.; Clardy J. Synthesis and in vitro DMPK profiling of a 1,2-dioxolane-based library with activity against Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2009, 19, 5657–5660. [DOI] [PubMed] [Google Scholar]
- Erhardt S.; Macgregor S. A.; McCullough K. J.; Savill K.; Taylor B. J. Model Studies of β-Scission Ring-Opening Reactions of Cyclohexyloxy Radicals. Org. Lett. 2007, 9, 5569–5572. [DOI] [PubMed] [Google Scholar]
- Dussault P. H.; Liu X. Lewis Acid-Mediated Displacements of Alkoxydioxolanes. Org. Lett. 1999, 1, 1391–1393. [DOI] [PubMed] [Google Scholar]
- Baumstark A. L.; Vasquez P. C.; Chen Y.-X. Synthesis and Thermolysis of Ketal Derivatives of 3-Hydroxy-1,2-dioxolanes. J. Org. Chem. 1994, 59, 6692–6696. [Google Scholar]
- Alkoxydioxolanes have also been prepared via conjugate addition of hydroperoxyacetals to α,β-unsaturated esters: Liu H.-H.; Jin H-X; Wu Y.-K. Synthesis and cleavage studies of a 1,2-dioxolane-type peroxide. Chin. J. Chem. 2004, 22, 1029–1033. [Google Scholar]
- Rieche A.; Schmitz E.; Grundemann E. Alkyl peroxides. XXIV. The structure of mesityl oxide peroxide. Chem. Ber. 1960, 93, 2443–8. [Google Scholar]
- Gibson D. H.; DePuy C. H. Free radical reactions of cyclopropanol. The formation of 1,2-dioxolane derivatives. Tetrahedron Lett. 1969, 10, 2203–2206. [Google Scholar]
- Baumstark A. L.; Vasquez P. C. Synthesis of pentasubstituted 3-hydroxy-1,2-dioxolanes. J. Org. Chem. 1992, 57, 393–395. [Google Scholar]
- Adam W.; Catalani L. H.; Griesbeck A. Diastereoselective ene reaction in the photooxygenation of the silyl cyanohydrins of α,β-unsaturated aldehydes. J. Org. Chem. 1986, 51, 5494–5496. [Google Scholar]
- Isayama S.; Mukaiyama T. Novel method for the preparation of triethylsilyl peroxides from olefins by the reaction with molecular oxygen and triethylsilane catalyzed by bis(1,3-diketonato)cobalt(II). Chem. Lett. 1989, 573–6. [Google Scholar]
- Reported in vitro antimalarial activity is based upon an average of two independent assays using the [3H]-hypoxanthine incorporation method with the P. falciparum strain NF54 obtained from MR4: ; Huber W.; Koella J. C. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993, 55, 257–261. [DOI] [PubMed] [Google Scholar]
- Desjardins R. E.; Canfield C. J.; Haynes J. D.; Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider B. B.; Rodini D. J.; Kirk T. C.; Cordova R. Dimethylaluminum chloride catalyzed ene reactions of aldehydes. J. Am. Chem. Soc. 1982, 104, 555–563. [Google Scholar]
- Isayama S. An efficient method for the direct peroxygenation of various olefinic compounds with molecular oxygen and triethylsilane catalyzed by a cobalt(II) complex. Bull. Chem. Soc. Jpn. 1990, 63, 1305–10. [Google Scholar]
- t-BuOOH activation of Co(II) catalysts for hydroazidations: Waser J.; Gaspar B.; Nambu H.; Carreira E. M. Hydrazines and Azides via the Metal-Catalyzed Hydrohydrazination and Hydroazidation of Olefins. J. Am. Chem. Soc. 2006, 128, 11693–11712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.