Abstract
Liver X receptors (LXRs) are transcription factors involved in the regulation of cholesterol homeostasis. LXR ligands have athero-protective properties independent of their effects on cholesterol metabolism. Platelets are involved in the initiation of atherosclerosis and despite being anucleate express nuclear receptors. We hypothesized that the athero-protective effects of LXR ligands could be in part mediated through platelets and therefore explored the potential role of LXR in platelets. Our results show that LXR-β is present in human platelets and the LXR ligands, GW3965 and T0901317, modulated nongenomically platelet aggregation stimulated by a range of agonists. GW3965 caused LXR to associate with signaling components proximal to the collagen receptor, GPVI, suggesting a potential mechanism of LXR action in platelets that leads to diminished platelet responses. Activation of platelets at sites of atherosclerotic lesions results in thrombosis preceding myocardial infarction and stroke. Using an in vivo model of thrombosis in mice, we show that GW3965 has antithrombotic effects, reducing the size and the stability of thrombi. The athero-protective effects of GW3965, together with its novel antiplatelet/thrombotic effects, indicate LXR as a potential target for prevention of athero-thrombotic disease.
Introduction
Liver X receptors (LXRs) belong to the nuclear receptor superfamily, and their natural ligands, oxysterols, are cholesterol derivatives.1 LXRs play key roles in cholesterol homeostasis by regulating the transcription of genes, such as cytochrome P450 7α-hydroxylase 1 (Cyp7a1) and apoliprotein E (ApoE), involved in cholesterol catabolism to bile acids2,3 and cholesterol efflux back to the liver,4–6 respectively. LXRs have also been shown to have anti-inflammatory7,8 and athero-protective effects after ligand stimulation.9,10
Platelets are anucleate blood cells with a central role in hemostasis but are also involved in inflammation and atherosclerosis.11–14 Their primary function is to prevent hemorrhage at sites of vascular injury, where exposed extracellular matrix proteins, such as collagen, interact with platelet surface receptors, resulting in platelet activation, aggregation, and thrombus formation. Although platelets are essential for the preservation of vascular integrity, inappropriate platelet activation, for example, at sites of atherosclerotic lesions, causes arterial thrombosis, leading to ischemic stroke and myocardial infarction, 2 of the main causes of morbidity and mortality in the industrialized world.15 Low-density lipoprotein is the main therapeutic target for the prevention of athero-thrombosis,16 and low-density lipoprotein-lowering medications, such as statins, appear additionally to display antiplatelet actions.17,18 Platelet function is also affected by cholesterol derivatives, although there is no consensus on whether these molecules have inhibitory or activatory roles.19
The initial entrapment of platelets on subendothelial collagen is mediated through the plasma protein von Willebrand factor (VWF), which binds simultaneously to collagen and the glycoprotein (GP) complex, GPIb-IX-V, on the platelet surface.20 These interactions are followed by more stable binding of collagen to platelet glycoprotein VI (GPVI),21 triggering intracellular signaling that leads to granule secretion and increased affinity of the surface integrin αIIbβ3 for its ligands, fibrinogen and VWF.22–27 Fibrinogen and VWF form bridges between adjacent platelets leading to platelet aggregation, which is reinforced by the release of secondary mediators, such as adenosine diphosphate (ADP) and thromboxane A2 (TXA2). ADP and TXA2 signal through G protein-coupled receptors present on the platelet surface, providing positive feedback loops to amplify the initial stimulation.28,29 Exposure of phosphatidylserine on the platelet surface combined with activation of coagulation pathways result in thrombin generation at the surface of activated platelets within the developing thrombus. Thrombin triggers signaling through G protein-coupled receptors that contributes to platelet activation.30 The thrombus, which serves to block the blood loss, is further stabilized through outside-in signaling after αIIbβ3 binding to its ligands.
Recent studies show that synthetic nonsteroidal LXR ligands, such as GW3965, reduce atherosclerosis in murine models of atherosclerosis, whereas plasma cholesterol or lipoprotein levels remain unaffected, suggesting that LXR-dependent pathways distinct from cholesterol transport could impact atherosclerosis.9,31 We and others have reported previously the presence of nuclear receptors in platelets.32–34 We therefore hypothesized that the athero-protective effects of LXR ligands may be mediated in part through platelets. In this study, we examined the role of LXR in platelets. Our results show that LXR-β is present in platelets and that LXR ligands inhibit platelet function and thrombus formation in vivo, suggesting a potential new axis for prevention of atherosclerosis and thrombosis based on acute nongenomic actions of this receptor in platelets.
Methods
Reagents
Collagen was obtained from Nycomed, bovine thrombin and arachidonic acid from Sigma-Aldrich, and collagen-related peptide (CRP) from Professor R. Farndale (University of Cambridge, Cambridge, United Kingdom). Nuclear receptor ligands, 15d-PGJ2 and T0901317 were purchased from Enzo Life Sciences and GW3965 from Tocris Bioscience. Primary anti-LXR-β (H-8), -LXR-α/β (S-20), -Spleen tyrosine kinase (Syk; C-20), -phospholipase C γ2 (PLC-γ2; Q-20), -β3 (C-20), and -PPAR-γ (E-8), antibodies used for immunoblotting, immunoprecipitation, and flow cytometry, were from Santa Cruz Biotechnology. Anti-LXR-β (ab25237) antibodies used in immunocytochemical analysis and flow cytometry were provided by Abcam. Anti-GPIb AlexaFluor-488-labeled antibody was from Emfret Analytics. Antiphosphotyrosine antibody 4G10 was obtained from Millipore and antibodies against specific phosphorylation sites from Epitomics. Phycoerythrin (PE)-Cy5 mouse antihuman CD62P and PE-Cy5 mouse IgG1 κ-isotype control used to measure P-selectin exposure were from BD Biosciences PharMingen. Polyclonal fluorescein isothiocyanate-labeled antifibrinogen was provided by Dako. All other reagents were from previously described sources.35
Human washed platelet preparation
Human blood was obtained from consenting healthy volunteers. A total of 50 mL of blood was collected into a syringe containing 3 mL of anticoagulant 4% (weight/volume) sodium citrate and mixed with 7 mL of acid citrate dextrose (ACD), and washed platelets were prepared by differential centrifugation as described previously.35 Platelets were resuspended in modified Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (134mM NaCl, 0.34mM Na2HPO4, 2.9mM KCl, 12mM NaHCO3, 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5mM glucose, and 1mM MgCl2, pH 7.3) and rested for 30 minutes at 30°C before experiments.
Platelet aggregation and dense granule secretion assays
Platelets (4 × 108 cells/mL) were incubated with luciferin at 37°C for 2 minutes and then stimulated with an agonist in an optical lumi-aggregometer (Chronolog) with continuous stirring (1200 rpm). Platelet aggregation was determined by measuring changes in optical density as described previously35 and dense granule secretion by measuring changes in the adenosine triphosphate (ATP) concentration using the luciferin-luciferase system kit (Chronolog).
Measurement of [Ca2+]i by spectrofluorimetry
Mobilization of calcium from intracellular stores was measured in platelets preloaded with the fluorescent dye Fluo-4NW as described previously.39 Platelets were stimulated with CRP or thrombin and calcium release was measured using a Fluoroskan ascent plate reader (Thermolab Systems) with excitation at 485 nm and emission measured at 530 nm.
Flow cytometric measurement of LXR-β within platelets
To measure LXR within platelets, washed platelets were fixed with 2% formyl saline, permeabilized using BD Phosflow perm buffer III (BD Biosciences), washed, resuspended in HEPES buffer saline, and incubated with antibodies to LXR-β. Platelets were then washed, resuspended in HBS, and incubated with Cy3-labeled secondary antibody (Invitrogen), fixed using 0.2% formyl saline and analyzed by flow cytometry. Negative controls were set using an appropriate isotype control.
Flow cytometric analysis: α-granule secretion and fibrinogen binding to integrin αIIbβ3
Flow cytometry was used to examine affinity up-regulation of the integrin αIIbβ3 and platelet α-granule secretion by detecting levels of fibrinogen binding to αIIbβ3 and P-selectin exposure on platelet surface, respectively. After stimulation with CRP, platelets were incubated at room temperature for 20 minutes with fluorescein isothiocyanate-labeled fibrinogen and PE/Cy5 antihuman-CD62P (P-selectin). Reactions were stopped by 100-fold dilution in 0.2% (volume/volume) formyl saline. Flow cytometric acquisition was performed using a FACSCalibur device, and data were collected from 5000 events analyzed using the CellQuest Pro Software Version 3.3 (BD Biosciences). Negative controls were set using an appropriate IgG1 κ-isotype matched control for the anti-CD62P antibody and ethyleneglycoltetraacetic acid (10μM) for the fibrinogen binding.
Immunoblotting and immunoprecipitation
Immunoblot analysis was performed using standard techniques as described previously.35 For immunoprecipitations, washed platelets (8 × 108 cells/mL) were pretreated with ethyleneglycoltetraacetic acid (1mM), indomethacin (10μM), and apyrase (2 U/mL), lysed, and proteins of interested were isolated using 1 μg/mL of appropriate antibodies. Proteins of interest were immunoprecipitated as described previously.35 Densitometry was performed to quantify the band intensities and glyceraldehyde-3-phosphate dehydrogenase levels or levels of the immunoprecipitated protein were used to normalize the data.
Tail-bleeding assay
Fifteen 7- to 8-week-old C57BL/6 mice (The Jackson Laboratory) were anesthetized using ketamine (80 mg/kg) and xylazine (5 mg/kg) administered via the intraperitoneal route before a tail biopsy. The time to cessation of bleeding was measured up to 20 minutes. All animal procedures were approved and licensed by the United Kingdom Home Office.
Intravital microscopy and laser-induced injury
Intravital microscopy and data analysis were performed as previously described.36 Briefly, 8 C57BL/6 mice were anesthetized by intraperitoneal injection of ketamine (125 mg/kg), xylazine (12.5 mg/kg), and atropine (0.25 mg/kg). Mouse circulation was accessed via a cannulus placed in the jugular vein, and platelets were marked with Alexa-488-conjugated anti-GPIb antibody. After exteriorization of the testicles and the surrounding cremaster muscle, injury on the cremaster arteriole wall was induced with a Micropoint Ablation Laser Paint (Andor Technology). Thrombi were observed using an upright Olympus BX microscope. Images were captured prior to and after the injury by a Hamamatsu charge-coupled device camera in 640 × 480 format and analyzed using Slidebook software Version 5.0 (Intelligent Imaging Innovations).
In vitro thrombus formation
Whole citrated blood was incubated with the lipophilic dye 3,3-dihexyloxacarbocyanine iodide and perfused through collagen-coated (400 μg/mL) Vena8Biochip (Cellix) at a shear rate of 20 dyn/cm2. Thrombi Z-images were taken every 30 seconds using a Nikon eclipse (TE2000-U) microscope, and thrombus fluorescence intensity was calculated using the Slidebook, Version 5 (Intelligent Imaging Innovations).
Statistical analysis
Normally distributed data were analyzed using analysis of variance and t test. The nonparametric Mann-Whitney U test was used to analyze non-normally distributed data.
Results
LXR is present in human platelets
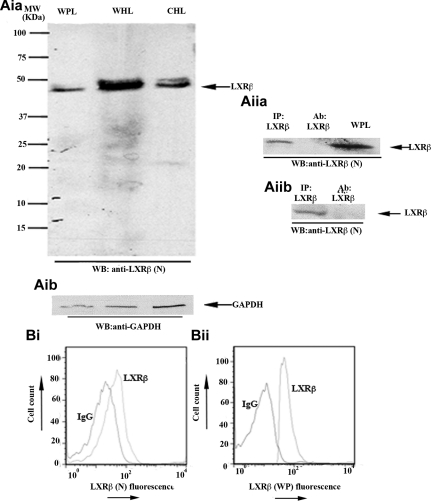
LXR is found in 2 isoforms, LXR-α and LXR-β. LXR-α is expressed mainly in liver and adipose tissue, whereas LXR-β is present in a number of tissues.37 Previous studies have reported that LXR-β reduced atherosclerosis in apoE−/− mice without up-regulating the expression of other genes involved in cholesterol metabolism, such as Cyp7a1, suggesting that this receptor has actions that are independent of cholesterol metabolism.38,42 In this study, we investigated the expression of LXR-β and its potential role in platelets. Western blot analysis, using a mouse monoclonal anti-LXR-β antibody raised against the N-terminus of the protein, revealed a protein band of approximately 45 kDa, suggesting the presence of LXR-β in human platelets (Figure 1Ai). This was further confirmed by immunoprecipitating LXR-β from platelet lysates using 2 additional anti-LXR antibodies raised against different parts of the protein (Figure 1Aii). The expression of LXR-β in permeabilized human (Figure 1B) or mouse (data not shown) platelets was also shown by flow cytometry using 2 different LXR-β antibodies.
Figure 1.
LXR-β is present in human platelets. (Aia-Aib) Whole platelet lysates (WPL) were immunoblotted for LXR-β (Aia) or glyceraldehyde-3-phosphate dehydrogenase (Aib). Using a mouse monoclonal anti-LXR-β antibody raised against the N-terminus of the protein (LXR-β(N)), a 45-kDa band was detected. Whole HeLa lysates (WHL) and cytosolic HeLa lysates (CHL) were used as positive controls. (Aiia-Aiib) LXR-β was immunoprecipitated (IP) from platelet lysates using antibodies raised against the C-terminus amino acid sequence of LXR-β (Aiia) or the whole protein (Aiib), and IP samples were Western-blotted for LXR-β. WPL and antibodies used for IP were used as positive and negative controls, respectively. (B) Human platelets were stained with mouse (Bi) or rabbit (Bii) anti-LXR-β antibodies or the same amount of IgG control, followed by equivalent PE-conjugated secondary antibodies and analyzed by flow cytometry. A shift in the fluorescence profile was observed when anti-LXR-β antibodies were used. MW indicates molecular weight.
LXR ligands inhibit platelet aggregation and [Ca2+] mobilization
Having shown that LXR is expressed in platelets, we explored its role in platelet function. The effect of the synthetic LXR-selective ligands GW3965 and T0901317 on platelet aggregation induced by a range of physiologic agonists was examined.
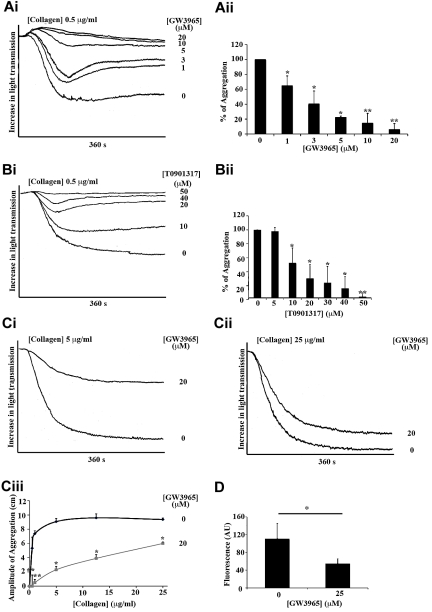
At low collagen concentrations (0.5 μg/mL), the structurally unrelated LXR ligands, GW3965 (Figure 2A) and T0901317 (Figure 2B), decreased platelet aggregation in a concentration-dependent manner when aggregation was monitored for 360 seconds (Figure 2A-B). An approximate 40% reduction in aggregation was observed at 1μM GW3965 (Figure 2A) or 10μM T0901317 (Figure 2B), whereas 20 or 50μM GW3965 or T0901317, respectively, decreased aggregation by 95%. Differences in the potencies of GW3965 and T0901317 to inhibit platelet aggregation are consistent with the reported lower potency of T0901317 relative to GW39654,43 (50% effective concentration, T0901317 > 50nM, GW3965 30nM; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Figure 2.
LXR ligands inhibited platelet aggregation and calcium mobilization stimulated by collagen or CRP. Washed human platelets (4 × 108 cells/mL) were preincubated with GW3965, T0901317, or DMSO (0.04% volume/volume) for 10 minutes and stimulated with collagen (A-C). Aggregation was measured as change in light transmission and monitored for 360 seconds. (Ai,Bi) Representative aggregation traces of platelets treated with a range of GW3965 (1-20μM) (Ai) or T0901317 (10-50μM) (Bi) concentrations or DMSO followed by collagen stimulation (0.5 μg/mL). (Aii,Bii) Data are plotted as percentage of aggregation (vehicle-treated representing 100% aggregation) and represent mean plus or minus SEM values. (C) Aggregation traces of platelets stimulated by 5 μg/mL collagen (Ci) or 25 μg/mL collagen (Cii) after pretreatment with 20μM GW3965 or DMSO. (Ciii) Inhibition of aggregation by 20μM GW3965 was measured over a range (0.5-25 μg/mL) of collagen. Data are plotted as amplitude of aggregation in centimeters at 360 seconds after addition of collagen concentrations. (D) Fluo-4NW-loaded platelets were incubated with GW3965 (20μM) or DMSO (0.04%) for 10 minutes and then stimulated with CRP (2.5 μg/mL) for 200 seconds, and intracellular mobilization of calcium was measured by spectrofluorimetry. n ≥ 3. *P < .05. **P < .01.
To gain insight into the potency of LXR ligands, the effects of GW3965 (Figure 2C) and T0901317 (supplemental Figure 1B) on aggregation stimulated by a range of collagen concentrations was examined. High GW3965 concentrations (eg, 20μM) elicited a significant inhibitory effect on aggregation induced by low (0.5 and 1 μg/mL), intermediate (3 and 5 μg/mL; Figure 2C), and high concentrations of collagen (10 and 25 μg/mL; Figure 2Cii-iii) at 360 seconds, although relatively low concentrations of GW3965 were only effective at low collagen concentrations (data not shown). Similar data were achieved when the GPVI receptor was stimulated by a specific synthetic ligand, the CRP (supplemental Figure 1B). Because platelet aggregation is dependent on calcium release from intracellular stores, the effect of GW3965 on GPVI-induced calcium mobilization was examined. A total of 20μM GW3965 reduced CRP-induced (2 μg/mL) calcium mobilization by approximately 50%, suggesting that LXR ligand actions affect initial steps of platelet activation (Figure 2D).
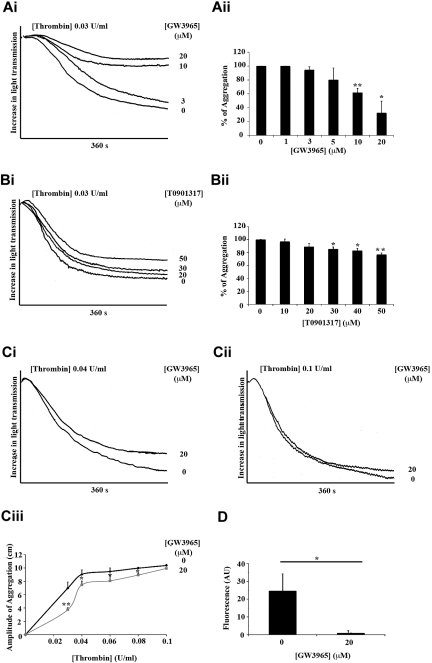
The effects of GW3965 and T0901317 on aggregation and calcium release induced by thrombin, a potent platelet agonist that signals through the protease-activated receptor-1 and protease-activated receptor-4, were also examined (Figure 3). Low GW3965 concentrations (1, 3, or 5μM) had only a subtle inhibitory effect on thrombin-induced aggregation (0.03 U/mL; Figure 3A), whereas high GW3965 concentrations (20μM) reduced aggregation by 65%. In line with data achieved from aggregation stimulated by collagen, T0901317 inhibited, to a lesser extent, thrombin-induced aggregation compared with inhibition caused by GW3965 (eg, high T0901317 concentrations reduced aggregation only by ∼ 20%; Figure 3B). At low thrombin concentrations (0.03 and 0.04 U/mL; Figure 3C), the effect of GW3965 was more evident, whereas inhibition was overcome almost completely with higher thrombin concentrations (0.06, 0.08, and 0.1 U/mL; Figure 3Cii-iii). Similar data were achieved when platelet aggregation was stimulated by arachidonic acid, a relatively weak platelet agonist that is metabolized to TXA2 and acts through TP receptors on platelet surface (supplemental Figure 1C). The use of collagen (Figure 2Ciii) and thrombin (Figure 3Ciii) concentrations that cause similar amounts of aggregation also allows comparison between the effects of GW3965 on collagen- and thrombin-induced aggregation. These results suggest that GW3965 is able to inhibit platelet aggregation induced by a range of agonists that signal through different receptors and pathways, although it is more potent when platelets are stimulated through GPVI. In agreement with data achieved from calcium mobilization stimulated by CRP, GW3965 also inhibited calcium release stimulated by thrombin (Figure 3D).
Figure 3.
LXR ligands diminished platelet aggregation and calcium mobilization stimulated by thrombin. Washed platelets were preincubated with GW3965, T0901317, or vehicle (DMSO 0.04% volume/volume) for 10 minutes and stimulated with thrombin for 360 seconds (A-C). (Ai,Bi) Representative aggregation traces of platelets treated with GW3965 (1-20μM) (Ai), T0901317 (10-50μM) (Bi), or vehicle followed by thrombin stimulation (0.03 U/mL). (Aii,Bii) Data are plotted as percentage of aggregation and represent mean values. (C) Aggregation traces of platelets stimulated by 0.04 U/mL (Ci) or 0.1 U/mL (Cii) thrombin after pretreatment with 20μM of GW3965 or DMSO. (Ciii) Inhibition of aggregation by 20μM GW3965 was measured over a range (0.03-1 U/mL) of thrombin. (D) Fluo-4NW-loaded platelets were incubated with GW3965 (20μM) or DMSO for 10 minutes and then stimulated with thrombin (0.08 U/mL) for 200 seconds, and intracellular mobilization of calcium measured by spectrofluorimetry. n ≥ 3. *P < .05. **P < .01.
GW3965 attenuates GPVI-stimulated activation of integrin αIIbβ3 and degranulation
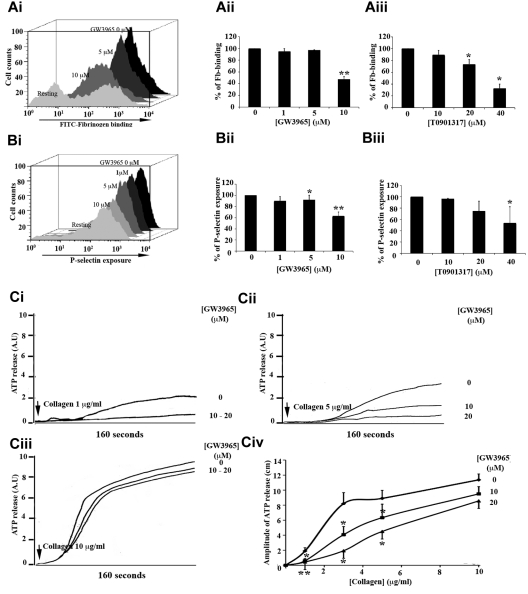
Because LXR ligands affected, to a greater extent, collagen- or CRP-induced aggregation, the impact of GW3965 and T0901317 on calcium-dependent functions occurring before aggregation, in response to these GPVI agonists, was investigated (Figure 4). Flow cytometry was used to measure binding of antifibrinogen antibody to platelets (Figure 4A) and P-selectin (Figure 4B) exposure on platelet surface, which were used as measures of activation of the integrin αIIbβ3 and α-granule secretion, respectively. CRP was used to stimulate platelets in this assay, as the fibrillar structure of collagen limits its use in flow cytometry. A total of 1 or 5μM GW3965 (Figure 4Aii,Bii) or 10μM T0901317 (Figure 4Aiii,Biii) displayed a minor inhibitory effect (< 5% reduction) on fibrinogen binding (Figure 4A) and P-selectin exposure (Figure 4B) when platelets were stimulated with 1 μg/mL CRP; but using higher concentrations of GW3965 (10μM) or T0901317 (40μM), the levels of fibrinogen and P-selectin on the platelet surface were reduced.
Figure 4.
GW3965 inhibited fibrinogen binding to αIIbβ3 and granule secretion. (A-B) Human washed platelets (4 × 108 cells/mL) were treated with GW3965 (1-10μM; Ai-ii,Bi-ii), T0901317 (10-40μM; Aiii,Biii), or vehicle, for 10 minutes stimulated with CRP (1 μg/mL) and analyzed by flow cytometry. Binding of antifibrinogen antibody to platelets and P-selectin exposure on platelet surface used as measures of αIIbβ3 activation (Ai) and α-granule secretion (Bi), respectively. (Aii-iii,Bii-iii) Data represent mean of median fluorescence values plus or minus SEM (n = 4). (C) ATP secretion traces from human platelets (4 × 108 cells/mL) treated with GW3965 (10 or 20μM) for 10 minutes and stimulated by 1 μg/mL (Ci), 5 μg/mL (Cii), or 10 μg/mL of collagen (Ciii). ATP release was monitored using a luciferin-luciferase assay. (Civ) Collagen concentration response curve. Data are shown as amplitude of the secretion response in centimeters. Arrows above traces indicate the point of collagen addition. n ≥ 3. *P < .05. **P < .01.
To investigate the role of LXR in dense granule secretion, ATP release from platelets stimulated over a concentration range of collagen was monitored. GW3965 abolished ATP release induced by low (1 μg/mL; Figure 4Ci,iv) collagen concentrations. Secretion stimulated by intermediate (3 and 5 μg/mL; Figure 4Cii,iv) collagen concentrations was reduced by approximately 30% to 50%, whereas the magnitude of inhibition was minimal at high (10 μg/mL; Figure 4Ciii-iv) collagen concentrations. The use of collagen as a platelet agonist in this assay allows comparisons with observations made on platelet aggregation. At 5 μg/mL collagen, GW3965 (20μM) reduced dense-granule secretion and aggregation by approximately 50% (Figure 4Civ) and 80% (Figure 2Ciii), respectively, whereas at 10 μg/mL collagen dense-granule secretion was decreased by 40% and aggregation by 60%, suggesting that the impact of GW3965 is more evident on aggregation. Indeed, GW3965 was found to inhibit α-granule secretion and in addition fibrinogen binding to integrin αIIbβ3. Together, these observations indicate that combination of smaller inhibitory effects on multiple aspects of platelet function results in the substantial inhibition of aggregation by LXR ligands.
GW3965 has antithrombotic actions
Having established that GW3965 modulates platelet function, we sought to determine the potential implication of LXR in hemostasis and thrombosis.
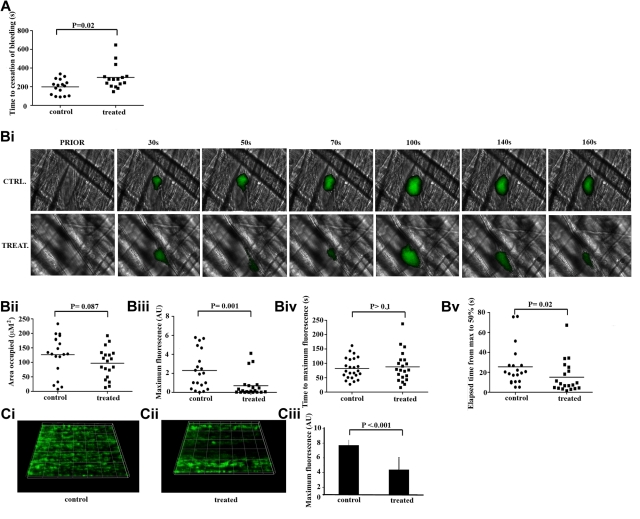
The effect of GW3965 on hemostasis was measured using a tail-bleeding assay. Vehicle-treated (0.08% volume/volume dimethyl sulfoxide [DMSO]) mice bled for approximately 200 seconds after tail biopsy (Figure 5A). Mean time to cessation of bleeding was extended by approximately 100 seconds in GW3965-treated mice (2 mg/kg), although a broad range of responses was observed. Three GW3965-treated mice bled substantially longer relative to controls, whereas some fell within the control bleeding time range. Similarly, a number of controls bled for a significantly shorter time compared with GW3965-treated samples. This is consistent with use of this assay in other studies43,44 and is indicative of a modest increase in bleeding time after GW3965 administration.
Figure 5.
GW3965 increased bleeding time and modulated platelet thrombus formation in vivo. (A) GW3965 (2 mg/kg) (n = 15) or DMSO (0.08% volume/volume) (n = 15) was administered intravenously to mice and time to cessation of bleeding, after a tail biopsy, was measured. Data represent individual mice; and horizontal lines, mean values. Statistical analysis was performed using the Mann-Whitney nonparametric T test. (B) GW3965 (2 mg/kg) or DMSO (0.08% volume/volume) was administered intravenously to mice, and platelets were fluorescently labeled by injection of Alexa488-conjugated anti-GPIb antibody. After laser-induced injury of the cremaster muscle arterioles, accumulation of platelets (shown in green) was assessed (Bi). The area covered by thrombi (Bii), maximum fluorescence value (Biii), time to peak fluorescence levels (Biv), and time elapsed between maximal and half-maximal fluorescence values (Bv) were measured. Graphs represent individual mouse data for 21 thrombi in 4 mice for each treatment. (C) Mouse whole blood mixed with the lipophilic dye 3,3-dihexyloxacarbocyanine iodide was treated with GW3965 (60μM) (Cii) or DMSO (0.1% volume/volume) (Ci) and perfused through collagen-coated (400 μg/mL) Vena8Biochip at a shear rate of 20 dyn/cm2. Thrombi were recorded through a series of images in the Z-plane through their full depth every 30 seconds using a Nikon eclipse (TE2000-U) microscope, and thrombus fluorescence intensity was calculated using the Slidebook, Version 5. Numerical data (Ciii) represent sum fluorescence intensities and are the average of 3 separate experiments.
The potential role of LXR in thrombosis was examined using an in vivo thrombosis assay, where the ability of fluorescently labeled platelets to form thrombi after laser-induced tissue injury was measured (supplemental Videos 1-2). Relatively large stable thrombi were formed after vessel injury in vehicle-treated mice (0.08% DMSO, CTRL in figure); but after treatment with GW3965 (2 mg/kg TREAT in figure), cyclical thrombus embolization and reformation were observed (Figure 5Bi). GW3965-treated thrombi occupied an area of comparable size to the equivalent area in vehicle-treated (Figure 5Bii); however, peak thrombus fluorescence levels were decreased more than 3-fold in GW3965-treated mice (Figure 5Biii), indicating that a significantly lower number of platelets was recruited to the forming thrombus. The initial kinetics of thrombus formation until peak fluorescence levels were similar between GW3965-treated and control mice (Figure 5Biv), but thrombi receded more rapidly in GW3965-treated animals, denoting that thrombi formed in the presence of the LXR ligand were less stable. Consistent with this, the elapsed time between maximal and half-maximal fluorescence values was 40% lower in GW3965-treated mice, which is indicative of a role for LXR in thrombotic disease (Figure 5Bv).
Because LXR ligands have been reported to affect endothelial cell function,45 we sought to investigate the possibility that the effects of GW3965 on thrombus formation are dependent on its actions on endothelium. To explore this further, 3,3-dihexyloxacarbocyanine iodide-labeled whole mouse blood was perfused through Vena8Biochip coated with collagen at a shear rate of 20 dyn/cm2 in the presence of vehicle or GW3965 (Figure 5C). GW3965 reduced the peak fluorescence levels, an indicator of the level of platelet recruitment to the thrombus, by approximately 50% compared with the vehicle-treated samples. Inhibition of thrombus formation in the absence of endothelial cells suggests that the observed effects of GW3965 on intravital thrombus formation are the result of diminished platelet function, although the possibility of potentially additional roles of endothelium in these effects cannot be ruled out. Collectively, these data indicate a potential role of platelet LXR in the regulation of hemostasis and thrombosis.
LXR modulates collagen-stimulated signaling in platelets
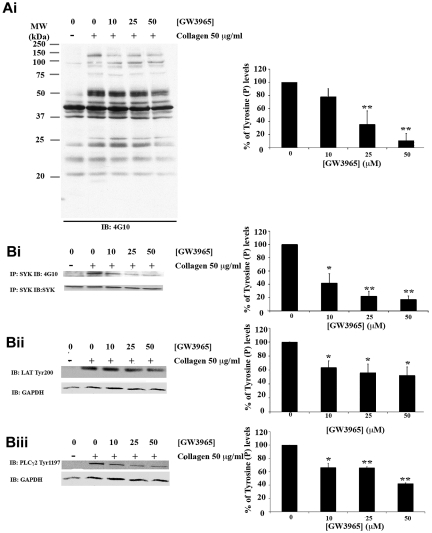
The inhibitory actions of GW3965 on multiple aspects of platelet function followed by impaired platelet responses in vivo suggest that the effects observed are the result of LXR interference in primary signaling responses after GPVI stimulation. Signaling events proximal to GPVI are characterized mainly by tyrosine phosphorylation cascades.21,24–27 The impact of GW3965 on these primary responses was explored by examination of total tyrosine phosphorylation levels in the presence of apyrase, indomethacin, and ethyleneglycoltetraacetic acid to block signaling through ADP receptors, TXA2 production, and aggregation, respectively. In collagen signaling studies, where signaling through positive mediators is blocked, to observe and quantify tyrosine phosphorylation of GPVI pathway components, the collagen concentration is increased.39 Platelets were therefore treated with increased LXR ligand concentrations before their stimulation with 50 μg/mL collagen and immunobloted using an antiphosphotyrosine antibody. Treatment with GW3965 resulted in inhibition of total tyrosine phosphorylation levels (Figure 6A). A total of 50μM of GW3965 reduced phosphorylation by 85%, whereas an approximate 20% inhibition was observed at 10μM GW3965.
Figure 6.
GW3965 inhibits tyrosine phosphorylation stimulated through GPVI. Platelets (8 × 108 cells/mL) pretreated with GW3965 (10-50μM) or DMSO (0.08% volume/volume) for 10 minutes and stimulated with collagen (50 μg/mL) for 90 seconds in the presence of ethyleneglycoltetraacetic acid (10μM), indomethacin (10μM), and apyrase (2 U/mL). For total tyrosine phosphorylation levels, lysates were immunoblotted with an antiphosphotyrosine antibody (Ai). For Syk phosphorylation levels (Bi), Syk was immunoprecipitated from lysates using an anti-Syk antibody and immunoblotted with an antiphosphotyrosine antibody. For phosphorylation of LAT (Bii) and PLC-γ2 (Biii), the phosphorylation of Tyr200 and Tyr1197, respectively, was examined using antiphospho-specific antibodies. Membranes were stripped and reprobed for glyceraldehyde-3-phosphate dehydrogenase (Ai,Bii,Biii) or Syk (Bi). Band intensities were quantified from 3 separate experiments (mean ± SEM) and normalized for protein loading. Bars represent the average percentage of tyrosine phosphorylation levels; 100% was defined as the activation achieved in DMSO (0.08% volume/volume)-treated platelets. *P < .05. **P < .01. MW indicates molecular weight; and IP, immunoprecipitation.
Syk,24 linker for activated T cells (LAT)25,26 and PLC-γ226,27 are key components of GPVI pathway and become tyrosine phosphorylated after GPVI stimulation. The impact of GW3965 on collagen-induced tyrosine phosphorylation of these proteins was therefore examined. Syk was immunoprecipitated from platelet lysates, and tyrosine phosphorylation levels were determined by immunoblot analysis using an antiphosphotyrosine antibody. Consistent with its inhibitory effect on total tyrosine phosphorylation levels, GW3965 was found to reduce Syk tyrosine phosphorylation (Figure 6Bi). Tyrosine phosphorylation levels of LAT and PLC-γ2 were assessed by immunoblot analysis of whole platelet lysates using antibodies against specific phosphorylation sites of these proteins. GW3965 reduced phosphorylation of LAT at Tyr200 (Figure 6Bii) and of PLC-γ2 at Tyr1197 (Figure 6Biii). A total of 10μM GW3965 inhibited Syk tyrosine phosphorylation by 60% and LAT or PLC-γ2 by approximately 35%. This difference in the magnitude of inhibition between Syk and LAT/PLC-γ2 phosphorylation could possibly be explained by the fact that only a specific phosphorylation site of LAT or PLC-γ2 was examined. Together, these observations suggest a potential involvement of LXR in signaling proximal to the GPVI receptor.
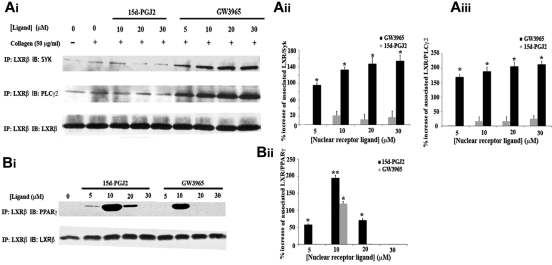
This hypothesis was reinforced after examining interactions of LXR with pathway components. LXR-β was isolated by immunoprecipitation from platelet lysates; and after immunoblot analysis, interactions with Syk or PLC-γ2 were investigated. Low levels of interactions of LXR with Syk or PLC-γ2 were observed in resting or collagen-stimulated cells (Figure 7A). LXR-Syk associations were increased by 90% after treatment with 5μM GW3965, whereas increasing GW3965 concentrations further enhanced these interactions. Likewise, LXR-PLC-γ2 associations were 1.5- or 2.5-fold increased by 5 or 30μM GW3965, respectively. These results suggest that the GW3965-induced LXR interactions with signaling molecules proximal to GPVI result in diminished platelet function, although we cannot rule out the possibility that a part of GW3965 effects could be LXR independent. We have shown previously that another nuclear receptor, peroxisome proliferator-activated receptor-γ (PPAR-γ), is also involved in the GPVI pathway and interacts with Syk and LAT on collagen stimulation, whereas its ligand, 15d-PGJ2, causes loss of these associations.35 Because crosstalk between these nuclear receptors affects their actions,40 PPAR-γ involvement in LXR-β interactions with GPVI pathway components was examined. 15d-PGJ2 did not affect significantly interactions of LXR-β with Syk or PLC-γ2, suggesting that PPAR-γ and LXR can independently interact with GPVI components (Figure 7B).
Figure 7.
LXR-β interacts with Syk, PLC-γ2, and PPAR-γ. Platelets (8 × 108 cells/mL) pretreated with GW3965 or 15d-PGJ2 (5-30μM) or DMSO (0.08% volume/volume) for 10 minutes and stimulated with collagen (50 μg/mL) for 90 seconds in the presence of ethyleneglycoltetraacetic acid (10μM), indomethacin (10μM), and apyrase (2 U/mL). LXR-β was immunoprecipitated from lysates separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with anti-Syk, anti-PLC-γ2 (A) or anti-PPAR-γ (B) antibodies. Membranes were stripped and reblotted for LXR-β. Band intensities were quantified from 3 separate experiments and normalized for LXR-β loading. Numerical data represent the percentage increase in the associations of LXR with Syk (Aii), PLC-γ2 (Aiii), or PPAR-γ (Bi), where 0% association was defined as the level of association in the untreated samples. *P < .05. **P < .01.
Based on the ability of PPARs to dimerize with LXRs,40 we explored potential associations between these receptors in platelets. Low concentrations of LXR (GW3965) or PPAR-γ (15d-PGJ2) ligands caused LXR and PPAR-γ to associate, indicating an ability of nuclear receptors to interact in the absence of genomic function in platelets, whereas these interactions were not observed at higher concentrations of the nuclear receptor ligands (Figure 7B). Collectively, these data suggest that an equilibrium exists between PPAR-γ/LXR dimers and PPAR-γ or LXR associated with components of the GPVI pathway, indicating that increased presence of LXR in the GPVI signaling complex results in pathway inhibition.
Discussion
Although cholesterol is a vital component of cell membranes, elevated cholesterol levels contribute to the development of atherosclerosis. LXRs are key regulators of cholesterol homeostasis,3–6 and LXR ligands have been shown to have athero-protective effects possibly through modulation of cholesterol catabolism to bile acids and cholesterol efflux from macrophages back to the liver.9,41 In this study, we suggest an alternative mechanism that could contribute to antiatherogenic effects of LXR ligands based on their ability to regulate platelet function.
Apolipoprotein E (apoE) is regulated directly by LXR, and it serves as extracellular acceptor of cholesterol during cholesterol efflux6,38; thus, its role in atherosclerosis has been explored previously. ApoE−/− mice display impaired ability of ApoE-containing lipoproteins to be taken up by the liver, resulting in high plasma cholesterol levels and development of severe atherosclerosis.41 Treatment of apoE−/− mice with LXR ligands has been shown to reduce the atherosclerotic lesions9,31,41; however, this was without affecting significantly cholesterol levels,9,31 suggesting that LXR ligands may have effects independent of their actions on cholesterol metabolism. The presence of LXR-β in platelets and the ability of GW3965 to inhibit platelet function stimulated through a range of physiologic agonists could indicate platelets as potential mediators of part of the observed antiatherogenic effects. This hypothesis is reinforced by the reported role of platelets in the initiation of atherosclerosis,12–13,46 through platelet interactions with endothelial cells facilitated by P-selectin and β3 integrins.13,46,47 Our findings that LXR ligands inhibit P-selectin exposure and activation of the integrin αIIbβ3 further support a potential extended role for GW3965 through acute actions on platelets.
Our results also show that GW3965 inhibits the ability of platelets to form thrombi in vivo, by affecting both the size and the stability of growing thrombi. This is of importance at sites of atherosclerotic lesions, where treatment with GW3965 could prevent platelet accumulation and thrombosis, which often precipitates stroke or myocardial infarction. GW3965 treatment allowed initial thrombi to form (a physiologic process necessary for tissue repair after vascular injury) but reduced by approximately 40% the stability of thrombi preventing in this way occlusion of the vessel. These observations are in line with the inhibitory effects of LXR ligands on multiple aspects on platelet function. An impaired platelet response to collagen in the presence of GW3965 results in reduced secretion and release of positive secondary mediators, such as ADP and TXA2, although an initial thrombus forms. Reduced presence of these mediators combined with lower thrombin production, however, prevents amplification loops to take place resulting in smaller and less stable thrombi.
Because platelets have no nuclei, the acute effects of LXR ligands in platelets are nongenomic. Platelets have been shown to express other nuclear receptor isoforms, such as PPAR-γ,33 which are able to modulate platelet function on ligand stimulation.33,35 In this study, we have shown that LXR is able to associate with PPAR-γ on treatment with LXR or PPAR-γ ligands, indicating that nuclear receptors retain their ability to dimerize in the anucleate platelet. We have shown previously that PPAR-γ interacts with Syk and LAT on collagen stimulation, whereas PPAR-γ ligands cause loss of these associations.35 The observation of LXR-β-PPAR-γ interactions only at lower concentrations of GW3965 or 15d-PGJ2 could possibly be explained by an antagonistic relationship between LXR-β/PPAR-γ dimers and interactions of LXR-β or PPAR-γ with GPVI pathway components. This may underscore a potential generic mechanism of nuclear receptor action in platelets based on bidirectional interactions of nuclear receptors with: (1) other nuclear receptors and (2) components of the GPVI pathway in the presence or absence of their ligands.
The inhibitory effects of GW3965 on calcium mobilization from internal stores after thrombin stimulation indicate that LXR ligands could modulate signaling downstream G protein-coupled receptors. Furthermore, the associations of LXR with Syk and PLC-γ2 could also reflect involvement of LXR in outside-in signaling through αIIbβ3. After binding to fibrinogen or VWF, αIIbβ3 triggers tyrosine phosphorylation-dependent signaling inside the platelet, leading to stabilization of the growing thrombus,48,49 with Syk56 and PLC-γ257 known to play roles in these events. Consistent with this, the stability of the thrombus in our in vivo thrombus formation model was affected significantly by GW3965.
Because of their key roles in cholesterol metabolism, LXRs have been targets for drug discovery. Although LXR ligands display promising anti-inflammatory and athero-protective effects, they have been shown to increase triglyceride levels,41 which are independent risk factors for atherosclerosis. These effects have, however, been attributed to activation of LXR-α,52 triggering research for development of LXR-β-specific receptors.55 A key future challenge will be to confirm whether GW3965 antiplatelet effects are specifically mediated through LXR-β. Because LXR-β is expressed in platelets, the potential future clinical use of LXR-β-selective ligands may therefore protect through actions on atherogenesis and platelet function.
In conclusion, we suggest that the genomic effects of LXR ligands on cholesterol metabolism combined with their nongenomic antiplatelet/antithrombotic effects make LXR-β a promising target for treatment of both initiation of atherosclerosis and progression to thrombosis.
Supplementary Material
Acknowledgments
This work was supported by the British Heart Foundation and Heart Research United Kingdom, and the Medical Research Council.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.S. designed the research, performed experiments, analyzed results, made figures, and wrote the paper; J.M.G. and L.A.M. designed research and wrote the paper; T.S., G.B., and P.S. performed experiments; and C.I.J. performed experiments, analyzed results, and made figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan Gibbins, Institute for Cardiovascular and Metabolic Research, Hopkins Building, Whiteknights campus, University of Reading, Reading, United Kingdom, RG6 6UB; e-mail: j.m.gibbins@reading.ac.uk.
References
- 1.Chawla A, Repa JJ, Evans RM, et al. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann JM, Kliewer SA, Moore LB, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272(6):3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 3.Peet DJ, Turley SD, Ma W, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93(5):693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 4.Repa JJ, Turley SD, Lobaccaro JA, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289(5484):1446–1447. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 5.Singaraja RR, Bocher V, James ER, et al. Human ABCA1 BAC transgenic mice show increased high density lipoprotein cholesterol and ApoAI-dependent efflux stimulated by an internal promoter containing liver X receptor response elements in intron 1. J Biol Chem. 2001;276(36):33969–33979. doi: 10.1074/jbc.M102503200. [DOI] [PubMed] [Google Scholar]
- 6.Repa JJ, Berge KE, Pomajzl C, et al. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277(21):18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 7.Joseph SB, Castrillo A, Laffitte BA, et al. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9(2):213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 8.Fowler AJ, Sheu MY, Schmuth M, et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120(2):246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 9.Joseph SB, McKilligin E, Pei L, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99(11):76049. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tangirala RK, Bischoff ED, Joseph SB, et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99(18):11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furman MI, Krueger LA, Linden MD, et al. Release of soluble CD40L from platelets is regulated by glycoprotein IIb/IIIa and actin polymerization. J Am Coll Cardiol. 2004;43(12):2319–2325. doi: 10.1016/j.jacc.2003.12.055. [DOI] [PubMed] [Google Scholar]
- 12.Theilmeier G, Michiels C, Spaepen E, et al. Endothelial von Willebrand factor recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia. Blood. 2002;99(12):4486–4493. doi: 10.1182/blood.v99.12.4486. [DOI] [PubMed] [Google Scholar]
- 13.Frenette PS, Johnson RC, Hynes RO, et al. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci U S A. 1995;92(16):7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindemann S, Tolley ND, Dixon DA. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154(3):485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9(2):154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 16.Sanz J, Moreno PR, Fuster V. The year in atherothrombosis. J Am Coll Cardiol. 2010;55(14):1487–1489. doi: 10.1016/j.jacc.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 17.Ali FY, Armstrong PC, Dhanji AR, et al. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arterioscler Thromb Vasc Biol. 2009;29(5):706–701. doi: 10.1161/ATVBAHA.108.183160. [DOI] [PubMed] [Google Scholar]
- 18.Sadowitz B, Maier KG, Gahtan V. Basic science review. Statin therapy: I. The pleiotropic effects of statins in cardiovascular disease. Vasc Endovascular Surg. 2010;44(4):241–245. doi: 10.1177/1538574410362922. [DOI] [PubMed] [Google Scholar]
- 19.Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80(1):361–365. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- 20.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84(2):289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 21.Gibbins JM, Okuma M, Farndale R, et al. Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor gamma-chain. FEBS Lett. 1997;413(2):255–259. doi: 10.1016/s0014-5793(97)00926-5. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji M, Ezumi Y, Arai M, Takayama H. A novel association of Fc receptor gamma-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J Biol Chem. 1997;272(38):23528–23531. doi: 10.1074/jbc.272.38.23528. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki-Inoue K, Tulasne D, Shen Y, et al. Association of Fyn and Lyn with the proline-rich domain of glycoprotein VI regulates intracellular signaling. J Biol Chem. 2002;277(24):21561–21566. doi: 10.1074/jbc.M201012200. [DOI] [PubMed] [Google Scholar]
- 24.Poole A, Gibbins JM, Turner MJ, et al. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16(9):2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbins JM, Briddon S, Shutes A, et al. The p85 subunit of phosphatidylinositol 3-kinase associates with the Fc receptor gamma-chain and linker for activitor of T cells (LAT) in platelets stimulated by collagen and convulxin. J Biol Chem. 1998;273(51):34437–34443. doi: 10.1074/jbc.273.51.34437. [DOI] [PubMed] [Google Scholar]
- 26.Gross BS, Lee JR, Clements JL, et al. Tyrosine phosphorylation of SLP-76 is downstream of Syk following stimulation of the collagen receptor in platelets. J Biol Chem. 1999;274(9):5963–5971. doi: 10.1074/jbc.274.9.5963. [DOI] [PubMed] [Google Scholar]
- 27.Oda A, Ikeda Y, Ochs HD. Rapid tyrosine phosphorylation and activation of Bruton's tyrosine/Tec kinases in platelets induced by collagen binding or CD32 cross-linking. Blood. 2000;95(5):1663–1670. [PubMed] [Google Scholar]
- 28.Daniel JL, Dangelmaier C, Jin J, et al. Molecular basis for ADP-induced platelet activation: evidence for three distinct ADP receptors on human platelets. J Biol Chem. 1998;273(4):2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- 29.Hirata M, Hayashi Y, Ushikubi F, et al. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991;349(6310):617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- 30.Vu TK, Hung DT, Wheaton VI, et al. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 31.Levin N, Bischoff ED, Daige CL, et al. Macrophage liver X receptor is required for anti-atherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25(1):135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 32.Moraes LA, Swales KE, Wray JA, et al. Non-genomic signaling of the retinoid X receptor through binding and inhibiting Gq in human platelets. Blood. 2007;109(9):3741–3744. doi: 10.1182/blood-2006-05-022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akbiyik F, Ray DM, Gettings KF, et al. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104(5):1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- 34.Ali FY, Davidson SJ, Moraes LA, et al. Role of nuclear receptor signaling in platelets: antithrombotic effects of PPARbeta. FASEB J. 2006;20(2):326–328. doi: 10.1096/fj.05-4395fje. [DOI] [PubMed] [Google Scholar]
- 35.Moraes LA, Spyridon M, Kaiser WJ, et al. Non-genomic effects of PPARgamma ligands: inhibition of GPVI-stimulated platelet activation. J Thromb Haemost. 2010;8(3):577–587. doi: 10.1111/j.1538-7836.2009.03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patil S, Gross PL, Stapleton M, et al. Platelet PECAM-1 inhibits thrombus formation in vivo. Blood. 2006;107(2):535–541. doi: 10.1182/blood-2005-04-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 2010;204(3):233–240. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
- 38.Laffitte BA, Repa JJ, Joseph SB, et al. LXRs control lipid-inducible expression of the apoliprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci U S A. 2001;16(5):507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tucker KL, Sage T, Stevens JM, et al. A dual role for integrin linked kinase in platelets: regulating integrin function and alpha-granule secretion. Blood. 2008;112(12):4523–4531. doi: 10.1182/blood-2008-03-148502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue L, Ye F, Gui C, et al. Ligand-binding regulation of LXR/RXR and LXR/PPAR heterodimerizations: SPR technology-based kinetic analysis correlated with molecular dynamics simulation. Protein Sci. 2005;14(3):812–822. doi: 10.1110/ps.04951405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradley MN, Hong C, Chen M, et al. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117(8):2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins JL, Fivush AM, Watson MA, et al. Identification of a non-steroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem. 2002;45(10):1963–1966. doi: 10.1021/jm0255116. [DOI] [PubMed] [Google Scholar]
- 43.Gratacap MP, Martin V, Valéra MC, et al. The new tyrosine-kinase inhibitor and anticancer drug dasatinib reversely affect platelet activation in vitro and in vivo. Blood. 2009;114(9):1884–1892. doi: 10.1182/blood-2009-02-205328. [DOI] [PubMed] [Google Scholar]
- 44.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Non-classical anti-factor VIII C2 domain antibodies are pathogenic in a murine in vivo bleeding model. J Thromb Haemost. 2009;7(4):658–664. doi: 10.1111/j.1538-7836.2009.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morello F, Saglio E, Noghero A, et al. LXR-activating oxysterols induce the expression of inflammatory markers in endothelial cells through LXR-independent mechanisms. Atherosclerosis. 2009;207(1):38–44. doi: 10.1016/j.atherosclerosis.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Nofer J-R, Brodde MF, Kehrel BE. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin Exp Pharmacol Physiol. 2010;37(7):726–735. doi: 10.1111/j.1440-1681.2010.05377.x. [DOI] [PubMed] [Google Scholar]
- 47.Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for GPIIbIIIa-dependent mechanisms and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin and GPIalpha. J Exp Med. 1998;187(3):329–339. doi: 10.1084/jem.187.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark EA, Shattil SJ, Ginsberg MH, et al. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin alpha IIb beta. J Biol Chem. 1994;269(46):28859–28864. [PubMed] [Google Scholar]
- 49.Arias-Salgado EG, Lizano S, Sarkar S, et al. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100(23):13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodside DG, Obergfell A, Leng L, et al. Activation of Syk protein tyrosine kinase through interaction with integrin beta cytoplasmic domains. Curr Biol. 2001;11(22):1799–1804. doi: 10.1016/s0960-9822(01)00565-6. [DOI] [PubMed] [Google Scholar]
- 51.Wonerow P, Pearce AC, Vaux DJ, et al. A critical role for phospholipase Cgamma2 in alpha IIb beta3-mediated platelet spreading. J Biol Chem. 2003;278(39):37520–37529. doi: 10.1074/jbc.M305077200. [DOI] [PubMed] [Google Scholar]
- 52.Alberti S, Schuster G, Parini P, et al. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J Clin Invest. 2001;107(5):565–573. doi: 10.1172/JCI9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quinet EM, Savio DA, Halpern AR, et al. Liver X receptor (LXR)-beta regulation in LXRbeta-deficient mice: implications for therapeutic targeting. Mol Pharmacol. 2006;70(4):1340–1349. doi: 10.1124/mol.106.022608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.