Abstract
The 2-fluoroalkoxy-substituted catechol-aporphines 6, 8a−f and 11-mono-hydroxyaporphines 11a−e were synthesized and found to have high in vitro affinity and selectivity for the dopamine D2 receptors. The catechol aporphines, 8b and 8d, and the monohydroxy aporphines, 11a−d, were identified as candidates for development as potential PET ligands.
Keywords: Aporphine, D2 agonist, neurological disorders, positron emission tomography, dopamine receptors
Dopamine is unarguably one of the most important neurotransmitters in the brain. Disturbances in the dopaminergic system, and especially irregularities in dopamine D2 receptor function, have been implicated in many different neurological and psychiatric disorders, including Parkinson's disease, Huntington's chorea, schizophrenia, attention deficit−hyperactivity disorder, Tourette's syndrome, restless leg syndrome, and addiction.1,2 Early diagnosis of these disorders is desirable, as early treatment would allow for a better outcome for the patient, by either slowing the progression of the disease or lessening the severity of the symptoms or future episodes. Physical symptoms tend to manifest themselves much later, after significant changes occur in the brain. Thus, identification of subtle changes in the brain early in the course of the disease, before a clinical diagnosis from physical symptoms can be made, would offer the best opportunity for early treatment.
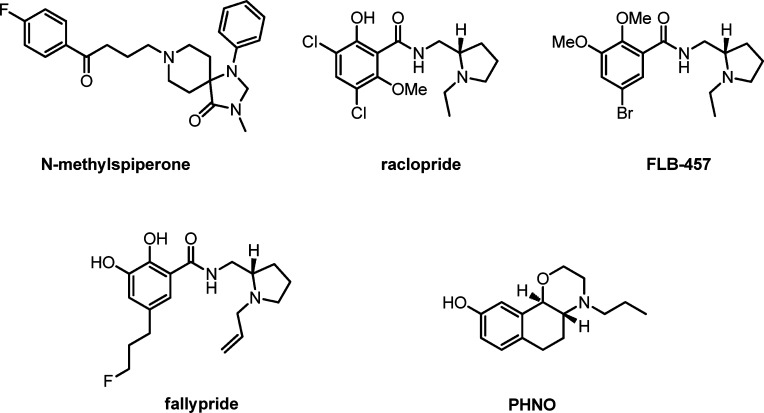
Noninvasive imaging of molecular and biological processes in living subjects with positron emission tomography (PET)3 and single photon emission computed tomography (SPECT)4,5 are invaluable tools for the investigation of human neurochemistry and neuropharmacology in vivo.6 Thus, extensive research efforts have been directed toward the development of PET radioligands suitable for probing the dopaminergic system. For example, the PET ligands [18F]-DOPA and the dopamine transporter ligand, [11C]-PE2I, have been used to quantify the presynaptic dopamine levels in patients suffering from Parkinson's disease.3 However, these radioligands do not elucidate the postsynaptic dopamine functions in neurological disorders. To gain more insight into dopamine D2 receptor function, several different D2 receptor radioligands have been developed to date. These include radioligands for striatal D2 binding, [11C]methylspiperone and [11C]raclopride, and high affinity ligands for extrastriatal binding, [11C]FLB457, [11C]cyclopropyl-FLB457, and [18F]fallypride (see Figure 1 for structures).7
Figure 1.
High affinity D2 ligands used in PET imaging.
Although these radioligands are invaluable tools for studying or diagnosing diseases, they have certain limitations. As mentioned above, the presynaptic radioligands do not directly provide information on postsynaptic dopamine function. Of the D2 radioligands discussed above, [11C]raclopride binding is reduced when the synaptic dopamine concentration is high. For others, selectivity may be an issue. For example, [11C]methylspiperone also has 5HT2 affinity, while the higher affinity ligands [11C]FLB457 and [18F]fallypride do not discriminate between D2 and D3 sites.8 It has been hypothesized that in schizophrenia and other DA-dependent neurological disorders, more D2 receptors exist in the D2high state9−12 and that D2high is the primary and common target for the antiparkinson action of dopamine agonists.13,14 However, all of the D2 radioligands discussed above are based on benzamide D2 antagonists, which do not discriminate between high affinity (D2high) and low affinity (D2low) states of the D2 receptor. Therefore, it is anticipated that an agonist tracer would be more sensitive to endogenous DA concentration changes than that of an antagonist tracer and, thus, will serve as a superior probe for quantifying endogenous DA concentration.15,16 There have been very few attempts to develop D2 agonist radiotracers to date. The agonist tracer [18F]F-PHNO was recently reported to have high binding affinities to D2 and D3 receptors in vitro as well as good brain penetration; however, in contrast to [3H]-(+)-PHNO (Figure 1), it did not perform well ex vivo.17 However, [11C]-(+)-PHNO proved to be a nonselective D2/D3 receptor agonist tracer with good brain uptake and favorable kinetics for PET in humans.18,19 As an alternative, aporphines exhibiting D2 receptor activity in the brain have been considered as potential agonist PET tracers. Several 11C- and 18F-labeled apomorphine analogues have been investigated for their in vivo binding potency to the D2 receptors and their distribution in brain and peripheral tissues of rats or monkey, such as N-[11C]methylnorapomorphine,20N-n-3-[18F]fluoropropylnorapomorphine,21,22N-n-2-[18F]fluoroethylnorapomorphine,22N-[11C]-propylnor-apomorphine (NPA),23 2-[11C]methoxy-NPA,24,25 and 2-chloro-N-[11C-propyl]norapomorphine.26
A need for even more potent dopamine ligands, with higher selectivity and oral availability, has led to the development of many novel aporphines.27 Furthermore, substituents in the 2-position of aporphines have been demonstrated to modulate the dopaminergic receptor potency and D2/D1 selectivity.28−33 In this report, several novel 2-fluoroalkoxy aporphines were synthesized and tested for dopamine receptor affinities and selectivities.
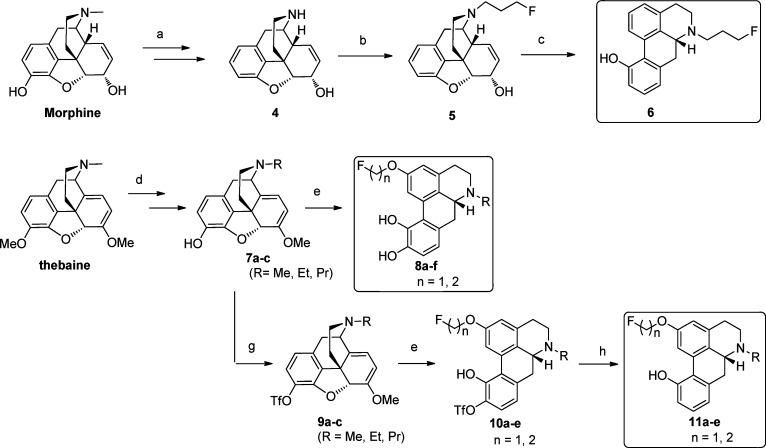
The synthesis of the 12 target molecules (6, 8a−f, and 11a−e) is shown in Scheme 1. 3-Deoxynormorphine 4 was prepared from morphine according to our published procedure in four steps.34N-Alkylation of 4 with 1-bromo-3-fluoropropane led to the N-substituted-3-deoxynormorphine 5. Acid-catalyzed rearrangement of 5 with methanesulfonic acid at 90−100 °C yielded the target compound 11-hydroxy-N-(3-fluoropropyl)aporphine 6. Starting from thebaine, N-ethyl and -propyl nororipavines 7b,c were prepared in four steps using our previously reported procedure.35 Acid-catalyzed rearrangement of oripavine 7a or nororipavines 7b,c with methanesulfonic acid at 90−95 °C36 in the presence of 2-fluoroethanol or 3-fluoropropanol yielded the corresponding fluorinated compounds 8a−f. N-n-Alkyl-3-O-[(trifluoromethyl)sulfonyl]nororipavines 9b,c were prepared in five steps from thebaine according the published procedure.37 3-O-[(Trifluoromethyl)sulfonyl] oripavine 9a was obtained in one step from oripavine.37 Acid-catalyzed rearrangement of 9a−c with methanesulfonic acid at 90−100 °C in the presence of either fluoroethanol or fluoropropanol yielded 10a−e. Pd/C-catalyzed reduction of the latter with Mg metal in MeOH at room temperature in the presence of NH4OAc37 provided the target compounds 2-(fluoroalkoxy)-11-hydroxy-N-n-alkylnoraporphines 11a−e. Alternatively, triflates 10a−e could be reduced using a Pd-triethylhydrosilane system to furnish 11-hydroxy aporphine derivatives 11a−e.38
Scheme 1. Synthesis of Aporphine Analogues 6, 8a−f, and 11a−e.
Reagents and conditions: (a) Ref (34). (b) 1-Bromo-3-fluoropropane, NaHCO3, EtOH/reflux. (c) MeSO3H, 95 °C. (d) Ref (35). (e) ROH, MeSO3H, 95 °C. (f) NaI, acetone, reflux. (g) PhNTf2, Et3N, CH2Cl2. (h) Pd/c, Mg, NH4OAc, MeOH.
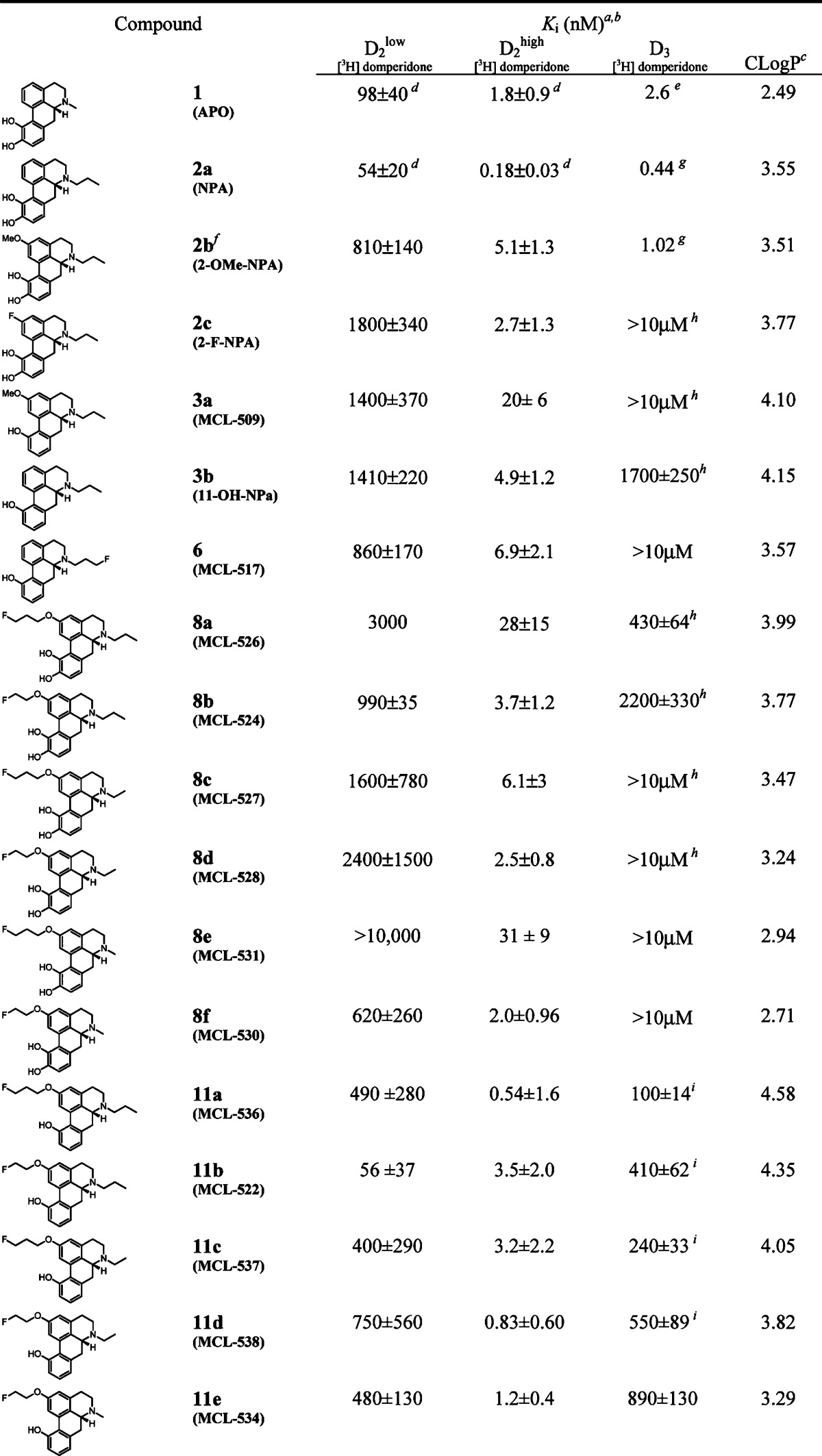
The receptor affinities of the 12 novel compounds 6, 8a−f, and 11a−e at D1 and D2 dopamine receptors were assessed using competitive radioreceptor binding assays with membrane-containing homogenates of rat corpus striatum tissue. Affinities to the D3 receptor were assessed using human D3 clones following procedures previously reported in detail14 (see the Supporting Information for details). However, the receptor affinities at D3 dopamine receptors for compounds 11a−d were assessed using rat clones following procedures reported in detail39 (see the Supporting Information for details). The results are summarized in Table 1.
Table 1. Affinities (Ki) at Dopamine D2 and D3 Receptorsb.
Source and radioligands: D1, rat striatum [3H]SCH23390; D2, rat striatum [3H] domperidone; D3, human D3 clone [3H]domperidone; errors are expressed as standard deviations.
Compounds have been tested and found to have low affinity to D1 (Ki > 5000) except for the following: 1 (Ki = 650 ± 310 nM), 2a (Ki = 490 ± 220 nM), 3b (Ki = 4300 ± 250 nM), 8f (Ki = 3700 ± 1200 nM), and 11e (Ki = 340 ± 44 nM), and no affinity to D1high, except for the following: 1 (Ki = 4.6 ± 1.2 nM), 2a (Ki = 1 ± 0.2 nM), and 2b (Ki = 8.1 ± 0.7nM); data for 1 and 2a obtained from ref (13).
Calculated using the chemical properties feature in CambridgeSoft ChemDraw Ultra, version 12.0.
Data from ref (13).
Data from ref (14).
For preparation, see ref (40).
See ref (25); HEK293T cell homogenate used with [3H]methylspiperone.
The following compounds were also found to have D3high affinity: 2c (Ki = 3.8 ± 2 nM), 3a (Ki = 130 ± 100 nM), 3b (Ki = 1.2 ± 1 nM), 8a (Ki = 1.1 ± 2 nM), 8b (Ki = 1.9 ± 1.5 nM), 8c (Ki = 230 ± 140 nM), and 8d (Ki = 250 ± 19 nM).
Source and radioligands: D3 rat clone [3H] domperidone; data provided by PDSP.
From the binding data shown in Table 1, we observed that the cold compounds 6, 8a−f, and 11a−e showed good to high affinity at D2high site, high selectivity of D2 versus D1, and in contrast to [11C]-(+)-PHNO17−19 and [18F]fallypride,8 exhibited low affinity or no affinity at all to the D3 site. N-Fluoropropyl aporphine 6 retained a similar binding affinity as N-propyl analogue 3b to D2high (6.9 and 4.9 nM, respectively). However, it has been previously shown that increasing the length of the N-substituent beyond three carbons causes D2 binding affinity to drop, thereby limiting the potential for improving binding affinity and selectivity by varying the labeled N-substituent.41 Placing a fluoroalkoxy group at position 2 would allow for more flexibility with respect to ligand design. Thus, a series of different N-n-propyl, ethyl, and methyl aporphines were systematically synthesized and evaluated, while varying the fluoropropanoxy and fluoroethoxy chains at position 2 (see Table 1). Likewise, the corresponding 10,11-dihydroxy (8a−f) and 11-hydroxy analogues (11a−e), all aimed at achieving the best combination of binding affinity, selectivity, and lipophilicity, were also evaluated.
We began by focusing on N-n-propyl catechol-aporphines, since these have been shown to have consistently higher D2 binding affinities and selectivities over their N-ethyl and N-methyl counterparts.41 Unfortunately, the 2-fluoropropanoxy analogue 8a exhibited a loss of D2high affinity as compared to NPA (2a), 2-MeO-NPA (2b),40 and 2-F-NPA (2c) (27 vs 5.1 to 2.7 nM range). However, in comparison, we found that by removing one carbon from the 2-substituent, 2-fluoroethoxy analogue (8b) restored D2high affinity (3.7 nM) without compromising the remaining DA receptor binding affinity profile.
We next focused our attention on N-ethyl analogues, with the expectation that we may be able to improve D2high binding affinities. In comparison to the N-propyl catechol-aporphine analogue 8a, the N-ethyl-2-fluoropropanoxy catechol-aporphine 8c afforded about a 4-fold increase in D2high affinity, while simultaneously showing higher selectivity against D3. The 2-fluoroethoxy analogue (8d) afforded a more than 2-fold improvement in D2high binding affinity, while retaining a similar binding affinity profile among the other dopamine receptors tested.
Encouraged by these findings, we investigated the N-methyl series. It was found that the 2-fluoropropanoxy catechol aporphine 8e exhibited a D2high affinity consistent with 8a, although, unlike 8a or even 8c, it did not exhibit any D3high affinity. The 2-fluoroethoxy analogue 8f afforded further improvement in D2high binding over the N-propyl and N-ethyl analogues 8b and 8d, again with no appreciable affinity to D3.
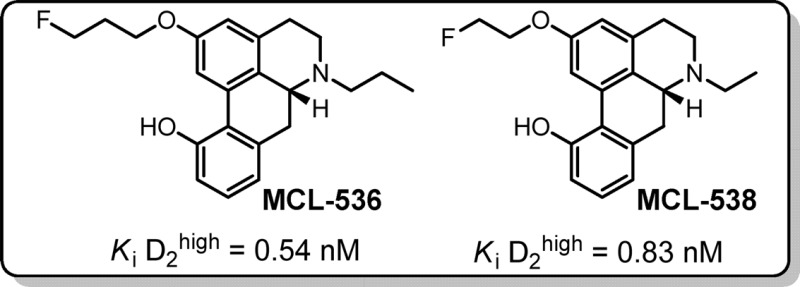
Finally, we investigated the series of 11-monohydroxy aporphines to determine the effect of the absence of the 10-hydroxy group on D2high binding affinities. It was reasoned that although 11-monohydroxyaporphines exhibited reduced D2high binding affinities as compared to the 10,11-dihydroxy analogues (see 3a, 3b, and 6 as compared to 1 and 2a−c, Table 1), they were also far less prone to oxidation than the catechol-aporphines and may thus be a viable consideration for development of imaging agents. Because catechol-aporphine 8f exhibited the highest D2high binding affinity among the catechol-aporphine derivatives, we began by synthesizing and testing its 11-monohydroxy analogue, 11e. We expected to obtain a more stable analogue, hopefully without significantly sacrificing D2high binding. We were pleased to find that the binding profile of analogue 11e exceeded our expectations, having a binding affinity to the D2high receptor in the same range as 8f. Encouraged by this promising result, we proceeded to synthesize and test other fluorinated 11-monohydroxy aporphines, which we hypothesized might also have analogous relationships in binding affinities to their parent catechol-aporphines as the 8f−11e pair. Another advantage to developing N-alkyl aporphines is that they are predicted to be more lipophilic than N-methyl derivatives, which may make them better candidates as potential PET ligands. Next, we tested N-propyl-2-fluoroethoxy-11-monohydroxy aporphine 11b, the analogue of catechol-aporphine 8b and found that, indeed, as in the 8f−11e pair, the D2high binding affinity was about the same. We were pleased to find that the N-ethyl 11-monohydroxy analogue 11d was found to have binding affinity on the order of 1 nM and exhibiting an improved binding affinity than its 10,11-dihydroxy analogue 8d. Next, we tested N-ethyl-2-fluoropropanoxy-11-monohydroxy aporphine 11c. As expected, it also exhibited an overall improved binding affinity to D2high as compared to its 10,11-dihydroxy analogue 8c. Unexpectedly, the last analogue, N-propyl-2-fluoropropanoxy-11-monohydroxy aporphine 11a, had the highest D2high binding affinity of any of the aporphines tested in this study. It was measured to have an average Ki value of 0.54 nM, which is at minimum an order of magnitude higher than its dihydroxy analogue 8a. Our goal of finding a fluorinated aporphine with high binding affinity and high selectivity to D2high was achieved. Having a series of fluorinated aporphines derivatives in hand, which exhibit binding affinities in the 0−2 nM range (8b,d,f; 11a−e), we can proceed with radiolabeling and in vivo PET studies.
A series of aporphines containing different N-substituents, substituents at the 2-position, 10,11-dihydroxy-, and 11-monohydroxy- groups have been systematically synthesized and tested for dopamine receptor binding affinity. Some conclusions could be made from the obtained binding data. It was found that 2-fluoroethoxy catechol-aporphines generally tended to have higher affinity to D2high than the 2-fluoropropanoxy analogues. In contrast to the generally accepted trend for nitrogen substituents in the aporphine series, smaller substituents on nitrogen (Me > Et > nPr) allowed for improved D2high binding and selectivity. Contrary to our expectations, removal of the 10-hydroxy group generally enhanced the binding affinity to the D2high site. This is an especially attractive characteristic since the 11-hydroxy aporphines are more resistant to oxidation and are orally active42 as compared to their 10,11-dihydroxy counterparts. Two of the 11-hydroxy aporphines, 11a and 11d, were found to have average subnanomolar affinity to the D2high binding site. Unlike the series of catechol-aporphines, no clear structure−activity relationships between the substituents and the binding affinities could be concluded in the 11-monohydroxy aporphine series. Finally, this new class of fluorinated aporphines could potentially prove to be valuable as therapeutics or as PET ligands. Such studies are currently in progress.
Acknowledgments
For compounds 11a−d, D3Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract #HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth, M.D., Ph.D., at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH (Bethesda MD). Thebaine, morphine, and oripavine were generously donated by Mallinckrodt Inc.
Supporting Information Available
Experimental details and characterization of all compounds and biological methods. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by a grant from the Branfman Family Foundation (J.L.N.) and NIH NIDA Research Training Grant T-32-DA007252 (A.W.S.).
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Bozzi Y.; Borrelli E. Dopamine in neurotoxicity and neuroprotection: What do D2 receptors have to do with it?. Trends Neurosci. 2006, 29, 167–174. [DOI] [PubMed] [Google Scholar]
- Marsden C. A. Dopamine: The rewarding years. Br. J. Pharmacol. 2006, 147, S136–S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ametamey S. M.; Horner M.; Schubiger P. A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [DOI] [PubMed] [Google Scholar]
- Innis R. B.; Seibyl J. B.; Scanley B. E.; Laruelle M.; Abi-Dargham A.; Wallace E.; Baldwin R. M.; Zea-Ponce Y.; Zoghbi S.; Wang S.; Gao Y.; Neumeyer J, L.; Charney D. S.; Hoffer P. B.; Marek K. L. Single photon emission computed imaging demonstrates loss of dopamine transporters in Parkinson's Disease. Proc. Natl. Acad. Sci. U.S.A. 1993, 90, 11965–11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibyl J. P.; Marek K.; Sheff K.; Zoghbi S.; Baldwin R. M.; Charney D. S.; van Dyck C. H.; Innis R. B. Iodine-123-beta-CIT and iodine-123-FPCIT SPECT measurement of dopamine transporters in healthy subjects and Parkinson’s patients. J. Nucl. Med. 1998, 3991500–1508. [PubMed] [Google Scholar]
- Neumeyer J. L.; Wang S.; Gao Y.; Milius R. A.; Kula N. S.; Campbell A.; Baldessarini R. J.; Zea-Ponce Y.; Baldwin R. M.; Innis R. B. N-ω-Fuoroalkyl Analogs of (1R)-2β-Carbomethoxy-3β-(4-iodophenyl)-tropane (β-CIT): Radiotracers for Positron Emission Tomography and Single Photon Emission Computed Tomography Imaging of Dopamine Transporters. J. Med. Chem. 1994, 37, 1558–1561. [DOI] [PubMed] [Google Scholar]
- Airaksinen A. J.; Nag S.; Finnema S. J.; Mukherjee J.; Chattopadhyay S.; Gulyas B.; Farde L.; Halldin C. [11C]Cyclopropyl-FLB457: A PET radioligand for low densities of dopamine D2 receptors. Bioorg. Med. Chem. 2008, 16, 6467–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsinga P. H.; Hatano K.; Ishiwata K. PET tracers for imaging of the dopaminergic system. Curr. Med. Chem. 2006, 13, 2139–2153. [DOI] [PubMed] [Google Scholar]
- Seeman P. All psychotic roads lead to increased dopamine D2high receptors: a perspective. Clin. Schizophrenia Relat. Psychoses 2008, 351–355. [Google Scholar]
- Seeman P.; Weinshenker D.; Quirion R.; Srivastava L. K.; Bhardwaj S. K.; Grandy D. K.; Premont R. T.; Sotnikova T. D.; Boksa P.; El-Ghundi M.; O'Dowd B. F.; George S. R.; Perreault M. L.; Männistö P. T.; Robinson S.; Palmiter R. D.; Tallerico T. Dopamine supersensitivity correlates with D2high states, implying many paths to psychosis. Proc. Natl. Acad. Sci. 2005, 102, 3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Dopamine D2high Receptors Measured Ex Vivo are Elevated in Amphetamine-Sensitized Animals. Synapse 2009, 63, 186–192. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine D2high Receptors on Intact Cells. Synapse 2008, 62, 314–318. [DOI] [PubMed] [Google Scholar]
- Seeman P. Antiparkinson therapeutic potencies correlate with their affinities at dopamine D2high receptors. Synapse 2007, 61, 1013–1018. [DOI] [PubMed] [Google Scholar]
- Seeman P.; Ko F.; Willeit M.; McCormick P.; Ginovart N. Antiparkinson concentrations of pramipexole and PHNO occupy dopamine D2High and D3High receptors. Synapse 2005, 58, 122–128. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J. Cereb. Blood Flow Metab. 2000, 20, 423–451. [DOI] [PubMed] [Google Scholar]
- Ross S. B.; Jackson D. M. Kinetic properties of the in vivo accumulation of 3H-(−)-N-n-propylnorapomorphine in mouse brain. Naunyn-Schmeideberg's Arch. Pharmacol. 1989, 340, 13–20. [DOI] [PubMed] [Google Scholar]
- Vasdev N.; Seeman P.; Garcia A.; Stableford W. T.; Nobrega J. N.; Houle S.; Wilson A. A. Syntheses and in vitro evaluation of fluorinated naphthoxazines as dopamine D2/D3 receptor agonists: Radiosynthesis, ex vivo biodistribution and autoradiography of [18F]F-PHNO. Nucl. Med. Biol. 2007, 34, 195–203. [DOI] [PubMed] [Google Scholar]
- Narendran R.; Slifstein M.; Guillin O.; Hwang Y.; Hwang D.-R.; Scher E.; Reeder S.; Rabiner E.; Laruelle M. Dopamine (D2/3) Receptor Agonist Positron Emission Tomography Radiotracer [11C]-(+)-PHNO is a D3 Receptor Preferring Agonist In Vivo. Synapse 2006, 60, 485–495. [DOI] [PubMed] [Google Scholar]
- Willeit M.; Ginovart N.; Kapur S.; Houle S.; Hussey D.; Seeman P.; Wilson A. A. High-Affinity States of Human Brain Dopamine D2/3 Receptors Imaged by the Agonist [11C]-(+)-PHNO. Biol. Psychiatry 2006, 59, 389–394. [DOI] [PubMed] [Google Scholar]
- Zijlstra S.; Vanderworp H.; Wiegman T.; Visser G. M.; Korf J.; Vaalburg W. Synthesis and in vivo distribution in the rat of a dopamine agonist: N-([11C]methyl)norapomorphine. Nucl. Med. Biol. 1993, 20, 7–12. [DOI] [PubMed] [Google Scholar]
- Zijlstra S.; Visser G. M.; Korf J.; Vaalburg W. Synthesis and in vivo distribution in the rat of several fluorine-18 labeled N-fluoroalkylaporphines. Appl. Radiat. Isot. 1993, 44, 651–658. [DOI] [PubMed] [Google Scholar]
- Morin I.; Gao Y.; Kula N. S.; Baldessarini R. J.; Neumeyer J. L. N-monofluoroalkylnoraporphines: Synthesis and binding dopamine receptor studies. Med. Chem. Res. 1992, 2, 354–360. [Google Scholar]
- Finnema S. J.; Seneca N.; Farde L.; Shchukin E.; Sovago J.; Gulyas B.; Wikstrom H. V.; Innis R. B.; Neumeyer J. L.; Halldin C. A Preliminary PET Evalulation of the New Dopamine D2 Receptor Agonist [11C]MNPA in Cynomolgus Monkey. Nucl. Med. Biol. 2005, 32, 353–360. [DOI] [PubMed] [Google Scholar]
- Hwang D.-R.; Narendran R.; Laruelle M. Positron-labeled dopamine agonists for probing the high affinity states of dopamine subtype 2 receptors. Bioconjugate Chem. 2005, 16, 27–31. [DOI] [PubMed] [Google Scholar]
- Skinbjerg M.; Namkung Y.; Halldin C.; Innis R. B.; Sibley D. R. Pharmacological characterization of 2-methoxy-N-propylnorapomorphine’s interactions with D2 and D3 dopamine receptors. Synapse 2009, 63, 462–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palner M.; McCormick P.; Gillings N.; Begtrup M.; Wilson A. A.; Knudsen G. M. Radiosynthesis and ex vivo evaluation of (R)-(−)-2-chloro-N-[1-11C-propyl]n-propylnorapomorphine. Nucl. Med. Biol. 2010, 37, 35–40. [DOI] [PubMed] [Google Scholar]
- Zhang A.; Zhang Y.; Branfman A. R.; Baldessarini R. J.; Neumeyer J. L. Advances in development of dopaminergic aporphinoids. J. Med. Chem. 2007, 50, 171–181. [DOI] [PubMed] [Google Scholar]
- Ramsby S.; Neumeyer J. L.; Grigoriadis D.; Seeman P. 2-Haloaporphines as potent dopamine agonists. J. Med. Chem. 1989, 32, 1198–1201. [DOI] [PubMed] [Google Scholar]
- Neumeyer J. L.; Gao Y.; Kula N. S.; Baldessarini R. J. Synthesis and dopamine receptor affinity of (R)-(−)-2-fluoro-N-n-propylnorapomorphine: a highly potent and selective dopamine D2 agonist. J. Med. Chem. 1990, 33, 3122–3124. [DOI] [PubMed] [Google Scholar]
- Søndergaard K.; Kristensen J. L.; Gillings N.; Begtrup M. Synthesis of (R)-(−)-2-fluoro norapomorphine—A precursor for the synthesis of (R)-(−)-2-fluoro-N-[11C]propylnorapomorphine for evaluation as a dopamine D2 agonist ligand for PET investigations. Eur. J. Org. Chem. 2005, 4428–4433. [Google Scholar]
- Zhang A.; Csutoras C.; Zong R.; Neumeyer J. L. Synthesis of 2-fluoro-11-hydroxy-N-propylnoraporphine: A potential dopamine D2 agonist. Org. Lett. 2005, 7, 3239–3242. [DOI] [PubMed] [Google Scholar]
- Si Y.-G.; Gardner M. P.; Tarazi F. I.; Baldessarini R. J.; Neumeyer J. L. Synthesis and binding studies 2-O- and 11-O-substituted N-alkylnoraporphines. Bioorg. Med. Chem. Lett. 2008, 18, 3971–3973. [DOI] [PubMed] [Google Scholar]
- Si Y.-G.; Gardner M. P.; Tarazi F. I.; Baldessarini R. J.; Neumeyer J. L. Synthesis and dopamine receptor affinities of N-alkyl-11-hydroxy-2-methoxynoraporphines: N-alkyl substituents determine D1 versus D2 receptor selectivity. J. Med. Chem. 2008, 51, 983–987. [DOI] [PubMed] [Google Scholar]
- Csutoras C; Zhang A.; Bidlack J. M.; Neumeyer J. L. An investigation of the N-demethylation of 3-deoxymorphine and the affinity of the alkylation products to μ, δ, and κ receptors. Bioorg. Med. Chem. Lett. 2004, 17, 2687–2690. [DOI] [PubMed] [Google Scholar]
- Si Y. G.; Gardner M. P.; Tarazi F. I.; Baldessarini R. J.; Neumeyer J. L. Synthesis and Dopamine Receptor Affinities of N-Alkyl-11-hydroxy-2-methoxynoraporphines: N-Alkyl Substituents Determine D1 versus D2 Receptor Selectivity. J. Med. Chem. 2008, 51, 983–987. [DOI] [PubMed] [Google Scholar]
- Sipos A.; Csutoras C.; Berenyi S.; Uustare A.; Rinken A. Synthesis and neuropharmacological characterization of 2-O-substituted apomorphines. Bioorg. Med. Chem. 2008, 16, 4563–4568. [DOI] [PubMed] [Google Scholar]
- Si Y. G.; Neumeyer J. L. Facile Synthesis of 2-Methoxy-11-hydroxyaporphine: A Potential D1 Receptor Ligand. Synthesis 2007, 24, 3787–3790. [Google Scholar]
- Hupp C. D.; Neumeyer J. L. Rapid Access to Morphinones: Removal of 4,5-Ether Bridge with Pd-catalyzed triflate reduction. Tetrahedron Lett. 2010, 51, 2359–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://pdsp.med.unc.edu/UNC-CH/Protocol/Book.pdf (accessed Nov 12, 2010).
- Gao Y.; Baldessarini R. J.; Kula N. S.; Neumeyer J. L. Synthesis and Dopamine Receptor Affinities of Enantiomers of 2-Substituted Apomorphines and Their N-n-Propyl Analogs. J. Med. Chem. 1990, 33, 1800–1805. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Ram V. J.; Campbell A.; Kula N. S.; Baldessarini R. J.; Neumeyer J. L. Synthesis and structural requirements on N-substituted norapomorphines for affinity and activity at dopamine D-1, D-2, and agonist receptor sites in rat brain. J. Med. Chem. 1990, 33, 39–44. [DOI] [PubMed] [Google Scholar]
- Campbell A.; Baldessarini R. J.; Gao Y.; Ram V. J.; Neumeyer J. L. R(−) and S(+) stereoisomers of 11-hydroxy-N-n-propylnoraporphine: Central dopaminergic behavioral activity in the rat. Neuropharmacology 1990, 29, 527–536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.