Abstract
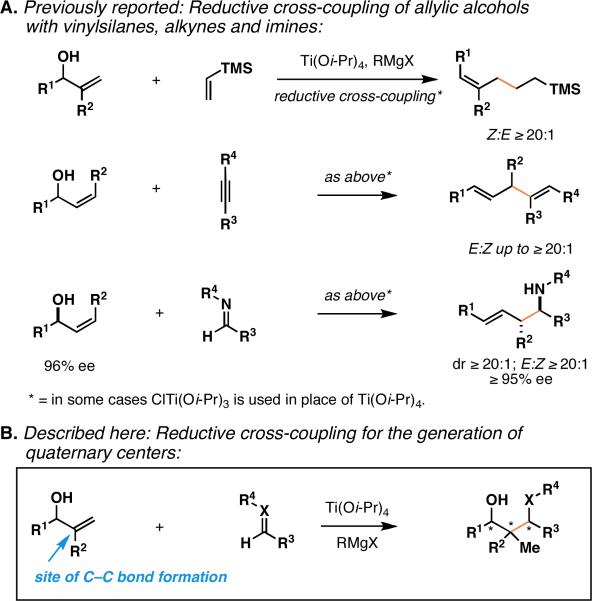
Regioselective titanium alkoxide-mediated reductive cross-coupling reactions of allylic alcohols with vinylsilanes and imines have previously been demonstrated to proceed with allylic transposition by formal metallo-[3,3]-rearrangement [thought to proceed by a sequence of: 1) directed carbometalation, and 2) syn-elimination]. While many examples have been described that support this reaction path, a collection of substrates have recently been identified that react by way of an alternative pathway, delivering a concise convergent route to coupled products bearing a quaternary center.
Keywords: Stereoselective synthesis, Reductive cross-coupling, Allylic alcohol, Titanium alkoxide, Quaternary center
Reductive cross-coupling is emerging as a powerful strategy for bimolecular C–C bond formation.1 Within this broad class of chemical reactions, metallacycle-mediated union of allylic alcohols with alkynes, vinylsilanes and imines, defines a suite of transformations that provide convergent stereoselective access to a variety of structural motifs not readily achieved by other convergent methods in organic/organometallic chemistry.2 As depicted in Figure 1A, intermolecular C–C bond formation is accomplished in concert with the stereoselective generation of isolated (Z)-trisubstituted alkenes, stereodefined 1,4-dienes, and complex homoallylic amines through processes that afford C–C bond formation at the sp2 carbon located distal to the allylic alcohol. Overall, these coupling reactions proceed with allylic transposition, deliver products containing stereodefined di- or trisubstituted alkenes, and can be performed in an enantioselective manner with transfer of stereochemical information from the allylic alcohol starting material to the functionalized product.3 Here, we describe recent observations in a subset of these coupling reactions where subtle changes in substrate structure result in a change in the regiochemical course of carbometalation. In short, these observations have culminated in the identification of reductive cross-coupling reactions of utility for the generation of quaternary carbon centers (Figure 1B). Due to the relative scarcity of intermolecular reactions suitable for the establishment of quaternary centers, and the well-accepted difficulties associated with their synthesis,4 these initial findings are of considerable interest, pointing to a new bimolecular class of reactions capable of forging these highly congested C–C bonds.
Figure 1.
Reductive cross-coupling reactions of allylic alcohols.
Our study of metallacycle-mediated reductive cross-coupling reactions of substituted allylic alcohols with alkynes,2a vinylsilanes2b and imines2c-f has defined a collection of novel stereoselective synthetic methods. Initial observations in this area pointed to a general and reproducible pattern of reactivity, where C–C bond formation occurs with allylic transposition. These observations were consistent with the proposition of a reaction pathway composed of: 1) directed carbometalation, and 2) syn-elimination (Figure 2A). While regioselective reactions of this ilk had previously been described, they were uniformly of limited synthetic utility, as bimolecular C–C bond formation was demonstrated to result in mixtures of alkene isomers.5 Our studies, focused on the use of a titanium alkoxide as the central metal component and an allylic alkoxide as the reactive allylic system, led to highly stereoselective coupling reactions that likely derive from a distinct and highly organized boat-like transition state for regioselective directed carbometalation (2 → 3), and a conserved mechanistic pathway for syn-elimination (3 → 4). Our early investigations have led to the identification of stereoselective reactions for the preparation of products bearing either an (E)-disubstituted, or (Z)-trisubstituted alkene, whereby C–C bond formation occurs distal to the allylic alcohol.
Figure 2.
Reductive cross-coupling of allylic alcohols/ethers: regioselection.
Interestingly, related studies in Zr-catalyzed carbomagnesiation by Hoveyda, define coupling processes that proceed by a unique regioselective path, where C–C bond formation occurs α- to the allylic alcohol, and delivers saturated products (7 and 8) by way of metallacycle 9 (Figure 2B).6 While being described over 15 years ago, this regio- and stereoselective intermolecular carbomagnesiation has, to our knowledge, not been demonstrated to be useful for the establishment of quaternary centers.7
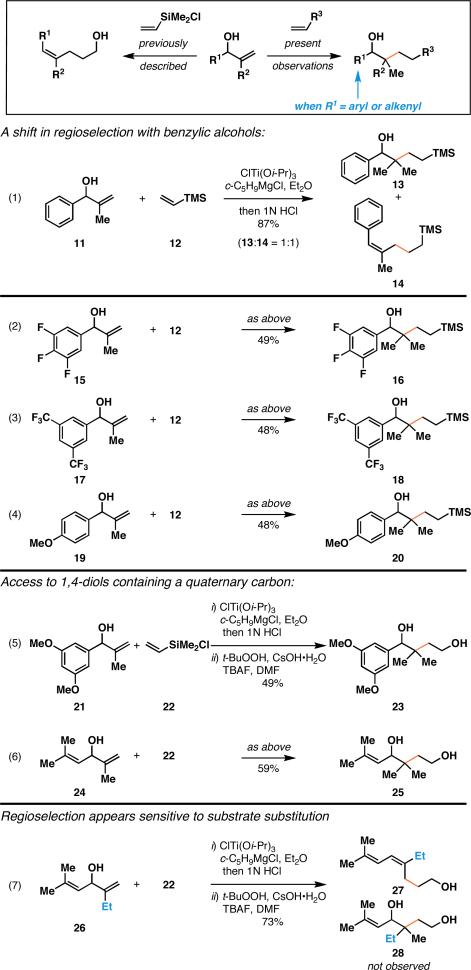
Initial study of allylic alcohol–vinylsilane reductive cross-coupling resulted in the elucidation of a useful reaction for the preparation of (Z)-trisubstituted olefins. In fact, the substitution pattern of the products from this process were similar to those anticipated from Claisen rearrangement, but possessed a stereochemistry not easily attained with this classic sigmatropic rearrangement.2b In subsequent studies of this transformation, we observed an unprecedented shift in regioselection, and accompanying mechanistic course of this subset of metallacycle-mediated cross-coupling reaction.
As depicted in eq 1 of Figure 3, reductive cross-coupling of allylic alcohol 11 with vinyltrimethylsilane proceeds in 87% yield, but delivers a mixture of regioisomeric products 13 and 14. While this cross-coupling reaction did not proceed in a regioselective manner, the differences in polarity associated with these two products made purification of product 13 trivial. As illustrated in eqs 2-4, related substrates 15, 17, and 19 could also be converted to products containing a quaternary center (16, 18, 20) in ca. 50% yield. Aiming to define a synthetically more useful coupling reaction, 1,4-diols could be prepared from a related reaction of allylic alcohol 21 with chlorodimethylvinyl silane 22. Here, reductive cross-coupling was followed by oxidation of the C–Si bond to deliver diol 23 in 49% yield (over two steps).
Figure 3.
Access to quaternary centers by allylic alcohol–vinylsilane reductive cross-coupling.
While these observations marked a dramatic shift in regioselection for reductive cross-coupling reactions of benzylic alcohols, similar results were obtained for the coupling of the 1,4-diene-3-ol substrate 24 with 22. After the two-step process of cross-coupling and oxidation, the 1,4-diol 25 was isolated in 59% yield.
Next, we aimed to realize a version of this coupling reaction that would be suitable for generating a stereodefined quaternary center. As depicted in eq 7 of Figure 3, these efforts targeted the coupling of substrate 26 with 22. While the only difference in substrate structure of 26 with respect to substrate 24 was the substitution of a Me- group for an Et-substituent, this coupling reaction of 26 with 22 delivered diene 27 in 73% yield; no evidence was found for the production of 28. Overall, this result indicated that the regioselection associated with coupling reactions of benzylic- and allylic- alcohols is quite sensitive to subtle perturbation of substrate structure.
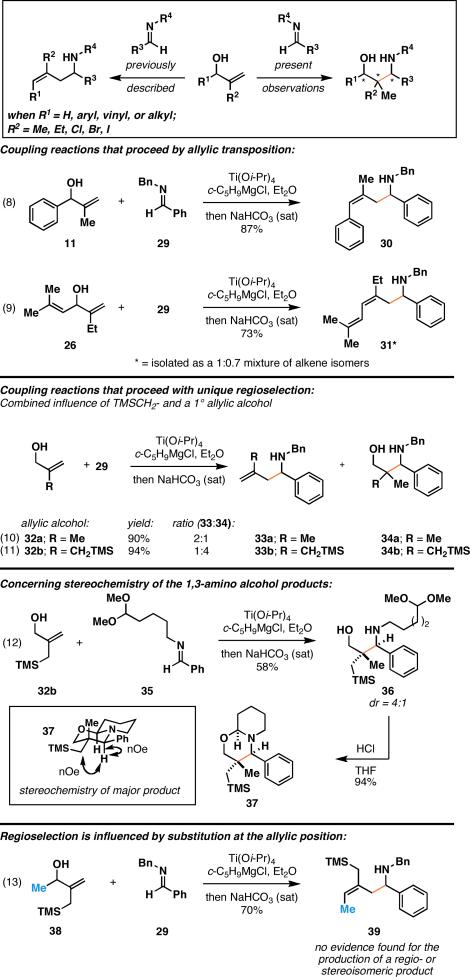
In the related coupling reactions of allylic alcohols with imines, a similar change in regioselection was not observed. As shown in eq 8 of Figure 4, coupling of the benzylic alcohol 11 with imine 29 proceeds in 87% yield, and delivers the homoallylic amine product 30. Unlike the coupling reaction of 11 with a vinylsilane, no evidence could be found for the formation of a product containing a quaternary center. Similarly, the coupling of allylic alcohol 26 with 29 also delivered homoallylic amine products (eq 9).
Figure 4.
Access to quaternary centers by allylic alcohol–imine reductive cross-coupling.
Interestingly, in this subset of reductive cross-coupling, if the starting material is a primary allylic alcohol, substantial quantities of isomeric products result. For example, coupling of 32a with 29 resulted in a 2:1 mixture of products 33a and 34a in 90% combined yield (eq 10). While the product containing a quaternary center was the minor product of this transformation, a subtle change in the structure of 32a resulted in a significant perturbation of reaction course. As depicted in eq 11, coupling of 32b with imine 29 resulted in the preferential formation of the 1,3-aminoalcohol product 34b over the homoallylic amine 33b (selectivity = 4:1).2f The stereochemistry of the major product formed was addressed as illustrated in eq 12. Here, coupling of 32b with imine 35 was followed by formation of the rigid bicyclic heterocycle 37. Subsequent nOe analysis indicated that the major product of this coupling reaction is as depicted in 36.
While the reaction of 32b with imines is selective for the formation of products containing a quaternary center, simple substitution of this starting material results in a shift of regioselection back to the preferential formation of homoallylic amine products. As depicted in eq 13, coupling of the secondary allylic alcohol 38 with imine 29 results in the formation of 39 in 70% yield, as a single olefin isomer. Here, no evidence was found for the production of a 1,3-aminoalcohol-containing product.2f
These studies have identified structural features that lead to a change in the regiochemical course of Ti(Oi-Pr)4-mediated reductive cross-coupling reactions between allylic alcohols and vinylsilanes or imines. The factors that influence each of these cross-coupling reactions are distinct. With vinylsilane-based coupling reactions, additional unsaturation (in the form of a benzylic alcohol or a 1,4-diene-3-ol system) shifts the regiochemical course of coupling. In these cases, substantial quantities of products containing a quaternary carbon are formed. With imine–allylic alcohol coupling, such additional unsaturation does not affect the regiochemical course of reductive cross-coupling. However, a different structural feature shifts regioselection in these reactions. Specifically, coupling of primary allylic alcohols with imines results in a substantial quantity of product containing a 1,3-aminoalcohol and accompanying quaternary center. This tendency is further enhanced with a substrate that contains a TMSCH2- substituent (32b), indicating a potential role of electronic factors in changing the regiochemical course of this coupling reaction. A mechanistic rationale in support of these subtle changes in regioselection as a function of substrate structure remains to be defined.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support of this work by the National Institutes of Health (GM80266 and GM80266-S1).
Footnotes
Supplementary Material
Experimental procedures, compound characterization, copies of spectral data is available free of charge via the Internet at http://#####.
References and notes
- 1.For recent reviews, see: Reichard HA, McLaughlin M, Chen MZ, Micalizio GC. Eur. J. Org. Chem. 2010:391. doi: 10.1002/ejoc.200901094. Olan A, Six Y. Tetrahedron. 2010;66:15. Wolan A, Six Y. Tetrahedron. 2010;66:3097. Montgomery J, Sormunen GJ. Top. Curr. Chem. 2007;279:1. Patman RL, Bower JF, Kim IS, Krische MJ. Aldrichimica Acta. 2008;41:95. Jeganmohan M, Cheng C-H. Chem. Eur. J. 2008;14:10876. doi: 10.1002/chem.200800904. Moslin RM, Miller-Moslin KM, Jamison TF. Chem. Commun. 2007:4441. doi: 10.1039/b707737h. Sato F, Urabe H, Okamoto S. Chem. Rev. 2000;100:2835. doi: 10.1021/cr990277l. Kulinkovich OG, de Meijere A. Chem. Rev. 2000;100:2789. doi: 10.1021/cr980046z.
- 2.For allylic alcohol–alkyne coupling, see: Kolundzic F, Micalizio GC. J. Am. Chem. Soc. 2007;129:15112. doi: 10.1021/ja075678u. For allylic alcohol–vinylsilane coupling, see: Belardi JK, Micalizio GC. J. Am. Chem. Soc. 2008;130:16870. doi: 10.1021/ja8074242. For allylic alcohol–imine coupling, see: Takahashi M, McLaughlin M, Micalizio GC. Angew. Chem. Int. Ed. 2009;48:3648. doi: 10.1002/anie.200900236. Tarselli MA, Micalizio GC. Org. Lett. 2009;11:4596. doi: 10.1021/ol901870n. Umemura S, McLaughlin M, Micalizio GC. Org. Lett. 2009;11:5402. doi: 10.1021/ol9022134. Yang D, Micalizio GC. J. Am. Chem. Soc. 2009;131:17548. doi: 10.1021/ja908504z. For related coupling reactions, see: Lysenko IL, Kim K, Lee HG, Cha JK. J. Am. Chem. Soc. 2008;130:15997. doi: 10.1021/ja806440m. Lysenko IL, Lee HG, Cha JK. Org. Lett. 2009;11:3132. doi: 10.1021/ol901006c.
- 3.Chen MZ, McLaughlin M, Takahashi M, Tarselli MA, Yang D, Umemura S, Micalizio GC. 2010 doi: 10.1021/jo101535d. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For recent reviews, see: Denissova I, Barrriault L. Tetrahedron. 2003;59:10105. Cozzi PG, Hilgraf R, Zimmermann N. Eur. J. Org. Chem. 2007;36:5969.
- 5.a Suzuki N, Kondakov DY, Takahashi T. J. Am. Chem. Soc. 1993;115:8485. [Google Scholar]; b Suzuki N, Kondakov DY, Kageyama M, Kotora M, Hara R, Takahashi T. Tetrahderon. 1995;51:4519. [Google Scholar]; c Takai K, Yamada M, Odaka H, Utimoto K, Fujii T, Furukawa I. Chem. Lett. 1995:315. [Google Scholar]; d Barluenga J, Rodríguez F, Álvarez-Rodrigo L, Zapico JM, Fañanás FJ. Chem. Eur. J. 2004;10:109. doi: 10.1002/chem.200305374. [DOI] [PubMed] [Google Scholar]
- 6.Houri AF, Didiuk MT, Xu Z, Horan NR, Hoveyda AH. J. Am. Chem. Soc. 1993;115:6614. [Google Scholar]
- 7.For examples where a related mode of reactivity in intramolecular settings was effective for the generation of quaternary centers, see: Degrado SJ, Adams JA, Hoveyda AH. Inorg. Chim. Acta. 2003;345:261.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.