Abstract
During infection with viruses that establish latency, the immune system needs to maintain lifelong control of the infectious agent in the presence of persistent Ag. By using a γ-herpesvirus (γHV) infection model, we demonstrate that a small number of virus-specific central-memory CD8+ T cells develop early during infection, and that virus-specific CD8+ T cells maintain functional and protective capacities during chronic infection despite low-level Ag persistence. During the primary immune response, we show generation of CD8+ memory T cell precursors expressing lymphoid homing molecules (CCR7, L-selectin) and homeostatic cytokine receptors (IL-7α, IL-2/IL-15β). During long-term persistent infection, central-memory cells constitute 20–50% of the virus-specific CD8+ T cell population and maintain the expression of L-selectin, CCR7, and IL-7R molecules. Functional analyses demonstrate that during viral persistence: 1) CD8+ T cells maintain TCR affinity for peptide/MHC complexes, 2) the functional avidity of CD8+ T cells measured as the capacity to produce IFN-γ is preserved intact, and 3) virus-specific CD8+ T cells have in vivo killing capacity. Next, we demonstrate that at 8 mo post-virus inoculation, long-term CD8+ T cells are capable of mediating a protective recall response against the establishment of γHV68 splenic latency. These observations provide evidence that functional CD8+ memory T cells can be generated and maintained during low-load γHV68 persistence.
Immunological memory is one of the hallmarks of the adaptive immune system and it can be functionally defined as the stronger protective response of the host to secondary Ag challenge (1, 2). It, thus, allows the immune system to respond more vigorously to infectious pathogens that have been encountered previously. Upon primary activation by Ag, CD8+ T cells follow a program of proliferation and differentiation into effectors that control the infection (3). After this expansion phase, the majority of Ag-specific CD8+ T cells undergo programmed cell death, leaving a population of memory CD8+ T cells (4). A rapid and efficient recall response is partially due to the ubiquitous presence of increased frequencies of memory T cells in peripheral tissues and secondary lymphoid organs (5). In addition, several characteristics of memory T cell populations contribute to enhanced secondary responses including the ability to self-renew in the absence of Ag, higher activation status and reduced costimulatory requirements, expression of lymphoid homing molecules and homeostatic cytokine receptors, higher frequency than naive precursors, rapid proliferation upon secondary stimulation, and the ability to maintain the integrity of the T cell repertoire (1, 6, 7). Nevertheless, some of these characteristics may be arbitrary (i.e., markers, Ref. 8) or not universal (i.e., long lived, Ref. 9). The heterogeneity of memory T cells in phenotype, function, and location has led to the idea that they can be divided in two major subsets: effector (CD62LlowCCR7low) and central-memory (CD62LhighCCR7high) cells based on their expression of lymphoid homing receptors (10).
Memory CD8+ T cells during viral infections play a major role in protection by rapid recognition and lysis of virus-infected cells. The contribution of memory CD8+ T cells to recall responses is well-defined during acute infections where Ag is cleared (11, 12). There is, however, little and contradictory information regarding memory generation during persistent infections. This is of special relevance because persistent infectious diseases are a major health concern worldwide (13, 14). The current paradigm, mostly derived from studies with lymphocytic choriomeningitis virus (LCMV)3 and HIV, is that persistent infection leads to some degree of CD8+ T cell effector dysfunction or deletion and lack of T cell memory formation (15–19). CD8+ T cell dysfunction during chronic infection has been raised as one reason for pathogen persistence (20). This loss of function in the presence of persistent Ag follows a progression in the sequence of cytotoxicity, IL-2, TNF-α, and IFN-γ production, exhaustion, and deletion (20, 21). However, these findings appear to be at odds with situations where viruses persist and CD8+ T cell functions are not apparently compromised (22–25), with reports of residual Ag presentation (26) or with a possible role for Ag in the maintenance of immunological memory (27). γ-herpesviruses (γHV) are characterized by the establishment of lifelong asymptomatic infection, the persistence in >90% of the human population, and the association with a large list of life-threatening conditions (28, 29). CD8+ T cells are thought to be the main effectors of long-term virus control (28, 30, 31). How do CD8+ T cell populations maintain control of infection without pathogen recrudescence during persistent infections? Humans encounter different pathogens through life of which a considerable number reach a persistent, parasitic, commensal, or a latency state and the human system has to provide lifelong protection. Herpesvirus infections are a clear example: initial infection with CMV or EBV may occur early in childhood, which demonstrates that the immune system is capable of controlling infection for 70 years or more. Although persistent infections induce a continual evolution of the function and numbers of T cells that are specific for the infecting pathogen, there seems to be a substantial difference between infections with impaired CD4+ T cell help and/or a high antigenic load such as HIV or LCMV-clone 13 and infections with intact CD4+ T cell help and low-load Ag persistence, such as herpesvirus infections (32, 33).
To understand more clearly how CD8+ memory T cells are generated, maintained, and function during low-load persistent viral infections, we studied their fate and function during the acute and long-term response to a γHV infection. Using a murine model of infection with γHV68, we show that Ag-specific CD8+ T cell function is maintained during long-term, low-level viral and Ag persistence.
Materials and Methods
Mice and viral infection
C57BL/6J and BALB/cJ mice were obtained from The Jackson Laboratory, Harlan Farms, or were bred at Columbus Children’s Research Institute (CCRI). γHV68, clone WUMS, was propagated and titered on monolayers of NIH3T3 fibroblasts. Mice were housed in BL2 containment under pathogen-free conditions. The Institutional Animal Care and Use Committee at the CCRI approved all the animal studies described here. Mice were anesthetized with 2,2,2,-tribromoethanol and intranasally inoculated with 1000 PFU γHV68 in 30 µl of HBSS.
Virus analysis
To determine the number of cells containing γHV68 genomes long-term postinfection, a combination of limiting dilution analysis and nested PCR (LDA-PCR) was used (34). Splenocytes were isolated, RBC were lysed, and the number of cells per spleen was determined. Cells were serially diluted with NIH3T3 cells in 96-well plates, lysed, and viral DNA was amplified by PCR using primers specific for γHV68 Orf50. A 2-µl aliquot of the product was reamplified using nested primers. The final product was analyzed by ethidium bromide staining of DNA after electrophoresis in 3% agarose gel. This procedure was able to consistently detect a single copy of target sequence. Twelve replicates were assessed for each cell dilution and linear regression analysis was performed to determine the reciprocal frequency (95% degree of confidence) of cells positive for γHV68 DNA. At least three independent experiments were used to determine the mean reciprocal frequency and SD of γHV68 DNA in splenocytes. Infectious center assays were performed as described (35).
Ag-presentation assays
Splenocytes were isolated and processed into single-cell suspensions using collagenase D (5 mg/ml) treatment for 45 min. Cells were next incubated with 2 mM PBS/EDTA for 10 min at room temperature to disrupt multicellular complexes. Cells were stained with an anti-CD11c Ab conjugated to magnetic beads (Miltenyi Biotec) and separation columns were used to purify cells into CD11c+ and CD11c− fractions. A total of 3 × 104 APCs from each fraction were plated with 1 × 105 γHV68-specific lacZ-inducible T cell hybridomas (49100.2 and 4943.4) (36) in 96-well plates in triplicate and incubated overnight. β-galactosidase activity was assessed in individual wells as described (36). Background stimulation was determined by using APCs from naive spleens as stimulators.
Flow cytometry analysis
Splenocytes were processed into single-cell suspensions as described above. Cells were stained with Fc-block (CD16/32) and then washed. The cells were then stained with a combination of the following γHV68-specific MHC tetramers: ORF6487–495/Db, ORF61524–531/Kb, ORF65131–140/ Dd and M291–99/Kd, and Abs against CD62L (MEL-14), CD8 (53-6.7), CD43 (1B11), CD122 (TM-b1), CD127 (A7R34), CD44 (IM7), and CD4 (GK1.5). All Abs were purchased from eBioscience with the exception of CD43 (BD Pharmingen). MHC tetramers were generated as described (37) or obtained from the National Institutes of Health Tetramer Core Facility. The surface expression of CCR7 was analyzed using the recombinant ligand CCL19 Ig followed by anti-human Fc-biotin and streptavidin-PE. This process can be slightly detrimental for the intensity of the tetramer staining. Flow cytometry data were acquired on FACSCalibur or BD LSR (BD Biosciences) and analyzed using FlowJo software. Gates were set using negative controls, isotype controls, and following the staining pattern of each marker on the bulk lymphocyte and CD8 population. For any given activation marker and tetramer combination, all the analysis gates are identical in size and position.
T cell avidity assays
Tetramer dilution
Single-cell suspensions were obtained as indicated above. Cells were plated in flasks coated with anti-mouse IgG plus IgM Abs (Jackson Immunochemicals) for 1 h to enrich for T cells. The nonadherent cells were incubated with Fc-block (CD16/32) and washed. A total of 2 × 106 cells were plated per well in 96-well plates and stained with ORF6487–495/Db at 2-fold dilutions for 1 h at room temperature (RT). Cells were washed and subsequently incubated with anti-CD8 Ab, washed, and then fixed with 1% paraformaldehyde. The results were expressed as the percentage of tetramer/CD8 double-positive cells over the log concentration of tetramer. To compare groups within each experiment, the data were normalized to the response at the highest concentration of tetramer. The affinity, KD, is derived from the slope by KD = 1/slope.
Tetramer decay
Cells were processed, enriched by panning and Fc-blocked as described above. After staining with ORF6487–495/Db and anti-CD8 Ab for 1 h at RT, cells were washed twice and incubated in an excess of anti-Db 28-14-8s purified Ab and incubated at 37°C to allow tetramer dissociation. The anti-Db Ab was used to block rebinding of the tetramer to the TCR. Decay was followed at intervals from 0 to 50 min at which times the cells were fixed with 1% paraformaldehyde. The results were expressed as the percentage of tetramer/CD8 double-positive cells over time. To compare groups within each experiment, the data were normalized to the percentage of maximum tetramer binding over time. The half-life was derived from the slope by t1/2= ln2/slope.
Intracellular cytokine staining
Single-cell suspensions were obtained as indicated above. A total of 2 × 106 cells/sample were incubated in complete medium in the presence of IL-2 (10 U/ml), brefeldin A (10 µg/ml), and 10-fold dilutions of purified ORF6487–495 peptide for 5 h at 37°C. As positive control, cells were stimulated with PMA/ionomycin. After Fc-blocking, the cells were stained with Abs anti-CD44 and anti-CD8, fixed, permeabilized, and stained with anti-IFN-γ or isotype control Abs. The data were expressed as the percentage of IFN-γ/CD8 double-positive cells over the log peptide concentration after subtracting the background obtained from the negative controls. The data were normalized to the response at the highest concentration of peptide. The EC50 was determined as the peptide concentration provoking a response halfway between baseline and maximum.
In vivo cytotoxicity assay
Single-cell suspensions obtained from naive spleens were pulsed with ORF6487–495 peptide or irrelevant influenza NP366–374 peptide (1 µg/106 cells) for 2 h at 37°C. The NP-pulsed control cells were stained with 50 nM CFSE and the relevant targets with 0.5 µM CFSE for 15 min at 37°C. The cells were thoroughly washed, combined in a 1:1 ratio, and a total of 0.5–1 × 107 cells were injected i.v. into mice. The presence of CFSE-positive cells was analyzed in spleen cell suspensions from the recipient mice 6 or 40 h later. Percent-specific killing was calculated according to the formula percent-specific killing = (1 – (ratio of naive recipients/ratio of infected recipients) × 100), where ratio = (number of CFSEhigh/number of CFSElow).
Adoptive cell transfers
Splenocytes from BALB/cJ mice at 8 mo post-γHV68 inoculation were processed into single-cell suspensions as described above and stained with anti-CD4 and CD8 Abs. Cells were purified using a FACSVantage with Diva option and 4 × 105 purified cells (purity 99%) were transferred i.v. into naive BALB/cJ mice. The recipient and control mice were infected with γHV68 24 h later. Viral latency was determined in the spleen on day 16.
Statistical analysis
The data were fitted to linear and nonlinear regression lines. The form with the highest R2 was used in further comparisons between groups. Early and late groups were compared using two-way ANOVA with group and replicate as main effects while controlling for concentration or time. The extra sum-of-squares F test was used to compare the models and reject or not the simpler null hypothesis model. Statistical analyses were run several times if there were outliers in the data. One analysis included all observations. A second analysis was run excluding outliers that were outside a range of 3 SDs. There was one observation dropped from the tetramer dilution studies, none from the tetramer decay studies, and one from the IFN-γ studies. The statistical analysis was performed using GraphPad Prism and SigmaStat software.
Results
γHV persistence: low antigenic load
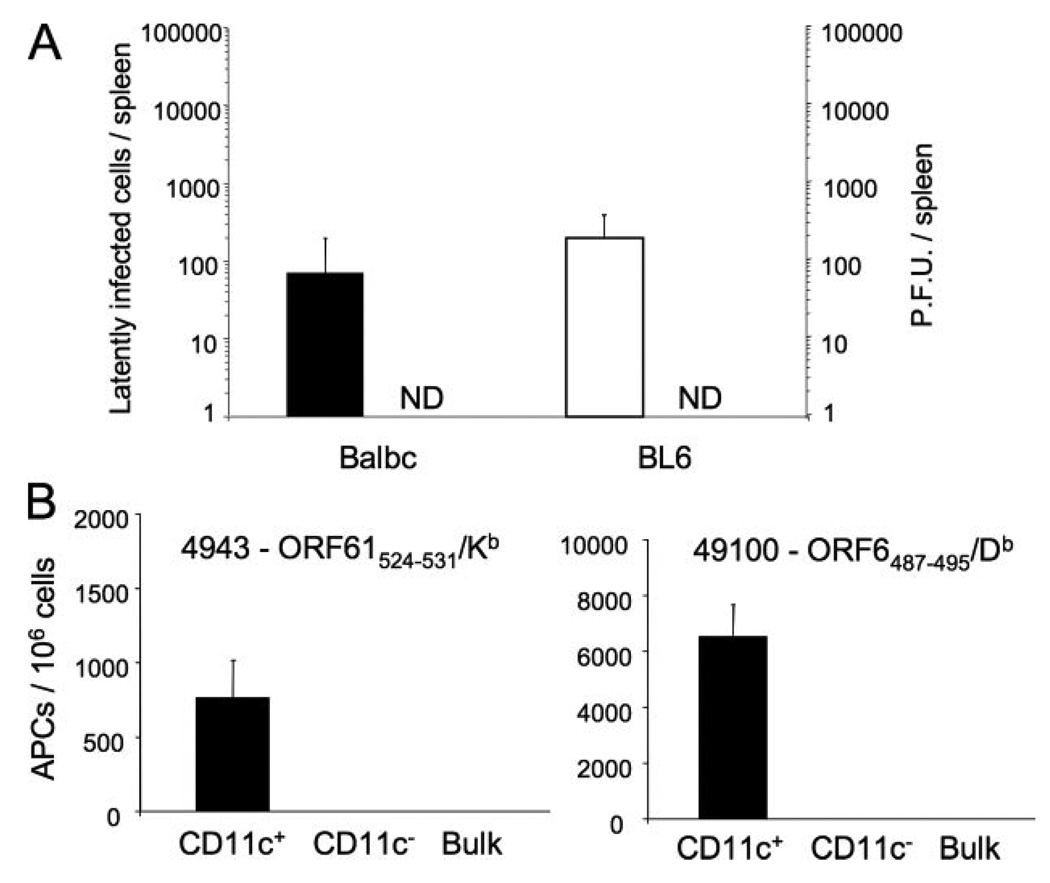
γHVs are characterized by the establishment of lifelong asymptomatic infection by, among other strategies, persisting in a latent state in memory B cells. There is, however, a low level of viral reactivation from latency that is thought to contribute to an active process of infection of new cell reservoirs and to the release of infectious viral particles (28, 34, 38). Thus, γHVs represent a good model to study immune responses during conditions of low-level Ag persistence. To study the low levels of viral latency during γHV68 persistent infection, we determined the viral load in the spleen during long-term γHV68 infection of mice. We used a sensitive LDA-PCR assay for γHV68 orf50 to detect the number of cells carrying viral DNA. The data show that there were consistently <500 latently infected cells per spleen in C57BL/6 and BALB/c mice at 5 months postinfection (m.p.i.) (Fig. 1A). In addition, we sought to determine the number of infectious viral particles at the same times postinfection using a plaque assay. The data show that no infectious virus was detected (Fig. 1A), indicating that there was lack of lytic virus or that the number of lytic viral particles at 5 m.p.i. is very low and thus, below the detection level of this assay. Altogether, these data indicate that the viral load during long-term γHV68 carrier state is very low and suggest that the levels of persistent Ag in the host must also be low. To determine the levels of persistent Ag during long-term infection, we used two lacZ+ -inducible T cell hybridomas specific for two class-I-restricted lytic-phase epitopes from the ssDNA-binding protein (ORF6487–495/Db) and from the large RNase reductase subunit (ORF61524–531/Kb) of γHV68. As shown in Fig. 1B, hybridoma stimulation with bulk spleen cells did not detect the presence of viral Ags on 4 m.p.i. splenocytes. However, enrichment of CD11c+ cell fraction using magnetic beads induced the stimulation of γHV68-specific hybridomas. No stimulatory capacity could be detected in the CD11c− population. Together, these data demonstrate the during persistent γHV infection, a small population of APCs, likely dendritic cells, is capable of presenting lytic cycle-associated viral Ags on the cell surface in the context of MHC molecules.
FIGURE 1.
Long-term infection is characterized by low viral titers and low Ag persistence. A, Analysis of γHV68 titers during long-term latency. The number of latently infected cells (left y-axis) in the spleen of C57BL/6 (■) and BALB/c (□) mice was determined using LDA-PCR assay at 5 m.p.i. The number of lytically infected cells (ND, not detected, right y-axis) was determined using a modified plaque assay. Three individual mice were independently analyzed at each time point; error bars represent SD. B, Analysis of γHV68 antigenic load in mice during long-term latency. Spleen cells from mice 4 m.p.i. were used as stimulators in an in vitro assay with two lacZ-inducible γHV68-specific T cell hybridomas to determine the number of APCs capable of presenting viral Ags on their cell surface. Left panel, Hybridoma 4943 specific for peptide ORF61/Kb; right panel, hybridoma 49100 specific for peptide ORF6/Db. Dendritic cells were purified by anti-CD11c Ab labeling and magnetic bead enrichment (75% purity).
Generation of central-memory CD8+ T cells during virus persistence
Previous studies have shown that there is a shift in the composition of the memory T cell pool from an effector-memory to a central-memory phenotype over time (11, 12, 39). However, during chronic LCMV infection, the presence of persistent Ag is thought to prevent T cell memory generation (15). We sought to gain a clearer understanding of the generation and heterogeneity of different subsets of memory CD8+ T cells in lymphoid organs and the respiratory tract during low-level Ag persistence using γHV68 infection as a model. We performed a temporal kinetic analysis of the evolution of γHV68-specific CD8+ T cells in two different mouse strains: C57BL/6 and BALB/c. To track Ag-specific T cells, we used tetramers against three different CD8+ T cell epitopes (lytic cycle: ORF61524–531/Kb and ORF6487–495/Db, latent cycle: M291–99/Kd). We analyzed the level of cell surface expression of the lymphoid homing molecule L-selectin (CD62L) and the activation-associated glycoform of CD43 (1B11) to distinguish subpopulations of effector and memory CD8+ T cells. Up-regulation of L-selectin expression on Ag-experienced cells is a common characteristic of memory T cells and their precursors (5, 12). The activation-associated glycoform of CD43 (1B11) has been extensively used to distinguish memory from effector T cells in mice (40, 41). Our data show that Ag-specific CD8+ T cells with a central-memory phenotype (CD62LhighCD43low) could be readily detected at 14 days postinfection (d.p.i.) in spleen and their frequency increased over time during the course of persistent infection (Fig. 2, A and B). Central-memory CD8+ T cells constituted between 15 and 50% of the γHV68-specific CD8+ T cells independent of the tissue or epitope analyzed during long-term infection (9 m.p.i.) (Fig. 2C). The kinetic analysis also shows that the effector CD8+ T cell population (CD62LlowCD43high) contracts from day 14 postinfection until day 90 (Fig. 2B). This process is accompanied by a progressive increase in the frequency of effector-memory T cells (CD62LlowCD43low). This shift in the composition of the T cells from effectors (CD43high) to memory cells (CD43low) stabilizes 3 mo postinfection and the frequency of total memory CD8+ T cells plateaus and remains remarkably stable until 18 mo postinfection, constituting 70–90% of the CD8+ T cells for any epitope analyzed. There is, however, a shift in the composition of the CD8+ memory T cell pool over time that starts 3 mo postinfection with central-memory T cells (CD62Lhigh CD43low) gradually increasing their frequency while the effector-memory subset (CD62LlowCD43low) contracts (Fig. 2B). Similar kinetics were found for ORF6487–495/Db-specific CD8+ T cells (data not shown). Altogether, the data show that virus-specific CD8+ T cells primarily had an effector-memory phenotype and were heterogeneous long-term post-virus inoculation in both lymphoid organs and the lung. In addition, a population of virus-specific CD8+ T cells resembling a central-memory phenotype could be detected at early stages postinfection and progressively increased its frequency over time.
FIGURE 2.
Memory CD8+ T cells predominate during long-term infection. Temporal kinetic analysis of the effector and memory CD8+ T cell response to γHV68. A, Representative dot plots showing the CD62L/CD43 distribution of a population of virus-specific CD8 T cells (ORF61524–531/Kb) at different times after infection (14 days, 3 and 18 mo). Note the progressive increase in CD62L+CD43− γHV68-specific CD8+ T cells. Cells have been previously gated as tetramer+CD8+B, Evolution of the different populations of Ag-specific CD8+ T cells during γHV68 infection. CD62L− CD43+ : ■; CD62L−CD43−: ▲; CD62L+CD43−: ●. Data are presented for ORF6487–495/Db-specific CD8+ T cells in spleen and lung. Similar kinetics were found for ORF61524–531/Kb-specific CD8+ T cells. C, All the Ag-specific CD8+ T cell populations analyzed in spleen and MLN were highly heterogeneous at 9 m.p.i. No significant differences were found between lytic-cycle epitopes (ORF61524–531/Kb and ORF6487–495/Db) and latent-cycle epitopes (M291–99/Kd). All the analyses were performed in C57BL/6 and BALB/c mice. Three individual mice were analyzed at each time point; error bars represent SD.
Our previous data suggest the possibility that memory CD8+ T cell precursors are being generated during the initial stages of the adaptive immune response to a γHV infection. To investigate this possibility, we analyzed the expression of two of the hallmarks of bona fide memory T cells on virus-specific CD8+ T cells: homeostatic-cytokine receptors (IL-7 and IL-15) (15, 39) and lymphoid homing molecules (L-selectin and CCR7) (5, 12). The strategy for the gating of these populations is described in Fig. 3. The analysis was performed 14 d.p.i., which marks the peak of γHV68 latency and of viral Ag expression (36, 42). As shown in Fig. 4, A and B, subsets of γHV68-specific CD8+ T cells expressing L-selectin, CCR7, and receptors for IL-7 and IL-2/IL-15β on their cell surface could be detected in bone marrow and spleen 2 wk post-virus inoculation. These data indicate that phenotypically “normal” memory CD8+ T cell precursors are being generated during the peak of the T cell response to a persistent γHV infection. It should be noted that the analysis of C57BL/6 mice at 14 d.p.i. shows two populations of tetramer-positive cells, one which is tetramer low and positive for L-selectin, IL-2/IL-15β, IL-7α, and CCR7, and one which is tetramer high and negative for the markers tested. Transient loss of tetramer binding and TCR/CD8 down-regulation has been observed after T cell activation (43–45) and could be explained by the asymmetric T cell lymphocyte division after Ag stimulation (46). To determine whether memory T cell precursors generated during primary infection had a central- or effector-memory phenotype and their anatomical distribution, we analyzed simultaneously the presence of L-selectin and IL-7R on ORF6487–495/Db-specific CD8+ T cells in different lymphoid tissues (Fig. 4C). Ag-specific CD8+ T cells consisted of cells with an effector phenotype (CD62LlowCD127low), central-memory phenotype (CD62LhighCD127high), and effector-memory phenotype (CD62LlowCD127high) in mediastinal lymph node (MLN), spleen, and bone marrow. The analysis shows that memory populations constitute a significant fraction of the cells in the lymphoid tissues analyzed. Although most of the memory cells are found in the spleen, Fig. 4D shows that their frequencies are higher in bone marrow and MLN than in spleen (CD62LhighCD127high: bone marrow 8.7%, MLN 10.7%, and spleen 3.3%; CD62LlowCD127high: bone marrow 9.8%, MLN 12.1%, and spleen 7.3%). Similar numbers and frequencies were obtained using CD43 and CD62L to divide the sub-populations of Ag-specific cells (data not shown). Taken together, these results provide strong evidence that during primary γHV68 infection CD8+ memory T cell precursors with a central-memory and effector-memory phenotype are being generated within lymphoid tissues.
FIGURE 3.
Flow cytometry analysis of Ag-specific CD8+ T cells. A, Representative staining showing tetramer vs CD8 dot plots for each one of the tetramers used in this study. B, Distribution of CD122 and CD127 staining on spleen cells; CD8+ T cells and tetramer+/CD8+ T cells. M291–99/ Kd staining on day 28 is shown. Gates were set using negative controls, isotype controls, and following the staining pattern of each marker on the bulk lymphocyte and CD8+ population. For any given activation marker, the analysis gates are identical in size and x-axis position. Numbers represent the percentage of positive cells in each quadrant/gate.
FIGURE 4.
CD8+ memory T cells are generated during the early phase of infection. A and B, Memory CD8+ T cell precursors are detected in bone marrow (A) and spleen (B) of 14 d.p.i. γHV68-infected C57BL/6 mice. The subset of Ag-specific cells precursors that express L-selectin, CD122, CD127, and CCR7 (x-axis) is shown inside the gate against the binding of ORF61524–531/Kb and ORF6487–495/Db (y-axis). The numbers indicate the percentage of cells inside the gate. The cells have been previously gated as CD8+tetramer+C and D, Central and effector-memory cells are present in lymphoid organs of infected mice 14 days after virus inoculation. The number (C) of CD8+ORF6487–495/Db+ lymphocytes expressing CD62LhighCD127high (central-memory cells), CD62LlowCD127high (effector-memory cells), and CD62LlowCD127low (effectors) and their frequency (D) among the CD8+ORF6487–495/Db+ lymphocyte population is shown in bone marrow, MLN, and spleen. Data correspond to six to nine individual mice per group. Error bars, SD.
Memory CD8+ T cells express homeostatic cytokine receptors and lymphoid homing molecules during virus persistence
Continuous Ag stimulation prevents the expression of homeostatic cytokine receptors and lymphoid homing molecules on CD8+ memory T cells during LCMV persistence, which partially contributes to explain their dysfunction (15). Thus, we analyzed the expression of L-selectin, CCR7, IL-2/IL-15β receptor, and IL-7R on γHV68–specific CD8+ T cells during long-term infection. The analysis of CD8+ T cells specific for two lytic cycle epitopes (ORF61524–531/Kb, ORF6487–495/Db) during long-term infection of C57BL/6 mice shows that virus-specific CD8+ T cells have down-regulated the expression of IL-2/IL-15β receptor, with only a small fraction of the tetramer-positive cells expressing CD122 on their cell surface (1–4%) (Fig. 5). However, the majority of the virus-specific CD8+ T cells show surface expression of the IL-7R (70–80%). In addition, a subpopulation of the Ag-specific CD8+ T cells express the lymphoid homing molecules L-selectin and CCR7 on their cell surface (20–30%). These data are in contrast with results from other chronic infections (15) and indicate that CD8+ T cells expressing molecular determinants of central-memory cells are maintained during persistent γHV68 infection. To determine whether the down-regulation of the IL-2/IL-15β receptor is specific for lytic cycle Ags or it also occurs on CD8+ T cells specific for latent cycle epitopes, we did a temporal kinetic analysis of γHV68–specific CD8 T cells in BALB/c mice comparing the response of a lytic-epitope (ORF65131–140/Dd) and a latent epitope (M291–99/Kd). As shown in Fig. 6, CD8+ T cells specific for the latent epitope M291–99/Kd maintain the expression of the IL-2/IL-15β receptor over time. Lytic epitope-specific CD8+ T cells, however, progressively down-regulate the surface expression of the IL-2/IL-15β receptor. Both populations of CD8+ T cells maintain the expression of IL-7R and L-selectin. Together, these data indicate that during long-term persistent γHV infection, memory CD8+ T cells retain the expression of homeostatic cytokine receptors (IL-7) and lymphoid homing molecules (L-selectin, CCR7) at their cell surface. However, CD8+ T cells specific for lytic cycle Ags (ORF65131–140/ Dd, ORF61524–531/Kb, and ORF6487–495/Db) specifically down-regulate CD122 surface expression.
FIGURE 5.
Lytic epitope-specific memory CD8+ T cells express L-se-lectin, CCR7, and IL-7α receptor but down-regulate IL-2/IL-15β receptor expression during long-term infection. Analysis of bone marrow and spleen cells from mice at 9 m.p.i. with γHV68. Cells were previously gated as CD8+tetramer+: ORF6487–495/Db is shown on A and ORF61524–531/Kb on B. The plots show tetramer staining (y-axis) vs memory markers (CD62L, CD122, CD127, and CCR7 on x-axis). Note that the expression of IL-2/IL-15β receptor is down-regulated while the rest of memory markers remain expressed at significant levels. The numbers indicate the percentage of cells inside the gate. Representative plots from three independent experiments with similar results are shown.
FIGURE 6.
Latent epitope-specific CD8+ T cells maintain the expression of lymphoid homing molecules and homeostatic cytokine receptors during long-term infection. Contour plots show the temporal evolution of memory CD8+ T cells in BALB/c mice for a latent epitope (M291–99/KdA) and for a lytic epitope (ORF65131–140/DdB). Spleen cells were previously gated as CD8+tetramer+. Tetramer staining is plotted on the y-axis and the specific memory marker on the x-axis (CD122, CD127, CD62L). Note that memory CD8+ T cells specific for the latent epitope maintain expression of the memory markers while cells specific for the lytic epitope progressively lose surface expression of IL-2/IL-15β receptor by day 90 after infection. Representative plots from three independent experiments with similar results are shown. C, Bar diagrams show the frequency of IL-2/IL-15β-expressing cells for the two epitopes described above. Error bars, SD. *, p < 0.05.
Ag-specific CD8+ T cells maintain TCR affinity during virus persistence
High-avidity CD8+ T cells are thought to play a major role in terminating viral infections (47, 48). The kinetics and affinity of TCR-peptide/MHC interactions drive the selection of TCR clones during the course of an immune response resulting in an overall increased affinity for Ag (49). This mechanistic model suggests that continuous Ag stimulation influences the CD8+ T cell pool by inducing changes in TCR usage or affinity that translate in the selection of high-affinity immunodominant clones (50). Other studies show that high-avidity CD8+ T cells are more susceptible to apoptosis or clonal senescence under conditions of excessive Ag load (51, 52) which may explain a decrease in immunodominance and clonal dominance during memory to herpesvirus persistence compared with primary responses (53). In addition, the down-regulation of the IL-2/IL-15B receptor expression on lytic Ag-specific CD8+ T cells during persistent γHV68 infection may be related to a decrease in clonal affinity. IL-15/IL-15R signaling mimics TCR cross-linking (54), activates CD8+ T cells (55), and mediates avidity maturation of CD8+ T cells at the single cellular level by increasing TCR functional affinity (56). Thus, it is possible that in the presence of low levels of persistent Ag, high-avidity memory CD8+ T cells are being continuously stimulated by both Ag and IL-15 and driven to clonal exhaustion.
High-avidity CD8+ T cells bind preferentially under conditions of limited peptide/MHC complex availability (57, 58). To compare the relative TCR affinity of ORF6487–495/Db-specific CD8+ T cells during early (14–35 days) and long-term infection (at least 3 m.p.i.), we performed a dose-response titration using tetramer dilution under conditions ranging from saturation to limiting availability (Fig. 7A). The data show that the normalized titration curves for early and late time points postinfection were superim-posable (Fig. 7B). The analysis of the data indicated the fit was the same for linear and nonlinear approaches. Thus, in this situation, the simpler linear model was chosen (R2-early: 0.9887, R2-late: 0.9674). The slopes, elevations, and y intercepts of both curves were not significantly different and a common slope (9.2409) and common y intercept for all the data (95.6591) could be determined. There was no effect of the time postinfection on the affinity of tetramer binding (KD-early: 0.1017, KD-late: 0.1157). These results indicate that there was no significant change in the average TCR peptide/MHC affinity over time between primary infection and γHV68 persistence.
FIGURE 7.
The TCR affinity of virus-specific CD8+ T cells does not change from primary infection to viral persistence. A and B, Measurement of TCR affinity in CD8+ORF6487–495/Db+ cells by peptide/MHC tetramer dilution analysis. Lymphocytes were obtained from spleens of mice infected 14 days before (early group) or 3 mo before (late group), pooled, enriched by panning, and stained with serial 2-fold dilutions of ORF6487–495/Db tetramer conjugated to APC at concentrations ranging from 1/50 to 1/100,000. All the cells were subsequently stained with anti-CD8 Ab. Data are representative from three independent experiments with similar results. A, Representative FACS plots of the CD8-ORF6487–495/Db staining. B, Graphic representation of the normalized frequency of tetramer binding and linear regression analysis for the experiment shown in A. To normalize the groups, the data were expressed as the percentage of maximum tetramer binding. Inset, Raw data. C and D, Measurement of TCR affinity in CD8+ORF6487–495/Db+ cells as determined by peptide/MHC tetramer dissociation. Lymphocytes were sampled and processed as above, stained with ORF6487–495/Db tetramer, washed, saturated with anti-Db Ab, and incubated at RT to allow for tetramer dissociation. At different time points cells were sampled and stained with anti-CD8 Ab. Data are representative from two independent experiments with similar results. C, Representative FACS plots of the CD8-ORF6487–495/Db staining at different time points during tetramer decay. D, Graphic representation of the normalized frequency of tetramer binding over time and nonlinear one-phase exponential decay regression analysis of the experiment shown in C. To normalize the groups, the data were expressed as the percentage of maximum tetramer binding. Inset, Raw data.
The half-life of the TCR-peptide/MHC complex is related to the strength of signaling (59, 60) and the rate of dissociation of peptide-MHC tetrameric reagents from the T cell surface is related to the off rate of the TCR-peptide/MHC interaction (49). Thus, we used tetramer decay analysis to compare the binding of ORF6487–495/Db to γHV68-specific CD8+ T cells during early and long-term infection by measuring the dissociation rate using flow cytometry (Fig. 7C). The normalized results show that there were no major differences in the rate of tetramer dissociation time between CD8+ T cells analyzed at early and late time points during infection (Fig. 7D). The mathematical analysis indicated a better fit for the nonlinear one-phase exponential decay function (R2-early: 0.9481, R2-late: 0.9510), so that the functional form was used in comparing groups. The comparison of fit indicated that the preferred model was one common curve fitting all data sets independently of group and a common half-life value was determined as 17.19 min. (K: 0.0403). Altogether, the tetramer dilution and tetramer decay analyses demonstrate that Ag-specific CD8+ T cells exhibit similar TCR-peptide/MHC interaction affinities during primary and persistent γHV68 infection. These data suggest that persistent Ag expression during γHV68 infection does not substantially modify the TCR affinity of CD8+ T cells.
Ag-specific CD8+ T cells maintain functional avidity during virus persistence
The functional avidity of CD8+ T cells is mediated by the interaction between TCR/CD8β and peptide/MHC complexes but also by several other mechanisms such as the contribution of accessory molecules in lipid raft recruitment of signaling proteins and regulatory pathways (61–63). Persistent viral infections are thought to lead to different degrees of CD8+ T cell dysfunction (15, 16, 33). This progressive decrease in T cell function during viral persistence has been partially attributed to triggering of inhibitory pathways (17, 64). To compare the functional capabilities of γHV68 primary and long-term CD8+ T cell responses, we sought to determine their effectiveness in IFN-γ secretion or in cytotoxic killing. These two functions were chosen because they characterize the early (cytotoxicity) and final (IFN-γ) stages of CD8+ T cell functional impairment (20).
We measured the capacity of γHV68-specific CD8+ T cells to secrete IFN-γ in response to saturating and limiting availability of peptide at early and late times postinfection. The data show intracellular IFN-γ staining of splenic ORF6487–495/Db-specific CD8+ T cells (Fig. 8A). The data points show that the normalized titration curves for early and late time points postinfection overlapped (Fig. 8B). A better fit of IFN-γ production data over peptide concentration was obtained with a nonlinear sigmoidal dose-response curve analysis (R2-early: 0.8096, R2-late: 0.9082). The comparison of fits indicated that the preferred model was one common curve fitting all data sets independently of group and a common peptide concentration provoking a response halfway between baseline and maximum was determined (EC50: 2.229 × 10−4). There were no significant differences in IFN-γ-secreting capacity of each CD8+ T cell population defined as the negative log of the peptide concentration that resulted in 50% maximal IFN-γ production. These data indicate that Ag persistence during γHV infection has no detrimental effect on the functional capacity of virus-specific CD8+ T cells to produce IFN-γ in response to antigenic stimulus.
FIGURE 8.
The functional avidity of virus-specific CD8+ T cells does not change from primary infection to viral persistence as measured by their IFN-γ production capacity. Lymphocytes were obtained from spleens of three to four mice infected 5 wk before (early group) or 3 mo before (late group), pooled, enriched by panning, and stimulated with different concentrations of ORF6487–495 peptide. All the cells were subsequently processed for IFN-γ intracellular cytokine staining and surface stained with anti-CD8 Ab. Data are representative from three independent experiments. A, Representative FACS plots of the intracellular IFN-γ staining at two peptide concentrations for the early and late groups. B, Graphic representation of the normalized frequency of tetramer binding over time and nonlinear sigmoidal dose response regression analysis of the experiment shown in A. To normalize the groups, the data were expressed as the percentage of maximum IFN-γ staining at 1 µg/ml. Inset, Raw data minus background (isotype-control intracellular staining).
We next compared the cytotoxic activity of ORF6487–495/Db-specific CD8+ T cells directly in vivo in mice 3 wk, 3 mo, and 8 mo postinfection. The elimination of ORF6487–495-loaded targets was assessed 6 and 40 h after transfer. Specific target elimination occurred with faster and more efficient kinetics in mice at 3 wk postinfection, which correlated with a higher frequency of ORF6487–495/Db-specific CD8+ T cells (Fig. 9). Mice at 3 and 8 mo postinfection had low specific killing rates 6 h after transfer (5–14% specific killing) but were capable of killing specific targets after a 40-h interval (65–88% specific killing). In addition, no major differences in specific killing were found between mice at 3 and 8 mo postinfection. It should be noted that 1) during long-term infection, the frequency of splenic ORF6487–495/Db-specific CD8+ T cells is 5- to 20-fold lower than during acute infection (Fig. 9) and 2) at 3 and 8 m.p.i., the γHV68-specific CD8+ T cell pool predominantly consists of effector-memory cells (as shown in Fig. 2). Altogether, these data indicate that CD8+ T cells during persistent γHV68 infection are capable of mounting a specific CTL response in vivo although with slower kinetics that during acute infection. The analysis of IFN-γ production and cytotoxicity provides evidence of the functional capacity of virus-specific CD8+ T cells during persistent infection.
FIGURE 9.
Long-term CD8+ T cells maintain cytotoxic capacity during persistent viral infection. Cytotoxicity was assessed by an in vivo CTL assays. Spleen cells from naive mice were loaded with γHV68 ORF6487–495 or with influenza NP peptides and stained with different amounts of CFSE before injection into naive control mice or into mice at different time points after γHV68 inoculation. Killing was calculated 6 h (left column) or 40 h (middle column) after transfer. Histograms show representative killing data from the analysis of individual naive mice and from mice at 3 wk, 3 mo, and 8 m.p.i. Bar diagrams show specific killing data from four to five individual mice per time point. The frequency of ORF6487–495/Db+CD8+ T cells was determined by FACS staining of spleen cells and is shown on the right column as representative dot plots and as a bar diagram. Error bars, SD.
CD8+ T cells generated during long-term viral persistence can mediate protection to γHV68 latency during a recall response
Memory T cells mediate faster and more effective responses to secondary pathogen challenge than naive T cells (1, 2). To determine whether T lymphocytes from γHV68–immune mice were capable of preventing the establishment of viral latency, we tested the capacity of CD8+ T cells from γHV68–infected mice to protect during a recall response. CD8+ T cells were FACS purified from BALB/c mice that were intranasally inoculated with 1000 PFU of γHV68 8 mo before. During the sorting procedure, B cells were excluded by CD19 staining to avoid carrying latently infected B cells in the purified populations. A total of 4 × 105 CD8+ T cells or a 1:1 mixture of CD4+ and CD8+ T cells were i.v. inoculated in 300 µl of PBS into naive BALB/c recipients. The recipient mice were intranasally inoculated with γHV68 the following day and viral splenic latency was analyzed at the peak of latently infected B cell expansion on day 16. The results show that adoptive transfer of CD8+ T cells from persistently infected mice reduces the number of γHV68–infected cells during the establishment of spleniclatency (Fig. 10). In addition, the data revealed that the addition of CD4+ T cells did not improve the recall response of CD8+ T cells as measured by protection. These data show that memory CD8+ T cells from γHV68–infected mice can respond to a secondary virus challenge by decreasing the number of latently infected cells in the spleen during the establishment of latency phase.
FIGURE 10.
Long-term CD8+ T cells can a mediate protective recall response to γHV latency. Spleen cells from BALB/c donor mice 8 m.p.i. were sorted by FACS. A total of 4 × 105 immune CD8+ or a 1:1 mixture of CD4+/CD8+ cells was adoptively transfer by i.v. injection into naive mice. Control mice received no cells. Mice were challenged with γHV68 24 h later. The number of latently infected spleen cells was determined by an infectious center assay on day 16 after challenge. Error bars represent SD.
Discussion
Persistent infectious diseases are a major health concern (13, 14). The general consensus is that chronic viral infections lead to some degree of CD8+ T cell effector dysfunction or deletion and lack of T cell memory formation (15–17). γHVs are very successful pathogens that persistently infect >90% of the human population and initial infection may occur early in childhood (28). In this report, we demonstrate that herpesvirus-specific central-memory CD8+ T cells develop during infection and that herpesvirus-specific CD8+ T cells maintain functional and protective capacities during long-term persistent infection.
Our results show that during persistent infection with γHV68, there are consistently less than 500 latently infected cells per spleen. Despite this very low level of viral latency, splenic dendritic cells but not B cells are capable of presenting viral lytic-phase epitopes to γHV68–specific CD8+ T cell hybridomas. This finding is unexpected as germinal center and memory B cells constitute the major viral reservoir during long-term latency (65, 66). It suggests that dendritic cells cross-present lytic-phase viral Ags during γHV persistence and supports the existence of continuous viral reactivation during the latency phase of infection (38, 67, 68). Reduced levels of latency and continuous presentation of lytic-epitope Ags have relevant implications for the maintenance of CD8+ T cell memory. Low-level latency together with low viral gene expression (69) probably represents a very efficient immune escape strategy and explains the low frequency of latent-phase epitope-specific CD8+ T cells during γHV68 persistence (70). Continuous presentation of lytic viral epitopes explains the delayed generation of central-memory CD8+ T cells and why effectors and effector-memory cells still constitute a large fraction of the CD8+ T cell response during long-term latency (50–80%, Figs. 2, 4, and 5). It is possible that these lytic epitope-specific CD8+ T cells partially represent secondary effectors or de novo generated effectors as result of virus reactivation. In this sense, it has been recently suggested that low-level TCR stimulation that occurs during HSV latency results in the maintenance of an activated but functional CD8–memory pool (71) and that newly recruited naive CD8+ T cells influence the heterogeneous CD8+ T cell response during polyoma virus persistence (72).
Our data show that memory CD8+ T cell precursors could be detected at early stages during primary infection and that effectormemory cells constitute the dominant CD8+ T cell subset once primary infection has been resolved. The kinetic analysis of the γHV68–specific CD8+ T cell response shows that activated CD8+ T cells (CD62LlowCD43high) contract over a period of 3 mo postviral inoculation. This slow process can be in part explained for the prolonged infectious mononucleosis-like syndrome in γHV68–infected mice (73) which results in exacerbated and prolonged CD8+ T cell activation and expansion (74, 75). In addition, γHV68 persistent replication and reactivation from latency (34, 76, 77) will contribute to keep virus-specific CD8+ T cells stimulated. Although the frequency of memory CD8+ T cells remains stable during long-term viral persistence, there is a shift in the ratio between effector-memory and central-memory subsets, with central-memory cells progressively increasing their frequency over time. Selective expression of IL-7R identifies CD8+ T cells that generate memory cells (78, 79). In addition to this marker, we have also identified memory precursors using the expression of the IL-15R and the lymphoid homing molecules L-selectin and CCR7 (15). Our data correlate with results from acute and persistent infections that demonstrate that memory cell precursors exist at the peak of the CD8+ T cell response (39, 79, 80). The generation and stability of CD8+ T cell memory or CD8+ T cell function during Ag persistence is poorly understood. It has been suggested that long-term T cell memory might not develop in conditions of chronic Ag stimulation (6). Infections with high load of persisting Ag result in the suppression of IL-7 and IL-15 receptor expression and down-regulation of lymphoid homing molecules on virus-specific CD8+ T cells, and this correlates with progressive T cell exhaustion (15, 20, 81). Our results show that IL-7R, L-selectin, and CCR7 expression are maintained on 20–70% of Ag-specific CD8+ T cells during viral persistence 9 m.p.i. This represents a small fraction of the whole CD8+ population compared with that of acute respiratory infections, where most of the CD8+ T cell population is constituted by memory cells (82). It is also possible that small differences on the level of expression of phenotypic markers between memory cells generated during acute infections or γHV68 persistence may be biologically relevant. Nevertheless, virus-specific CD8+ T cells with characteristics of memory cells are maintained during long-term persistent γHV68 infection, which suggests that T cells may not need to fully rest from Ag exposure during conditions of low-level Ag persistence to differentiate into memory T cells expressing lymphoid homing molecules and homeostatic cytokine receptors. It should be noted that differences in Ag load, T cell precursor frequency, or TCR avidity can also influence the expression of various phenotypic markers (such as CD62L and IL-7R) and the capacity to generate a diversity of cytokines (such as IFN-γ, TNF-α, and IL-2), suggesting that the phenotype of memory T cells is ultimately a consequence of molecular events occurring during the Ag-driven phase of the response (83, 84).
IL-15 is implicated in CD8+ T cell proliferation, survival, and avidity (56, 85, 86). Our data indicate that IL-2/IL-15β receptor expression is down-regulated on lytic epitope-specific CD8+ T cells during long-term γHV68 persistence, which contrasts with its expression on latent epitope-specific CD8+ T cells. Interestingly, EBV infectious mononucleosis induces a permanent deficit in IL-15R expression on CD8+ T cells (87). It has been shown that γHV68–specific CD8+ T cell maintenance can be independent of IL-15 (88). The functional implications of impaired IL-15 signaling during γHV infections are unclear and we could not find functional deficiencies at the level of IFN-γ production and cytotoxic capacity on lytic epitope-specific CD8+ T cells. However, functional and phenotypic differences between latent and lytic Ag-specific CD8+ T cells have been described elsewhere (89–91).
The interaction between herpesviruses and the host presents several specific characteristics that are relevant to the analysis of the CD8+ T cell response: 1) continuous presence of persistent Ag may impact the evolution of the cognate response, 2) γHV persistence is characterized by low-level viral latency and antigenic load, and T cells should compete for this limited resource, and 3) antigenic mutation is not likely to affect the T cell response. Our findings indicate that Ag persistence during γHV68 infection does not modify TCR affinity and it has no detrimental effect on the functional capacity of virus-specific CD8+ T cells to produce IFN-γ or to kill in response to antigenic stimulus. These results are in concordance with data from murine CMV infection that suggest that CD8+ T cell avidity does not change over time (92) and with studies that indicate that the TCR memory repertoire to persistent herpesvirus infections, although heterogeneous, can be remarkably stable (50, 93). Altogether, these findings derived from herpesvirus infections argue against the idea that the continuous presence of Ag will eventually drive T cells into some degree of functional exhaustion (16, 20, 21). These differences are likely to be explained by the low-level Ag persistence during herpesvirus infections. In this sense, during HSV latency in neurons, virus-specific CD8+ T memory cells retain their capacity to produce IFN-γ (94). Although the TCR usage between T cells during primary and memory γHV responses is similar, there is a shift in clonotypes during memory formation (95). γHV68 persistence has also been associated with enhanced effector functions when compared with nonpersistently infected mice (96). These conflicting results may reflect differences in the experimental systems and differences between human and murine studies. Regardless, these studies shed light on the idea that CD8+ T cells during herpesvirus persistence maintain their functional ability to control infection and suggest that low-level Ag persistence may not intrinsically be a detrimental factor for T cell function. It should also be noted that this process might be mediated by the continuous recruitment of naive T cells during viral persistence (72). Our analysis of CD8+ T cell protection using adoptive transfers allowed us to determine the specific contribution of long-term T cell populations to the control of viral latency. Although Ag persistence has been linked to poor CD8+ T cell cytotoxic and recall responses (15), this was not the case during γHV68 infection. CD8+ T cells from persistently infected mice were capable of mounting cytotoxic responses in vivo, albeit with slower kinetics than during acute infection. These differences can be explained by the reduced frequency of Ag-specific CD8+ T cells and the contraction of effector cells after 3 m.p.i. In addition, long-term CD8+ T cells were capable of eliciting protection against the establishment of viral latency after adoptive transfer, which supports the maintenance of CD8+ T cell function during γHV68 persistence. Our findings indicate that protective memory can be generated during herpesvirus infections and suggest that the model of CD8+ T cell linear differentiation that postulates central-memory T cells as the end of a maturation process (12) also applies with an extended time frame to infections with low levels of persistent Ag. These findings have important implications for our understanding of immune control and protection against pathogens or Ags that persist in the host and for the design of vaccination strategies.
Acknowledgments
We thank C. McAllister and the Morphology Core for assistance with FACS sorting, J. Haynes and the Biostatistics Core for help with the statistical analysis, J. Cyster for CCL19 Ig, C. Walker for critical comments to the manuscript, and M. Blackman for her continued support.
Footnotes
This work was supported in part by National Institutes of Health Grant AI-59603 and by Columbus Children’s Research Institute.
Abbreviations used in this paper: LCMV, lymphocytic choriomeningitis virus; γHV68, murine γ-herpesvirus 68; LDA-PCR, limiting dilution nested PCR; RT, room temperature; d.p.i., days postinfection; m.p.i., months postinfection; MLN, mediastinal lymph node.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu. Rev. Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 2.Woodland DL. Cell-mediated immunity to respiratory virus infections. Curr. Opin. Immunol. 2003;15:430–435. doi: 10.1016/s0952-7915(03)00067-0. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- 5.Lefrancois L, Masopust D. T cell immunity in lymphoid and non-lymphoid tissues. Curr. Opin. Immunol. 2002;14:503–508. doi: 10.1016/s0952-7915(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol. Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 8.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol. Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 9.Robertson JM, MacLeod M, Marsden VS, Kappler JW, Marrack P. Not all CD4+ memory T cells are long lived. Immunol. Rev. 2006;211:49–57. doi: 10.1111/j.0105-2896.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 11.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J. Exp. Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, Von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;3:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 13.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rappuoli R. From Pasteur to genomics: progress and challenges in infectious diseases. Nat. Med. 2004;10:1177–1185. doi: 10.1038/nm1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 18.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 19.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8 + T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 20.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 22.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8+ T cell responses from primary to persistent phases of Epstein-Barr virus infection. J. Exp. Med. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hislop AD, Kuo M, Drake-Lee AB, Akbar AN, Bergler W, Hammerschmitt N, Khan N, Palendira U, Leese AM, Timms JM, et al. Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. J. Clin. Invest. 2005;115:2546–2555. doi: 10.1172/JCI24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickinson AB, Callan MF, Annels NE. T-cell memory: lessons from Epstein-Barr virus infection in man. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2000;355:391–400. doi: 10.1098/rstb.2000.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemball CC, Lee ED, Vezys V, Pearson TC, Larsen CP, Lukacher AE. Late priming and variability of epitope-specific CD8+ T cell responses during a persistent virus infection. J. Immunol. 2005;174:7950–7960. doi: 10.4049/jimmunol.174.12.7950. [DOI] [PubMed] [Google Scholar]
- 26.Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray D. A role for antigen in the maintenance of immunological memory. Nat. Rev. Immunol. 2002;2:60–65. doi: 10.1038/nri706. [DOI] [PubMed] [Google Scholar]
- 28.Rickinson AB, Kieff E. Epstein-Barr Virus. In: Fields DMKBN, Howley PM, editors. Fields Virology. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 29.Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus immunoevasion and tumorigenesis: two sides of the same coin? Annu. Rev. Microbiol. 2003;57:609–639. doi: 10.1146/annurev.micro.57.030502.090824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munz C. Immune response and evasion in the host-EBV interaction. In: Robertson ER, editor. Epstein-Barr Virus. Norfolk: Caister Academic Press; 2005. pp. 197–231. [Google Scholar]
- 31.Doherty PC, Christensen JP, Belz GT, Stevenson PG, Sangster MY. Dissecting the host response to a γ-herpesvirus. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2001;356:581–593. doi: 10.1098/rstb.2000.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, et al. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 34.Flano E, Kim IJ, Moore J, Woodland DL, Blackman MA. Differential γ-herpesvirus distribution in distinct anatomical locations and cell subsets during persistent infection in mice. J. Immunol. 2003;170:3828–3834. doi: 10.4049/jimmunol.170.7.3828. [DOI] [PubMed] [Google Scholar]
- 35.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Flano E, Usherwood EJ, Surman S, Blackman MA, Woodland DL. Lytic cycle T cell epitopes are expressed in two distinct phases during MHV-68 infection. J. Immunol. 1999;163:868–874. [PubMed] [Google Scholar]
- 37.Altman JD, Moss PH, Goulder PR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 38.Grundhoff A, Ganem D. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Invest. 2004;113:124–136. doi: 10.1172/JCI200417803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 40.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 41.Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, Baum LG, Pearce BD, Ahmed R. Dynamic regulation of T cell immunity by CD43. J. Immunol. 2002;168:6022–6031. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- 42.Flano E, Woodland DL, Blackman MA, Doherty PC. Analysis of virus-specific CD4+ T cells during long-term gammaherpesvirus infection. J. Virol. 2001;75:7744–7748. doi: 10.1128/JVI.75.16.7744-7748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniels MA, Devine L, Miller JD, Moser JM, Lukacher AE, Altman JD, Kavathas P, Hogquist KA, Jameson SC. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity. 2001;15:1051–1061. doi: 10.1016/s1074-7613(01)00252-7. [DOI] [PubMed] [Google Scholar]
- 44.Kambayashi T, Assarsson E, Chambers BJ, Ljunggren HG. IL-2 down-regulates the expression of TCR and TCR-associated surface molecules on CD8+ T cells. Eur. J. Immunol. 2001;31:3248–3254. doi: 10.1002/1521-4141(200111)31:11<3248::aid-immu3248>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Drake DR, 3rd, Ream RM, Lawrence CW, Braciale TJ. Transient loss of MHC class I tetramer binding after CD8+ T cell activation reflects altered T cell effector function. J. Immunol. 2005;175:1507–1515. doi: 10.4049/jimmunol.175.3.1507. [DOI] [PubMed] [Google Scholar]
- 46.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 47.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee HG. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 50.Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander-Miller MA, Leggatt GR, Sarin A, Berzofsky JA. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J. Exp. Med. 1996;184:485–492. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderton SM, Radu CG, Lowrey PA, Ward ES, Wraith DC. Negative selection during the peripheral immune response to antigen. J. Exp. Med. 2001;193:1–11. doi: 10.1084/jem.193.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davenport MP, Fazou C, McMichael AJ, Callan MF. Clonal selection, clonal senescence, and clonal succession: the evolution of the T cell response to infection with a persistent virus. J. Immunol. 2002;168:3309–3317. doi: 10.4049/jimmunol.168.7.3309. [DOI] [PubMed] [Google Scholar]
- 54.Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc. Natl. Acad. Sci. USA. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weng NP, Liu K, Catalfamo M, Li Y, Henkart PA. IL-15 is a growth factor and an activator of CD8 memory T cells. Ann. NY Acad. Sci. 2002;975:46–56. doi: 10.1111/j.1749-6632.2002.tb05940.x. [DOI] [PubMed] [Google Scholar]
- 56.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Rα-mediated avidity maturation of memory CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 58.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J. Exp. Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 60.Lyons DS, Lieberman SA, Hampl J, Boniface JJ, Chien Y, Berg LJ, Davis MM. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 61.Konig R. Interactions between MHC molecules and co-receptors of the TCR. Curr. Opin. Immunol. 2002;14:75–83. doi: 10.1016/s0952-7915(01)00300-4. [DOI] [PubMed] [Google Scholar]
- 62.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat. Immunol. 2006;7:803–809. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 63.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 64.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8+ T cell responses. Nat. Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 65.Flano E, Kim IJ, Woodland DL, Blackman MA. γ-herpesvirus latency is preferentially maintained in splenic germinal center and memory B cells. J. Exp. Med. 2002;196:1363–1372. doi: 10.1084/jem.20020890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim IJ, Flano E, Woodland DL, Lund FE, Randall TD, Blackman MA. Maintenance of long term γ-herpesvirus B cell latency is dependent on CD40–mediated development of memory B cells. J. Immunol. 2003;17(1):886–892. doi: 10.4049/jimmunol.171.2.886. [DOI] [PubMed] [Google Scholar]
- 67.Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 2005;79:1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim IJ, Burkum CE, Cookenham T, Schwartzberg PL, Woodland DL, Blackman MA. Perturbation of B cell activation in SLAM-associated protein-deficient mice is associated with changes in gammaherpesvirus latency reservoirs. J. Immunol. 2007;178:1692–1701. doi: 10.4049/jimmunol.178.3.1692. [DOI] [PubMed] [Google Scholar]
- 69.Virgin HWt, Presti RM, Li XY, Liu C, Speck SH. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J. Virol. 1999;73:2321–2332. doi: 10.1128/jvi.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Usherwood EJ, Roy DJ, Ward K, Surman SL, Dutia BM, Blackman MA, Stewart JP, Woodland DL. Control of gamma-herpesvirus latency by latent antigen-specific CD8+ T cells. J. Exp. Med. 2000;192:943–952. doi: 10.1084/jem.192.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. Latent virus influences the generation and maintenance of CD8+ T cell memory. J. Immunol. 2006;177:8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vezys V, Masopust D, Kemball CC, Barber DL, O’Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blackman MA, Flano E, Usherwood E, Woodland DL. Murine γ-herpesvirus-68: a mouse model for infectious mononucleosis? Mol. Med. Today. 2000;6:488–490. doi: 10.1016/s1357-4310(00)01813-x. [DOI] [PubMed] [Google Scholar]
- 74.Tripp RA, Hamilton-Easton AM, Cardin RD, Nguyen P, Behm FG, Woodland DL, Doherty PC, Blackman MA. Pathogenesis of an infectious mononucleosis-like disease induced by a murine γ-herpesvirus: role for a viral superantigen? J. Exp. Med. 1997;185:1641–1650. doi: 10.1084/jem.185.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flano E, Hardy CL, Kim IJ, Frankling C, Coppola MA, Nguyen P, Woodland DL, Blackman MA. T cell reactivity during infectious mononucleosis and persistent gammaherpesvirus infection in mice. J. Immunol. 2004;17(2):3078–3085. doi: 10.4049/jimmunol.172.5.3078. [DOI] [PubMed] [Google Scholar]
- 76.Gangappa S, Kapadia SB, Speck SH, Virgin HWt. Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice. J. Virol. 2002;76:11460–11468. doi: 10.1128/JVI.76.22.11460-11468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim I-J, Flano E, Woodland DL, Blackman MA. Antibody-mediated control of persistent γ-herpesvirus infection. J. Immunol. 2002;168:3958–3964. doi: 10.4049/jimmunol.168.8.3958. [DOI] [PubMed] [Google Scholar]
- 78.Holmes S, He M, Xu T, Lee PP. Memory T cells have gene expression patterns intermediate between naive and effector. Proc. Natl. Acad. Sci. USA. 2005;102:5519–5523. doi: 10.1073/pnas.0501437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 80.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 81.Chen G, Shankar P, Lange C, Valdez H, Skolnik PR, Wu L, Manjunath N, Lieberman J. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98:156–164. doi: 10.1182/blood.v98.1.156. [DOI] [PubMed] [Google Scholar]
- 82.Hikono H, Kohlmeier JE, Ely KH, Scott I, Roberts AD, Blackman MA, Woodland DL. T-cell memory and recall responses to respiratory virus infections. Immunol. Rev. 2006;211:119–132. doi: 10.1111/j.0105-2896.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 83.Kedzierska K, La Gruta NL, Turner SJ, Doherty PC. Establishment and recall of CD8+ T-cell memory in a model of localized transient infection. Immunol. Rev. 2006;211:133–145. doi: 10.1111/j.0105-2896.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 84.La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J. Immunol. 2004;172:5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- 85.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J. Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- 87.Sauce D, Larsen M, Curnow SJ, Leese AM, Moss PA, Hislop AD, Salmon M, Rickinson AB. EBV-associated mononucleosis leads to long-term global deficit in T-cell responsiveness to IL-15. Blood. 2006;108:11–18. doi: 10.1182/blood-2006-01-0144. [DOI] [PubMed] [Google Scholar]
- 88.Obar JJ, Crist SG, Leung EK, Usherwood EJ. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J. Immunol. 2004;173:2705–2714. doi: 10.4049/jimmunol.173.4.2705. [DOI] [PubMed] [Google Scholar]
- 89.Catalina MD, Sullivan JL, Brody RM, Luzuriaga K. Phenotypic and functional heterogeneity of EBV epitope-specific CD8+ T cells. J. Immunol. 2002;168:4184–4191. doi: 10.4049/jimmunol.168.8.4184. [DOI] [PubMed] [Google Scholar]
- 90.Hislop AD, Gudgeon NH, Callan MF, Fazou C, Hasegawa H, Salmon M, Rickinson AB. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J. Immunol. 2001;167:2019–2029. doi: 10.4049/jimmunol.167.4.2019. [DOI] [PubMed] [Google Scholar]
- 91.Obar JJ, Crist SG, Gondek DC, Usherwood EJ. Different functional capacities of latent and lytic antigen-specific CD8 T cells in murine gammaherpesvirus infection. J. Immunol. 2004;172:1213–1219. doi: 10.4049/jimmunol.172.2.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 93.Levitsky V, de Campos-Lima PO, Frisan T, Masucci MG. The clonal composition of a peptide-specific oligoclonal CTL repertoire selected in response to persistent EBV infection is stable over time. J. Immunol. 1998;161:594–601. [PubMed] [Google Scholar]
- 94.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Callan MF, Fazou C, Yang H, Rostron T, Poon K, Hatton C, McMichael AJ. CD8+ T-cell selection, function, and death in the primary immune response in vivo. J. Clin. Invest. 2000;106:1251–1261. doi: 10.1172/JCI10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Obar JJ, Fuse S, Leung EK, Bellfy SC, Usherwood EJ. Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J. Virol. 2006;80:8303–8315. doi: 10.1128/JVI.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]