1. DISEASE CHARACTERISTICS
1.1 Name of the disease (synonyms)
1.2 OMIM# of the disease
145600.
1.3 Name of the analyzed genes or DNA/chromosome segments
1.4 OMIM# of the gene(s)
180901 (RYR1), 114208 (CACNA1S).
1.5 Mutational spectrum
30 functionally confirmed causative point mutations (RYR1), about 200 MH-associated mutations in RYR1, few mutations in CACNA1S (of minor importance).5, 6, 7, 8
1.6 Analytical methods
Sequence analysis of the entire coding region (RYR1: 16 000 bp); sequence analysis of selected exons, mutation scanning of the entire coding region, mutation scanning of selected exons (direct sequencing, DHPLC, MLPA, restriction enzyme analysis).9, 10, 11, 12, 13
1.7 Analytical validation
>95% specificity, depending on the correctness of the phenotype.
1.8 Estimated frequency of the disease (incidence at birth (‘birth prevalence') or population prevalence)
Genetic incidence: 1:3000–1:10 000.14
1.9 If applicable, prevalence in the ethnic group of investigated person
Not applicable.
1.10 Diagnostic setting

Comment D:
Meaningful but not yet approved.
2. TEST CHARACTERISTICS
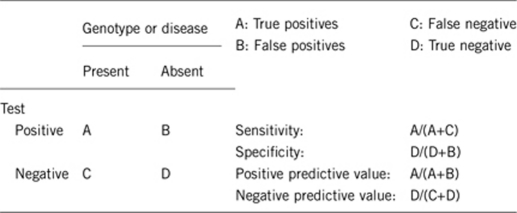
2.1 Analytical sensitivity
(proportion of positive tests if the genotype is present)
100%.
2.2 Analytical specificity
(proportion of negative tests if the genotype is not present)
100%.
2.3 Clinical sensitivity
(proportion of positive tests if the genotype is present)20, 21
>70%.
2.4 Clinical specificity
(proportion of negative tests if the disease is not present)20, 21
The clinical specificity can be dependent on variable factors, such as age or family history. In such cases, a general statement should be given, even if a quantification can only be made case by case.
>95%.
2.5 Positive clinical predictive value
(life time risk to develop the disease if the test is positive)20, 21
Life-time risk to develop the disease when patient is exposed to ‘trigger' anesthetics is >75%.
2.6 Negative clinical predictive value
(probability not to develop the disease if the test is negative)20, 21
Assume an increased risk based on family history for a non-affected person. Allelic and locus heterogeneity may need to be considered.
Index case in that family had been tested:
∼90%.
Index case in that family had not been tested:
∼15%.
3. CLINICAL UTILITY
3.1 (Differential) diagnosis: The tested person is clinically affected
Besides MH, a number of RYR1 mutations have been described to be associated with rare congenital myopathies. These include myopathies with cores (central core disease (CCD): MIM #11700; specific forms of multiminicore disease (MmD): MIM#255320) or central nuclei (centronuclear myopathy: MIM#160150) that are associated with a wide range of phenotypes. CCD is closely linked with MH, as both disorders share the same gene locus. In the other congenital myopathies, RYR1 mutations are rather an exception, but the MH risk must also be considered high until more information becomes available.22, 23, 24, 25, 26, 27
In contrast to this, there are disorders or syndromes that are very similar to classical MH, for example, serotonin syndrome or neuroleptic malignant syndrome. Other myopathies, such as Duchenne or Becker's muscular dystrophy, may induce ‘MH-like' symptoms under general anesthesia due to other pathophysiological pathways than for MH.19
3.1.1 Can a diagnosis be made other than through a genetic test?17, 18, 19, 28, 29
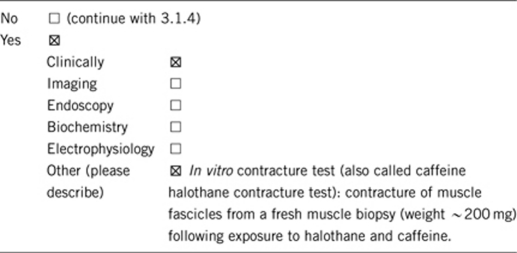
3.1.2 Describe the burden of alternative diagnostic methods to the patient?
Patients need surgery (open muscle biopsy). Contracture test must be performed within few hours of harvest. IVCT/CHCT can be performed only in special MH centers.1, 2
3.1.3 How is the cost effectiveness of alternative diagnostic methods to be judged?
Cost for muscle biopsy and IVCT: about €2000 in Europe. In the USA, the test is the CHCT version with a cost of about $6000.
3.1.4 Will disease management be influenced by the result of a genetic test?

3.2 Predictive setting: The tested person is clinically unaffected but carries an increased risk based on family history
MH predisposed persons are clinically unaffected until exposed to MH trigger agents.
Only patients who are tested MH positive need a special anaesthetic management during surgery. MH negative tested persons can be handled like the ‘normal' patients; regardless of the family history.
3.2.1 Will the result of a genetic test influence lifestyle and prevention?1, 2, 5
Yes.
If the test result is positive (please describe):
The patient gets an anesthesia-warning card to alert the anesthesiologist to problems during anesthesia. The card should always be carried by the holder. Extreme caution when exercising in hot environment and with extreme exercise.
If the test result is negative (please describe):
For clinical reasons of safety the patient needs to undergo a negative confirmation test (muscle biopsy, halothane/caffeine provocation test).
3.2.2 Which options in view of lifestyle and prevention does a person at-risk have if no genetic test has been done (please describe)?
Person should be alternatively tested with the IVCT/CHCT (muscle biopsy) to determine the MH risk. Otherwise the patient should consider himself at risk for MH.
3.3 Genetic risk assessment in family members of a diseased person
50%.
3.3.1 Does the result of a genetic test resolve the genetic situation in that family?
Yes, if a mutation is found. If the index patient does not have a mutation, MH susceptibility is still a possibility because of heterogeneity.
3.3.2 Can a genetic test in the index patient save genetic or other tests in family members?
Yes.
3.3.3 Does a positive genetic test result in the index patient enable a predictive test in a family member?
Yes.
3.4 Prenatal diagnosis
3.4.1 Does a positive genetic test result in the index patient enable a prenatal diagnostic?
Prenatal diagnosis is not yet approved solely for MH diagnosis but would be a promising option and would make sense if the familial MH mutation is known. PND could increase patient safety, for example, for the newborn of MH positive parents (MH-positive mother or father, newborn is 50% at risk) under the situation of a Cesarean section. In this case MH triggering agents (given under general anesthesia) must be avoided even if the mother is negative.
On the other hand, prenatal diagnosis is an important option for other congenital RYR1-related disorders and if such an indication is given the search for MH-associated mutations could be taken under consideration.
4. IF APPLICABLE, FURTHER CONSEQUENCES OF TESTING
Please assume that the result of a genetic test has no immediate medical consequences. Is there any evidence that a genetic test is nevertheless useful for the patient or his/her relatives? (Please describe)
Yes. If a mutation associated with CCD or MmD is found, the patient should be followed for evidence of muscle weakness. Prenatal counseling is advised in such cases.
Family members should be treated as MH susceptible until IVCT/CHCT test is done with a negative result.
Acknowledgments
This work was supported by the EuroGentest, an EU-FP6 supported NoE, contract number 512148 (EuroGentest Unit 3: ‘Clinical genetics, community genetics and public health', Workpackage 3.2).
The authors declare no conflict of interest.
References
- European Malignant Hyperthermia Group (EMHG) , http://www.emhg.org .
- Malignant Hyperthermia Association of the United States (MHAUS) , http://www.mhaus.org .
- McCarthy TV, Healy JM, Heffron JJ, et al. Localization of the malignant hyperthermia susceptibility locus to human chromosome 19q12-13.2. Nature. 1990;343:562–564. doi: 10.1038/343562a0. [DOI] [PubMed] [Google Scholar]
- Monnier N, Procaccio V, Stieglitz P, Lunardi J. Malignant-hyperthermia susceptibility is associated with a mutation of the alpha-1-subunit of the human dihydropyridine-sensitive L-type voltage-dependent calcium-channel receptor in skeletal muscle. Am J Hum Genet. 1997;60:1316–1325. doi: 10.1086/515454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwyler A, Deufel T, McCarthy T, West S. Guidelines for molecular genetic detection of susceptibility to malignant hyperthermia. Br J Anaesth. 2001;86:283–287. doi: 10.1093/bja/86.2.283. [DOI] [PubMed] [Google Scholar]
- McCarthy TV, Quane KA, Lynch PJ. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum Mutat. 2000;15:410–417. doi: 10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Sambuughin N, Holley H, Muldoon S, et al. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the North American population. Anesthesiology. 2005;102:515–521. doi: 10.1097/00000542-200503000-00007. [DOI] [PubMed] [Google Scholar]
- Stewart SL, Hogan K, Rosenberg H, Fletcher JE. Identification of the Arg1086His mutation in the alpha subunit of the voltage-dependent calcium channel (CACNA1S) in a North American family with malignant hyperthermia. Clin Genet. 2001;59:178–184. doi: 10.1034/j.1399-0004.2001.590306.x. [DOI] [PubMed] [Google Scholar]
- Ibarra MC, Wu S, Murayama K, et al. Malignant hyperthermia in Japan: mutation screening of the entire ryanodine receptor type 1 gene coding region by direct sequencing. Anesthesiology. 2006;104:1146–1154. doi: 10.1097/00000542-200606000-00008. [DOI] [PubMed] [Google Scholar]
- Rueffert H, Olthoff D, Deutrich C, et al. Mutation screening in the ryanodine receptor 1 gene (RYR1) in patients susceptible to malignant hyperthermia who show definite IVCT results: identification of three novel mutations. Acta Anaesthesiol Scand. 2002;46:692–698. doi: 10.1034/j.1399-6576.2002.460610.x. [DOI] [PubMed] [Google Scholar]
- Levano S, Vukcevic M, Singer M, et al. Increasing the number of diagnostic mutations in malignant hyperthermia. Hum Mutat. 2009;30:590–598. doi: 10.1002/humu.20878. [DOI] [PubMed] [Google Scholar]
- Girard T, Litman RS. Molecular genetic testing to diagnose malignant hyperthermia susceptibility. J Clin Anesth. 2008;20:161–163. doi: 10.1016/j.jclinane.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Stowell KM, Pollock N. DNA analysis and malignant hyperthermia susceptibility. Anaesth Intensive Care. 2008;36:305–307. doi: 10.1177/0310057X0803600302. [DOI] [PubMed] [Google Scholar]
- Monnier N, Krivosic-Horber R, Payen JF, et al. Presence of two different genetic traits in malignant hyperthermia families: implication for genetic analysis, diagnosis, and incidence of malignant hyperthermia susceptibility. Anesthesiology. 2002;97:1067–1074. doi: 10.1097/00000542-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Ording H. Incidence of malignant hyperthermia in Denmark. Anesth Analg. 1985;64:700–704. [PubMed] [Google Scholar]
- Brady JE, Sun LS, Rosenberg H, Li G. Prevalence of malignant hyperthermia due to anesthesia in New York State, 2001–2005. Anesth Analg. 2009;109:1162–1166. doi: 10.1213/ane.0b013e3181ac1548. [DOI] [PubMed] [Google Scholar]
- Ording H, Brancadoro V, Cozzolino S, et al. In vitro contracture test for diagnosis of malignant hyperthermia following the protocol of the European MH Group: results of testing patients surviving fulminant MH and unrelated low-risk subjects. The European Malignant Hyperthermia Group. Acta Anaesthesiol Scand. 1997;41:955–966. doi: 10.1111/j.1399-6576.1997.tb04820.x. [DOI] [PubMed] [Google Scholar]
- Larach MG, Localio AR, Allen GC, et al. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology. 1994;80:771–779. doi: 10.1097/00000542-199404000-00008. [DOI] [PubMed] [Google Scholar]
- Wappler F. Anesthesia for patients with a history of malignant hyperthermia. Curr Opin Anaesthesiol. 2010;23:417–422. doi: 10.1097/ACO.0b013e328337ffe0. [DOI] [PubMed] [Google Scholar]
- Rosenberg H, Davis M, James D, et al. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21. doi: 10.1186/1750-1172-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman RS, Rosenberg H. Malignant hyperthermia: update on susceptibility testing. JAMA. 2005;293:2918–2924. doi: 10.1001/jama.293.23.2918. [DOI] [PubMed] [Google Scholar]
- Zhou H, Lillis S, Loy RE, et al. Multi-minicore disease and atypical periodic paralysis associated with novel mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul Disord. 2010;20:166–173. doi: 10.1016/j.nmd.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmshurst JM, Lillis S, Zhou H, et al. RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann Neurol. 2010;68:717–726. doi: 10.1002/ana.22119. [DOI] [PubMed] [Google Scholar]
- Treves S, Jungbluth H, Muntoni F, Zorzato F. Congenital muscle disorders with cores: the ryanodine receptor calcium channel paradigm. Curr Opin Pharmacol. 2008;8:319–326. doi: 10.1016/j.coph.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Robinson RL, Brooks C, Brown SL, et al. RYR1 mutations causing central core disease are associated with more severe malignant hyperthermia in vitro contracture test phenotypes. Hum Mutat. 2002;20:88–97. doi: 10.1002/humu.10098. [DOI] [PubMed] [Google Scholar]
- Carpenter D, Robinson RL, Quinnell RJ, et al. Genetic variation in RYR1and malignant hyperthermia phenotypes. Br J Anaesth. 2009;103:538–548. doi: 10.1093/bja/aep204. [DOI] [PubMed] [Google Scholar]
- Ducreux S, Zorzato F, Ferreiro A, et al. Functional properties of ryanodine receptors carrying three amino acid substitutions identified in patients affected by multi-minicore disease and central core disease, expressed in immortalized lymphocytes. Biochem J. 2006;395:259–266. doi: 10.1042/BJ20051282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larach MG. Standardization of the caffeine halothane muscle contracture test. North American Malignant Hyperthermia Group. Anesth Analg. 1989;69:511–515. [PubMed] [Google Scholar]
- Rosenberg H, Dirksen RA, Sambuughin N.Malignant hyperthermia susceptibilityin Baskin P, Pagan R (ed): GeneReviews , http://www.genetests.org .


