Abstract
The most common form of childhood congenital muscular dystrophy, Type 1A (MDC1A), is caused by mutations in the human LAMA2 gene that encodes the laminin-α2 subunit. In addition to skeletal muscle deficits, MDC1A patients typically show a loss of peripheral nerve function. To identify the mechanisms underlying this loss of nerve function, we have examined pathology and cell differentiation in sciatic nerves and ventral roots of the laminin-α2-deficient (Lama2−/−) mice, which are models for MDC1A. We found that, compared with wild-type, sciatic nerves of Lama2−/− mice had a significant increase in both proliferating (Ki67+) cells and premyelinating (Oct6+) Schwann cells, but also had a significant decrease in both immature/non-myelinating [glial fibrillary acidic protein (GFAP)+] and myelinating (Krox20+) Schwann cells. To extend our previous work in which we found that doxycycline, which has multiple effects on mammalian cells, improves motor behavior and more than doubles the median life-span of Lama2−/− mice, we also determined how nerve pathology was affected by doxycycline treatment. We found that myelinating (Krox20+) Schwann cells were significantly increased in doxycycline-treated compared with untreated sciatic nerves. In addition, doxycycline-treated peripheral nerves had significantly less pathology as measured by assays such as amount of unmyelinated or disorganized axons. This study thus identified aberrant proliferation and differentiation of Schwann cells as key components of pathogenesis in peripheral nerves and provided proof-of-concept that pharmaceutical therapy can be of potential benefit for peripheral nerve dysfunction in MDC1A.
INTRODUCTION
The most common form of childhood congenital muscular dystrophy, Type 1A (MDC1A), is caused by recessive mutations in the human LAMA2 gene. Human LAMA2 (mouse Lama2) encodes laminin-α2, a component of the trimeric laminin-211 that is abundant in skeletal muscle, motor nerves and brain (1,2). Loss of motor nerve function, as well as pathology in skeletal muscle and brain white matter, is a characteristic finding in this devastating disease (3–6). In MDC1A patients, for example, motor nerve conduction velocity is reduced (7), likely due to abnormal myelination of the peripheral motor nerves. Similarly, in laminin-α2-deficient mice, which are models for human MDC1A, hindlimb paralysis develops during the second month of life, sciatic nerve conduction velocity is reduced and amyelinated axon bundles are abundant (8,9). The mechanisms underlying this peripheral nerve dysfunction are not fully understood and methods to ameliorate pathology are lacking. For this study, therefore, we analyzed cellular and molecular mechanisms of peripheral nerve dysfunction and tested doxycycline as a pharmaceutical approach to ameliorate nerve pathology.
Previous studies of laminin-α2 mutant mice have identified altered patterns of Schwann cell proliferation and differentiation within peripheral nerves and demonstrated that peripheral nerve dysfunction is independent of the skeletal muscle pathology. Electron microscopy and DNA-labeling studies, for example, have suggested that Schwann cell numbers, differentiation and/or morphology are affected in the peripheral nerves of dy/dy, dy2J/dy2J and nmf417/nmf417 mice, which carry different spontaneous or chemically induced mutations that are now known to affect laminin-α2 expression or function (2,9–15). Muscle-specific expression of laminin-α2 in dyW/dyW mice, which are laminin-α2-deficient due to targeted inactivation of the Lama2 gene, corrects pathology in skeletal muscles, but does not correct motor nerve dysfunction (16). Thus, nerve pathology due to laminin-α2-deficiency is independent of muscle pathology.
One significant pathogenetic mechanism by which loss of laminin-α2 leads to neuromuscular dysfunction appears to be inappropriate induction of cell death in skeletal muscles (17–21). Previous studies from our group demonstrated that either body-wide inactivation of Bax (a promoter of cell death) or transgenic muscle-specific overexpression of Bcl-2 (an inhibitor of cell death) ameliorated muscle pathology and increased the median lifespan of Lama2−/− mice from ∼30 to >100 days (17,20). Our studies also raised the possibility that increased cell death could play an additional role in nerve pathogenesis, because the onset of hindlimb paralysis in Lama2−/− mice was delayed by body-wide inactivation of Bax, but not by skeletal muscle-specific overexpression of Bcl-2 (17,20). We also found that treatment with doxycycline, which has multiple effects in mammalian cells including inhibition of death, significantly improves motor behavior, decreases inflammation and muscle cell death and more than doubles the lifespan of Lama2−/− mice (18), though we did not examine peripheral nerves in our earlier study of doxycycline-treated Lama2−/− mice.
For this study, we examined cellular and molecular mechanisms that underlie peripheral nerve dysfunction by analyzing molecular markers that identify Schwann cells at distinct stages of differentiation in Lama2−/− and wild-type mice. In addition, we extended our previous work with skeletal muscle to determine if doxycycline treatment had beneficial effects on peripheral nerves in Lama2−/− mice. As in our previous work (17–21), we used laminin-α2 knockout dyW/dyW mice (9,16), as models for human MDC1A. The results identified aberrant patterns of Schwann cell differentiation in Lama2−/− mice and showed that doxycycline treatment partially ameliorated peripheral nerve pathology.
RESULTS
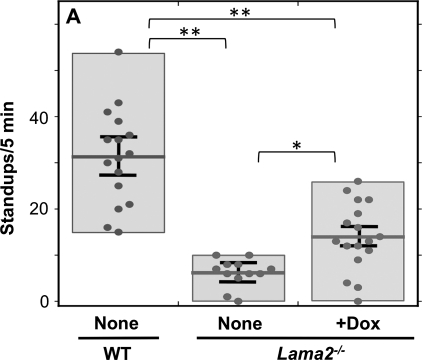
To examine the mechanisms of motor nerve pathology and the potential effects of doxycycline treatment on peripheral neuropathology in Lama2−/− mice, we first analyzed motor behavior of new cohorts of wild-type, untreated Lama2−/− and doxycycline-treated Lama2−/− mice as in our previous work (18). As in previous studies (18,22), we used the frequency with which mice stood up on their hindlimbs when placed in a new cage as a measure of motor function. We found that at 4 weeks after birth, untreated Lama2−/− mice stood up less than one-fifth as often as wild-type mice, consistent with impaired ability of their hindlimbs to function in weight-bearing activity. In contrast, we found that doxycycline-treated Lama2−/− mice had on average approximately twice as much spontaneous standing behavior as the untreated Lama2−/− mice, though the activity level of doxycycline-treated Lama2−/− mice nonetheless remained below that of untreated wild-type mice (Fig. 1) or doxycycline-treated wild-type mice (18) (data not shown). Of the 17 doxycycline-treated Lama2−/− mice that we examined, 14 showed improvement in motor behavior compared with the average of the untreated Lama2−/− mice, whereas three did not show an improvement (Fig. 1). The overall improvement in standing behavior that we found here was qualitatively similar to the finding in our earlier study that focused on muscle pathology (18). Thus, we confirmed that doxycycline treatment significantly improved the motor behavior of Lama2−/− mice.
Figure 1.
Doxycycline treatment improved the motor behavior of Lama2−/− mice. Exploratory behavior, measured by the number of times the mice stood up on their hind limbs during the first 5 min in a new cage, was, as indicated, significantly decreased in 4-week-old Lama2−/− mice (center, n= 12) compared with wild-type mice (WT, leftmost, n= 16); and doxycycline treatment significantly increased the motor behavior of Lama2−/− mice (rightmost, n= 17). Each dot represents a measurement from a different mouse; the boxes show the range of measurements; the horizontal bars are the average values; and error bars = SE. Note that 3 of the 17 doxycycline-treated Lama2−/− mice did not appear to show improved motor behavior. See text for discussion. *P < 0.05; **P < 0.01.
To begin to quantify Lama2−/− neuropathology, we first determined cross-sectional areas and numbers of cell nuclei in stained transverse sections of sciatic nerves obtained from 4-week-old wild-type, untreated Lama2−/− and doxycycline-treated Lama2−/− mice (Table 1). We found that the average cross-sectional areas of untreated and doxycycline-treated Lama2−/− sciatic nerves were approximately equal, though both had significantly smaller cross-sections than wild-type nerves. The average numbers of nuclei per sciatic nerve cross-section were approximately the same in wild-type, untreated Lama2−/− and doxycycline-treated Lama2−/− nerves (Table 1). However, because the cross-sectional areas of the Lama2−/− nerves were smaller than wild-type nerves, the density of cell nuclei was significantly higher in both the treated and the untreated Lama2−/− nerves than in wild-type nerves.
Table 1.
Properties of sciatic nerves from wild-type, untreated Lama2−/− and doxycycline-treated Lama2−/− mice
| Sciatic nerve source | Cross-sectional area (mm2) | Density of nuclei (nuclei/mm2) | Total number of nuclei per cross-section |
|---|---|---|---|
| Wild-type | 0.13 ± 0.014 (8) | 3030 ± 214 (8) | 381 ± 35 (8) |
| Lama2−/− untreated | 0.095 ± 0.009* (9) | 3830 ± 323** (9) | 348 ± 33 (9) |
| Lama2−/− + doxycycline | 0.096 ± 0.009* (10) | 4093 ± 394** (10) | 369 ± 40 (10) |
Data are given as average ± SE (n).
*P < 0.05, **P < 0.01 vs. wild-type (t-test). All other comparisons were not significant.
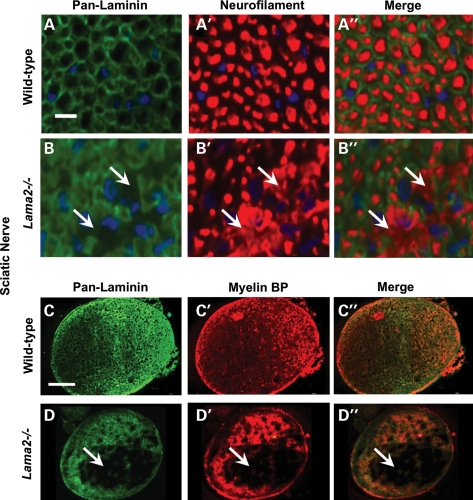
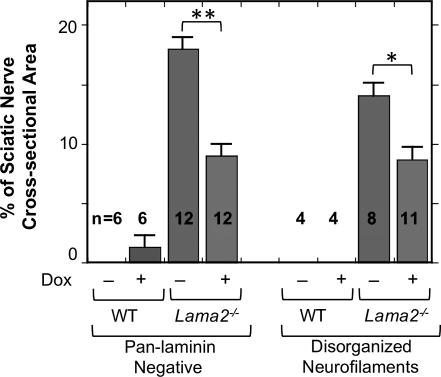
We next analyzed the pathological changes at the cellular level in Lama2−/− sciatic nerves and found that doxycycline treatment significantly, albeit partially, ameliorated pathology as measured by the amount of abnormal axonal morphology (Figs 2 and 3). We first double immunostained sciatic nerves with (i) an anti-neurofilament antibody to identify axons and (ii) a ‘pan-laminin’ antibody that reacted with multiple laminin subunits including laminins α1, α2, β1 and γ1 to identify endoneurial basal lamina associated with Schwann cells. In wild-type mice, all axons identified by neurofilament staining were well separated and organized (Fig. 2A′) and all axons were associated with laminin-positive basal lamina (Fig. 2A). In contrast, sciatic nerves in Lama2−/− mice showed areas in which axonal neurofilaments were disorganized (arrows, Fig. 2B′) and laminin was absent (arrows, Fig. 2B). We quantified axonal neurofilament disorganization and found that the percentage of the sciatic nerve cross-sectional area with disorganized neurofilaments was significantly less in doxycycline-treated than in untreated Lama2−/− mice, indicating partial amelioration of pathology, though doxycycline treatment did not fully restore the wild-type pattern (Fig. 3).
Figure 2.
Pathology in sciatic nerves from Lama2−/− mice. Transverse sections of sciatic nerves from 4-week-old Lama2−/− mice were double immunostained either (i) with an antibody that reacted with multiple laminins (pan-laminin) and an antibody specific for neurofilaments (rows A and B) or (ii) with the pan-laminin antibody and an antibody specific for myelin basic protein (MBP, rows C and D). At high magnification (rows A and B), axons in both wild-type and Lama2−/− sciatic nerve were outlined by the pan-laminin staining, whereas staining of neurofilaments in Lama2−/−, but not wild-type, nerves revealed the areas of disorganized axonal neurofilaments (arrows in B). At low magnification, wild-type nerves showed staining for both pan-laminin and MBP that was distributed throughout the cross-section of the nerve (row C), whereas the Lama2−/− sciatic nerves showed large areas devoid of staining for both pan-laminin and MBP (row D). See Figure 3 for quantitation. Scale bar in A = 10 µm for rows A and B; Scale bar in C = 100 µm for rows C and D.
Figure 3.
Doxycycline treatment decreased two markers of pathology in sciatic nerves of Lama2−/− mice. As indicated, quantitative immunohistology was used to measure the percentage of sciatic nerve cross-sectional areas that either lacked pan-laminin staining (as in Fig. 2D) or showed disorganized neurofilament staining (as in Fig. 2B′). As indicated, nerves from 4-week-old, doxycycline-treated Lama2−/− mice had significantly less pathology than nerves from untreated Lama2−/− mice of the same age. Nerves from wild-type mice had little or no pathology. *P < 0.05; **P < 0.01. Error bars = SE.
We then carried out a similar study in which we double immunostained sciatic nerves for pan-laminin and myelin basic protein. Though most of the axons within sciatic nerves from Lama2−/− mice showed staining with both the pan-laminin and the myelin basic protein antibodies, there were also areas, sometimes quite large, within the nerve that lacked staining for both laminin(s) and myelin basic protein (Fig. 2, rows C and D). When quantified, we found that the percentage of the sciatic nerve cross-sectional area that lacked both pan-laminin and myelin basic protein staining was significantly less in doxycycline-treated than in untreated Lama2−/− mice, again indicating partial amelioration of pathology, though the wild-type pattern was not fully restored (Fig. 3).
Taken together, these studies showed that, on average, ∼15% of the cross-sectional area of sciatic nerves from 4-week-old Lama2−/− mice showed pathological changes including poorly organized axons (i.e. abnormal neurofilament staining pattern), abnormal basal lamina (i.e. absence of pan-laminin staining) and non-myelination (i.e. absence of myelin basic protein). Pathological areas shared all three of these abnormalities and the pathological regions we identified by immunostaining corresponded to amyelinated regions identified on adjacent trichrome-stained sections (not shown). Furthermore, as measured by these markers, the amount of pathology in sciatic nerves of the Lama2−/− mice was significantly reduced by doxycycline treatment.
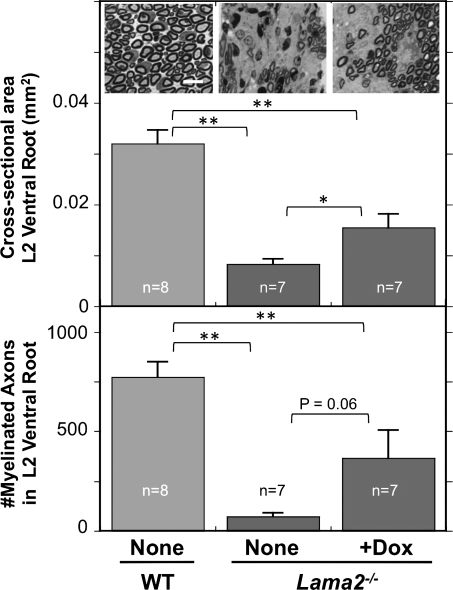
We next examined pathology and the possible effect of doxycycline in L2 ventral roots which are a source of motor axons for the sciatic nerve (23). Previous work had shown that axonal myelination and sorting in the ventral roots is highly dependent on laminin-211 (24). We dissected ventral roots from a subset of the 4-week-old mice that were also used for analyses of behavior and sciatic nerve pathology, including eight wild-type, seven untreated Lama2−/− mice and seven doxycycline-treated Lama2−/− mice. The seven samples from doxycycline-treated Lama2−/− mice included samples from four mice that showed improved standup behavior (average of 20.8 ± 2.3 standups/5 min) and, for comparison, from the three mice that did not show improved behavior (average of 8.3 ± 2.3 standups/5 min; Fig. 1). Upon measuring cross-sectional areas, we found that ventral roots in wild-type mice were significantly larger than those in untreated Lama2−/− mice and that doxycycline-treated Lama2−/− mice had intermediate-sized ventral roots that were significantly larger than those of untreated Lama2−/− mice, but still smaller than wild-type (Fig. 4). Upon quantifying axon numbers, we found that the L2 ventral roots of wild-type mice contained 617 ± 63 (average ± SE; n= 8) axons, all of which were well separated and myelinated (Fig. 4). In contrast, the ventral roots of untreated Lama2−/− mice contained only 57 ± 15 (n= 7) well-myelinated axons. This number was significantly fewer than in wild-type and indicated a very severe pathology in which a large majority of the axons were not well myelinated (Fig. 4). Doxycycline treatment increased the average number of myelinated axons in the ventral roots to 293 ± 110 (n= 7) for the seven Lama2−/− mice that we examined (Fig. 4). At P = 0.06, this increase did not quite reach the standard 5% level of significance.
Figure 4.
Ventral roots of Lama2−/− mice were smaller and had fewer myelinated axons. Sections of L2 ventral roots from 4-week-old-untreated wild-type (WT and none, leftmost), untreated Lama2−/− (none, center) and doxycycline-treated Lama2−/− (+Dox, rightmost) mice were stained with toluidine blue and used to determine both the cross-sectional area (top) and the total number of myelinated axons in each ventral root (bottom). At 4 weeks of age, the L2 ventral roots from Lama2−/− mice were significantly smaller and had <10% as many myelinated axons as wild-type. Ventral roots from doxycycline-treated Lama2−/− mice were significantly larger than in untreated Lama2−/− mice, though still smaller than wild-type. Doxycycline treatment also increased the average number of myelinated axons in ventral roots from Lama2−/− mice, though this increase did not reach quite reach significance at P = 0.06 because three of the seven doxycycline-treated mice that were examined did not show improvement in ventral root pathology, sciatic nerve pathology or standup behavior (see text for details). Inset photos show representative sections of ventral roots from a wild-type mouse, an untreated Lama2−/− mouse and one of the four doxycycline-treated Lama2−/− mice in which axon number was increased; scale bar = 15 µm. *P < 0.05; **P < 0.01. Error bars = SE.
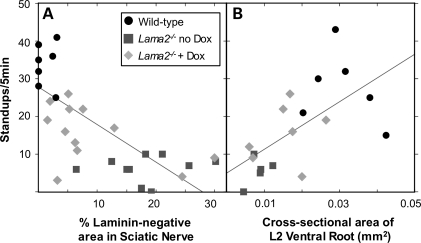
When further examining these seven doxycycline-treated samples, however, we noted that the ventral roots from the three Lama2−/− mice with poor standup behavior had a very low number of myelinated axons (29 ± 8.4; mean ± SE) similar to that of untreated Lama2−/− mice, whereas the four doxycycline-treated Lama2−/− mice that had improved standup behavior also had significantly more myelinated axons (491 ± 111, P < 0.02 vs. group with poor standup behavior, t-test). The three mice that did not show improved standup behavior upon doxycycline treatment also tended to have more sciatic nerve pathology (not shown). These observations suggested that standup frequency should decrease as the amount of peripheral nerve pathology increased, an idea confirmed by linear correlation analyses. In particular, standup behavior tended to decrease when there was an increase in the extent of sciatic nerve pathology as measured either by an increase in the amount of the laminin-negative area (Fig. 5A, r = 0.69, P < 0.01) or neurofilament disorganization (data not shown, r = 0.56, P < 0.01). Standup behavior also tended to decrease when there was an increase in ventral root pathology as measured by a decrease in the cross-sectional area (Fig. 5B, r = 0.62, P < 0.01) or the number of myelinated axons (data not shown, r = 0.67, P < 0.01).
Figure 5.
Relationship between motor behavior and extent of pathology in sciatic nerves and ventral roots. Linear correlation analysis was used to examine the possible relationship of motor behavior measured by the standup assay (as in Fig. 1) with either (A) the extent of sciatic nerve pathology measured by area lacking laminin (as in Figs 2 and 3) or (B) the extent of ventral root pathology measured by the cross-sectional area (as in Fig. 4). Values from wild-type (circles), untreated Lama2−/− (squares) and doxycycline-treated Lama2−/− (diamonds) mice were plotted, and the linear correlation (line) was calculated for the total group. For (A), the correlation coefficient was r = 0.69 (P < 0.01); and for (B), r = 0.62 (P < 0.01). Both analyses indicated that standup behavior decreased with increased pathology. See text for additional discussion.
To probe the cellular mechanisms of pathology and the possible effects of doxycycline, we returned to sciatic nerves and examined the expression patterns of differentiation stage-specific markers of Schwann cells. Schwann cell dysfunction had previously been implicated in the pathological changes in motor nerves of laminin-α2-deficient dy/dy, dy2J/dy2J, nmf417/nmf417 (2,10–12,14–16), but molecular markers of Schwann cell differentiation had not been studied in Lama2−/− mice. In particular, we examined expression of (i) GFAP, a marker of immature and non-myelinating Schwann cells; (ii) Oct6, a marker of pre-myelinating Schwann cells and (iii) Krox20, a marker of myelinating Schwann cells (25–28). As above, we examined sciatic nerves from untreated wild-type, untreated Lama2−/− and doxycycline-treated Lama2−/− mice.
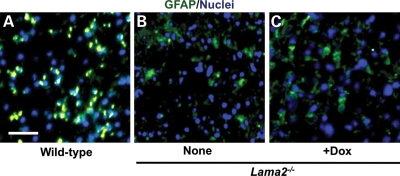
Our first analyses, of GFAP expression, suggested that Schwann cell differentiation status might be altered in Lama2−/− sciatic nerves. We found that ∼25–30% of all cells in sciatic nerves from 4-week-old wild-type mice expressed GFAP with a density of 865 ± 105 GFAP-positive cells/mm2 (mean ± SE, n= 8; Fig. 6). In contrast, GFAP-positive cells were only ∼10–15% of the cells in sciatic nerves from 4-week-old Lama2−/− mice whether untreated (466 ± 96 GFAP-positive cells/mm2, n= 6; P < 0.05 compared with wild-type) or treated with doxycycline (443 ± 57 GFAP-positive cells/mm2, n= 8; P < 0.01 compared with wild-type). Thus, the abundance of immature and non-myelinating Schwann cells, as defined by GFAP expression, was decreased to ∼50% of the wild-type level in Lama2−/− sciatic nerves, and doxycycline treatment did not alter this decrease.
Figure 6.
More cells that expressed GFAP, a marker of non-myelinating Schwann cells, were found in wild-type than in Lama2−/− sciatic nerves. Transverse sections of sciatic nerves from 4-week-old untreated wild-type (A), untreated Lama2−/− (B, none) and doxycycline-treated Lama2−/− (C, +Dox) mice were stained for GFAP (green) and nuclei (blue). As shown by the representative photos of entire nerve cross-sections, GFAP-positive cells were abundant and distributed throughout the wild-type nerve, but were less abundant in both untreated and doxycycline-treated Lama2−/− nerves. See text for quantitation. Scale bar in A = 40 µm.
To examine a possible mechanism underlying this decrease in GFAP-positive cells, we next examined the level of ongoing cell death in Lama2−/− sciatic nerves. A previous study had shown that GFAP-expressing cells are decreased in the motor nerves of mice with Schwann cell-specific knockout of laminin-γ1 (Lamg1), apparently due to increased apoptotic cell death (29); and we had previously shown that apoptotic cell death is increased in the skeletal muscles of Lama2−/− mice (18,21). We found in nerves, however, that terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive nuclei were very rare, amounting to no more than ∼0.2% of all nuclei (i.e. at most two positive nuclei per cross-section) in 4-week-old Lama2−/− sciatic nerves and were not significantly more abundant than in wild-type sciatic nerves. We examined nerve sections from five wild-type, as well as four untreated and five doxycycline-treated Lama2−/− mice with similar results. In contrast, a study of Schwann cell-specific laminin-γ1 knockout mice found that ∼3% of sciatic nerve nuclei were TUNEL-positive in 4-week-old mice (29). With the TUNEL assay, therefore, we found no difference in the rate of cell death in sciatic nerves from 4-week-old wild-type and Lama2−/− mice.
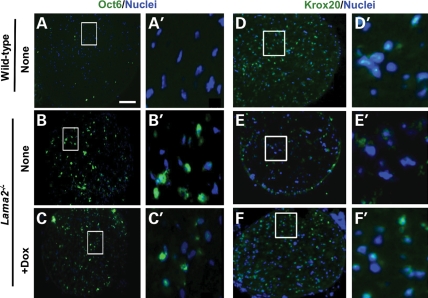
Our additional analyses of Oct6 and Krox20 expression identified further alterations among Schwann cells in Lama2−/− sciatic nerves. In particular, we found that Oct6-positive cells were more abundant in sciatic nerves from 4-week-old Lama2−/− mice than in the corresponding wild-type nerves (Figs 7A–C and 8), whereas Krox20-positive cells were less abundant in Lama2−/− than in wild-type nerves (Figs 7D–F and 8). The decrease in Krox20-positive cells was similar to that of the GFAP-positive cells. In addition, doxycycline treatment further increased the abundance of Oct6-positive cells and also increased the abundance of Krox20-positive cells in Lama2−/− sciatic nerves. As a result, the percentage and density of Oct6-positive cells was several-fold higher in doxycycline-treated Lama2−/− sciatic nerves than in wild-type nerves, and the frequency and density of Krox20-positive cells were significantly increased after doxycycline treatment (Figs 7 and 8).
Figure 7.
Expression of the Schwann cell differentiation stage-specific markers, Oct6 and Krox20, in wild-type and Lama2−/− sciatic nerves. Transverse sections of sciatic nerves from 4-week-old mice were stained as indicated either for Oct6 and nuclei or for Krox20 and nuclei. Panels in left-hand columns are at low magnification and the right-hand columns show the outlined regions at higher magnification. Lama2−/− nerves, both untreated and doxycycline-treated, appeared to have many more Oct6-positive cells than wild-type nerves, whereas Krox20-positive cells appeared to be more abundant in wild-type and doxycycline-treated Lama2−/− nerves than in untreated Lama2−/− nerves. See Figure 8 for quantitation. Scale bar in A = 100 µm for low magnification panels (A–C and D–F) and 10 µm for high magnification panels (A′–C′ and D′–F′).
Figure 8.
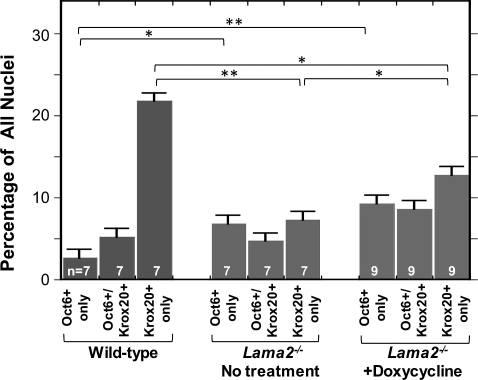
Altered expression of the Schwann cell differentiation stage-specific markers Oct6 and Krox20 in Lama2−/− sciatic nerves. Sciatic nerve sections were double immunostained for Oct6, a marker for pre-myelinating Schwann cells, and Krox20, a marker for myelinating Schwann cells. The percentage of cells that expressed Oct6 but not Krox20 (Oct6 only), both Oct6 and Krox20 (Oc6+/Krox20+) or Krox20 but not Oct6 (Krox20 only) was determined as indicated in sections of sciatic nerves from 4-week-old untreated wild-type (leftmost three bars), untreated Lama2−/− (central three bars) and doxycycline-treated Lama2−/− (rightmost three bars) mice. Untreated Lama2−/− mice had a significantly decreased percentage of Krox20-positive/Oct6-negative cells (i.e. mature/myelinating Schwann cells) compared with wild-type mice, but doxycycline treatment significantly increased the percentage of these cells. Both untreated and doxycycline-treated Lama2−/− nerves had increased percentages of Oct6-positive/Krox20-negative cells (i.e. premyelinating Schwann cells) compared with wild-type. *P < 0.05; **P < 0.01. Error bars = SE.
Using double immunostaining, we identified cells that coexpressed Oct6 and Krox20, as well as cells that expressed only Oct6 or only Krox20 in wild-type, untreated Lama2−/− and doxycycline-treated Lama2−/− sciatic nerves. The relative abundances of the three cell types varied in the different types of mice (Figs 7 and 8) as expected from our single immunostaining results. Each category of mouse had a distinct expression profile. Wild-type nerves had a very high percentage of Oct6-negative/Krox20-positive cells (myelinating Schwann cells), whereas the untreated Lama2−/− sciatic nerves had a higher percentage of Oct6-positive (premyelinating) cells and the doxycycline-treated Lama2−/− sciatic nerves had both more Oct6-positive and more Krox20-positive cells.
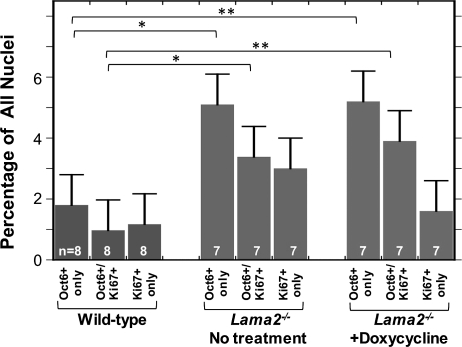
To determine whether the increased number of Oct6-positive cells in Lama2−/− sciatic nerves could be due to increased proliferation, we used double immunostaining for Oct6 and Ki67, a marker for proliferating cells, to compare cell proliferation in wild-type, untreated Lama2−/− and doxycycline-treated Lama2−/− sciatic nerves (Fig. 9). The results showed that Ki67-positive proliferating cells were relatively rare in 4-week-old wild-type sciatic nerves, but were significantly more abundant in both untreated and doxycycline-treated Lama2−/− sciatic nerves (Fig. 9). We found cells that coexpressed Oct6 and Ki67, as well as cells that expressed Oct6 alone or Ki67 alone. These experiments also confirmed the increased abundance of Oct6-positive cells in Lama2−/− compared with wild-type sciatic nerves and showed that about one-third of these Oct6-positive cells were also Ki67-positive (Fig. 9). Doxycycline treatment, however, did not significantly change the abundance of proliferating Ki67-positive cells in Lama2−/− sciatic nerves.
Figure 9.
Increased percentage and proliferation of Oct6-positive cells in Lama2−/− sciatic nerves. Sciatic nerve sections from 4-week-old mice were double immunostained for Oct6, a marker for pre-myelinating Schwann cells, and Ki67, a marker for proliferating cells. The percentage of cells that expressed (i) Oct6 but not Ki67 (Oct6 only), (ii) both Oct6 and Ki67 (Oct6+/Ki67+) or (iii) Ki67 but not Oct6 (Ki67 only) was determined in untreated wild-type (leftmost three bars), untreated Lama2−/− (central three bars) and doxycycline-treated Lama2−/− (rightmost three bars) sciatic nerves. When compared with wild-type, significantly higher percentages of Oct6-only (non-proliferative, premyelinating Schwann cells) and double-positive Ki67+/Oct6+ (proliferating, premyelinating Schwann cells) cells were found in both untreated and doxycycline-treated Lama2−/− sciatic nerves. *P < 0.05; **P < 0.01. Error bars = SE.
DISCUSSION
We found that peripheral nerve pathology in Lama2−/− mice was characterized by aberrant differentiation of Schwann cells and partially ameliorated by treatment with doxycycline. In particular, we found that when compared with wild-type, Lama2−/− sciatic nerves had more premyelinating (Oct6-positive/Krox20-negative) Schwann cells and more proliferating cells, but also had fewer mature/myelinating (Oct6-negative/Krox20-positive) and fewer immature/non-myelinating (GFAP-positive) Schwann cells. These patterns were consistent with inhibition of Schwann cell maturation and myelination in the Lama2−/− sciatic nerves. In addition, we found that doxycycline treatment partially ameliorated peripheral nerve pathology in Lama2−/− mice. Though doxycycline treatment did not affect the abundance of immature/non-myelinating Schwann cells or the abnormally high rate of proliferation in Lama2−/− sciatic nerves, we found that doxycycline (i) increased the abundance of both pre-myelinating and myelinating Schwann cells, (ii) increased motor activity of the treated mice and (iii) decreased the extent of pathology in peripheral nerves.
In this study, we used molecular markers to define the aberrant Schwann cell differentiation and proliferation patterns in peripheral nerves of Lama2dyW/dyW knockout mice. For example, our results showed that there is an increase in Oct6-positive/Krox20-negative Schwann cells in Lama2−/− sciatic nerves. Oct6 is a marker for premyelinating and promyelinating (i.e. after contact is made with an axon) Schwann cells (27) that are at a stage between the earlier immature and final myelinating stages of Schwann cell differentiation. In contrast, we found that two other populations of Schwann cells, immature/non-myelinating (GFAP-positive) (25) and mature/myelinating (Krox20-positive) (26) were decreased in Lama2−/− sciatic nerves. Taken together, our results suggest that in the absence of laminin-α2, Schwann cell maturation and myelination of motor axons are partially inhibited, whereas, perhaps as a compensatory response, there is an increase in proliferation and a subsequent accumulation of Schwann cells at earlier pre- and/or pro-myelinating stages of differentiation. It is possible, though not yet investigated, that the decrease in immature/non-myelinating Schwann cells that we found might be associated with peripheral sensory nerve pathology in Lama2−/− mice (15), because previous studies have found that the loss of non-myelinating Schwann cells, e.g. by cell-type-specific inactivation of erbB or laminin-γ1, leads to sensory nerve pathology (30,31).
The mechanism by which the loss of laminin-α2 leads to only a partial, rather than total, failure of myelination also remains to be determined. Laminin-α2 appears to have both a structural role in formation of the Schwann cell basal lamina and a signaling role through dystroglycan and integrin receptors (2,32) and loss of either or both functions could contribute to pathology. Complete trimeric laminins that do not require laminin-α2, e.g. laminin-411, continue to be expressed in laminin-α2-deficient nerves (24), and may partially carry out the functions of laminin-α2, thus limiting the extent of neuropathology. This idea is supported by the finding that complete loss of all Schwann cell laminins due to laminin-γ1 inactivation produces a much more extensive loss of myelination (33). Our studies suggest that the laminin-negative areas in the Lama2−/− sciatic nerves lack laminins α1, β1 and γ1, but the mechanism underlying focal loss of additional laminins is unknown. Additional studies with isoform-specific antibodies are needed to further define the molecular changes within the pathological and non-pathological nerve regions; and studies with the reagents used here of additional strains of laminin-deficient mice are needed to determine the generality of our finding. Molecular studies of ventral roots, which showed a much more severe loss of myelinated axons than did sciatic nerves, are also needed. The pathological regions that lacked laminin(s) and myelin basic protein but had disorganized neurofilaments that we characterized in Lama2−/− sciatic nerves corresponded with the regions of unsorted axons or amyelination noted, for example, on trichrome or H&E stained sections both by us (not shown) and in previous studies (9,24,32).
Our molecular marker analyses of Schwann cells in Lama2−/− knockout mice were consistent with and extended previous studies of mouse strains that are laminin-α2-deficient due to spontaneously arising (dy/dy and dy2J/dy2J) or chemically induced (nmf417/nmf417) mutations (2,9–15). For example, a 3H-thymidine labeling study showed that Schwann cell proliferation is much higher in dy2J/dy2J than in wild-type L4 ventral roots at 2 months after birth (14). Similarly, using Ki67 as a marker for proliferating cells, we found increased proliferation in the sciatic nerves of 4-week-old Lama2−/− mice compared with wild-type. The previous study also found approximately equal numbers of nuclei in sciatic nerves from 2-month-old dy2J/dy2J and wild-type mice (14), and we too found approximately equal numbers of nuclei in sciatic nerves from 4-week-old Lama2−/− and wild-type mice (Table 1).
In addition, previous morphological studies have consistently found that peripheral nerves of laminin-α2 mutant mice have regions with abnormal myelination and axon organization, as well as decreased numbers of mature myelinating Schwann cells (2,9–11,13,15,16,24,34). Our molecular marker studies similarly showed that sciatic nerves of 4-week-old Lama2−/− knockout mice had fewer myelinating (Krox20-positive) Schwann cells, but our studies also extended the previous work by demonstrating that Lama2−/− knockout mice had fewer immature/non-myelinating (GFAP-positive) and more premyelinating (Oct6-positive) Schwann cells. Laminin-α2 deficiency also causes myelination deficits in the central nervous system; and, in dy/dy mice, immature oligodendrocyte progenitors accumulate in adult brains (35), an outcome similar to the accumulation of premyelinating Schwann cells that we found in sciatic nerves of Lama2−/− knockout mice.
The current study leaves open the question of the role of cell death in peripheral nerve pathology. We found increased proliferation, but no increase in total number, of Schwann cells in Lama2−/− sciatic nerves. In the 4-week-old mice that we examined here, however, we were not able to identify a significant number of TUNEL-positive nuclei in Lama2−/− sciatic nerves. Thus, it was not possible to compare the rates of cell death in wild-type vs. Lama2−/− nerves or after doxycycline treatment. It is possible that cell death might have been more frequent at earlier stages of development as suggested by an earlier study (13), but our study was not designed to examine this issue. Progeny of proliferating Schwann cells might be lost via a mechanism that either does not result in TUNEL-positive nuclei or occurs a slow a rate that does not produce a detectable level of the short-lived TUNEL-positive nuclei. In contrast to the lack of TUNEL-positive nuclei in nerves, we and others previously found that up to ∼1–2% of the nuclei in Lama2−/− skeletal muscles were TUNEL-positive, which was significantly higher than in wild-type muscles (18,36). Also, in mice with laminin-γ1-null Schwann cells, ∼3% of the nuclei are TUNEL-positive, indicating a high level of ongoing cell death (33). However, knockout of laminin-γ1 eliminates all functional laminins, whereas laminin-α2-deficient nerves retain functional laminins (e.g. LN-411) that likely ameliorate pathology (32).
In two studies, we found that pathology in both skeletal muscle (18) and peripheral nerves (this study) was partially ameliorated by doxycycline, but the cellular and molecular mechanism(s) by which doxycycline ameliorates pathology remains to be determined. Doxycycline is also beneficial in a mouse model of oculopharyngeal muscular dystrophy (37). Doxycycline and related molecules produce a large number of molecular and cellular changes in mammalian cells and tissues (18,38–41). In Lama2−/− skeletal muscle, doxycycline treatment may be beneficial by reducing inflammation and cell death (18). Doxycycline can also inhibit matrix metalloproteases (41), an activity that could affect the course of peripheral neuropathology (42). With regard to Schwann cells, doxycycline could act directly on immature cells to halt early death or to activate differentiation pathways, thereby making additional immature cells available for entrance into the myelination pathway. Because the pathology in peripheral nerves appears to be largely independent of pathology in skeletal muscles (16), it may be the doxycycline acts through different mechanisms in different tissues.
Doxycycline treatment is one of a number of experimental therapeutic strategies that have been shown to be beneficial in laminin-α2 deficiency. Among these, additional strategies are genetic inhibition of the mitochondrial cell death pathway (17,21); transgenic expression of laminin-α1 (43), laminin-α2 (16) or a modified agrin protein that can replace laminin (22); proteasome inhibition (44); feeding a 50% protein diet (45); inactivating the complement system or treating with prednisolone (46) and treating with insulin-like growth factor 1 (47) or clenbuterol (48). Furthermore, agrin or laminin-α1 can lessen pathology in laminin-α2-deficient mice even when expression begins after pathology is in progress (49,50); and transgenic expression of laminin-α1 also lessens nerve pathology (51). Combinations of treatments that work through different mechanisms, e.g. strengthening extracellular attachments and inhibiting cell death, might prove to be more effective than any single treatment.
Doxycycline clearly has limits as a pharmaceutical treatment of laminin-α2 deficiency, both because it produces only partial amelioration of pathology in the mouse models and because it can have significant deleterious effects upon long-term administration in humans, particularly in young children (41,52,53). It should be possible, however, to improve upon doxycycline as a pharmaceutical treatment as further understanding of the mechanisms of pathology is gained and additional therapeutic targets are identified. A pharmacological therapy that significantly ameliorates pathology in both the nerves and the muscles in human MDC1A could be of considerable benefit to patients; and our study suggests that such a pharmaceutical therapy may be feasible.
MATERIALS AND METHODS
Mice
Heterozygous Lama2dy-W/+ mice, which carry the targeted dy-W mutation in the Lama2 gene (16), were obtained from Dr Eva Engvall and have been maintained in our laboratory for >5 years by breeding with C57BL/6J mice (17,18,20,21). Mice obtained from crosses of Lama2+/− heterozygotes were genotyped at weaning by PCR (17).
Doxycycline treatment
Doxycycline (Sigma-Aldrich, St Louis, MO, USA) was administered to mothers and newborns in drinking water at 6 mg/ml in 5% sucrose beginning on the day of birth (18). Untreated litters received either normal water or 5% sucrose in water; and we found no significant differences in measured outcomes between Lama2−/− mice that received normal water and those that received 5% sucrose in water (18). Because of the necessity of administering doxycycline through drinking water, entire litters were randomly assigned to either the doxycycline or the untreated group. Mice were monitored daily and weighed at least once per week. Tissues were dissected from untreated and doxycycline-treated control (Lama2+/+ or Lama2+/−) and experimental (Lama2−/−) mice at 28 days after birth. Hindlimb function was assayed by counting how often mice stood up on their hindlimbs during their first 5 min in a new cage (18,22). Protocols were approved by the Institutional Animal Use and Care Committee of the Boston Biomedical Research Institute.
Ventral root histology
L2 ventral roots were dissected, fixed with 2% glutaraldehyde, 2% paraformaldehyde for 24 h, post-fixed in 1% OsO4, dehydrated through a series of alcohol solutions and embedded in Epon 812. Transverse semi-ultrathin sections (1.0 μm) were made through the center of each specimen and the sections were stained with 0.5% toluidine blue for light microscopy.
Immunohistochemistry
Sciatic nerves were collected in phosphate-buffered saline (PBS), embedded in OCT (Tissue Tek, Torrance, CA, USA) and snap-frozen on dry ice. Indirect immunofluorescence was performed on 10-μm thick cryosections fixed in cold methanol or 3.7% paraformaldehyde for 10 min, rinsed three times in PBS and blocked with either 3% normal goat serum (Vector Laboratories, Burlingame, CA, USA) or 3% rat serum (Vector Laboratories). The following primary antibodies were used: rabbit polyclonal antibody (pAb) that reacts with multiple laminin subunits including laminins α1, β1 and γ1 used at 1:50 dilution (Pan-laminin; L-9393; Sigma-Aldrich); mouse monoclonal antibody (mAb) SMI31 which reacts with phosphorylated neurofilament H, a neurofilament form that is generally restricted to axons, used at 1:1000 (SMI-31R; Covance, Princeton, NJ, USA); goat pAb anti-OCT6 used at 1:50 (sc-11661; Santa Cruz Biotech, Santa Cruz, CA, USA); rabbit pAb anti-Krox20 used at 1:100 (PRB-236P; Covance); rat mAb anti-myelin basic protein used at 1:500 (MAB386; Millipore, Billerica, MA, USA); mouse mAb anti-GFAP used at 1:1000 (MAB360, Millipore) and rabbit pAb anti-Ki67 antibody used at 1:1000 (VP-K451; Vector Laboratories). Secondary antibodies included: goat anti-rabbit Alexa 488 (A11008; Invitrogen, Carlsbad, CA, USA); goat anti-rabbit Alexa 594 (A11012; Invitrogen); goat anti-mouse Alexa 488 (A11001; Invitrogen); goat anti-mouse Alexa 594 (A11005; Invitrogen); donkey anti-goat Alexa 488 (A11055; Invitrogen) and goat anti-rat cy-3 (112-166-003; Jackson Immunoresearch, West Grove, PA, USA). Sections were examined with a fluorescent microscope (Nikon Eclipse E800). Cross-sectional areas of individual sciatic nerves were determined using the SPOT Advanced v4.6 program (Diagnostic Instruments, Sterling Heights, MI, USA). TUNEL assay was performed using with the ApopTag system (Millipore).
Statistics
Results are presented as the average ± SE, and the reported ‘n’ values are the numbers of different individual mice from which data were obtained. Statistical significance was assessed by the appropriate unpaired two-tailed t-test, Welch alternate t-test or non-parametric Mann–Whitney U-test, and linear correlations were examined using the InStat program (v3.1a, GraphPad Software, San Diego, CA, USA).
FUNDING
This work was supported by the National Institutes of Health (5R01HL064641, 1U54HD060848) and the Muscular Dystrophy Association of the United States (114981).
ACKNOWLEDGEMENTS
We thank Dr Gabriel Corfas (Children's Hospital and Harvard Medical School, Boston, MA, USA) and Dr Joshua R. Sanes (Harvard University) for helpful conversations and Suzanne White (Histology Core Facility, Beth Israel Deaconess Medical Center, Boston, MA, USA) for help with preparation of nerve sections.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Patton B.L., Miner J.H., Chiu A.Y., Sanes J.R. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J. Cell Biol. 1997;139:1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patton B.L., Wang B., Tarumi Y.S., Seburn K.L., Burgess R.W. A single point mutation in the LN domain of LAMA2 causes muscular dystrophy and peripheral amyelination. J. Cell Sci. 2008;121:1593–1604. doi: 10.1242/jcs.015354. [DOI] [PubMed] [Google Scholar]
- 3.Muntoni F., Voit T. The congenital muscular dystrophies in 2004: a century of exciting progress. Neuromuscul. Disord. 2004;14:635–649. doi: 10.1016/j.nmd.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Reed U.C. Congenital muscular dystrophy. Part I: a review of phenotypical and diagnostic aspects. Arq. Neuropsiquiatr. 2009;67:144–168. doi: 10.1590/s0004-282x2009000100038. [DOI] [PubMed] [Google Scholar]
- 5.Collins J., Bönnemann C.G. Congenital muscular dystrophies: toward molecular therapeutic interventions. Curr. Neurol. Neurosci. Rep. 2010;10:83–91. doi: 10.1007/s11910-010-0092-8. [DOI] [PubMed] [Google Scholar]
- 6.Rocha C.T., Hoffman E.P. Limb-girdle and congenital muscular dystrophies: current diagnostics, management, and emerging technologies. Curr. Neurol. Neurosci. Rep. 2010;10:267–276. doi: 10.1007/s11910-010-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shorer Z., Philpot J., Muntoni F., Sewry C., Dubowitz V. Demyelinating peripheral neuropathy in merosin-deficient congenital muscular dystrophy. J. Child Neurol. 1995;10:472–475. doi: 10.1177/088307389501000610. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa M., Miyagoe-Suzuki Y., Ikezoe K., Miyata Y., Nonaka I., Harii K., Takeda S. Schwann cell myelination occurred without basal lamina formation in laminin α2 chain-null mutant (dy3K/dy3K) mice. Glia. 2001;35:101–110. doi: 10.1002/glia.1075. [DOI] [PubMed] [Google Scholar]
- 9.Guo L.T., Zhang X.U., Kuang W., Xu H., Liu L.A., Vilquin J.T., Miyagoe-Suzuki Y., Takeda S., Ruegg M.A., Wewer U.M., Engvall E. Laminin α2 deficiency and muscular dystrophy; genotype-phenotype correlation in mutant mice. Neuromuscul. Disord. 2003;13:207–215. doi: 10.1016/s0960-8966(02)00266-3. [DOI] [PubMed] [Google Scholar]
- 10.Bray G.M., Aguayo A.J. Quantitative ultrastructural studies of the axon Schwann cell abnormality in spinal nerve roots from dystrophic mice. J. Neuropathol. Exp. Neurol. 1975;34:517–530. doi: 10.1097/00005072-197511000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Stirling C.A. Abnormalities in Schwann cell sheaths in spinal nerve roots of dystrophic mice. J. Anat. 1975;119:169–180. [PMC free article] [PubMed] [Google Scholar]
- 12.Bray G.M., Perkins S., Peterson A.C., Aguayo A.J. Schwann cell multiplication deficit in nerve roots of newborn dystrophic mice. A radioautographic and ultrastructural study. J. Neurol. Sci. 1977;32:203–212. doi: 10.1016/0022-510x(77)90235-0. [DOI] [PubMed] [Google Scholar]
- 13.Jaros E., Bradley W.G. Development of the amyelinated lesion in the ventral root of the dystrophic mouse. Ultrastructural, quantitative and autoradiographic study. J. Neurol. Sci. 1978;36:317–339. doi: 10.1016/0022-510x(78)90041-2. [DOI] [PubMed] [Google Scholar]
- 14.Perkins C.S., Bray G.M., Aguayo A.J. Persistent multiplication of axon-associated cells in the spinal roots of dystrophic mice. Neuropathol. Appl. Neurobiol. 1980;6:83–91. doi: 10.1111/j.1365-2990.1980.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 15.Jaros E., Jenkinson M. Quantitative studies of the abnormal axon–Schwann cell relationship in the peripheral motor and sensory nerves of the dystrophic mouse. Brain Res. 1983;258:181–196. doi: 10.1016/0006-8993(83)91141-1. [DOI] [PubMed] [Google Scholar]
- 16.Kuang W., Xu H., Vachon P.H., Liu L., Loechel F., Wewer U.M., Engvall E. Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. J. Clin. Invest. 1998;102:844–852. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girgenrath M., Dominov J.A., Kostek C.A., Miller J.B. Inhibition of apoptosis improves outcome in a model of congenital muscular dystrophy. J. Clin. Invest. 2004;114:1635–1639. doi: 10.1172/JCI22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girgenrath M., Beermann M.L., Vishnudas V.K., Homma S., Miller J.B. Pathology is alleviated by doxycycline in a laminin-alpha2-null model of congenital muscular dystrophy. Ann. Neurol. 2009;65:47–56. doi: 10.1002/ana.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller J.B., Girgenrath M. The role of apoptosis in neuromuscular diseases and prospects for anti-apoptosis therapy. Trends Mol. Med. 2006;12:279–286. doi: 10.1016/j.molmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Dominov J.A., Kravetz A.J., Ardelt M., Kostek C.A., Beermann M.L., Miller J.B. Muscle-specific BCL2 expression ameliorates muscle disease in laminin-alpha2-deficient, but not dystrophin-deficient, mice. Hum. Mol. Genet. 2005;14:1029–1040. doi: 10.1093/hmg/ddi095. [DOI] [PubMed] [Google Scholar]
- 21.Vishnudas V.K., Miller J.B. Ku70 regulates Bax-mediated pathogenesis in laminin-alpha2-deficient human muscle cells and mouse models of congenital muscular dystrophy. Hum. Mol. Genet. 2009;18:4467–4477. doi: 10.1093/hmg/ddp399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moll J., Barzaghi P., Lin S., Bezakova G., Lochmüller H., Engvall E., Müller U., Ruegg M.A. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature. 2001;413:302–307. doi: 10.1038/35095054. [DOI] [PubMed] [Google Scholar]
- 23.Hui K., Kucera J., Henderson J.T. Differential sensitivity of skeletal and fusimotor neurons to Bcl-2-mediated apoptosis during neuromuscular development. Cell Death Diff. 2008;15:691–699. doi: 10.1038/sj.cdd.4402294. [DOI] [PubMed] [Google Scholar]
- 24.Yang D., Bierman J., Tarumi Y.S., Zhong Y.P., Rangwala R., Proctor T.M., Miyagoe-Suzuki Y., Takeda S., Miner J.H., Sherman L.S., Gold B.G., Patton B.L. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J. Cell Biol. 2005;168:655–666. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jessen K.R., Morgan L., Stewart H.J., Mirsky R. Three markers of adult non-myelin-forming Schwann cells, 217c(Ran-1), A5E3 and GFAP: development and regulation by neuron–Schwann cell interactions. Development. 1990;109:91–103. doi: 10.1242/dev.109.1.91. [DOI] [PubMed] [Google Scholar]
- 26.Zorick T.S., Syroid D.E., Arroyo E., Scherer S.S., Lemke G. The transcription factors SCIP and Krox-20 mark distinct stages and cell fates in Schwann cell differentiation. Mol. Cell. Neurosci. 1996;8:129–145. doi: 10.1006/mcne.1996.0052. [DOI] [PubMed] [Google Scholar]
- 27.Arroyo E.J., Bermingham J.R., Jr., Rosenfeld M.G., Scherer S.S. Promyelinating Schwann cells express Tst-1/SCIP/Oct-6. J. Neurosci. 1998;18:7891–7902. doi: 10.1523/JNEUROSCI.18-19-07891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svaren J., Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–1551. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu W.M., Feltri M.L., Wrabetz L., Strickland S., Chen Z.L. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J. Neurosci. 2005;25:4463–4472. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S., Rio C., Ji R.R., Dikkes P., Coggeshall R.E., Woolf C.J., Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat. Neurosci. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- 31.Yu W.M., Yu H., Chen Z.L., Strickland S. Disruption of laminin in the peripheral nervous system impedes nonmyelinating Schwann cell development and impairs nociceptive sensory function. Glia. 2009;57:850–859. doi: 10.1002/glia.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yurchenco P.D., Patton B.L. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 2009;15:1277–1294. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z.L., Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J. Cell Biol. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada E., Mizuhira V., Nakamura H. Abnormalities of the sciatic nerves of dystrophic mice, with reference to nerve counts and mean area of axons. Bull. Tokyo Med. Dent. Univ. 1975;22:25–43. [PubMed] [Google Scholar]
- 35.Relucio J., Tzvetanova I.D., Ao W., Lindquist S., Colognato H. Laminin alters fyn regulatory mechanisms and promotes oligodendrocyte development. J. Neurosci. 2009;29:11794–11806. doi: 10.1523/JNEUROSCI.0888-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyagoe Y., Hanaoka K., Nonaka I., Hayasaka M., Nabeshima Y., Arahata K., Nabeshima Y., Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- 37.Davies J.E., Wang L., Garcia-Oroz L., Cook L.J., Vacher C., O'Donovan D.G., Rubinsztein D.C. Doxycycline attenuates and delays toxicity of the oculopharyngeal muscular dystrophy mutation in transgenic mice. Nat. Med. 2005;11:672–677. doi: 10.1038/nm1242. [DOI] [PubMed] [Google Scholar]
- 38.Alano C.C., Kauppinen T.M., Valls A.V., Swanson R.A. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc. Natl Acad. Sci. USA. 2006;103:9685–9690. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sapadin A.N., Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Yao J.S., Shen F., Young W.L., Yang G.Y. Comparison of doxycycline and minocycline in the inhibition of VEGF-induced smooth muscle cell migration. Neurochem. Int. 2007;50:524–530. doi: 10.1016/j.neuint.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Granillo G.A., Rodriguez-Granillo A., Milei J. Effect of doxycycline on atherosclerosis: from bench to bedside. Recent Pat. Cardiovasc. Drug Discov. 2011;6:42–54. doi: 10.2174/157489011794578419. [DOI] [PubMed] [Google Scholar]
- 42.Liu H., Kim Y., Chattopadhyay S., Shubayev I., Dolkas J., Shubayev V.I. Matrix metalloproteinase inhibition enhances the rate of nerve regeneration in vivo by promoting dedifferentiation and mitosis of supporting Schwann cells. J. Neuropathol. Exp. Neurol. 2010;69:386–395. doi: 10.1097/NEN.0b013e3181d68d12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gawlik K., Miyagoe-Suzuki Y., Ekblom P., Takeda S., Durbeej M. Laminin alpha1 chain reduces muscular dystrophy in laminin alpha2 chain deficient mice. Hum. Mol. Genet. 2004;13:1775–1784. doi: 10.1093/hmg/ddh190. [DOI] [PubMed] [Google Scholar]
- 44.Carmignac V., Quéré R., Durbeej M. Proteasome inhibition improves the muscle of laminin α2 chain-deficient mice. Hum. Mol. Genet. 2011;20:541–552. doi: 10.1093/hmg/ddq499. [DOI] [PubMed] [Google Scholar]
- 45.Zdanowicz M.M., Slonim A.E., Bilaniuk I., O'Connor M.M., Moyse J., Teichberg S. High protein diet has beneficial effects in murine muscular dystrophy. J. Nutr. 1995;125:1150–1158. doi: 10.1093/jn/125.5.1150. [DOI] [PubMed] [Google Scholar]
- 46.Connolly A.M., Keeling R.M., Streif E.M., Pestronk A., Mehta S. Complement 3 deficiency and oral prednisolone improve strength and prolong survival of laminin alpha2-deficient mice. J. Neuroimmunol. 2002;127:80–87. doi: 10.1016/s0165-5728(02)00104-2. [DOI] [PubMed] [Google Scholar]
- 47.Lynch G.S., Cuffe S.A., Plant D.R., Gregorevic P. IGF-I treatment improves the functional properties of fast- and slow-twitch skeletal muscles from dystrophic mice. Neuromuscul. Disord. 2001;11:260–268. doi: 10.1016/s0960-8966(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 48.Hayes A., Williams D.A. Examining potential drug therapies for muscular dystrophy utilising the dy/dy mouse: I. Clenbuterol. J. Neurol. Sci. 1998;157:122–128. doi: 10.1016/s0022-510x(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 49.Meinen S., Barzaghi P., Lin S., Lochmüller H., Ruegg M.A. Linker molecules between laminins and dystroglycan ameliorate laminin-alpha2-deficient muscular dystrophy at all disease stages. J. Cell Biol. 2007;176:979–993. doi: 10.1083/jcb.200611152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gawlik K.I., Durbeej M. Transgenic overexpression of laminin alpha1 chain in laminin alpha2 chain-deficient mice rescues the disease throughout the lifespan. Muscle Nerve. 2010;42:30–37. doi: 10.1002/mus.21616. [DOI] [PubMed] [Google Scholar]
- 51.Gawlik K.I., Li J.Y., Petersén A., Durbeej M. Laminin alpha1 chain improves laminin alpha2 chain deficient peripheral neuropathy. Hum. Mol. Genet. 2006;15:2690–2700. doi: 10.1093/hmg/ddl201. [DOI] [PubMed] [Google Scholar]
- 52.Gordon P.H., Moore D.H., Miller R.G., Florence J.M., Verheijde J.L., Doorish C., Hilton J.F., Spitalny G.M., MacArthur R.B., Mitsumoto H., et al. Western ALS Study Group. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 53.Minagar A., Alexander J.S., Schwendimann R.N., Kelley R.E., Gonzalez-Toledo E., Jimenez J.J., Mauro L., Jy W., Smith S.J. Combination therapy with interferon beta-1a and doxycycline in multiple sclerosis: an open-label trial. Arch. Neurol. 2008;65:199–204. doi: 10.1001/archneurol.2007.41. [DOI] [PubMed] [Google Scholar]