Abstract
An improved synthesis of a water-soluble deep-cavity cavitand (octa-acid, 1) is presented. Previously (Gibb, C. L. D. & Gibb, B. C., J. Am. Chem. Soc., 2004, 126, 11408–11409) we documented access to host 1 in eight (non-linear) steps starting from resorcinol; a synthesis that required four steps involving chromatographic purification. Here we reveal a modified synthesis of host 1. Consisting of seven (non-linear) steps, this new synthesis involves only one chromatographic step, and avoids a minor impurity observed in the original approach. This improved synthesis will therefore be useful for the laboratories that are investigating the properties of these types of host.
Keywords: host, cavitand, water-soluble, octa-acid, supramolecular chemistry
Results and Discussion
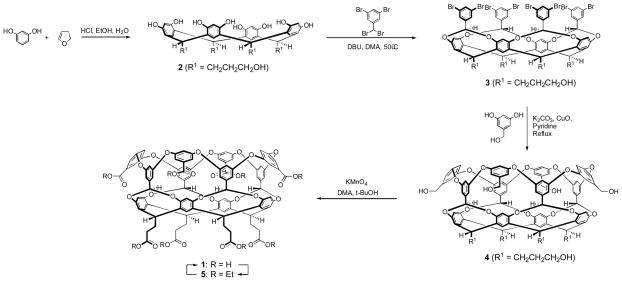
Deep-cavity cavitand 1, the so-called ‘octa-acid’ (Scheme 1), is a water-soluble host that is central to our studies of aqueous-based supramolecular systems (1). Its ease of synthesis and propensity to dimerize into supramolecular nano-capsules has proven to be of considerable interest both to our own studies(2, 3), and to a number of other research groups who are independently studying it (4–7). The host is a bowl-shaped amphiphile composed of a concave hydrophobic pocket, a wide hydrophobic rim, and a convex outer surface ‘coated’ with eight water-solubilizing carboxylic acid groups. Because of these structural features, in aqueous solution the host is essentially monomeric, but the presence of a suitable guest molecule triggers dimerization of the host and encapsulation of the guest (or guests) (2, 3, 8). Driven by the hydrophobic effect, this assembly occurs for molecules as small as propane (9) and other small hydrocarbons (10), as large as steroids (1), and as polar as triethylene glycol derivatives (11). The significant kinetic stability of the capsule has also allowed it to demonstrate interesting function, including as a yoctoliter reaction flask for controlling the course of photochemical reactions (12–19), and a means to bring about the kinetic resolution of constitutional isomers (20).
Scheme 1.
Synthesis of octa-acid 1.
Previously (1) we documented access to octa-acid 1 in eight overall steps (longest linear sequence six-steps) starting from the resorcinol condensation with dihydrofuran to give resorcinarene 2 (structure in Scheme 1). However, since this report we have noted the presence of some cavitand impurities that arise in the synthesis. Although not affecting the binding properties of the octa-acid 1, these have caused some concern and also attenuate the overall reported yield of 9%. Furthermore, the fact that the workup of four steps required chromatography reduces the appeal of the synthesis somewhat. With these points noted, and with octa-acid 1 and related derivatives becoming increasingly utilized, we have been keen to make the synthesis of 1 more efficient. Consequently, we report here on a modified synthesis of octa-acid 1 that consists of seven total steps including a six-step linear sequence. The overall yield of this shorter process is 8%, but this value represents pure octa-acid 1. Furthermore, in this new synthesis only one chromatographic step is required. In combination these improvements have increase the accessibility of octa-acid and other such deep-cavity cavitands in our laboratory. We therefore believe that others who study these hosts will benefit from this modified synthesis.
The overarching idea behind the synthesis was to make the isolation of each intermediate as facile as possible, and if needed, add one chromatography step near the end of the synthesis to ensure maximum purity of the final product. The new synthetic route is shown in Scheme 1. The first step, the acid catalyzed condensation of resorcinol and dihydrofuran to give resorcinarene 2, was originally reported to occur in 61% yield on the 20 g scale (21). In studying the variables in this reaction we however observed that modifying the addition of dihydrofuran to the reaction solution increased the yield of 2 to 83%. The four-fold “bridging” of 2 to form octabromide 3 (Scheme 3) requires 3,5-dibromobenzal bromide, a compound that was originally synthesized in 72% yield (two steps). More specifically, metal halogen exchange of 1,3,5-tribromobenzene n-BuLi and quenching the resulting lithiate with dimethylformamide (DMF) gave 3,5-dibromobenzaldehyde, and subsequent bromination with BBr3 gave the corresponding benzal bromide(1, 22). Recently however, we identified a commercial source of 3,5-dibromo-benzaldehyde (Beta Pharma. Inc.) that eliminated the synthesis and (chromatographic) purification of this material. In addition, we devised a modification to the workup of the bromination step that avoided the chromatographic purification of the benzal bromide but still allowed it to be isolated in 92% yield after crystallization. These modifications provided rapid access to the benzal bromide in the tens of grams scale.
We did not identify an improved synthesis of octabromide 3 (45% yield), but we did determine an efficient means to isolate this highly insoluble compound. Previously, crude 3 was directly treated with benzyl bromide/NaH to generate the soluble tetra-benzyl ether that was isolated using chromatography. The protecting group was subsequently removed at a later stage in the synthesis. Wishing to improve on this procedure, we determined a work-up that took advantage of the insolubility of 3 so that the pure product could be obtained from a slow precipitation process. This avoided the benzylation step and its attendant chromatographic purification, but without prior benzylation the workup of the subsequent eight-fold ‘weaving’ reaction to make octol 4 presented a new problem: how to handle the very insoluble 4 to attain a sufficiently pure product for the synthesis of octa-acid 1. After considerable investigation we ultimately identified a workup procedure that allowed the isolation of 4 in 75–80% purity. NMR (in DMSO-d6) suggested that the impurities at this stage were not deep-cavity cavitands but intermediates in the ‘weaving’ process and/or incorrectly ‘woven’ products and could be readily removed at the end of the synthesis. Hence, this 75–80% pure 4 was considered satisfactory for the next step in the synthesis. That noted, 4 is itself a potentially useful intermediate for the synthesis of different cavitands, so we determined that the corresponding pure octa-acetate could be isolated in 75% yield by heating a solution of 4 in acetic anhydride, and that this could then be converted into pure 4 by treatment with aqueous dimethylacetamide and lithium hydroxide (92% yield).
Returning to the formation of 1, potassium permanganate was directly used to oxidize the crude 4 to give octa-acid 1 of an estimated 75–80% purity. 1H NMR analysis of the crude mixture revealed that it contained trace amounts of the same impurity occasionally observed in the earlier synthesis (1).
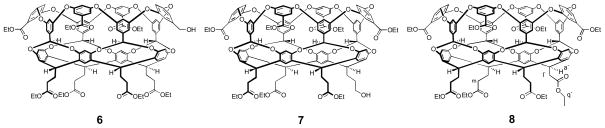
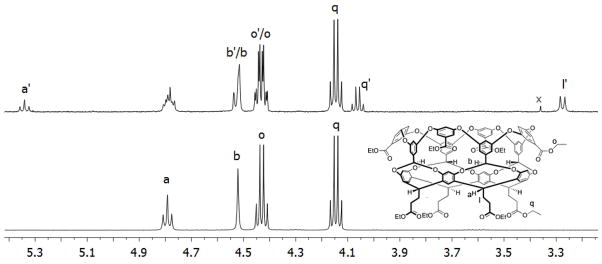
Octa-acid 1 is difficult to purify on the 100–1000 mg scale, and so to obtain pure 1 we carried out an esterification/hydrolysis procedure. Upon esterification with EtOH/HCl, chromatography of the crude mixture revealed and separated three deep-cavity cavitands in an approximate 7:2:1 ratio (overall 75–80% yield), as well the 20–25% of non-deep-cavity cavitand impurities. 1H NMR analysis of the cavitands revealed two points. First, the respectively products were the anticipated octa-ester 5 (Scheme 1), and the hepta-ester mono-ols 6, and 7 (Figure 1) arising from incomplete oxidation. Second, each product contained a minor cavitand impurity (< 5%). In an attempt to increase the yield of 5, the reaction time and equivalents of KMnO4 in the prior oxidation of 4 were increased, but these changes did not ultimately increase its yield at the expense of 6 or 7. However, these experiments did indicate increased amounts of the minor products, and this was confirmed by oxidizing crude 4, isolating the crude octa-acid 1, and then oxidizing the sample a second time. Esterification of this crude reaction mixture and chromatographic and NMR analysis revealed the sample consisted mostly of 5 and its attendant impurity, and very little 6 and 7. In this sample, it was not possible to completely resolve cavitand 5 from its respective minor product by column chromatography. However, partial separation was achieved to yield pure 5 (in overall 54% yield from 4) and the minor product in ca. 80–90% purity. A combination of MS and NMR identified the minor product. Figure 2 shows a portion of its 1H NMR spectrum along with that of pure octa-ester 5. The NMR of the former contains signals essentially identical to 5 but also characteristic shifted signals for: methine signal a (a′ at 5.35 ppm), methylene signal for q (q′ at 4.05 ppm), and methylene signal l (not shown is l′ at 3.25 ppm).
Figure 1.
Chemical structures of esters 6, 7 and 8.
Figure 2.
Partial 1H NMR spectra of: pure octa-ester 5 (lower spectrum) and minor product of 80–90% purity (upper spectrum). x indicates unknown trace impurity..
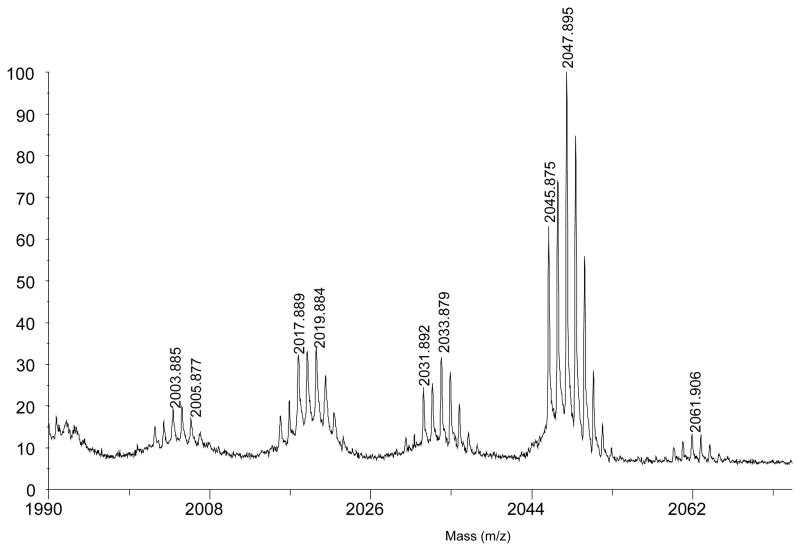
These observed signals could be explained by a shortening of one or more of the pendant groups of the cavitand, and MALDI-MS proven this to be the case. Thus the MS spectrum of 80–90% pure sample (Figure 3), showed a small peak for 5 (2061.9, [M + Ag]+), a major peak corresponding to a cavitand missing one methylene group (2047.9, [M + Ag]+), as well as three other peaks of decreasing intensity that differ by 14 Daltons (2033.9, 2019.9 and, 2003.9) (23). Thus, the major impurity of octa-ester 5 is octa-ester 8 (Figure 1), but also formed are cavitands in which two, three and four pendant groups have been shortened. Considering the amount of 6, 7 and the truncated versions of 5, 6 and 7 that is formed in the oxidation of 4, we were interested in examining alternatives to the oxidation conditions. However, of the many variations we considered none proved as effective as the KMnO4/DMA/t-BuOH combination that had been previously identified. Regardless, the final step in the synthesis of 1, i.e., the hydrolysis of pure octa-ester 5, occurred without incident (91% yield).
Figure 3.
MALDI-TOF MS of enriched pendant-shortened cavitands (matrix: AgNO3 + TFA).
We believe that in larger-scale syntheses of octa-acid using the original procedure, both hepta-acid mono-ols and the pendant group shortened derivatives are present in small quantities. Spectroscopically the former are very hard to identify, whilst the latter were collectively evident by small signals analogous to those shown in Figure 2. However, the presence of trace quantities of these compounds is unlikely to significantly affect the observed binding and assembly properties of octa-acid 1. Likewise, their similar molecular masses to 1 mean that it is unlikely that they significantly affect stoichiometry calculations for binding studies. Regardless, having as the penultimate step in the synthesis a workup that requires chromatography does allow these impurities to be removed and hence give essentially pure octa-acid 1 in an overall 8% yield.
In summary, we have devised a slightly shorter, but considerably more efficient synthesis of cavitand 1 that allows for the removal of trace quantities of cavitand side-products. In addition, we have devised a route to the isolation of pure 4; a cavitand that is an important intermediate in the synthesis of many cavitands with alternative outer coats that can bestow water solubility. We will report on these in due course, but the more efficient synthesis of 1, a procedure that allows the ready isolation of gram quantities of the host, should be of utility to those interested in the fascinating binding and assembly properties of octa-acid.
Experimental
General
3,5-dibromobenzaldehyde and 3,5-dihydroxybenzyl alcohol were purchased from Beta Pharma. Inc.; other reagents were purchased from Aldrich and were used without purification. DMA was dried over 4 Å molecular sieves and degassed before use; other solvents were used directly without additional purification. All reactions were performed under N2. NMR spectra were recorded on a Varian Inova 500 MHz spectrometer. Chemical shifts are reported relative to CDCl3 (7.26 ppm) or (CD3)2SO (2.50 ppm). MALDI-MS spectra were collected using a Bruker-Daltonics MALDI-TOF Autoflex III mass spectrometer. Elemental analysis was performed by Atlantic Microlab Inc.
Dodecanol 2
The synthesis of this compound has been previously reported (see reference 21) but we now use a slightly modified procedure. Typically 20 g (182 mmol, 1 equiv) of resorcinol was dissolved in 120 mL of methanol and 30 mL of 37% HCl under N2 and the solution was cooled to 0 °C. To this stirring solution, 13.8 mL (182 mmol, 1 equiv) of 2,3-dihydrofuran was then added via syringe over a 10 min period. The stirring reaction was maintained at 0 °C for 30 min. The mixture was subsequently heated to 50 °C for 5 days. During this time, a considerable amount of precipitate was formed. After this time the reaction mixture was allowed to cool to rt, the solid filtered off and taken up in 0.5 L of distilled water and sonicated. The solid was again filtered off, washed again with distilled water and dried at RT then 120 °C (27.10 g, yield: 83%).
3,5-dibromobenzal bromide
To a dry one liter round-bottomed flask containing 500 mL of dichloromethane was added 60g (227.4 mmol, 1.0 equiv) of 3,5-dibromo- benzaldehyde. To this stirring solution was added 23.8 mL (251.8 mmol, 1.1 equiv) of BBr3. The mixture was stirred at rt for 24 h (precipitate observed). Subsequently, 250 mL of water was added slowly to quench excess BBr3 and dissolve the water-soluble boronic acid side-product (Caution: quenching liberates HBr gas). The water and organic layers were separated and the organic phase washed twice with water (2 × 300 mL), and before Na2SO4 and active charcoal were added to the organic layer to dry and decolorize the solution. The solvent was removed under reduced pressure and the solid dried for 30 min under high vacuum. Crystallization from hexane afforded the benzal bromide as colorless crystals (85.4 g, yield: 92%).
Octa Bromide 3
To a dry one liter flask containing 500 mL of degassed DMA was added 10.0 g (13.87 mmol, 1.0 equiv) of dodecanol 2 and 27.2 g (66.71 mmol, 4.8 equiv) of 3,5-dibromobenzal bromide. After adding 16.6 mL (111.0 mmol, 8.0 equiv.) of DBU, the stirred reaction mixture was heated to 50 °C for 2 days. The DMA was subsequently removed under reduced pressure and the residue dried overnight under high vacuum. 500 mL of CHCl3 was then added to dissolve the crude mixture and the solution washed with water (3 × 300 mL). Particulate matter in the aqueous phase was discarded. The organic layer was dried quickly with anhydrous Na2SO4 (< 5 min) and filtered. A prolonged drying process resulted in the precipitation of product. The volume of solution was reduced under reduced pressure to ca. 150 mL and refrigerated (5 °C) for 12 h. The pure product, having precipitate from the solution, was filtered, washed with CHCl3, and dried at 120 °C (10.7 g, yield: 45%). Mp > 250°C. 1H NMR (500 MHz, DMSO) δ 7.93 (s, 8H), 7.72 (s, 4H), 7.12 (s, 4H), 5.50 (s, 4H), 4.71 (t, J = 10.0, 4H), 4.49 (t, J = 6.2, 4H), 3.53 (q, J = 8.3, 8H), 2.49 – 2.45 (m, 8H), 1.57 – 1.43 (m, 8H). MS (MALDI): Calcd. 1813 [M + Ag]+, Found: 1813 [M + Ag]+. Anal. Calcd. for C68H56Br8O12: C, 47.92; H, 3.31 Found: C, 47.58; H, 3.35.
Crude Octol 4
For ten minutes, N2 gas was bubbled through a stirring suspension of 6.84 g (4.0 mmol, 1.0 equiv) of octa-bromide 3, 3.36 g (24.0 mmol, 6.0 equiv) of 3,5-dihydroxybenzyl alcohol, and 6.62 g (48.0 mmol, 12 equiv) of K2CO3 in 300 mL of pyridine. After this time, 3.80 g (48.0 mmol, 12 equiv) of CuO nano-powder was added and the mixture was heated to a vigorous reflux by sand bath (a normal reflux was not sufficient to bring about reaction) for 3 weeks. After the solvent was removed by reduced pressure, the crude material was dried on a high vacuum line for 1 hour (excessive drying and/or exposing the mixture to air for extended periods led to greatly reduced yields). Subsequently, 250 mL of THF was added and the reaction mixture sonicated for 30 min. The mixture was then filtered through THF-wet Celite and the solvent of the filtrate evaporated under reduced pressure to give a brown crude solid. This material was dried at rt overnight under high vacuum. To the dried solid was added 50 mL of CHCl3 and suspension sonicated for 20 min. The solid obtained after filtration (the filtrate should be green) was suspended once again in CHCl3, sonicated for 20 min, and dried under vacuum for overnight at 120 °C to give ~ 3.9 g crude octol 4 as an off-white powder (yield by weight: 65%, ca. 75% purity by NMR, therefore, 45% estimated yield).
Octa-acetate of octol 4
To 10 mL of Ac2O was added 1g (0.62 mmol) of crude octol 4. The mixture was heated to 100 °C (oil bath) for 16 h. The homogeneous solution was then cooled to rt and the solvent removed under high vacuum. The residue was dried for 2 h and purified by chromatography (0.91 g, yield: 75%). Rf = 0.25 (CHCl3/Acetone, 15:1, v/v). Mp > 250 °C. 1H NMR (500 MHz, CDCl3) δ 7.20 (d, J = 2.0, 8H), 7.14 (s, 4H), 6.98 (t, J = 2.2, 4H), 6.54 (t, J = 2.0, 4H), 6.51 (d, J = 2.2, 8H), 6.01 (s, 4H), 5.20 (s, 8H), 4.82 (t, J = 8.2, 4H), 4.53 (s, 4H), 4.16 (t, J = 6.5, 8H), 2.32 (q, J = 8.0, 8H), 2.12 (s, 12H), 2.07, (s, 12H), 1.71 – 1.65 (m, 8H). MS (MALDI): Calcd. 2061 [M + Ag]+, Found: 2061 [M + Ag]+. Anal. Calcd. for C112H96O32: C, 68.85; H, 4.95 Found: C, 68.18; H, 4.94.
Pure Octol 4
To 910 mg (0.46 mmol, 1 equiv) of octa-acetate 5 in 60 mL DMA was added 10 ml of H2O and 625 mg (14.9 mmol, 32 equiv) of LiOH·H2O. The solution was heated to 50 °C for 24 h. The solvent was removed under high vacuum and the residue dried at rt for 16 h. 30 mL of 1 M HCl and 120 mL of H2O was added to the resulting solid and the mixture sonicated for 30 min. Filtration and washing with solid with distilled water gave octol 4, which was dried at rt for 16 h. 10 mL of Acetone was then added to this solid and the mixture sonicated for 2 min and allowed to stand for 2 h. Filtration and drying of the solid under high vacuum (at 120 °C) afforded pure octol 4 (690 mg, yield: 92%).
Crude Octa-acid 1
To a solution of 3.5 g (2.16 mmol, 1.0 equiv) of crude octol 4 in 300 mL degassed DMA and 300 mL of t-BuOH was added 9.57 g (60.55 mmol, 28.0 equiv) of KMnO4. The resulting purple solution was stirred at rt for 2 d. The reaction mixture was filtered and the solid washed thoroughly with 4 × 200 mL distilled water. The combined filtrate was evaporated under high vacuum and dried at rt for 16 h. 20 mL of 20% HCl was then added to the solid and the suspension sonicated for 5 min. Following filtration, the solid was shaken with 150 mL of distilled water. Filtration and washing with water gave crude octa-acid 1 which was dried at 120 °C for overnight (~ 3.4 g).
Octa-Ester 5 (Hepta-Esters 6 and 7)
HCl gas was bubbled through a solution of 2.5 g of crude octa-acid 1 in 150 mL of Ethanol for ~2 minutes. To this suspension was added 100 mL of CHCl3 and the solution heated up to reflux for 4 days. The solvent was then removed and 10 mL of Ethanol added to the residue, the resulting suspension shaken, and then filtered. The resulting off-white solid was dried at 120 °C for 16 h. Chromatography of the crude product afforded three main products: octa-ester 5 (1.26 g), hepta-ester-rim-OH 6 (0.38 g), and hepta-ester-feet-OH 7 (0.17 g). Octa-ester 5: Rf = 0.33 (CHCl3/Acetone, 40:1, v/v). mp > 250 °C. 1H NMR (500 MHz, CDCl3) δ 7.88 (d, J = 2.2, 8H), 7.19 (s, 4H), 7.02 (t, J = 2.1, 4H), 6.78 (t, J = 2.2, 4H), 6.52 – 6.50 (m, 8H), 5.97 (s, 4H), 4.79 (t, J = 8.3, 4H), 4.52 (s, 4H), 4.43 (q, J = 7.1, 8H), 4.14 (q, J = 7.1, 8H), 2.58 (q, J = 7.7, 8H), 2.31 (t, J = 7.4, 8H), 1.44 (t, J = 7.1, 12H), 1.26 (t, J = 7.1, 12H). MS (MALDI): Calcd. 2061 [M + Ag]+, Found: 2061 [M + Ag]+. Anal. Calcd. for C112H96O32: C, 68.85; H, 4.95 Found: C, 69.09; H, 4.90. Hepta-ester-rim-OH 6: Rf = 0.37 (CHCl3/Acetone, 15:1, v/v). mp > 250 °C. 1H NMR (500 MHz, CDCl3) δ 7.89 – 7.87 (m, 6H), 7.23 (d, J = 1.9, 2H), 7.19 (s, 4H), 7.01 (m, 4H), 6.78 (m, 3H), 6.54 (m, 1H), 6.51 (m, 8H), 6.01 (s, 1H), 5.98 (d, J = 2.4, 3H), 4.84 (s, 2H), 4.80 (m, 4H), 4.53 (s, 4H), 4.43 (q, J = 7.1, 6H), 4.15 (qd, J = 7.1, 1.1, 8H), 2.59 (q, J = 7.5, 8H), 2.32 (t, J = 7.0, 8H), 1.44 (t, J = 7.1, 9H), 1.26 (td, J = 7.1, 1.2, 12H). MS (MALDI): Calcd. 2019 [M + Ag]+, Found: 2019 [M + Ag]+. Anal. Calcd. for C110H94O31: C, 69.10; H, 4.96 Found: C, 68.21; H, 5.05. Hepta-ester-feet-OH 7: Rf = 0.24 (CHCl3/Acetone, 15:1, v/v). mp > 250 °C. 1H NMR (500 MHz, CDCl3) δ 7.90 – 7.86 (m, 8H), 7.24 (s, 2H), 7.17 (s, 2H), 7.02 (m, 4H), 6.78 (m, 4H), 6.54 – 6.50 (m, 8H), 5.97 (s, 4H), 4.82 – 4.74 (m, 4H), 4.54 (s, 1H), 4.52 (s, 3H), 4.43 (qd, J = 7.1, 2.2, 8H), 4.18 – 4.10 (m, 6H), 3.75 (q, J = 5.9, 2H), 2.72 – 2.46 (m, 6H), 2.46 – 2.24 (m, 8H), 1.59 – 1.51 (m, 2H), 1.44 (td, J = 7.1, 1.5, 12H), 1.26 (m, 9H). MS (MALDI): Calcd. 2019 [M + Ag]+, Found: 2019 [M + Ag]+. Anal. Calcd. for C110H94O31: C, 69.10; H, 4.96 Found: C, 68.44; H, 4.96.
Pure Octa-acid 1
To 430 mg (0.22 mmol, 1 equiv) of octa-ester 5 in 43 mL DMA was added 2.65 ml of 2.0 M (5.3 mmol, 24 equiv) aqueous LiOH. The solution was heated to 50 °C and small amounts of distilled water added until the precipitate fully dissolved. The resulting clear solution was stirred at 50 °C for 24 h. After this time the solution was filtered, the solvent removed under high vacuum, and the residue dried for 2 h. Subsequently, 5 mL of 20% HCl was added to the solid and the suspension sonicated for 1 min before a further 40 mL of water was added and the suspension shook. Filtration and washing with water gave octa-acid 1, which was dried at rt for 3 h. 5 mL of Acetone was then added to the solid, the mixture sonicated for 2 min, and then left to stand for 2 h. Filtration, and drying at 120 °C for 48 h under high vacuum afforded pure octa-acid 1 (370 mg, yield: 91%).
Acknowledgments
BCG, SL and CLDG acknowledge the financial support of the National Institutes of Health (GM074031). SWI acknowledges a fellowship from the Louisiana Board of Regents Graduate Fellows Program.
References
- 1.Gibb CLD, Gibb BC. J Am Chem Soc. 2004;126:11408–11409. doi: 10.1021/ja0475611. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Gibb BC. Chem Commun. 2008:3709–3716. doi: 10.1039/b805446k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibb BC. In: Organic Nano-Structures. Atwood JL, Steed JW, editors. John Wiley and Sons; 2007. [Google Scholar]
- 4.Chen JYC, Jayaraj N, Jockusch S, Ottaviani MF, Ramamurthy V, Turro NJ. J Am Chem Soc. 2008;130:7206–7207. doi: 10.1021/ja801667w. [DOI] [PubMed] [Google Scholar]
- 5.Podkoscielny D, Gadde S, Kaifer AE. J Am Chem Soc. 2009;131:12876–12877. doi: 10.1021/ja9045108. [DOI] [PubMed] [Google Scholar]
- 6.Saitoha M, Fukaminatoa T, Irie M. Journal of Photochemistry and Photobiology A: Chemistry. 2009;207:28–31. [Google Scholar]
- 7.Baldridge A, Samanta SR, Jayaraj N, Ramamurthy V, Tolbert LM. J Am Chem Soc. 2010;132:1498–1499. doi: 10.1021/ja908870k. [DOI] [PubMed] [Google Scholar]
- 8.Ewell J, Gibb BC, Rick SW. J Phys Chem B. 2008;112:10272–10279. doi: 10.1021/jp804429n. [DOI] [PubMed] [Google Scholar]
- 9.Gibb CLD, Gibb BC. J Am Chem Soc. 2006;128:16498–16499. doi: 10.1021/ja0670916. [DOI] [PubMed] [Google Scholar]
- 10.Gibb CLD, Gibb BC. Chem Commun. 2007:1635–1637. doi: 10.1039/b618731e. [DOI] [PubMed] [Google Scholar]
- 11.Gibb CLD, Gibb BC. Tetrahedron. 2009;65:7240–7248. doi: 10.1016/j.tet.2009.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaanumalle LS, Gibb CLD, Gibb BC, Ramamurthy V. J Am Chem Soc. 2004;126:14366–14367. doi: 10.1021/ja0450197. [DOI] [PubMed] [Google Scholar]
- 13.Kaanumalle LS, Gibb CLD, Gibb BC, Ramamurthy V. J Am Chem Soc. 2005;127:3674–3675. doi: 10.1021/ja0425381. [DOI] [PubMed] [Google Scholar]
- 14.Kaanumalle LS, Nithyanandhan J, Pattabiraman M, Jayaraman N, Ramamurthy V. J Am Chem Soc. 2004;126:8999–9006. doi: 10.1021/ja049492w. [DOI] [PubMed] [Google Scholar]
- 15.Kaanumalle LS, Gibb CLD, Gibb BC, Ramamurthy V. Org Biomol Chem. 2007;5:236–238. doi: 10.1039/b617022f. [DOI] [PubMed] [Google Scholar]
- 16.Natarajan A, Kaanumalle LS, Jockusch S, Gibb CLD, Gibb BC, Turro NJ, Ramamurthy V. J Am Chem Soc. 2007;129:4132–4133. doi: 10.1021/ja070086x. [DOI] [PubMed] [Google Scholar]
- 17.Gibb CLD, Sundaresan AK, Ramamurthy V, Gibb BC. J Am Chem Soc. 2008;130:4069–4080. doi: 10.1021/ja7107917. [DOI] [PubMed] [Google Scholar]
- 18.Sundaresan AK, Gibb CLD, Gibb BC, Ramamurthy V. Tetrahedron. 2009;65:7277–7288. doi: 10.1016/j.tet.2009.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundaresan AK, Kaanumalle LS, Gibb CLD, Gibb BC, Ramamurthy V. Dalton Trans. 2009;20:4003–4011. doi: 10.1039/b900017h. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Gan H, Hermann AT, Rick SW, Gibb BC. Nature Chemistry. 2010;2:847–852. doi: 10.1038/nchem.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibb BC, Chapman RG, Sherman JC. J Org Chem. 1996;61:1505–1509. [Google Scholar]
- 22.Laughrey ZR, Gibb CLD, Senechal T, Gibb BC. Chem Eur J. 2003;9:130–139. doi: 10.1002/chem.200390008. [DOI] [PubMed] [Google Scholar]
- 23.These masses correspond to the largest signal in each envelope and correspond to the theoretical isotope distribution for each cavitand, with the exception of the weakest signal at 2003.9. In this case, noise contributes to the mis-shaping of the isotope distribution envelop relative to the theoretical.