Abstract
Autosomal recessive Stargardt disease (STGD1) is a macular dystrophy caused by mutations in the ABCA4 (ABCR) gene. The disease phenotype that is most recognized in STGD1 patients, and also in the Abca4−/− mouse (a disease model), is lipofuscin accumulation in retinal pigment epithelium. Here, we tested whether delivery of the normal (wt) human ABCA4 gene to the subretinal space of the Abca4−/− mice via lentiviral vectors would correct the disease phenotype; that is, reduce accumulation of the lipofuscin pigment A2E. Equine infectious anemia virus (EIAV)-derived lentiviral vectors were constructed expressing either the human ABCA4 gene or the LacZ reporter gene under the control of the constitutive (CMV) or photoreceptor-specific (Rho) promoters. Abca4−/− mice were injected subretinally with 1 µl (~5.0 × 105 TU) of each EIAV vector in one eye at postnatal days 4 and 5. An injection of saline, an EIAV-null vector, or an uninjected contralateral eye served as a control. Mice were killed at various times after injection to determine photoreceptor (PR) transduction efficiency and A2E concentrations. EIAV-LacZ vectors transduced from 5 to 20% of the PRs in the injected area in mice. Most importantly, a single subretinal injection of EIAV-CMV-ABCA4 to Abca4−/− mouse eyes substantially reduced disease-associated A2E accumulation compared to untreated and mock-treated control eyes. Treated eyes of Abca4−/− mice accumulated 8–12 pmol per eye (s.d. = 2.7) of A2E 1 year after treatment, amounts comparable to wt controls, whereas mock-treated or untreated eyes had 3–5 times more A2E (27–39 pmol per eye, s.d. = 1.5; P = 0.001–0.005). Although extrapolation to humans requires caution, the high transduction efficiency of both rod and cone photoreceptors and the statistically significant reduction of A2E accumulation in the mouse model of STGD1 suggest that lentiviral gene therapy is a potentially efficient tool for treating ABCA4-associated diseases.
Keywords: Stargardt disease, ABCA4, EIAV lentivirus, mouse model, A2E
Introduction
Autosomal recessive Stargardt disease (STGD1; MIM 248200) is arguably the most common hereditary recessive macular dystrophy (estimated frequency of 1:8000–10 000 in the US).1 It is characterized by a highly variable age of onset and clinical course. Most cases present with juvenile to young-adult onset, insidious to rapid central visual impairment, progressive bilateral atrophy of the foveal retinal pigment epithelium (RPE) and photoreceptors, and the frequent appearance of yellow-orange flecks distributed around the macula and/or the mid-retinal periphery.2,3
Several laboratories independently described ABCA4 (initially called ABCR) in 1997 as the causal gene for arSTGD.4–6 Subsequently, several cases were reported where ABCA4 mutations segregated with retinal dystrophies of significantly different phenotype, such as autosomal recessive cone-rod dystrophy7,8 and autosomal recessive retinitis pigmentosa (arRP).7,9,10 Disease-associated ABCA4 alleles have shown an extraordinary heterogeneity,4,11–15 with > 500 disease-associated ABCA4 variants identified to date. The empirical estimates suggest that the carrier frequency of ABCA4 alleles in general population is at least 5%.12,16,17 This finding, that at least 1/20 people carry a disease-associated ABCA4 allele, has significant implications for the amount of retinal pathology attributable to ABCA4 variation.
The ABCA4 protein was first described in 1976 as an abundant component of photoreceptor outer segment disk rims.18,19 Hence, it was called a Rim protein until the encoding gene, ABCA4, was cloned and characterized as a member of the ATP-binding cassette transporter superfamily, which suggested a transport function of some substrate in photoreceptor outer segments.4,6 All-trans-retinal, an isoform of the rhodopsin chromophore, was identified as a potential substrate of ABCA4 by its ability to stimulate ATP hydrolysis by reconstituted ABCA4 protein in vitro, suggesting that retinal could also be the in vivo substrate for ABCA4.20 Studies of Abca4 knock-out (Abca4−/−) mice have fully supported this hypothesis, and have led to the concept that ABCA4 is a ‘flippase’ of a complex of all-trans-retinal and phosphatidylethanolamine (PE).21 Recent experimental data confirmed this hypothesis.22
Mice lacking a functional Abca4 gene have demonstrated delayed dark adaptation, increased all-trans-retinal following light exposure, elevated PE in rod outer segments, and a striking deposition of a major lipofuscin fluorophore (A2E) in RPE.21 Based on these findings, it was suggested that the ABCA4-mediated retinal degeneration may result from adverse effects of lipofuscin accumulation in the RPE, with secondary photoreceptor degeneration due to loss of the RPE support role.21 A2E, a pyridinium bis-retinoid, derived from two molecules of vitamin A aldehyde and one molecule of ethanolamine, has been characterized as one of the major components of retinal pigment epithelial lipofuscin,23 although others have been described more recently.24 Evidence indicates that bis-retinoid compounds such as A2E can damage cells by initiating photo-oxidative processes and/or by a detergent-like perturbation of membranes.25 Accumulation of lipofuscin in the macular region of RPE is characteristic to aging eyes and is the hallmark of both STGD1 and age-related macular degeneration. Together, these data define ABCA4 as a ‘rate-keeper’ of retinal transport in the visual cycle with an auxiliary, but not an essential, role in this process, because individuals completely lacking the functional protein (for example, some patients with RP-like phenotype) maintain some vision for years. Over time, however, even mild dysfunction of ABCA4 affects vision irreparably.
Genetic and functional studies have defined ABCA4 as an important, but difficult target for therapeutic applications. Suggested treatment strategies include light restriction, limiting dietary intake of vitamin A, slowing down the visual cycle with small molecule drugs and compounds modifying the functional (ATP-binding and hydrolysis) activity of ABCA4.26 However, as each of these approaches will have either indirect or limited effects on the ABCA4 function, these would only delay or modify the disease progress.
Somatic gene therapy has demonstrated success in several animal models of (recessive) retinal degeneration. 27,28 Most well known reports have been that a recombinant adeno-associated virus (rAAV) carrying wild-type Rpe65 (rAAV-Rpe65) improved visual function in the Briard dog model of childhood blindness.29–31 Similar successes have been reported for the Rpe65−/− and Lrat−/− mouse models with AAV and lentiviral vectors.32–35 Delivering a normally functioning gene to photoreceptors harboring mutant ABCA4 via gene therapy should therefore be considered as a possible ‘cure’ for ABCA4-associated diseases because: (1) all ABCA4-associated diseases are recessive, caused by a variable insufficiency exceeding 50%, so that adding a functional gene could fully restore visual function; (2) degeneration of the retinal cells in all ABCA4-associated diseases is relatively delayed, allowing a reasonable time window for therapeutic intervention.
Lentiviral vectors offer several advantages for gene therapy applications in general, as well as in the specific case of ABCA4-associated diseases. First and foremost, lentiviruses are capable of delivering genes stably and permanently into the genome of transduced cells in vivo.36–38 Secondly, they can transduce nondividing cells, a crucial requirement for terminally differentiated cells such as photoreceptors.39 Thirdly, they can carry relatively large expression cassettes, which is essential in this case because the human ABCA4 cDNA is almost 7 kb which exceeds the capacity of the commonly used AAV vectors.
Here, we describe the utility of equine infectious anemia virus (EIAV)-based lentiviral gene therapy in the mouse model of ABCA4-associated diseases leading to a substantial modulation of the disease phenotype.
Results
Photoreceptor transduction efficiency with EIAV-based vectors and constitutive promoters in mice
The efficiency of rodent photoreceptor transduction by lentiviruses has been an area of much debate, despite early reports of success in rats.40,41 Subsequent studies have reported rather low efficiencies of PR transduction by lentiviruses, especially in adult animals, which have raised concerns in their suitability as efficient tools for gene therapy in mouse models.37,42 In this study we have extensively evaluated the PR transduction efficiency of EIAV-based lentiviral vectors (Figure 1) in mice. Although the Abca4−/− mouse is currently the only animal model for STGD1, the ultimate goal of these studies was to assess the possibility of treating STGD1 in humans. In mice, subretinal injections of ~1 µl of the EIAV-CMV-LacZ vector (~5.0 × 105 transducing units (TU)) or the HIV-EF1α-LacZ vector (3.0 × 105 TU) were performed at postnatal days 4 and 5 (P4–5). Mice were killed 14 days later and retinal sections were examined for reporter gene expression after X-gal staining (Figure 2). A single injection routinely resulted in effectively transduced areas amounting to 20–30% of the entire retina (Figure 2a). EIAV-derived constructs routinely targeted at least 5% of photoreceptors, RPE and, occasionally, Mueller cells (Figure 2b), whereas the transduction was mainly restricted to RPE in the case of the HIV-derived vectors (Figure 2c).
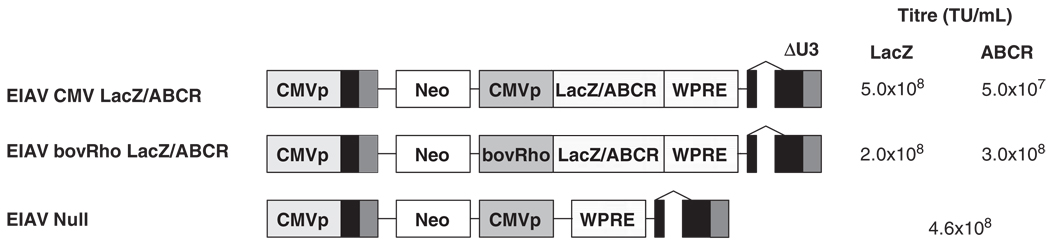
Figure 1.
Schematic representation of the equine infectious anemia virus (EIAV) vectors used in this study including the titer of each vector in transducing units per ml (TU ml−1). CMVp, the cytomegalovirus immediate/early promoter; Neo, Neomycin open reading frame; bovRho, 0.3 kb fragment from the bovine rhodopsin promoter; LacZ, β-galactosidase reporter gene, ABCR, human ABCR/ABCA4 cDNA; WPRE, modified Woodchuck hepatitis virus post-transcriptional regulatory element.
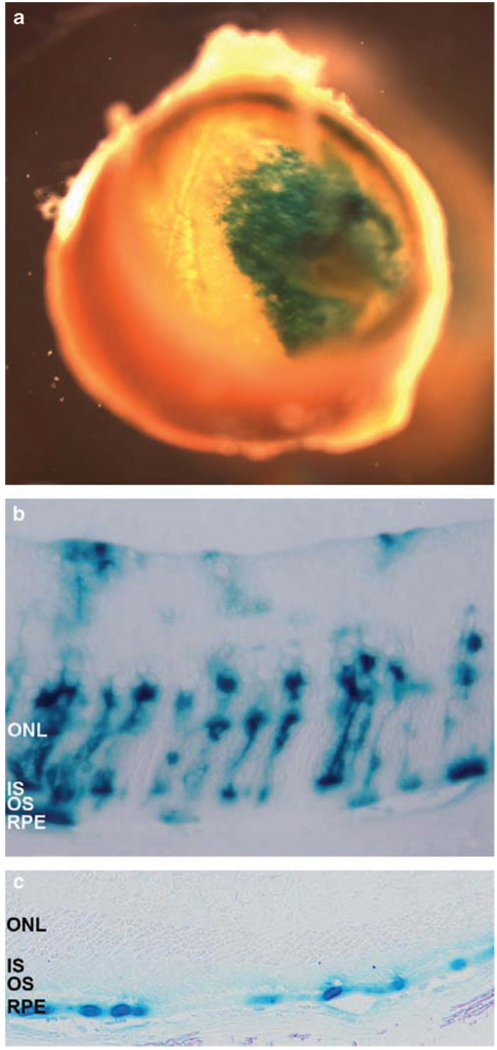
Figure 2.
X-gal staining of the posterior segment (a) and Epon-embedded sections of mouse retinas transduced with the EIAV-CMV-LacZ vector (b) or the HIV-EF1α-LacZ vector (c). (a) A single injection of the EIAV-CMV-LacZ vector transduced ~20–30% of the entire retina. (b and c) Retinal sections of 18 days old mice, injected subretinally with 1 µl of viral solution containing 3–5 × 105 TU of either EIAV-CMV-LacZ (b) or HIV-EF1α-LacZ (c) 14 days earlier. EIAV-derived vector transduced ~5% of photoreceptors, retinal pigment epithelium (RPE) and occasionally Mueller cells (b), whereas transduction was restricted to RPE cells with the HIV-derived vector (c). Representative images of n = 10 eyes (a and b) and n = 6 eyes (c) are shown.
In summary, we determined that the EIAV-based vectors are substantially more efficient in transducing PRs in mouse models than HIV-based vectors (at least those used in our study).
Evaluation of photoreceptor transduction efficiency with EIAV-based vectors using PR-specific promoters
Rod opsin (Rho) promoters have been widely used to efficiently drive and restrict the expression of genes in photoreceptors.43,44 As the ABCA4 gene is expressed only in PRs in vivo, we tested if the bovine Rho (bRho) promoter43,44 when used in EIAV-based vectors carrying the LacZ reporter gene could couple the high efficiency of photoreceptor transduction with expression restricted to PRs. Experiments in mice were designed exactly as described above for EIAV vectors with the control of the constitutive (CMV) promoter, where 1 µl (2.0 × 105 TU) of EIAV-Rho-LacZ constructs (Figure 1) were injected subretinally to Abca4−/− mice at P4–5, followed by examination of the retinas by X-gal staining, Epon sectioning and microscopy. Figure 3 shows that the reporter gene was expressed in mouse photoreceptors with high efficiency and at the injected site; up to one-fifth of all photoreceptors in parts of the injected area expressed LacZ (~20%; s.d. = 5.5, s.e.m. = 2.4, n = 5) and the expression was limited to photoreceptors, the site of ABCA4 expression in vivo.
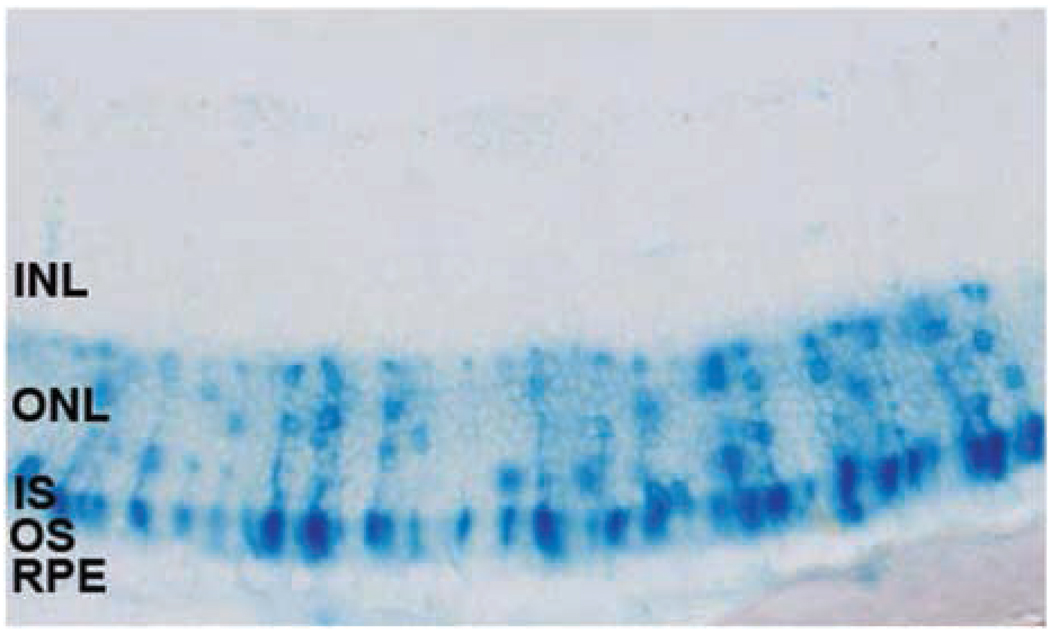
Figure 3.
X-gal staining of Epon section of a mouse retina following subretinal delivery of the equine infectious anemia virus (EIAV)-bRho-LacZ vector. The newborn mice (P4–5) were subretinally injected with 2.0 × 105 TU of the EIAV-bRho-LacZ virus and X-gal staining was carried out 14 days later. The X-gal staining was restricted to the photoreceptor cell layer in all animals (n = 5).
Efficacy of EIAV ABCR vectors in the Abca4−/− mouse model
The Abca4−/− mouse was first reported by Weng and colleagues.21 These mice reproduced two main features of STGD1 in humans: (1) an enhanced accumulation of the major fluorophore of the RPE lipofuscin, A2E, in the RPE cells21,45,46 and (2) a delayed dark adaptation.47 We produced an Abca4−/− mouse strain by a homozygous targeted deletion of exon 1 of the murine Abca4 gene (data not shown) and confirmed the phenotype of enhanced A2E accumulation. Specifically, at measured time points of 6–12 months of age, our Abca4−/− mice presented with 8- to 10-fold excess of A2E in the RPE cells as compared to wild-type (wt) controls (see Figure 6 in the next section). The data on delayed dark adaptation and reduction in the a-wave component of the full field electroretinogram (ERG) was less prominent in our strain (data not shown) than reported previously.21,47 As we could not obtain robust differences using ERG to determine the kinetics of dark adaptation in these mice, ERG was only used to assess whether there was significant injury or toxicity to the photoreceptors from the subretinal injections.
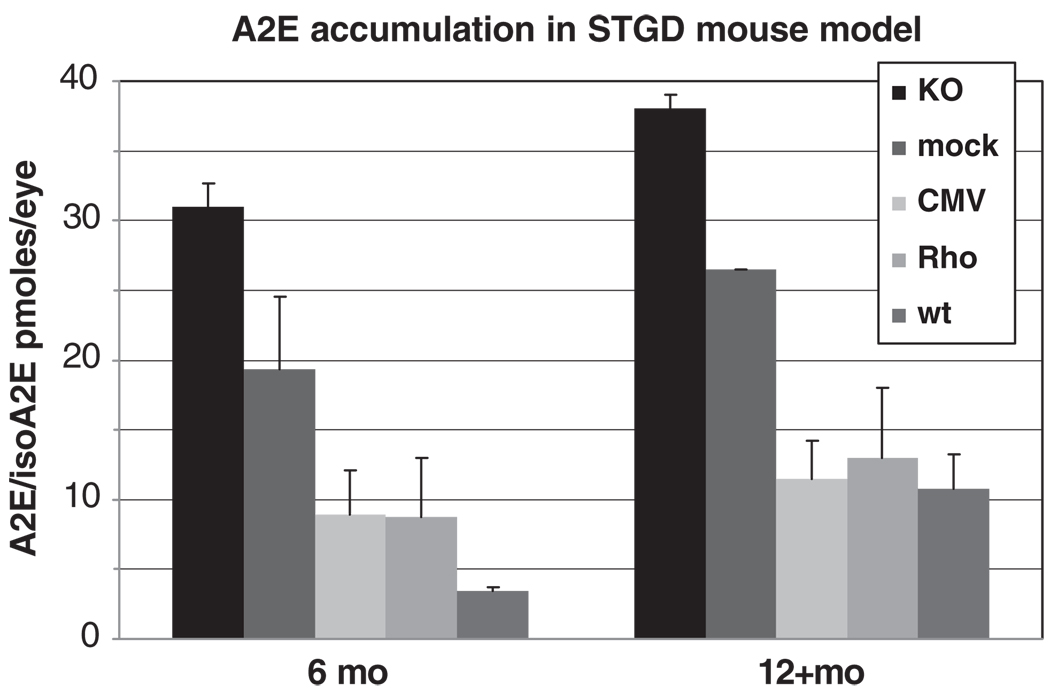
Figure 6.
A2E/isoA2E content in mouse eyes at 6 and 12+ months time points. KO, untreated Abca4−/− mice; mock, Abca4−/− animals injected with equine infectious anemia virus (EIAV)-null virus; CMV, Abca4−/− mice treated with EIAV-CMV-ABCA4 construct; Rho, Abca4−/− mice treated with EIAV-Rho-ABCA4 constructs; wt, wild type animals. Each bar (data point) represents n = 4–20 eyes with s.d. values shown.
The transgene expression from the EIAV-ABCA4 vectors following subretinal delivery to Abca4−/− mice was first determined by a reverse transcriptase–polymerase chain reaction (RT–PCR) on total RNA isolated from vector-treated eyes 2 weeks and 2 months after the injection (Figure 4). The results confirmed the expression of ABCA4 in the Abca4−/− mice retinas for at least up to 2 months post subretinal delivery of either the EIAV-CMV-ABCA4 or the EIAV-bRho-ABCA4 vectors.
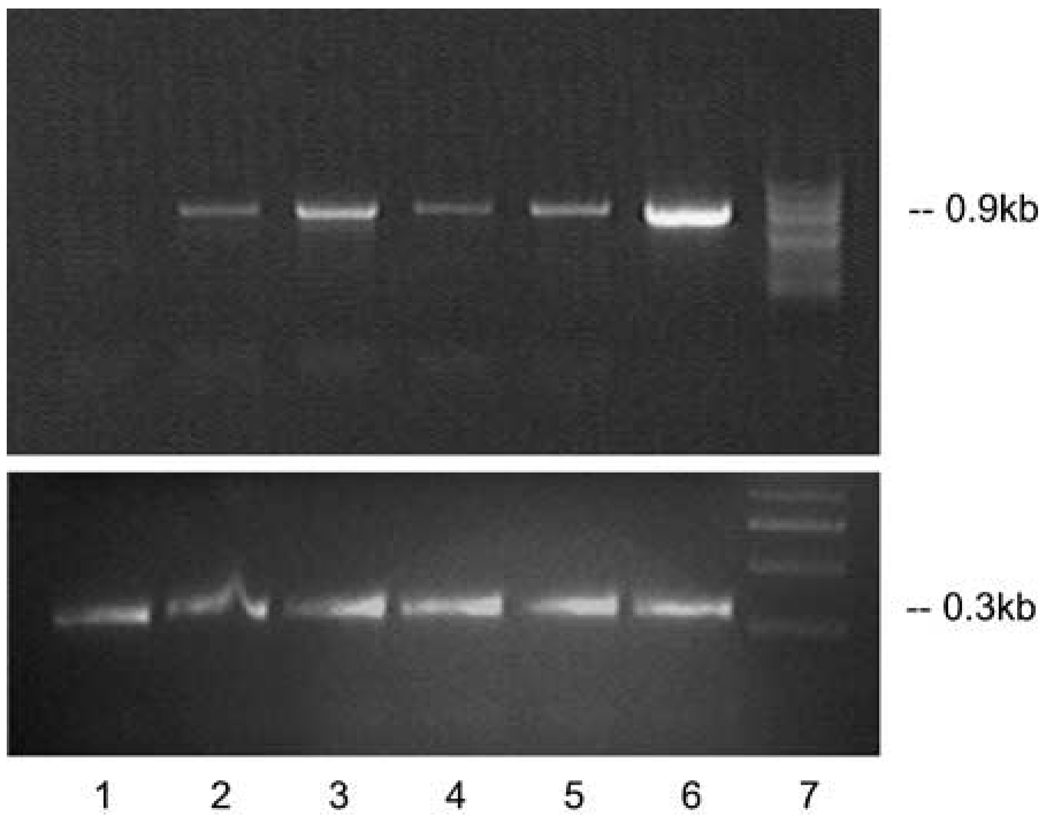
Figure 4.
Reverse transcriptase (RT)–PCR on the total RNA from eyecups of Abca4−/− mice treated with equine infectious anemia virus (EIAV)-ABCA4 vectors 2 weeks (lanes 2, 3) and 2 months (lanes 4–6) after subretinal injections. Upper panel: lane 1, untreated Abca4−/− mouse eyes; lane 2, Abca4−/− mouse eyes 2 weeks after EIAV-CMV-ABCR injection; lane 3, Abca4−/− mouse eyes 2 weeks after EIAV-bRho-ABCR injection; lane 4, Abca4−/− mouse eyes 2 months after EIAV-CMV-ABCR injection; lane 5, the same experiment as in lane 4 with a different isolate of the EIAV-CMV-ABCR virus; lane 6, Abca4−/− mouse eyes 2 months after EIAV-bRho-ABCR injection; lane 7, marker. Lower panel shows amplification of a 0.3 kb fragment of the actin gene from the same RNA isolates.
Next, we determined the pattern of human ABCA4 protein expression in the EIAV-CMV-ABCA4 treated mice 2 weeks post vector delivery using the monoclonal antibody for the ABCA4 protein (Rim 3F4).20 The human ABCA4 protein is localized to PR outer segments and to the RPE of the Abca4−/− mouse retina (Figure 5). The ABCA4 histological data perfectly correlates with the LacZ expression data for the EIAV-CMV-LacZ vector, where expression was seen in PRs and in RPE (Figure 2b). Together, these data confirm the expression of the ABCA4 gene in the photoreceptors of Abca4−/− mice for up to 2 months following subretinal delivery of the EIAV-CMV-ABCA4 or EIAV-Rho-ABCA4 vectors.
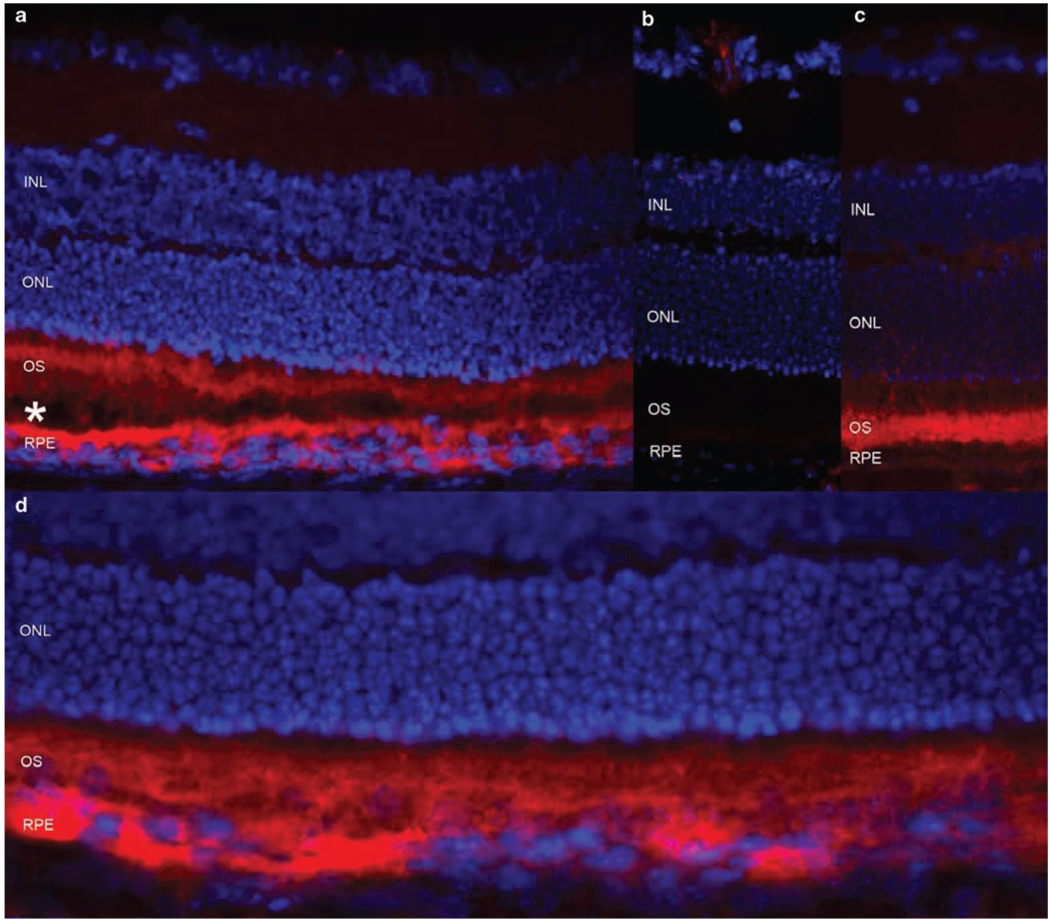
Figure 5.
Expression of the human and murine ABCA4 protein in the mouse retina. (a) The Abca4−/− mouse retina treated with the EIAV-CMV-ABCA4 virus, showing the human ABCA4 in photoreceptor (PR) outer segments (OS) and in the RPE (in red). OS and RPE cell layers are slightly detached (shown by *) to further separate the staining in the PR outer segments and RPE cells. (b) Untreated Abca4−/− mouse, lacking any ABCA4/Abca4. (c) Wild-type mouse showing the endogenous mouse Abca4 protein in PR outer segments. (d) The same experiment as in (a), showing the extent of the expression of the human ABCA4 protein in the treated area with no detachment. INL, inner nuclear layer; ONL, outer nuclear layer; OS, outer segments of photoreceptors; RPE, retinal pigment epithelium.
Subretinal delivery of the human ABCA4 gene reduces A2E accumulation in Abca4−/− mice
We determined the therapeutic efficacy of an EIAV-based gene therapy approach by injecting EIAV-CMV-ABCA4 and EIAV-Rho-ABCA4 vectors into the subretinal space of one eye in Abca4−/− mice whereas the contralateral eye was either left untreated (KO) or injected with EIAV-null vector (mock). Eyes were harvested and analyzed for their A2E content at 6 and 12+ months after vector delivery (Figure 6).
At 6 months of age, the untreated Abca4−/− mice have ~31 pmol per eye (s.d. = 1.7) of A2E/isoA2E, compared to ~19 pmol per eye (s.d. = 5.2) for mock-treated (a nonsignificant difference; P = 0.08 by Student’s t-test) and ~9 pmol per eye (s.d. = 3.2) for animals injected with therapeutic viruses (a significant difference; P = 0.001 with double-sided t-test). The A2E accumulation is kept under control by the EIAV-ABCA4 vectors; that is, it increases slowly similar to the wt mice reaching the same levels by the age of 1 year (P = 0.005). The statistically significant difference between experimental (ABCA4-treated) and control (mock-treated) groups were confirmed by pair-wise (t-test) and multiple comparisons (ANOVA; P = 0.008). In summary, these data clearly indicate a therapeutic effect, most reasonably based on what must be a partial restoration of ABCA4 function. Elevated A2E accumulation in human and mouse eyes is caused by dysfunctional ABCA4;21,25,26 therefore, reduction of A2E accumulation rates is a direct measure of the ABCA4 function.
ERG evaluation of photoreceptor status
Subretinal surgery causes a transient local retinal detachment and some photoreceptor damage both of which can interfere with the visual cycle and consequently influence A2E accumulation. In order to determine whether subretinal surgery itself was a significant cause of the observed reduction of A2E in the treated P4–5 Abca4−/− mice, we examined ERG before euthanizing the animals in each experiment. ERG was somewhat reduced in all animals who received subretinal injections, but it was significantly lower in the EIAV-null injected (mock-treated) controls than in the therapeutically treated mice (Supplementary Table 1). The ERG difference between mock-treated and untreated animals is statistically more significant (P < 1 × 10−8, Student’s t-test), than the difference between animals treated with therapeutic vectors and the untreated ones (P = 0.0006). Therefore, the significant difference in A2E content in the mice treated with EIAV-ABCA4 compared to the mock-treated animals cannot be explained by greater photoreceptor pathology. On the contrary, the significantly lower reduction of ERG in the EIAV-ABCA4 treated mice compared to the mock-treated controls clearly indicates that the surgical trauma is not a major factor in the observed therapeutic effect; that is, in the statistically significant difference in A2E reduction in the treatment group vs the control group.
Discussion
Development of successful treatments for inherited retinal disease caused by congenital defects is a major challenge. Recessive diseases, arising from insufficient gene activity which disrupts a metabolic pathway (such as the visual cycle), are particularly amenable to somatic gene therapy. Here, we demonstrate that defects of the visual cycle leading to vision loss can be successfully treated by direct gene therapy with EIAV-based lentiviral vectors. Contrary to some prevailing concerns, the EIAV lentiviral vectors efficiently transduce PRs in newborn mice. The precisely quantifiable measure of ABCA4 function, accumulation of A2E/isoA2E (as measured by high performance liquid chromatography (HPLC)), was significantly reduced after treating a mouse model of STGD1 (Abca4−/−) with subretinal injections of EIAV-ABCR constructs.
The Abca4−/− mouse is currently the only available animal model for STGD1. The murine phenotype resembles STGD1 in humans in that the KO mice accumulate the major fluorophore of lipofuscin, A2E, at a 5- to 10-fold higher rate (Figure 6).21,45 Another reported measurable feature is the delayed recovery time after photobleach.21 The mouse strain used in this study, although made in a practically identical fashion (deletion of the exon 1 of the mouse Abca4 gene), did not exhibit this phenotype as prominently as previously reported. Therefore, as the Abca4−/− mice do not present with any other clinically relevant phenotypic feature, we chose A2E accumulation, the direct measure of the protein function, also as the measure of the efficiency of the therapeutic intervention. Injection of the human ABCA4 gene under both constitutive and PR-specific promoters in the subretinal space of the Abca4−/− mice substantially and reproducibly reduced A2E accumulation. The effect was efficiently sustained and resulted in A2E levels comparable to those in wt mice at 12 months of age.
The reported low efficiency of PR transduction with lentiviruses in (adult) rodents has been considered a major limiting factor for gene therapy of eye diseases with lentiviral vectors. Although the early experiments with rats were successful,40,41 they were not confirmed subsequently, especially in adult rodent models.37,42 Here, we confirm a relatively efficient (5–20%) expression of the reporter gene (LacZ) driven from both constitutive and PR-specific promoters after subretinal injection of mice at P4–5 (Figure 2).
Efficient reduction of A2E/isoA2E accumulation was obtained by the transfer of the human ABCA4 gene with EIAV-based lentivectors to the subretinal space of Abca4−/− mice. The consistent observation that there was a more profound therapeutic effect than expected from the size of the injected area (~25–30% of the entire retina, Figure 2a) was surprising but could be explained by several, possibly interacting, factors. The subretinal injection causes retinal detachment and possibly some photoreceptor loss, which reduces the amount of A2E reaching RPE. This is the most plausible explanation for the observed small reduction of A2E accumulation in the EIAV-null injected mice. This hypothesis receives support from the ERG data, which reflects the number of functional photoreceptors. However, this mechanism cannot explain the much more significant therapeutic effect in the EIAV-ABCA4-treated mice, because their ERGs were larger than those of the mock-injected mice whereas their A2E levels were significantly lower. Another possibility is that the efficiently transduced areas may contain more ABCA4 than physiologically necessary, thereby eliminating A2E precursors more efficiently and compensating for the neighboring retina. The human protein could also be more efficient in the mouse than the endogenous murine protein, or the expression of the ABCA4 protein in the RPE could also reduce A2E levels, for example, by exporting A2E from RPE cells. Our preliminary experiments with the EIAV-ABCA4 vectors with the VMD2 promoter, which expresses ABCA4 only in the RPE, showed approximately two times less A2E accumulation than mock-injected animals (data not shown) suggesting that ABCA4 expression in the RPE may have an additional effect on A2E levels.
The comparison of the mock-injected mice with the animals treated with ABCA4-containing constructs provides strong evidence that the lentiviral-mediated gene transfer has a therapeutic effect. The possibility that the observed effect on A2E accumulation is an artifact and not a direct result of EIAV-ABCR transduction is further contraindicated by two major lines of evidence: first, the mock-injection experiments, where subretinal injections were performed with either the same amount of solution containing no virus, or an EIAV-null virus, reduced A2E accumulation much less than experiments with most efficient viruses (Figure 6). In addition, the same effect, that is, a slight (1/4–1/3) reduction of A2E was also observed with several lentiviral constructs containing ABCA4 under less efficient promoters (that is, PDEb; data not shown), suggesting that any mechanical damage to the retina or even an injection of a less efficient construct will not have the strong suppressing effect on A2E accumulation observed with therapeutic constructs. The PR transduction and A2E accumulation data were highly reproducible between experiments.
No obvious side effects were detected in treated animal models. We avoided examining the animals, especially the P4–5 mice, immediately after surgery. It would not be surprising, if biomicroscopy was performed right after surgery, to observe cells in the vitreous but evidence of prolonged and/or increasing inflammation was never detected. Examinations of the surface of the eyes of the mice within the first week after surgery did not reveal signs of inflammation or infection. Postmortem histology also revealed no inflammatory cells in the vicinity of the injected area or elsewhere in the retina. The major concern one would have with this procedure, if extended to humans, is that it requires detaching the macula retina, the site of the major pathology and the structure causing most of the vision loss in Stargardt disease. This detachment can be done without vitrectomy; instead, a small hole in the paramacular neural retina is produced by current methods, which could be further improved.
The therapeutic reduction of A2E by subretinal treatment with EIAV-based vectors could be used alone, or in combination with nonviral treatments. For example, we and others have been able to reduce A2E accumulation with compounds slowing the visual cycle48–50 or by reducing the light reaching the retina,51 suggesting a complementary therapeutic role of several approaches. Other pharmacological agents could also be applied, such as those inhibiting apoptosis. For example, several studies have suggested that intraocularly delivered neurotrophic factors (such as BDNF, CNTF and others) can delay photoreceptor degeneration in several animal models.52 In most cases, however, effects of these treatments last a limited time and require repeated administrations. A lentiviral vector, which integrates the therapeutic gene into the host chromosome, should last permanently and not require repeated treatments. The ability to integrate into the host genome has however, been considered a cause of potentially severe side effect(s) in cases where retroviral and lentiviral vectors are used as delivery vehicles for therapeutic genes. The most publicized case involves gene therapy of X-linked severe combined immunodeficiency (X-SCID) by gammaretroviral vectors, where a functional copy of the IL2Rγ gene was delivered into the host genome. Although the trial was very effective in treating the disease, it resulted in vector integration in close proximity to genes that act as proto-oncogenes, resulting in insertional mutagenesis.53 However, unlike the MLV vectors used in the X-SCID trial, the EIAV-based lentiviral vectors described here do not preferentially integrate into the promoter regions or the 5′ ends of the transcription units. It has been shown that EIAV vectors preferentially integrate within active genes favoring AT-rich regions with a similar profile to that of HIV-1 vectors.54 Furthermore, it has recently been demonstrated that the genotoxicity associated with lentiviral vectors is very low.55
In conclusion, treatment of mouse models with lentiviral vectors carrying a reporter gene and/or the therapeutic gene, ABCA4, results in high-level and sustained expression of a transgene/protein in the injected area. The resulting efficient reduction of A2E accumulation in the mouse model of STGD1 with EIAV-based vectors allows for optimism in transferring the technology to patients diagnosed with Stargardt macular dystrophy and other ABCA4-associated diseases.
Materials and methods
Animals
All animal experiments were approved by Columbia University (IACUC) and conformed to the standards of the American Veterinary Medical Association Panel on Euthanasia and recommendations of the Association of Research for Vision and Ophthalmology. Animals were maintained under normal cyclic lighting. Abca4−/− mice were generated in collaboration with Bristol-Myers Squibb and Lexicon Genetics (The Woodlands, TX, USA) and genotyped as described previously.45 Specifically, all Abca4−/− and wt mice used in this study were homozygous for the Rpe65 Leu450 variant.45 Typically, subretinal injections with lentiviral vectors were performed in mice on P4–5 in all experiments.
Construction of viral vectors
The human immunodeficiency virus-based vector (HIV-EF1α-LacZ) was generously provided by R Pawliuk and P Leboulch (Genetix Pharmaceuticals, Cambridge, MA, USA). The configurations of the minimal EIAV vectors used in this study are shown in Figure 1. The human ABCA4 cDNA was a generous gift from Hui Sun and Jeremy Nathans (HHMI, Johns Hopkins Medical Center).20 The ABCA4 cDNA was cloned into the EIAV-CMV-LacZ vector (from the pONY8 series described previously)56 to replace LacZ to create the EIAV-CMV-ABCR construct. Subsequently, the constitutive hCMV promoter was replaced with the minimal (0.3 kb) photoreceptor-specific bRho promoter43,44 in both constructs to obtain EIAV-bRho-LacZ and EIAV-bRho-ABCR vectors. A detailed description of the specific cloning strategies is available on request.
EIAV viral vector production
Recombinant EIAV vectors were produced using the transient vector production method by three plasmid cotransfections of HEK293T cells using lipofectamine (Invitrogen, Carlsbad, CA, USA) as described previously57 except that the transfections were carried out in 10 layer cell factories and media was changed 6–8 h after sodium butyrate addition and vector harvested 42 h post transfection and concentrated by double centrifugation process. The following quantities of plasmid were used per cell factory: 440 µg genome plasmid, 220 µg EIAV synthetic gag/pol (pESynGP; Wilkes et al., manuscript in preparation) and 8.8 µg VSV-G envelope (pHCMVG).58
The titers of the viral vector stocks were determined by the integration (DNA) assay. HEK293T cells were transduced and passaged three times post transduction. Negative (mock-transduced) and positive (reference vector) controls were included in each assay. Cells were harvested at day 10 and total DNA extracted using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA). The DNA concentration was analyzed by spectrophotometer and adjusted to 30 ng µl−1. Samples were analyzed in duplicate using 150 ng DNA per 25 µl reaction. The PCR mix contained 1× TaqMan Universal Master Mix with AmpErase UNG (Applied Biosystems, Foster City, CA, USA), 300 nm EIAV forward (5′-ATTGGGAGACCCTTTGACATTG), and reverse (5′-ACCAGTAGTTAATTTCTGAGACCCTTGTA) primers and 200 nm probe (5′-FAMCACCTTCTCTAACTTCTTGAGCGCCTTGCTTAMRA). A stock of plasmid DNA containing the amplicon was used to prepare standards of known copy number and a standard curve. DNA prepared from HEK293T cells containing a single EIAV (pONY8.4ZCG) vector copy, cloned by limiting dilution and verified by Southern blotting, was used as an internal reference standard. Samples were transferred to a 96-well optical plate and amplified using a TaqMan 7900HT quantitative PCR machine using the cycling parameters: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The plasmid standard dilution series was used to plot a regression curve of Ct plotted against copies of EIAV packaging signal. The predicted number of transducing units per ml (TU ml−1) of each sample was calculated from the regression equation and normalized to the pONY8.4ZCG reference standard. Titers were adjusted to account for the number of cells at the time of transduction and the dilution and volume of the vector applied.
Reverse transcriptase–polymerase chain reaction
In-vivo expression of the human ABCA4 in treated Abca4−/− animals was confirmed by RT–PCR. Whole mouse eyes were harvested either 2 weeks or 2 months after vector delivery and frozen in liquid nitrogen. The eye cups (two per tube) were crushed and homogenized in 350 µl ALT buffer (containing 1% fresh 2-mercaptoethanol) with the Qiagen Qiashredder. Total RNA was isolated with the Qiagen mini RNeasy kit according to the manufacturer’s protocol (Qiagen). RT–PCR reactions were carried out with equal amounts of total RNA using the SuperScript III One-Step RT–PCR System (Invitrogen) with Platinum Taq DNA polymerase (Invitrogen). At the end of the reaction 20 µl of each product was separated on a 1.2% agarose gel. Primers for the human ABCA4 gene were as follows: forward, ABCA4 5708-F 5′-GGAAGAACCTGTTTGCCATGGTGGT-3′; reverse, ABCA4 6613-R 5′-TTCTAGTTTAGGGGCTTCCTGCTGG-3′.
LacZ expression
After euthanasia, eyes were quickly removed and fixed in 3% glutaraldehyde overnight at 4 °C After washing, the anterior segments, lens and vitreous were removed and the eye cups washed with phosphate buffered saline (PBS). The eye cups were reacted with X-gal reagents (1 mg ml−1 X-gal, 5 mm potassium ferrocyanide, 5 mm potassium ferricyanide, 2 mm MgCl2, 0.02% Nonidet P-40 diluted from 20% stock, 0.01% Na deoxycholate diluted from 10% stock, in PBS) overnight at room temperature on a rotator and finally incubated for 1 h at 37 °C in a closed chamber. The eye cups were rinsed in PBS until the solution no longer turned yellow and then postfixed in 3% glutaraldehyde overnight at 4 °C.
Immunohistochemistry for ABCA4
Mouse eyes were embedded in Optimal Cutting Temperature (OCT) embedding compound and frozen in liquid nitrogen immediately after euthanasia. Cryo sectioning was performed, followed by fixation with 4% paraformaldehyde for 10 min. Sections were washed in PBS and dried at room temperature for one hour, then hydrated with PBS for 10 min. The sections were permeabilized with 0.2% Triton X100 for 10 min at room temperature. The MOM (Mouse on Mouse) basic kit from Vector (BMK-2202) was used for immunolabeling. Sections were blocked with MOM Ig blocking reagent for one hour at room temperature, washed in PBS, immersed in working solution of MOM diluent for 5 min, incubated with ABCA4 monoclonal antibody Rim 3F4 (a generous gift from Robert Molday, University of British Columbia, Vancouver, Canada), washed in PBS, incubated with MOM biotinylated anti-mouse immunoglobulin G reagent for 10 min at room temperature, washed in PBS, incubated with secondary antibody Alexa 594 antistreptavidin 1:500 in MOM diluent for 30 min at room temperature and washed, dried and mounted in Vectashield mounting medium plus 4′,6-diamidino-2-phenylindole dihydrochloride.
Electroretinogram
ERG was recorded from the corneas of mice anesthetized by intraperitoneal xylazine/ketamine and the pupils dilate with epinephrine and cyclogel. A cotton wick or a Burian–Allen contact lens electrode designed for mice (Hansen Ophthalmic Development Lab, Coralville, IA, USA) was used to detect the ERG with a reference gold electrode in the mouth and a needle ground electrode in the tail. The mouse was mounted on an electronically controlled heat blanket (TC 1000, CWE Inc, Ardmore, PA, USA) that monitored core body temperature by a rectal thermometer in order to maintain it at 37 °C. Mice were dark adapted overnight and prepared under deep red light for testing. Light stimuli were obtained from a ganzfeld stimulator coupled to a Grass photic stimulator (Grass Technologies, West Warwick, RI, USA). The mouse rested on the heat blanket on a block with its head within the ganzfeld. The ERG responses were recorded with an AD instruments Power Lab Scope system (AD Instruments, Colorado Springs, CO, USA) and a Mac computer. ERGs were obtained from Abca4−/− mice treated with therapeutic and null vectors and untreated animals and compared to Abca4+/+ mice. The b-wave amplitudes of the ERG were measured from the negative peak of the a-wave to the positive peak of the b-wave.
Quantification of A2E/isoA2E in mouse eyes
Tissue extraction and HPLC analysis was carried out as previously described.46 Posterior eye cups were pooled and homogenized in PBS using a tissue grinder. An equal volume of a mixture of chloroform/methanol (2:1) was added and the sample was extracted three times. To remove insoluble material, extracts were filtered through cotton and passed through a reversed phase (C18 Sep-Pak, Millipore) cartridge with 0.1% trifluoroacetic acid (TFA) in methanol. After removing solvent by evaporation under gas, the extract was dissolved in methanol containing 0.1% TFA, for HPLC analysis. For quantification of A2E, a Waters 600E HPLC was used with a C18 column (4 × 150 mm) with the following gradient of acetonitrile in water (containing 0.1% TFA): 90–100% (0–10 min), 100% acetonitrile (10–20 min). A flow rate of 0.8 ml min−1 was used and the solution was monitored at 430 nm. An injection volume 10 µl was used. Extraction and injection for HPLC was performed under dim red light. Levels of A2E and iso-A2E were determined by reference to external standards of HPLC-purified A2E/iso-A2E.
Statistical analyses
Data are presented as means and standard deviation (s.d.) and error of the mean (s.e.m.). Pair-wise comparisons with t-test and multiple comparisons with ANOVA were used to determine statistical significance.
Supplementary Material
Acknowledgements
We thank Dr SP Goff, Dr EA Pierce, Dr I Verma, Dr P Leboulch, Dr R Pawliuk, Dr S Rose, Dr C Barber, Dr T Nagasaki and Dr T Schoen for helpful discussions. We are also grateful to Dr J Nathans, Dr H Sun, Dr R Pawliuk and Dr RS Molday for valuable reagents and advice. This work was supported in part by funds from Foundation Fighting Blindness, National Neurovision Research Institute, Manning Family Foundation, Mr and Mrs Michael Schneeweiss, NIH EY13435 (RA), EY12951 (JRS) and unrestricted grant to the Department of Ophthalmology, Columbia University from Research to Prevent Blindness Inc.
Footnotes
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
References
- 1.Blacharski P. In: Retinal dystrophies and degenerations. Newsome DA, editor. New York: Raven Press; 1988. pp. 135–159. [Google Scholar]
- 2.Anderson KL, Baird L, Lewis RA, Chinault AC, Otterud B, Leppert M, et al. A YAC contig encompassing the recessive Stargardt disease gene (STGD) on chromosome 1p. Am J Hum Genet. 1995;57:1351–1363. [PMC free article] [PubMed] [Google Scholar]
- 3.Stargardt K. Über familiäre, progressive Degeneration in der Maculagegend des Auges. Albrecht von Graefes Arch Ophthalmol. 1909;71:534–550. [Google Scholar]
- 4.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997a;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 5.Azarian SM, Travis GH. The photoreceptor rim protein is an ABC transporter encoded by the gene for recessive Stargardt’s disease (ABCR) FEBS Lett. 1997;409:247–252. doi: 10.1016/s0014-5793(97)00517-6. [DOI] [PubMed] [Google Scholar]
- 6.Illing M, Molday LL, Molday RS. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem. 1997;272:10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- 7.Cremers FP, van de Pol DJ, van Driel M, den Hollander AI, van Haren FJ, Knoers NV, et al. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt’s disease gene ABCR. Hum Mol Genet. 1998;7:355–362. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- 8.Rozet JM, Gerber S, Souied E, Perrault I, Chatelin S, Ghazi I, et al. Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur J Hum Genet. 1998;6:291–295. doi: 10.1038/sj.ejhg.5200221. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Mir A, Paloma E, Allikmets R, Ayuso C, del Rio T, Dean M, et al. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998;18:11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 10.Rozet JM, Gerber S, Ghazi I, Perrault I, Ducroq D, Souied E, et al. Mutations of the retinal specific ATP binding transporter gene (ABCR) in a single family segregating both autosomal recessive retinitis pigmentosa RP19 and Stargardt disease: evidence of clinical heterogeneity at this locus. J Med Genet. 1999;36:447–451. [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis RA, Shroyer NF, Singh N, Allikmets R, Hutchinson A, Li Y, et al. Genotype/Phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. Am J Hum Genet. 1999;64:422–434. doi: 10.1086/302251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maugeri A, van Driel MA, van de Pol DJ, Klevering BJ, van Haren FJ, Tijmes N, et al. The 2588G → C mutation in the ABCR gene is a mild frequent founder mutation in the Western European population and allows the classification of ABCR mutations in patients with Stargardt disease. Am J Hum Genet. 1999;64:1024–1035. doi: 10.1086/302323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishman GA, Stone EM, Grover S, Derlacki DJ, Haines HL, Hockey RR. Variation of clinical expression in patients with Stargardt dystrophy and sequence variations in the ABCR gene. Arch Ophthalmol. 1999;117:504–510. doi: 10.1001/archopht.117.4.504. [DOI] [PubMed] [Google Scholar]
- 14.Fumagalli A, Ferrari M, Soriani N, Gessi A, Foglieni B, Martina E, et al. Mutational scanning of the ABCR gene with double-gradient denaturing-gradient gel electrophoresis (DG-DGGE) in Italian Stargardt disease patients. Hum Genet. 2001;109:326–338. doi: 10.1007/s004390100583. [DOI] [PubMed] [Google Scholar]
- 15.Rivera A, White K, Stohr H, Steiner K, Hemmrich N, Grimm T, et al. A comprehensive survey of sequence variation in the ABCA4 (ABCR) gene in Stargardt disease and age-related macular degeneration. Am J Hum Genet. 2000;67:800–813. doi: 10.1086/303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatsenko AN, Shroyer NF, Lewis RA, Lupski JR. Late-onset Stargardt disease is associated with missense mutations that map outside known functional regions of ABCR (ABCA4) Hum Genet. 2001;108:346–355. doi: 10.1007/s004390100493. [DOI] [PubMed] [Google Scholar]
- 17.Jaakson K, Zernant J, Kulm M, Hutchinson A, Tonisson N, Glavac D, et al. Genotyping microarray (gene chip) for the ABCR (ABCA4) gene. Hum Mutat. 2003;22:395–403. doi: 10.1002/humu.10263. [DOI] [PubMed] [Google Scholar]
- 18.Papermaster DS, Converse CA, Zorn M. Biosynthetic and immunochemical characterization of large protein in frog and cattle rod outer segment membranes. Exp Eye Res. 1976;23:105–115. doi: 10.1016/0014-4835(76)90194-9. [DOI] [PubMed] [Google Scholar]
- 19.Papermaster DS, Schneider BG, Zorn MA, Kraehenbuhl JP. Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J Cell Biol. 1978;78:415–425. doi: 10.1083/jcb.78.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- 21.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 22.Beharry S, Zhong M, Molday RS. N-retinylidene-phosphatidy-lethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR) J Biol Chem. 2004;279:53972–53979. doi: 10.1074/jbc.M405216200. [DOI] [PubMed] [Google Scholar]
- 23.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci. 1999;40:2988–2995. [PubMed] [Google Scholar]
- 24.Fishkin N, Jang YP, Itagaki Y, Sparrow JR, Nakanishi K. A2-rhodopsin: a new fluorophore isolated from photoreceptor outer segments. Org Biomol Chem. 2003;1:1101–1105. doi: 10.1039/b212213h. [DOI] [PubMed] [Google Scholar]
- 25.Sparrow JR. RPE lipofuscin: formation, properties and relevance to retinal degeneration. In: Tombran-Tink J, Barnstable CJ, editors. Retinal Degenerations: Biology, Diagnostics and Therapeutics. Totowa, NJ: Humana Press; 2007. pp. 213–236. [Google Scholar]
- 26.Allikmets R. Stargardt disease: from gene discovery to therapy. In: Tombran-Tink J, Barnstable CJ, editors. Retinal Degenerations: Biology, Diagnostics and Therapeutics. Totowa, NJ: Humana Press; 2007. pp. 105–118. [Google Scholar]
- 27.Allocca M, Tessitore A, Cotugno G, Auricchio A. AAV-mediated gene transfer for retinal diseases. Expert Opin Biol Ther. 2006;6:1279–1294. doi: 10.1517/14712598.6.12.1279. [DOI] [PubMed] [Google Scholar]
- 28.Campochiaro PA. Gene therapy for retinal and choroidal diseases. Expert Opin Biol Ther. 2002;2:537–544. doi: 10.1517/14712598.2.5.537. [DOI] [PubMed] [Google Scholar]
- 29.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 30.LeMeur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Therapy. 2007;14:292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- 31.Narfstrom K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM, et al. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci. 2003;44:1663–1672. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- 32.Bemelmans AP, Kostic C, Crippa SV, Hauswirth WW, Lem J, Munier FL, et al. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 2006;3:e347. doi: 10.1371/journal.pmed.0030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dejneka NS, Surace EM, Aleman TS, Cideciyan AV, Lyubarsky A, Savchenko A, et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther. 2004;9:182–188. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- 35.Batten ML, Imanishi Y, Tu DC, Doan T, Zhu L, Pang J, et al. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gouze E, Pawliuk R, Gouze JN, Pilapil C, Fleet C, Palmer GD, et al. Lentiviral-mediated gene delivery to synovium: potent intra-articular expression with amplification by inflammation. Mol Ther. 2003;7:460–466. doi: 10.1016/s1525-0016(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 37.Kostic C, Chiodini F, Salmon P, Wiznerowicz M, Deglon N, Hornfeld D, et al. Activity analysis of housekeeping promoters using self-inactivating lentiviral vector delivery into the mouse retina. Gene Therapy. 2003;10:818–821. doi: 10.1038/sj.gt.3301948. [DOI] [PubMed] [Google Scholar]
- 38.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 39.Kingsman SM. Lentivirus: a vector for nervous system applications. Ernst Schering Res Found Workshop. 2003;43:179–207. doi: 10.1007/978-3-662-05352-2_11. [DOI] [PubMed] [Google Scholar]
- 40.Miyoshi H, Takahashi M, Gage FH, Verma IM. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi M, Miyoshi H, Verma IM, Gage FH. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol. 1999;73:7812–7816. doi: 10.1128/jvi.73.9.7812-7816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gruter O, Kostic C, Crippa SV, Perez MT, Zografos L, Schorderet DF, et al. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Therapy. 2005;12:942–947. doi: 10.1038/sj.gt.3302485. [DOI] [PubMed] [Google Scholar]
- 43.Lem J, Applebury ML, Falk JD, Flannery JG, Simon MI. Tissue-specific and developmental regulation of rod opsin chimeric genes in transgenic mice. Neuron. 1991;6:201–210. doi: 10.1016/0896-6273(91)90356-5. [DOI] [PubMed] [Google Scholar]
- 44.Zack DJ, Bennett J, Wang Y, Davenport C, Klaunberg B, Gearhart J, et al. Unusual topography of bovine rhodopsin promoter-lacZ fusion gene expression in transgenic mouse retinas. Neuron. 1991;6:187–199. doi: 10.1016/0896-6273(91)90355-4. [DOI] [PubMed] [Google Scholar]
- 45.Kim SR, Fishkin N, Kong J, Nakanishi K, Allikmets R, Sparrow JR. Rpe65 Leu450Met variant is associated with reduced levels of the retinal pigment epithelium lipofuscin fluorophores A2E and iso-A2E. Proc Natl Acad Sci USA. 2004;101:11668–11672. doi: 10.1073/pnas.0403499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci USA. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, Travis GH. Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1685–1690. [PubMed] [Google Scholar]
- 48.Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 49.Radu RA, Mata NL, Nusinowitz S, Liu X, Sieving PA, Travis GH. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. Proc Natl Acad Sci USA. 2003;100:4742–4747. doi: 10.1073/pnas.0737855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maiti P, Kong J, Kim SR, Sparrow JR, Allikmets R, Rando RR. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry. 2006;45:852–860. doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- 51.Sieving PA, Chaudhry P, Kondo M, Provenzano M, Wu D, Carlson TJ, et al. Inhibition of the visual cycle in vivo by 13-cis retinoic acid protects from light damage and provides a mechanism for night blindness in isotretinoin therapy. Proc Natl Acad Sci USA. 2001;98:1835–1840. doi: 10.1073/pnas.041606498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 54.Hacker CV, Vink CA, Wardell TW, Lee S, Treasure P, Kingsman SM, et al. The integration profile of EIAV-based vectors. Mol Ther. 2006;14:536–545. doi: 10.1016/j.ymthe.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 56.Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL, et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet. 2001;10:2109–2121. doi: 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- 57.Wong LF, Ralph GS, Walmsley LE, Bienemann AS, Parham S, Kingsman SM, et al. Lentiviral-mediated delivery of Bcl-2 or GDNF protects against excitotoxicity in the rat hippocampus. Mol Ther. 2005;11:89–95. doi: 10.1016/j.ymthe.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 58.Yee J, Miyanohara A, LaPorte P, Bouic K, Burns JC, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.