Abstract
A lesson from dominantly inherited forms of diverse neurodegenerative diseases, including amyotrophic lateral sclerosis, spinocerebellar ataxia and Huntington’s disease, is that the selective dysfunction or death of the neuronal population most at risk in each disease is not mediated solely by mutant derived damage within the target neurons. The disease-causing toxic process, which in each case is caused by mutation in a gene that is widely or ubiquitously expressed, involves mutant damage within the non-neuronal glial cells of the central nervous system - especially astrocytes and microglia. Disease mechanism is non-cell autonomous, with toxicity derived from glia as a prominent contributor to driving disease progression and in some instances even disease initiation.
Introduction
The classic view of neurotoxicity in neurodegenerative diseases is based upon the idea that a specific neuronal population is especially vulnerable to a cumulative toxic burden, e.g., in dominantly inherited examples of disease from intraneuronal damage from accumulation of a toxic mutant protein. Chronic damage combined with normal aging drives this deleterious action to a threshold that overwhelms the neuron’s defensive mechanisms, triggering degeneration and neuronal death or both (reviewed in ref. 1). An initial view was that this mechanism would be cell-autonomous, that is, independent of mutant damage accumulated within other cell types which interact with the affected neurons.
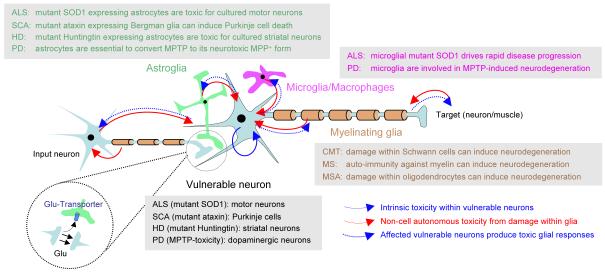
Evidence from genetic (or chemical) mimics in mice of diverse human neurodegenerative diseases including amyotrophic lateral sclerosis (ALS), spinocerebellar ataxia (SCA), Huntington’s disease (HD), Parkinson’s disease (PD) and multiple system atrophy (MSA) have, however, shaken this classic view. There is now powerful evidence for non-cell autonomous mechanisms in which neurodegeneration is strongly influenced by toxicity or mutant protein expression in both neuronal and non-neuronal cells in the neighborhood of the vulnerable neurons, especially the CNS glial cells, astrocytes2-5, oligodendrocytes6 and microglia7-9, each of which have intimate contact with neurons (Fig. 1).
Figure 1.
Insights from animal models of diverse human neurodegenerative diseases suggest that disease mechanisms are non-cell autonomous requiring the convergence of damage within the vulnerable neurons and their neighboring glial cells. Glial-derived toxicity can strongly influence disease progression (e.g. in ALS7,8) or can even contribute to disease initiation (e.g. in SCA3,44). Glutamate (Glu) mediated excitotoxicity is a prime example of neuron-glial toxicity that has been proposed to be a significant component in ALS27,28, SCA3,44 and HD2,55.
The major question for glial involvement and its contribution to non-cell autonomous mechanisms in diseases that have classically been thought of as primary “neuro-”degenerative diseases, is whether interactions within or between glial or neuronal cells are necessary for, or contribute to, the neurodegenerative process, as opposed to toxicity arising solely within vulnerable neurons. Three different pathways of glial involvement in non-cell autonomous degeneration of the vulnerable neurons can be imagined: i) toxicity within the affected neurons could stimulate damaging responses from glia that are not directly damaged by this toxicity or their own synthesis of the mutant protein; ii) mutant protein expression (or toxicity) in glial cells could disturb a normal glial response, amplifying initial damage to the vulnerable neurons; or iii) mutant expression (or toxicity) within glia could disturb normal glial function, thus becoming a primary source of neurotoxicity, potentially independent of mutant (or toxic) effects within the neurons at risk. The latter two possibilities blur the lines between diseases previously thought as of primary neuronal origin (e.g., ALS, SCA, HD, PD and partly also MSA) and those that are of primary glial origin, accompanied by secondary neurodegeneration (including multiple sclerosis (MS) and certain forms of Charcot-Marie-Tooth disease (CMT), both related either to myelinating oligodendrocytes or Schwann cells, respectively) (Fig. 1). We focus here on examples, especially ALS and SCA7, where molecular genetic methods in mice have demonstrated a non-cell autonomous disease mechanism in which damage within glia, especially astyrocytes and microglia, plays an essential role in toxicity.
Astrocytes: interlinked gatekeepers of glutamate
Astrocytes provide essential services to the neurons they support, including roles in synapse formation, maintenance and plasticity, as well as regulating cerebral blood flow (reviewed in ref. 10,11). They transport various nutrients and metabolic precursors to neurons (via the malate-aspartate shuttle) and are central to extracellular potassium homeostasis via their potassium channels located at synapses and at astrocytic “end-foot” processes juxtaposed around capillaries. They are probably best known for their essential roles in the catabolism of several amino acids, especially rapid recovery of glutamate, the primary excitatory neurotransmitter in the CNS, from synaptic clefts by astrocytic glutamate transporters (GLT-1/EAAT2 and GLAST/EAAT1) and for returning glutamate (in the form of glutamine) to neurons (via the glutamate-glutamine shuttle).
Less appreciated is that astrocytes are not just single cells: they are networked together by a series of gap junctions that, among other things, propagate calcium waves throughout the linked astrocyte network (reviewed in ref. 10,11). This could become important when considering how they might contribute to spread damage from an initiating focal point.
Microglia: resident macrophages of the CNS
Microglia, the macrophages of the CNS, play well established roles in the programmed elimination of neural cells during development and in maintaining normal neuronal survival by removing toxic cellular debris (reviewed in ref. 12). Their major role in the adult CNS is linked to neuroinflammation in disease and injury. Their surveillance systems lie in wait for damage signals which stimulate their activation, migration to the site of damage, and their proliferation. As major components of the inflammatory response, which is known to be a double-edged sword with both neurotoxic and neurotrophic effects, microglia are mobilized in essentially all examples of disease and injury to the CNS (reviewed in ref. 12).
Astrocytes and microglia as major contributors in ALS
ALS is the most prominent adult motor neuron disease, characterized by progressive, fatal paralysis from premature degeneration and death of upper brain and lower spinal cord motor neurons (reviewed in ref. 13). Although most instances occur as sporadic disease without an apparent genetic origin, approximately 10% (referred to as familial ALS) are caused by dominant mutation. Sporadic and familial ALS produce similar pathologies with selective loss of the same set of vulnerable motor neurons. About a quarter of the inherited cases are due to missense mutations in the ubiquitously expressed enzyme superoxide dismutase 1 (SOD1), whose enzymatic activity is to destroy the highly reactive oxygen radical, superoxide (reviewed in ref. 13).
Mimics of familial ALS have been produced for half a dozen of the >115 disease associated SOD1 mutants. Expression of mutant but not a comparable increase in synthesis of wild type SOD1 generates age-dependent, progressive, fatal paralysis in mice and rats when mutant synthesis is either ubiquitously (from transgenes using the authentic SOD1 transcriptional promoter13-15) or broadly neuronal and glial expressed (using transgenes carrying the prion protein (Prp) promoter16) (Fig. 2a). A consensus has emerged that disease results from an acquired toxicity of the mutant protein, not loss of enzymatic activity13,17. Indeed, complete absence of SOD1 does not compromise life span in laboratory mice nor provoke motor neuron disease18.
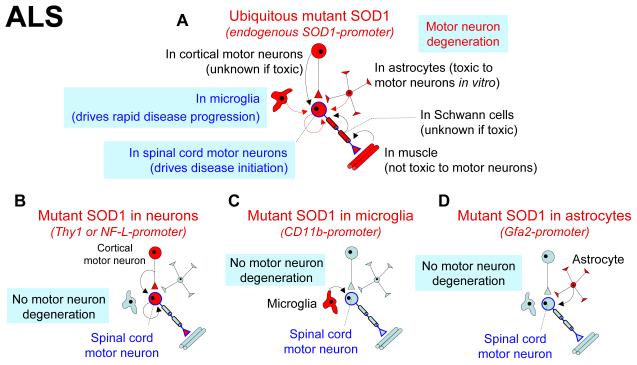
Figure 2.
Non-cell autonomous neurotoxicity in ALS. (a) Selective mutant SOD1 silencing demonstrated that mutant damage within motor neurons drives disease initiation8,22,23, while mutant expression within neighboring microglia underlies rapid disease progression7,8. The in vivo contributions of mutant SOD1 expression in astrocytes or Schwann cells are not yet established, while mutant expression in muscle does not contribute to disease56. Consistent with non-cell autonomous mechanisms, selective mutant expression in either motor neurons19,20 (b), astrocytes29 (c) or microglia7 (d) alone are not sufficient to induce motor neuron degeneration.
Absence of degeneration or disease after mutant SOD1 synthesis uniquely within neurons, either by the Thy119 or the NF-L (neurofilament-light)20 promoters, provided the initial evidence that disease mechanism probably does not arise strictly from mutant damage within the vulnerable motor neurons (Fig. 2b). Analysis of chimeric mice that were mixtures of normal cells and cells expressing high levels of mutant SOD1 provided even more direct evidence for a non cell autonomous disease mechanism. Motor neurons surrounded by higher numbers of normal neighbors survived longer without degeneration despite expressing high levels of mutant SOD1, while genetically normal motor neurons acquired damage from their mutant expressing neighbors21.
Disease onset was delayed and survival extended following partial removal within the CNS of an ubiquitously expressed mutant SOD1 transgene only from motor neurons (using a motor neuron specific Cre transgene)8 (Fig. 2a). Despite this, diminished mutant synthesis within motor neurons left disease progression almost unaffected. So too did viral-mediated siRNA knock-down that within the CNS suppressed mutant SOD1 selectively within motor neurons. Despite strongly slowed onset22,23, disease progression was slightly accelerated22. Thus, mutant synthesis within motor neurons is a central contributor to disease initiation, but is only a minor player in mediating disease progression.
So what about the contribution to disease of mutant SOD1 expression within astrocytes and microglia? Both ALS mice and patients develop prominent features of neuroinflammation (reviewed in refs. 10,12,13) including astrogliosis and microgliosis24,25 and minocycline, a tetracycline derivative with anti-inflammatory activity, extends survival in ALS mice26.
The clearest evidence is for mutant damage developed within microglia as a contributor to disease progression (Fig. 2a). Although partial excision of an ubiquitously expressed mutant SOD1 transgene from cells of the myeloid lineage (including microglia) produced no slowing of disease onset, survival was strongly extended through dramatic slowing of disease progression8. Similarly, replacing the entire myeloid lineage (including microglia) by transplantation of normal bone marrow cells into mutant SOD1 mice that could not make their own myeloid cells (due to absence of the PU.1 transcription factor) had no effect on disease onset, but extended survival by slowing disease progression7. Similar transplantation to replace the entire myeloid lineage with mutant SOD1 cells, including all microglia, did not produce disease in an otherwise wild type mouse (Fig. 2c), conclusively demonstrating that the mutant expression within microglia/macrophages is not sufficient for motor neuron disease, but does drive rapid disease progression7.
Astrocytic glutamate transporters are of prime importance in protecting motor neurons against glutamate excitotoxicity. Altered glutamate handling is one of the few firm mechanistic links between sporadic and mutant SOD1 mediated ALS. In both mutant SOD1 animals14,15 and human patients27, astrocytic GLT-1/EAAT2 glutamate transporters (and activity) are focally lost. This is likely to be of functional consequence for disease. Indeed, upregulating the GLT-1/EAAT2 transporter by transcriptional induction with beta-lactam antibiotics, including ceftriaxone, extended survival in ALS mice28 and this approach is now the basis of an ongoing clinical trial.
Although the effect on disease course from mutant SOD1 reduction within astrocytes has not yet been reported (for example, by selective gene excision), expression of mutant SOD1 at high levels only within astrocytes (using the GFAP-derived Gfa2-promoter) produced reactive astrocytosis, but was not sufficient to induce motor neuron degeneration29 (Fig. 2d). When coupled with similar absence of neurodegeneration after high level expression of mutant SOD1 within all microglia7 (see above; Fig. 2c), it seems likely that mutant damage within both glial cell types rapidly accelerates disease progression7,8. On the other hand, mutant damage within the motor neurons is a key aspect driving disease initiation (Fig. 2a).
Mechanisms for microglial and astrocytic mediated neurotoxicity in ALS
What is the nature of mutant SOD1 damage developed within microglia or astrocytes? Among candidates that can induce motor neuron toxicity (at least in vitro) are FasLigand/FasR30, NOS/NO30, NGF/p7531, TNFα32 and glutamate-excitotoxicity10. Enhanced synthesis of TNFα by activated mutant microglia32 was initially attractive. This now seems unlikely since deletion of TNFα does not affect mutant SOD1 mediated disease33. Neither is microglial (nor astrocytic for that matter) production of nitric oxide (NO) a likely contributor, since deletion of iNOS, the inducible nitric oxide synthase, does not affect disease course34.
Microglia are the source of NADPH oxidase, the main reactive oxygen species-producing enzyme during inflammation. This multimeric oxidase is expressed by all phagocytes and is indispensable for protection against infectious microorganisms. It is also upregulated in familial and sporadic ALS and in mutant SOD1 mice35. While this could simply reflect the strong microgliosis accompanying mutant SOD1 mediated disease, deletion from mice of the catalytic subunit (gp91phox) extended survival of mutant SOD1 mice35 to exactly the same extent as did replacement of all mutant-expressing myeloid cells with normal ones7. It is thus likely that microglial derived NADPH oxidase dependent reactive oxygen species (ROS) and the oxidative damage arising from that ROS is at least a part of the microglial-derived component of non-cell autonomous disease.
As for the contribution of astrocytes, co-cultures of primary mutant SOD1 expressing astrocytes4,5 with primary motor neurons purified from embryos5 or generated in larger numbers by differentiation of mouse embryonic stem cells4,5 have demonstrated that mutant astrocytes diminish motor neuron survival over a two week period (relative to similar cultures with normal glial cells). Astrocyte-derived toxicity is i) selective for motor neurons, with no effect on sensory neurons or interneurons5, ii) can be conferred by astrocyte conditioned media5 and iii) acts on wild type as well as mutant SOD1 motor neurons4,5. The toxic species remains unidentified, however, and no signs of increased excitotoxicity could be detected in these in vitro systems5. Nevertheless, the reduced accumulation of GLT-1/EAAT2 that is almost universally seen in human ALS27 and animal models14,15 strongly supports an in vivo, astrocyte-derived excitotoxic component to disease.
Lastly, induction of chromogranin A synthesis in reactive astrocytes as well as neurons, combined with the proposal for an unusual, chromogranin-mediated secretion of mutant SOD136 in ALS mice, suggests that astrocytes and motor neurons may also drive disease progression through production of extracellular mutant SOD1. Consistent with an influence of extracellular mutant SOD1, both active and passive immunization to misfolded, mutant SOD1 can extend survival in ALS mice37. Since extracellular mutant SOD1 is a potent activator of microglial cells36, this provides a non-cell autonomous, feed-forward mechanism highlighting how reaction to actions from one cell type can accelerate toxicity to another once damage has initiated.
SCA7: Glia as a primary source of Purkinje cell toxicity
Spinocerebellar ataxias (SCAs) are neurological disorders characterized by the common feature of cerebellar neurodegeneration leading in affected patients to progressive motor incoordination (reviewed in ref. 38). The most affected cells are the large, complex cerebellar Purkinje neurons. Intimate non-neuronal neighbors to these neurons are the Bergmann glia, the cerebellum’s specialized astrocytes which use long finger-like processes to enwrap the huge dendritic trees of Purkinje cells (reviewed in ref. 39). Mutations in at least 25 genes cause similar ataxias, six of which (SCA1, 2, 3, 6, 7 and 17) represent dominant, polyglutamine repeat expansions (polyQ) in each of 6 different genes.
SCA7 is caused by polyQ expansion in the gene encoding ataxin-7. An initial mouse demonstrated that ataxia can be induced through a strictly cell autonomous mechanism: selective expression of mutant ataxin-7 solely in Purkinje cells (using the Pcp2-promoter) produced a mild ataxia and neurodegeneration at advanced ages40. This was not too unexpected since the polyQ expanded mutant protein, SCA1-linked ataxin-1, had already been shown to produce strong ataxia and prominent Purkinje cell degeneration when expressed from the same Purkinje cell specific Pcp2-promoter41. More robust ataxin-7 mediated disease was achieved with a pan-neuronal (PDGF-B) promoter to direct synthesis of mutant ataxin-7 in Purkinje cells and all their interacting neurons42 (Fig. 3a). An even stronger ataxic phenotype with neurodegeneration was achieved by a knock-in strategy of mutant ataxin-7 which recapitulated endogenous ataxin-7 levels and widespread neuro/glial expression patterns including within Purkinje cells43.
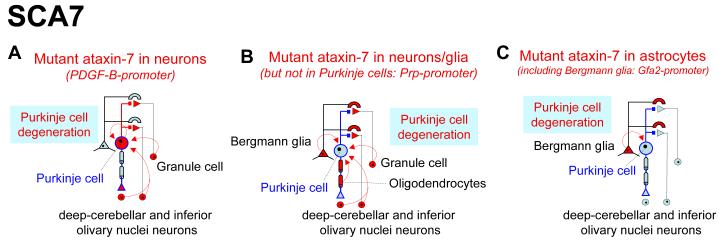
Figure 3.
Non-cell autonomous, glial derived toxicity can lead to degeneration of Purkinje neurons in SCA7. Although moderate Purkinje cell degeneration can be induced by neuronal expression of mutant ataxin-742 (a), expression of mutant ataxin-7 in neurons and glia, but not the Purkinje neurons themselves (b), or in the astrocytic Purkinje cell associated Bergmann glia alone3 (c), is sufficient to provoke Purkinje cell degeneration44.
This was just the beginning of the story, however. Additional mice were constructed in which mutant (polyQ expanded) ataxin-7 was expressed from the prion protein (Prp) promoter. In these mice, mutant ataxin-7 was expressed in many neurons and glia, but was conspicuously absent from Purkinje neurons44 (Fig. 3b). Nevertheless, the mice developed severe Purkinje degeneration and accompanying ataxia44. Since damage to Bergmann glia identified in the Prp-promoted mice was reminiscent of that also found in human SCA7 patients and in the mutant ataxin-7 knock-in mice3,43, La Spada and colleagues tested whether mutant ataxin-7 expressed only in cerebellar Bergmann glia and other astrocytes (using the GFAP-derived Gfa2-promoter) was sufficient for Purkinje toxicity3 (Fig. 3c). Ataxin-7 with expanded polyQ, but not wild type ataxin-7, caused ataxia and degeneration of Purkinje neurons that did not express the mutant3 and did so without generating additional neurological deficits. Moreover, Purkinje cell degeneration was remarkably similar to that induced from much broader expression of ataxin-7 produced with the Prp-promoter44, demonstrating that toxicity could originate from Bergmann glial cells alone3.
A central supporting role for Bergmann glia, which contain large amounts of the glutamate transporter GLAST/EAAT1, is enwrapping the glutamatergic synapses that granule cells and inferior olivary nuclei neurons make with Purkinje cell dendrites39. In the affected cerebellar regions of presymptomatic Gfa2- and Prp-driven mutant ataxin-7 mice, transporter-mediated uptake of glutamate at these Purkinje cell synapses was impaired3, as inferred from reduction in the GLAST/EAAT1 glutamate transporter and its mRNA. Glutamate uptake was also reduced in mutant Bergmann glial cultures, cerebellar slices and cerebellar synaptosomes3.
The evidence in mice makes it clear that damage to Purkinje neurons can be mediated by mutant ataxin-7 synthesis solely within those neurons or solely within their neighboring support cells, the Bergmann glia. In the real disease setting, the mutant is expressed in both neuronal and glial partners (and a lot of other cells too). Mechanistically, the simplest view is that the enormous size of the Purkinje cell dendritic tree and the correspondingly staggering numbers of glutamatergic synapses place Purkinje cells at risk to even the smallest disturbances of glutamate uptake and consequent excitotoxicity. Disease mechanism, therefore, leading to ataxia and Purkinje cell degeneration in SCA7 is non-cell autonomous with toxicity originating both from damage within Purkinje cells and within neighboring glial and neuronal cells as direct contributors to disease initiation and propagation.
Non-cell autonomous neurotoxicity in PD and MSA
Parkinson’s disease (PD) is characterized by focal loss of dopaminergic neurons of the substantia nigra, producing the well known tremors and progressive stiffness. While most disease is sporadic, several genetic causes are known, including dominant mutations in, or even increased synthesis of, α-synuclein, a highly abundant presynaptic protein (reviewed in ref. 45). Intraneuronal accumulations of α-synuclein are found in multiple diseases including sporadic PD and diffuse Lewy body disease (reviewed in ref. 45) while in multiple system atrophy (MSA), whose clinical presentation includes Parkinsonism, ataxia and autonomic failure, α-synuclein-containing inclusions are actually more prominent in oligodendrocytes than in neurons which is used as postmortem diagnostic confirmation of MSA (reviewed in ref. 46). Although increased intraneuronal synthesis of α-synuclein was initially demonstrated to damage those neurons47, the potential for non-cell autonomous damage from α-synuclein deposits came from demonstration that similar inclusions could form by elevated expression selectively within the axon-ensheathing oligodendrocytes, thereby inducing secondary neurodegeneration of the associated neurons6.
While direct tests similar to those used in ALS or SCA in mice to establish non-cell autonomous mechanism have not yet been reported in models of either PD or MSA, clear evidence for non-cell autonomous mechanism has come from chemically induced PD. MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) can induce a Parkinsonian syndrome in humans and rodents almost indistinguishable from PD (reviewed in ref. 45). Toxicity to substantia nigral neurons requires conversion of MPTP into MPP+, a conversion that itself requires the (mostly) astrocytic enzyme monomine oxidase B (MAO-B). Once released from astrocytes, MPP+ is taken up by specific transporters that are (mostly) only expressed by the vulnerable neurons (reviewed in ref. 45). A microglial role in amplifying initial damage was suggested by robust microgliosis (reviewed in ref. 48) that includes elevation of iNOS9. This role of microglia is of mechanistic importance for disease, since iNOS null mice are more resistant to MPTP toxicity9. Further, both in vivo and in cell co-cultures the inflammatory modulator minocycline reduces MPTP neurotoxicity and (at least in vitro) only in the presence of microglia49. Thus, at least in this example of chemically induced PD, neurotoxicity requires the convergence of actions within astrocytes, microglia and the target neurons.
Non-cell autonomous neurotoxicity in HD
Huntington’s disease (HD) is a dominant, fatal, progressive disease characterized by prominent, age dependent degeneration and death of striatal medium spiny neurons. Clinical presentation includes movement disorders (chorea and bradykinesia), psychiatric symptoms, and cognitive deficits, with a typical disease course of 15-20 years after a midlife onset. HD is caused by polyQ expansion in the widely expressed huntingtin gene, which includes both neuronal and non-neuronal cells. The preferential loss of striatal neurons is by no means confined to this neuronal pool: other cells include cortical pyramidal neurons and by end stage there is much wider cell loss in many other brain regions (reviewed in ref. 50).
HD disease mechanism is non-cell autonomous and based upon pathological cell-cell interactions, at least in rodents. This was demonstrated by the selective transgene activation within specific neuronal cell types51-52 (achieved by Cre recombinase-mediated excision of a transcriptional stop cassette embedded in a single copy transgene that, after excision, encodes Huntington exon1 containing an expanded polyQ). Progressive motor deficits and striatocortical neuropathology were observed only when mutant huntingtin expression was activated (with nervous system specific nestin-Cre)51 in multiple neuronal (and glial) cell types, including striatal medium spiny neurons, cortical interneurons and cortical pyramidal neurons but not when its synthesis was restricted (using Emx1-Cre)51 to cortical pyramidal neurons or (using Dlx5/6-Cre)52 to striatal medium spiny neurons alone.
A direct test for a contribution from mutant huntingtin damage within glia cannot be easily posed using this gene activation method; reports using the gene excision approach pioneered in mutant SOD1–mediated ALS are eagerly awaited. Nevertheless, substantial evidence makes microglial and astrocytic roles likely. Progressive reactive microgliosis is an established feature of disease in humans53 and multiple mouse models. The inflammatory modulator minocycline delays disease in mice generated by wide spread expression of mutant Huntington exon 1 (R6/2), accompanied by decreased accumulation of microglial derived iNOS activity54. In the same mouse model, mutant Huntington expression is in many cell types, including astrocytes. Mutant huntingtin accumulates in astroglial nuclei of diseased brains, accompanied by decreased levels of the GLT-1/EAAT2 glutamate transporter and transporter activity in HD mouse models2,55. Perhaps most importantly, in mouse neuron-astrocyte co-cultures, mutant astrocytes increase neuronal vulnerability to excitotoxicity2.
Conclusion
Since most neurodegenerative disease-linked mutant proteins are widely expressed, it is likely that their expression outside the vulnerable neurons, especially within glial cells, contributes to most disease mechanisms. Mutant products within glial cells drive toxicity to neighboring neurons either by release of toxic components or by mutant mediated reduction in one or more neuronal support functions. In mutant SOD1-linked ALS, although mutant SOD1 expression in motor neurons is required for disease initiation, neurotoxicity is produced by damage within and released by the neighboring mutant astrocytes, while damage within microglia drives rapid disease progression. Even more directly, in SCA7, Purkinje cell degeneration can be induced by mutant ataxin-7 expression solely in the neighborhood of these cells, with astrocytic Bergmann glia a primary source of in vivo neurotoxicity.
Although the exact targets of glial-mediated toxicity in these non-cell autonomous neurodegenerative disease mechanisms remain unproven, glutamate excitotoxicity mediated by reduced astrocytic-derived glutamate transport at synapses is the most promising candidate. Damage from mutant astrocytes and microglia could also impair dendritic signaling (in turn inducing synaptic pruning) or damage the required contributions of axon-ensheathing myelinating glial cells in axonal maintenance (with a consequent disturbance in axonal transport).
Disease mechanisms in many (if not all) of the major neurodegenerative diseases are all but certain to be non-cell autonomous, including a crucial glial involvement. Glial involvement central to disease pathogenesis has raised the likelihood that disease onset and progression may be driven by mutant damage acting within different cell types, points now established for inherited ALS. This realization is of high importance for the design of disease therapies, especially when considering development of stem-cell based approaches. Instead of the extremely challenging task of functionally replacing the lost neurons, supply of normal (or therapeutically modified) glial cells (at least some of which will probably track to sites of initial damage) may represent a more feasible alternative for diluting the toxic action of mutant glia around the remaining neurons, as well as bolstering the supporting role(s) provided by undamaged, normal glia.
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- CMT
Charcot-Marie-Tooth disease
- CNS
Central nervous system
- GLT1
Glutamate transporter (EAAT2)
- GLAST
Glutamate transporter (EAAT1)
- HD
Huntington’s disease
- iNOS
inducible nitric oxide synthase
- MS
Multiple sclerosis
- MSA
Multiple system atrophy
- PD
Parkinson’s disease
- PolyQ
polyglutamine repeats
- ROS
Reactive oxygen species
- SOD1
Superoxide dismutase 1
- SCA
Spinal cerebellar atrophy
- TNFα
Tumor necrosis factor alpha
- Glu
Glutamate
References
- 1.Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat. Med. 2004;10:1055–63. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 2.Shin JY, et al. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J. Cell. Biol. 2005;171:1001–12. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Custer SK, et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat. Neurosci. 2006;9:1302–11. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]
- 4.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat. Neurosci. 2007;10:608–14. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagai M, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 2007;10:615–22. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yazawa I, et al. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–59. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Beers DR, et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16021–6. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boillee S, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–92. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 9.Liberatore GT, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5:1403–9. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 10.Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2006;2:679–89. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- 11.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–62. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 13.Boillee S, Velde C. Vande, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Bruijn LI, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions: An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1997;18:327–38. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 15.Howland DS, et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc. Natl. Acad. Sci. U.S.A. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, et al. Coincident thresholds of mutant protein for paralytic disease and protein aggregation caused by restrictively expressed superoxide dismutase cDNA. Neurobiol. Dis. 2005;20:943–52. doi: 10.1016/j.nbd.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Bruijn LI, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–4. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 18.Reaume AG, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–7. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 19.Lino MM, Schneider C, Caroni P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J. Neurosci. 2002;22:4825–32. doi: 10.1523/JNEUROSCI.22-12-04825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J. Neurosci. 2001;21:3369–74. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clement AM, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–7. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 22.Ralph GS, et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat. Med. 2005;11:429–33. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- 23.Miller TM, et al. Virus-delivered small RNA silencing sustains strength in amyotrophic lateral sclerosis. Ann. Neurol. 2005;57:773–6. doi: 10.1002/ana.20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henkel JS, Beers DR, Siklos L, Appel SH. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol. Cell. Neurosci. 2006;31:427–37. doi: 10.1016/j.mcn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Henkel JS, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann. Neurol. 2004;55:221–35. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- 26.Zhu S, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–8. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 27.Bristol LA, Rothstein JD. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Ann. Neurol. 1996;39:676–9. doi: 10.1002/ana.410390519. [DOI] [PubMed] [Google Scholar]
- 28.Rothstein JD, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 29.Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J. Neurosci. 2000;20:660–5. doi: 10.1523/JNEUROSCI.20-02-00660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raoul C, et al. Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6007–12. doi: 10.1073/pnas.0508774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pehar M, et al. Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J. Neurochem. 2004;89:464–73. doi: 10.1111/j.1471-4159.2004.02357.x. [DOI] [PubMed] [Google Scholar]
- 32.Weydt P, Yuen EC, Ransom BR, Moller T. Increased cytotoxic potential of microglia from ALS-transgenic mice. Glia. 2004;48:179–82. doi: 10.1002/glia.20062. [DOI] [PubMed] [Google Scholar]
- 33.Gowing G, Dequen F, Soucy G, Julien JP. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase 1 mutations. J. Neurosci. 2006;26:11397–402. doi: 10.1523/JNEUROSCI.0602-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Son M, Fathallah-Shaykh HM, Elliott JL. Survival in a transgenic model of FALS is independent of iNOS expression. Ann. Neurol. 2001;50:273. doi: 10.1002/ana.1104. [DOI] [PubMed] [Google Scholar]
- 35.Wu DC, Re DB, Nagai M, Ischiropoulos H, Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12132–7. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urushitani M, et al. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat. Neurosci. 2006;9:108–18. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 37.Urushitani M, Ezzi SA, Julien JP. Therapeutic effects of immunization with mutant superoxide dismutase in mice models of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2495–500. doi: 10.1073/pnas.0606201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taroni F, DiDonato S. Pathways to motor incoordination: the inherited ataxias. Nat. Rev. Neurosci. 2004;5:641–55. doi: 10.1038/nrn1474. [DOI] [PubMed] [Google Scholar]
- 39.Bellamy TC. Interactions between Purkinje neurones and Bergmann glia. Cerebellum. 2006;5:116–26. doi: 10.1080/14734220600724569. [DOI] [PubMed] [Google Scholar]
- 40.Yvert G, et al. Expanded polyglutamines induce neurodegeneration and transneuronal alterations in cerebellum and retina of SCA7 transgenic mice. Hum. Mol. Genet. 2000;9:2491–506. doi: 10.1093/hmg/9.17.2491. [DOI] [PubMed] [Google Scholar]
- 41.Burright EN, et al. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–48. doi: 10.1016/0092-8674(95)90273-2. [DOI] [PubMed] [Google Scholar]
- 42.Yvert G, et al. SCA7 mouse models show selective stabilization of mutant ataxin-7 and similar cellular responses in different neuronal cell types. Hum. Mol. Genet. 2001;10:1679–92. doi: 10.1093/hmg/10.16.1679. [DOI] [PubMed] [Google Scholar]
- 43.Yoo SY, et al. SCA7 knockin mice model human SCA7 and reveal gradual accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. Neuron. 2003;37:383–401. doi: 10.1016/s0896-6273(02)01190-x. [DOI] [PubMed] [Google Scholar]
- 44.Garden GA, et al. Polyglutamine-expanded ataxin-7 promotes non-cell-autonomous purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J. Neurosci. 2002;22:4897–905. doi: 10.1523/JNEUROSCI.22-12-04897.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 46.Croisier E, Graeber MB. Glial degeneration and reactive gliosis in alpha-synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol. (Berl) 2006;112:517–30. doi: 10.1007/s00401-006-0119-z. [DOI] [PubMed] [Google Scholar]
- 47.Masliah E, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–9. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 48.Hirsch EC, et al. The role of glial reaction and inflammation in Parkinson’s disease. Ann. N.Y. Acad. Sci. 2003;991:214–28. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 49.Du Y, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14669–74. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 2000;23:217–47. doi: 10.1146/annurev.neuro.23.1.217. [DOI] [PubMed] [Google Scholar]
- 51.Gu X, et al. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 2005;46:433–44. doi: 10.1016/j.neuron.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 52.Gu X, et al. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington’s disease. Mol. Neurodegener. 2007;2:8. doi: 10.1186/1750-1326-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sapp E, et al. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J. Neuropathol. Exp. Neurol. 2001;60:161–72. doi: 10.1093/jnen/60.2.161. [DOI] [PubMed] [Google Scholar]
- 54.Chen M, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 55.Lievens JC, et al. Impaired glutamate uptake in the R6 Huntington’s disease transgenic mice. Neurobiol. Dis. 2001;8:807–21. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- 56.Miller TM, et al. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:19546–51. doi: 10.1073/pnas.0609411103. [DOI] [PMC free article] [PubMed] [Google Scholar]