Abstract
Strategies to protect against sexual transmission of HIV include the development of products formulated for topical application, which limit the toxicities associated with systemic oral pre-exposure prophylaxis. Following several clinical trial failures, attention is now focused on antiretroviral (ARV) agents. Highly potent ARV topical formulations provide a female-controlled, targeted, and feasible option for HIV prevention. A recently completed tenofovir gel trial was the first to demonstrate significant protection against HIV acquisition. Topical ARVs have the advantage of delivering high concentration of drug at the site of transmission of HIV, with low systemic absorption. Sustained-release formulations, such as intravaginal rings, will likely improve adherence and can be designed to provide controlled and continuous delivery of ARV combinations. Further studies to test alternative dosing strategies and pharmacokinetic/pharmacodynamic relationships in the genital tract will provide valuable information as the field strives to improve upon the promising tenofovir gel trial results.
Keywords: Topical microbicides, Antiretroviral drugs, Genital tract, Tenofovir, Dapivirine, Maraviroc, Human immunodeficiency virus, Herpes simplex virus, Intravaginal ring, Adherence, Biomarkers, Pharmacokinetics, Pharmacodynamics, Pre-exposure prophylaxis
Introduction
Despite increasing access to antiretroviral (ARV) therapy, the HIV epidemic continues to increase with a worldwide prevalence now estimated to be 33.3 million people. In 2009, there were an estimated 2.6 million new HIV infections and 1.8 million AIDS-related deaths [1]. In the developing world, the majority of new infections are transmitted heterosexually, and women are affected at a disproportionately higher rate, resulting in a concomitant epidemic of vertical transmission. In sub-Saharan Africa, for example, one third of all pregnant women are infected with HIV [2••]. Despite significant efforts over the past decade, the development of an HIV vaccine remains elusive.
Prior to this year, few randomized controlled trials targeted at HIV prevention have shown significant success. Male circumcision significantly decreased the risk of HIV acquisition among men [3], although the direct benefits to women are currently unclear and implementation is a global public health challenge. Consistent condom use is the most effective method of prevention, however, social barriers limit its impact on the HIV epidemic. Specifically, women may be unable to negotiate male condom use, may wish to become pregnant, or may perceive that condoms are unnecessary because they are in a mutually monogamous and safe relationship [4]. Moreover, female condoms have not been widely accepted. Thus, topical (also referred to as microbicides) or oral pre-exposure prophylaxis (PrEP) strategies are urgently needed to empower women in the battle against HIV and other sexually transmitted infections (STIs).
Oral PrEP with Truvada®, a fixed-dose combination of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC), was recently shown in the Pre-exposure Prophylaxis Initiative (iPrEx) study to provide protection against HIV infection among high-risk men who have sex with men, although adherence was suboptimal [5]. Similar oral PrEP regimens are being studied in women. However, the ability to achieve much higher local concentrations with topically formulated products, concerns regarding toxicity, and the risk of resistance associated with long-term systemic ARV exposure suggest that topical delivery may be a superior option for HIV prevention in women. This notion is supported by the observation that tenofovir (TFV) gel provided unanticipated protection against herpes simplex virus type 2 (HSV-2) in the Center for the AIDS Program of Research in South Africa (CAPRISA) 004 trial, whereas oral Truvada® failed to protect men from HSV-2 in the iPrEX study. This may reflect the higher drug levels achieved with topical compared to systemic TFV formulations [2••, 6].
First-generation vaginal microbicides consisted of surfactants (Nonoxynol-9 [N-9] and C31G), buffering agents designed to maintain the protective acidic environment of the female genital tract (BufferGel), and anionic polymers that blocked HIV attachment and entry, but were only active at relatively high concentrations (μg range) with even higher concentrations required in the presence of semen (Cellulose Sulfate [CS], PRO 2000, Carraguard) [7]. These six microbicide candidates were tested as gel formulations in eleven randomized controlled trials and failed to demonstrate effectiveness in preventing HIV acquisition in women. In fact, N-9 and CS were found to potentially increase the risk of HIV infection [8–10]. Attention is now focused on formulating more potent ARVs as gels, films, or intravaginal rings (IVRs). Many of the drugs in development, such as TFV, are also used for systemic treatment of HIV infection. While this approach simplifies the regulatory pathway for licensure, it also raises concerns about selecting for resistant viruses that are more difficult to treat if a woman becomes infected while using a topical agent. A recently completed TFV gel study is the first microbicide trial to demonstrate significant protection against HIV acquisition and represents a major breakthrough in advancing vaginal ARV microbicides as an HIV prevention strategy for women [2••].
The Next Generation of Microbicides: ARVs in Clinical Trials
TFV, an acyclic nucleotide analogue, is phosphorylated intracellularly to TFV diphosphate (TFV-DP), which is a competitive inhibitor of HIV-1 reverse transcriptase and terminates the propagating DNA chain. Preclinical studies demonstrated in vitro activity against HIV and safety in cell culture, tissue explant, and murine models [10–12]. TFV effectively blocked transmission of simian-human immunodeficiency virus (SHIV) in nonhuman primates (NHP) when applied as an intravaginal gel [13•]. The CAPRISA 004 study was a double blind, randomized placebo-controlled phase 2b study performed in rural and urban South Africa, where the prevalence of HIV infection in young women is as high as 51% [2••]. Sexually active HIV-negative women (n=889) were randomized to 1% TFV gel (40 mg) or universal hydroxyethylcellulose (HEC) placebo gel and were instructed to apply one dose of gel intra-vaginally within 12 h before sex and a second dose as soon as possible within 12 h after sex. Most participants reported using product within 2 h before and after sex. TFV gel reduced HIV acquisition by an estimated 39% overall, and by 54% in women who reported >80% gel adherence. TFV gel was found to be highly acceptable and safe. No TFV-related resistance was detected in 35 women who seroconverted to HIV during the study.
An unanticipated finding was a 51% reduction in HSV-2 infection rate in the group of women randomized to TFV gel use, which was independent of the effect on HIV. This most likely reflects the markedly higher levels of intracellular TFV-DP achieved in the genital tract following vaginal as compared to oral TFV dosing [6], which may be sufficient to inhibit viral DNA polymerase and prevent HSV-2 replication. TFV inhibits HSV-2 infection of human explant tissue at concentrations >100 μg/mL (Mesquita and Herold, unpublished). HSV-2 infection is associated with an increased risk of HIV-1 acquisition of 3.1-fold in women [14]. Thus, a microbicide that protects against both HIV and HSV-2 may impact HIV acquisition to a greater extent than a microbicide that affects HIV alone. If these results are confirmed in larger trials, TFV gel may be the first licensed product for HIV prevention in women. A phase 2b study is ongoing and two confirmatory phase 3 trials are planned (Table 1).
Table 1.
ARVs in ongoing or planned clinical trials
| Category | Compound | Trial phase | Trial name | Clinicaltrials.gov identifiera | n | Study description |
|---|---|---|---|---|---|---|
| Nucleoside reverse transcriptase inhibitors | Tenofovir | 1 | MTN 002 | NCT00540605 | 16 | Maternal single-dose PK and placental transfer of TFV gel among healthy term gravidas |
| 1 | MTN 006 | NCT00984971 | 18 | Safety, acceptability, and PK of TFV gel applied rectally in men and women, as compared to oral TFV | ||
| 1 | MTN 007 | NCT01232803 | 60 | Safety and acceptability of TFV gel applied rectally in men and women | ||
| 1 | MTN 008 | NCT01136759 | 105 | Expanded safety of TFV gel in pregnancy and lactation | ||
| 2 | MTN 001 | NCT00592124 | 144 | Adherence and PK of oral and vaginal preparations of TFV | ||
| 2b | MTN 003 (VOICE) | NCT00705679 | 5000 | Safety and effectiveness of TFV gel, TDF tablet, and TDF/emtricitabine tablet | ||
| 3 | FACTS 001 | 3150 | Confirmatory study of TFV gel effectiveness against HIV and HSV-2 in expanded populations, including adolescents | |||
| 3 | MDP 302 | 3750 | Confirmatory study of TFV gel effectiveness in expanded populations, including adolescents | |||
| Nonnucleoside reverse transcriptase inhibitors | Dapivirine | 1/2 | IPM 020 | NCT00799058 | 128 | Dapivirine gel safety and PK |
| 3 | IPM 009a | 3000 | Dapivirine IVR safety and efficacy | |||
| IPM 009b | 3000 | |||||
| MIV-150 | 1 | TBD | Safety of MIV-150/zinc acetate gel | |||
| Entry inhibitors | 2G12, 2F5, 4E10 | 1 | MABGEL 1 | 30 | Safety and PK of monoclonal antibodies to HIV viral envelope in gel vehicle | |
| Combinations | Dapivirine/maraviroc | 1 | IPM 025 | NCT01242579 | 40 | Safety of maraviroc, dapivirine, and maraviroc-dapivirine combination gel |
| 1 | IPM 026/MTN 013 | TBD | Safety and PK of maraviroc, dapivirine, and maraviroc-dapivirine combination silicone IVRs |
Clinicaltrials.gov, a registry of United States and international trials
CAPRISA Center for the AIDS Programme of Research in South Africa, FACTS Follow-on Africa Consortium for Tenofovir Studies, HSV-2 herpes simplex virus, type 2, IPM International Partnership For Microbicides, IVR intravaginal ring, MABGEL monoclonal antibodies gel, MDP Microbicides Development Programme, MTN Microbicide Trials Network, PK pharmacokinetics, TBD to be determined, TDF tenofovir disoproxil fumarate, TFV tenofovir, VOICE vaginal and oral interventions to control the epidemic
Microbicide Trial Network (MTN) study 003, which is actively enrolling and anticipated to be completed in 2013, is a double blind, randomized study examining the safety and effectiveness of either daily topical or oral PrEP. Women are first randomized to receive either topical or oral PrEP. Within the topical group, women are then assigned to either 1% TFV or placebo gel. Women assigned to the oral group receive TDF (prodrug of TFV), Truvada®, or placebo. The study aims to enroll 5000 women, with 1000 in each of the five groups. The primary endpoint is prevention of HIV infection, as measured by seroconversion. The trial is not powered for a head-to-head comparison of topical versus oral PrEP, but should provide data on both strategies. In addition to HIV endpoints, MTN 003 will also examine adherence, HSV-2 incidence, and drug resistance.
The two planned phase 3 trials are the Follow-on African Consortium for Tenofovir Studies (FACTS 001) and Microbicide Development Programme (MDP) 302 study. FACTS 001 will examine effectiveness of 1% TFV gel in preventing HIV and HSV-2 infections in women aged 16 to 30 years at six trial sites across South Africa. The MDP is planning a study in five African countries to assess the effectiveness of a single dose of TFV or placebo gel applied prior to sex compared to the CAPRISA 004 regimen. Women assigned to the single-dose regimen will be instructed to apply the gel before sex, but if gel is not applied prior to sex, it should be applied immediately after sex. If a single dose provides comparable protection to the CAPRISA 004 regimen, it would be less expensive and more convenient for women to use.
The next most advanced candidate topical product is dapivirine. Dapivirine is a highly potent nonnucleoside reverse transcriptase inhibitor (NNRTI), which binds to a hydrophobic pocket on the HIV reverse transcriptase enzyme. Dapivirine has activity against multiple clades of HIV, including NNRTI-resistant strains [15]. Phase 1/2 clinical trials of dapivirine gel at various concentrations (0.001%–0.05%) in HIV-negative, sexually active women have shown high safety, tolerability, and acceptability [16, 17]. Following vaginal gel application, dapivirine was detectable in blood, but at much lower levels than when administered orally, and accumulation did not occur over a 42-day period when administered twice daily [16]. Dapivirine concentrations in vaginal fluids in the 0.05% gel groups were 4–5 logs greater than the 50% inhibitory concentration (IC50) for dapivirine against HIV-1 in vitro. Furthermore, dapivirine levels remained elevated in vaginal fluids for over 24 h after application of the last dose, a finding that supports a daily dosing regimen [17]. Dapivirine gel is currently in phase 1/2 trials.
IVR formulations of dapivirine are being advanced in parallel as women may have a preference for rings, as demonstrated by the high user acceptability of contraceptive IVRs. Dapivirine IVRs were found to be highly acceptable and safe, with the most common adverse event being mild vaginal bleeding [18, 19]. A phase I trial recently completed in 24 women compared 28 consecutive day use of dapivirine matrix and reservoir silicone IVRs with a placebo IVR [19]. Adverse events occurred with similar frequency in the three study groups. Dapivirine from both ring types was distributed throughout the lower genital tract drug at high concentrations with minimal systemic absorption. Maximum concentration (Cmax) and area under the concentration-time curve (AUC) values were significantly higher with the matrix than reservoir IVR. A compartmental transport model using MRI imaging has demonstrated that IVRs can adequately provide inhibitory concentrations of dapivirine throughout the vaginal canal within 24 h [20]. Two phase 3 dapivirine matrix ring trials are in development and will include a total of 6000 African women (Table 1).
ARVs in Preclinical and Early Clinical Development
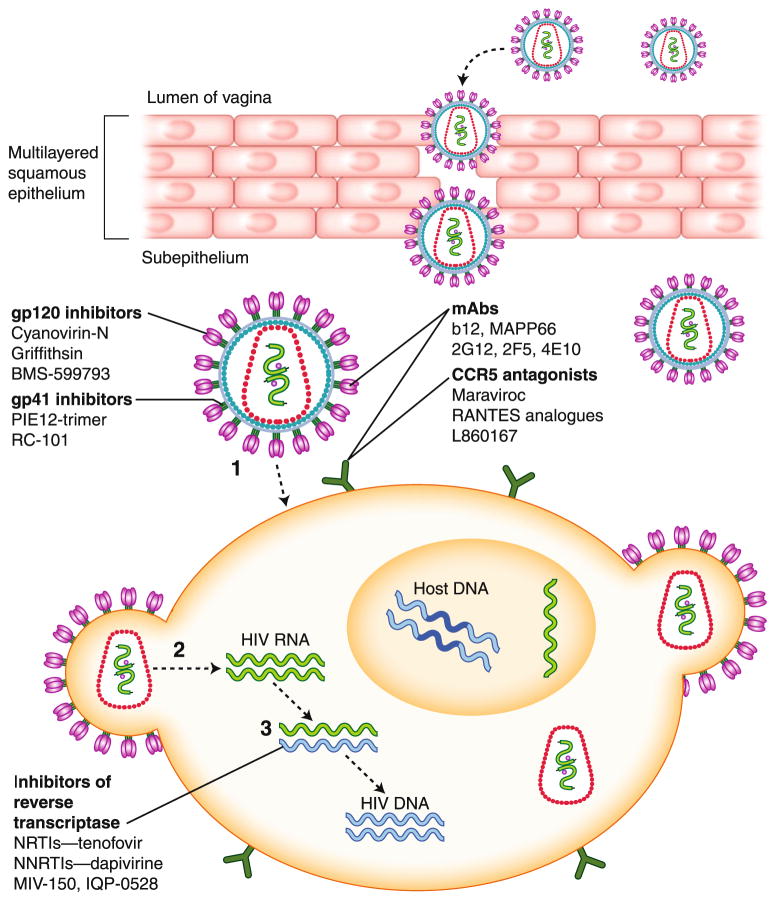
Although substantial preclinical and early phase clinical studies were completed with UC-781 [21, 22], its development has been discontinued because of difficulties with the optimization of formulations. However, two other NNRTIs are moving forward (Fig. 1 and Table 2).
Fig. 1.
Antiretroviral (ARV) microbicides and mechanism of action within the female genital tract. ARVs inhibit HIV infection of target cells at various steps including viral attachment (1), fusion (2), and reverse transcription (3). CCR5, chemokine (C–C) motif receptor 5; mAbs, monoclonal antibodies; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleotide reverse transcriptase inhibitor; RANTES, regulated upon activation, normal T-cell expressed and secreted
Table 2.
ARV microbicides in preclinical development
| Category | Mechanism of action | Compound | Stage of development |
|---|---|---|---|
| Nonnucleoside reverse transcriptase inhibitors | Bind to a hydrophobic pocket within reverse transcriptase to block DNA polymerization | UC-781 | Development has been discontinued |
| IQP-0528 | Preclinical | ||
| Entry inhibitors | |||
| CCR5 antagonists | Bind to CCR5 to inhibit binding of coreceptor to gp120, preventing virus entry into cells | RANTES analogues | Preclinical |
| L860167 | Preclinical | ||
| gp120 inhibitors | Bind to gp120 to prevent binding of virus to cells | Cyanovirin-N | Preclinical |
| Griffithsin | Preclinical | ||
| BMS-599793 | Preclinical | ||
| mAbs | Bind to a range of different targets on viral gp120 or gp41, or to cellular receptors (eg, CD4, CCR5, CXCR4) to prevent entry of HIV into cells | b12 | Preclinical |
| MAPP66 | Preclinical | ||
| D-peptide | Targets gp41 pocket | PIE12-Trimer | Preclinical [49] |
| Retrocyclin | Binds to heptad repeat 2 (HR2) region of gp41 | RC-101 | Preclinical [50] |
CCR5 chemokine (C–C) motif receptor 5, CXCR4 chemokine (C–X–C motif) receptor 4, mAbs monoclonal antibodies, RANTES regulated upon activation, normal T-cell expressed and secreted
IQP-0528 is a small molecular weight pyrimidinedione compound that is unique because of its high potency and low toxicity (selectivity indices greater than 1 million), dual mechanism of action (NNRTI and entry inhibitor), and low cost of synthesis [23]. IQP-0528 inhibits clinical isolates representative of multiple clades, inhibits cell-free and cell-associated virus transmission, and displays no toxicity to lactobacilli, human cells, or tissue. The drug has been formulated both as a gel and IVR and is currently being advanced into NHP studies.
MIV-150 is an NNRTI being developed by the Population Council as part of a combination topical prevention strategy. MIV-150 exhibits potent activity against clinical isolates that are resistant to NNRTIs, NRTIs, and protease inhibitors. It has a direct virucidal effect and inactivates free HIV virions in vitro at submicromolar concentrations [24]. MIV-150 demonstrated potent activity against SHIV infection in NHP [25]. A phase 1 gel trial is planned.
Drugs that target HIV entry are also in development for topical prevention. Maraviroc is a potent inhibitor of CCR5 viral entry and thus is optimal for disruption of sexual transmission, where R5 viruses predominate. Maraviroc achieved one of the highest female genital tract exposures of all ARVs evaluated to date. Cervicovaginal fluid exposures exceeded those of plasma by a median of 1.9-fold and 2.7-fold after single and repeated oral dosing, respectively [26]. A recent study found that topical vaginal application of maraviroc protected macaques from R5-tropic SHIV-162P3 infection [27]. Maraviroc gel and ring formulations are being evaluated alone and in combination with dapivirine (Table 1).
Cyanovirin-N (CV-N) acts by binding to high-mannose oligosaccharides predominantly in the C2-C4 region of the HIV-1 gp120 protein [28] to inhibit the conformational change required for virus-target cell attachment and subsequent fusion. CV-N demonstrated potent activity in the low nanomolar range against cell-free and cell-associated virus, and inhibited infection of ectocervical explants and virus dissemination by migratory cells [29]. The efficacy of a gel-based formulation was demonstrated in a SHIV89.6P vaginal challenge model [30]. In addition to gel formulations, efforts have focused on the development of a live vaginal CV-N–based microbicide by introducing the gene for CV-N into lactobacilli.
A related carbohydrate-binding protein with potentially even greater potency and safety is griffithsin (GRFT), which was originally isolated from the red algae Griffithsia. GRFT blocks HIV binding and entry with an average EC50 of 40 pM, and is active in preventing infection of human cervical explants and against primary sexually transmitted HIV-1 isolates from clades A, B, and C [31]. Importantly, GRFT did not induce an inflammatory response in cervical explants and had no mitogenic activity on cultured human lymphocytes [31]. In contrast, CV-N displayed modest mitogenic activity and triggered an increase in release of select cytokines and chemokines after 3 days in culture [29]. Thus, GRFT, which has been manufactured using transgenic plant technology [31], may provide a safe, highly potent and novel strategy to prevent HIV infection. Formulations are currently being developed and evaluated.
PSC-RANTES, an N-terminally modified analogue of a natural chemokine ligand of CCR5 [32], is a highly potent entry inhibitor for CCR5-using HIV. Its inhibitory mechanism involves the durable intracellular sequestration of CCR5 [32]. Studies in the rhesus vaginal challenge model demonstrated that blocking CCR5 by PSC-RANTES provided high-level protection against vaginal challenge with the SHIV162P4 isolate [33]. Concern that manufacturing costs might limit the utility of PSC-RANTES led to the production of two PSC-RANTES derivatives (5P12-RANTES and 6P4-RANTES) that appear stable, lack the ability to induce cell proliferation [34], and are active in the macaque challenge model [35].
Advances in Microbicide Formulation
Adherence and acceptability issues associated with coitally dependent gels, characteristic of the first-generation microbicides, fostered the development of coitally independent sustained-release formulations, such as films and rings [36]. Many women have a strong preference for rings compared to gel formulations for vaginal administration, citing discretion and ease of use as major advantages [37]. The high user acceptability of current vaginal ring products coupled with the potential ability to deliver ARVs for up to 90 days, suggest that IVRs may contribute substantially in the prevention of HIV.
IVRs are torus-shaped polymeric devices either loaded with drug throughout the polymer matrix (monolithic or matrix type) or in the core which is surrounded by a non-medicated sheath of the polymer (core or reservoir type). The polymer backbone plays a critical role in designing controlled-release IVRs, as the interactions of the drug with the backbone will affect the release kinetics and stability of the drug [38•]. IVRs of differing copolymer composition and diameter that are in development for microbicide delivery include silicone, ethylene vinyl acetate (EVA), and polyurethane.
There are three vaginal ring devices currently approved for commercial use by the FDA: Estring and Femring, which are silicone elastomer rings for hormone replacement, and NuvaRing, an EVA ring for contraception. Thermoplastic polyurethanes, while not yet in clinical use as vaginal products, are one of the most versatile materials used in biomedical applications. The processing temperature, chemical, and mechanical properties can be modified to control the kinetics of drug release of both hydrophilic and hydrophobic drugs [39]. IVR devices are currently being studied for controlled and continuous delivery of TFV, dapivirine, IQP-0528, MIV-150, and maraviroc.
Combination Microbicides
Vaginal delivery of ARV combinations will likely provide greater activity, protect against drug-resistant isolates that may be in circulation, and limit the risk of selecting for resistance if seroconversion occurs while using product, and thus may improve upon the protection observed with TFV gel. The evidence that single-dose nevirapine is associated with the development of drug resistance in the context of prevention of mother-to-child transmission of HIV supports the need for combination strategies. TFV in combination with emtricitabine has been shown to prevent transmission in macaques following vaginal challenge with SHIV [13•]. Several groups are conducting studies to deliver maraviroc as a component of a combination IVR product (eg, TFV and maraviroc).
In addition to ARV ring combinations, other drugs are being considered for combination with HIV-specific agents to provide contraception and protection against other STIs. The addition of hormones to an ARV ring could provide parallel protection against HIV and pregnancy. A silicone ring that delivers acyclovir for HSV prevention has advanced to a clinical trial (Keller, Herold, and Smith, ongoing) and a phase 2 trial is in development to test the effect of a gel containing carrageenans to inhibit incident human papillomavirus infections (Einstein, Keller, Herold, et al.).
Need for Improved In Vivo Assays to Predict Safety and Efficacy
Early-phase microbicide studies have not consistently predicted the outcome of efficacy trials. Not only did several products fail to protect against HIV, but N-9, C31G, and CS were associated with higher rates of HIV acquisition [8, 9, 40], highlighting the need for more predictive safety biomarkers. Studies by our group and others have provided insight into the biological mechanisms that likely contributed to the increased risk of HIV and have led to incorporation of new assays to evaluate microbicides. These mechanisms include disruption of the epithelial barrier, induction of an inflammatory response, and loss of protective antimicrobial peptides [10].
In expanded phase 1 safety studies, we found that 14 daily vaginal applications of 0.5% PRO 2000 or 1% TFV gel did not trigger an inflammatory response or induce sustained loss in protective immune mediators (eg, defensins, secretory leukocyte protease inhibitor, etc.), which is consistent with the demonstrated safety of both drugs in vitro and in clinical trials [2••, 10, 12, 41–43]. We also observed no reduction in the endogenous antimicrobial activity of genital tract secretions against bacteria or viruses (eg, E. coli and HSV), which may provide a biomarker of functional soluble mucosal immunity. In addition to incorporating these candidate safety biomarkers into phase 1 studies, it has been suggested that the size and duration of current phase 1/2 trials may be inadequate to identify conventional clinical safety signals [44].
Pharmacokinetics and Pharmacodynamics
The success or failure of a microbicide is likely to be determined by the complex interaction between pharmacokinetics (PK), viral kinetics, host susceptibility, and possible drug-induced toxicity [45•]. Drug partitions within multiple compartments, including the vaginal lumen, vaginal and cervical tissue, target cells, and blood, and the relative concentrations in each compartment, will depend on multiple factors including hydrophobicity and metabolism. For example, TFV is rapidly converted to TFV-DP, which is retained intracellularly. PK may also be modified by genital tract secretions, mucus, pH, semen, and the act of coitus [7, 46•]. For luminally active drugs, such as the first-generation microbicides, measuring drug levels in the vaginal lumen may be informative, whereas for intracellularly active drugs, such as TFV, measuring tissue or intracellular levels of the active metabolite may be most useful. However, obtaining tissue biopsies in clinical trials is not always feasible and the assays are more complex. For example, reproducibly infecting vaginal or cervical tissue has proven more difficult than colorectal tissue, in part reflecting the more variable and limited numbers of immune cells in biopsies from the female genital tract.
Therefore, we have focused on developing surrogate markers of tissue PK and pharmacodynamics (PD). The approach we are testing is whether drug levels and anti-HIV activity in genital tract secretions collected by cervicovaginal lavage (CVL) or vaginal swab could prove to be predictive of tissue levels (PK) and antiviral activity (PD). We have established assays to measure the antiviral activity in CVL by spiking the samples with HIV (or HSV-2) diluted in semen (or control buffer) and then infecting target cells. The antiviral activity reflects the biological activity of extracellular drug as well as endogenous antimicrobial activity of female genital tract secretions [41, 46•, 47]. This assay provides a direct assessment of PD for drugs that act luminally, such as PRO 2000, and an indirect PD measurement for drugs that act intracellularly (TFV) or that target the cell surface (maraviroc).
We have applied this assay to clinical studies with PRO 2000 [46•, 47] and TFV [41]. Results obtained from the PRO 2000 studies would have predicted the negative outcome of the phase 3 trial [43]. Specifically, while CVL obtained from women who applied PRO 2000 gel provided significant protection against HIV and HSV when spiked with virus in medium or buffer alone [47], the protective effect was lost when virus was introduced in seminal plasma [7]. Parallel results were obtained in a subsequent postcoital study in which no significant protective effect against HIV or HSV-2 was observed in postcoital CVL obtained from women who applied 0.5% PRO 2000 gel [46•]. In addition, less PRO 2000 was recovered in the postcoital samples compared to the concentration recovered following gel application in the absence of sex. We could not determine if the drug was redistributed within the genital tract, bound to semen, or lost due to leakage. However, these results underscore the need for PK/PD studies with postcoital sampling, particularly in the setting of intermittent gel application. A PK/PD study following vaginal intercourse in the setting of coitally dependent or daily TFV gel application is being considered, which should provide important information regarding optimal dosing schedules.
In a recently completed 14-day study, CVL obtained from participants who applied TFV gel once daily demonstrated consistently higher anti-HIV levels than subjects who applied placebo gel, adjusting for time and baseline levels. Moreover, anti-HIV activity correlated significantly with the concentration of TFV; 30 of 34 (88%) CVL specimens with a TFV level >1000 ng/mL were associated with >80% inhibition. TFV persisted in CVL several days after last gel application, which could reflect the prolonged half-life of drug in the vaginal lumen and/or transport of dephosphorylated drug from intracellular stores (where the phosphorylated moiety has a prolonged half-life) back into the extracellular space and/or vaginal lumen [41]. These findings, as well as the CAPRISA 004 trial results, demonstrate the importance of incorporating robust pharmacology sampling into future prevention trials. Examination of CVL samples from the CAPRISA 004 trial also demonstrated a compelling trend between higher TFV CVL concentrations and lower HIV infection rates [6].
The extent to which PK/PD measurements in CVL correlate with protection will depend, in part, on the relationship between extracellular and intracellular drug compartments. This is currently being evaluated in samples collected in ongoing TFV (MTN 001) and dapivirine (IPM 020) trials in which drug levels in both compartments (CVL and tissue) are being measured. If drug concentrations in CVL correlate with activity, and if there is a relationship between luminal and tissue levels, then CVL may provide a more realistic and reproducible approach than biopsies for measuring PK/PD in future clinical trials. These assays may also provide an objective measure of adherence to product.
Conclusions
The success of CAPRISA 004 creates new challenges, including preparing for implementation, navigating an uncertain regulatory environment, and the need to study different populations. CAPRISA 008 is a 3-year study in development that will assess the feasibility and effectiveness of providing TFV gel in family planning clinics. CAPRISA 009 is a study in development that will provide care, treatment, and resistance monitoring for CAPRISA 004 trial participants who acquired HIV infection [48]. Outcomes for those who receive ARV treatment that includes TFV will be compared with those whose regimens exclude TFV. Other ongoing and planned studies include safety in pregnancy and during lactation; comparison of adherence and PK of oral and vaginal TFV formulations; studies in younger women; and studies with rectally applied gel in both men and women (Table 1).
The results from CAPRISA 004 and the iPrEX studies, if confirmed, highlight the complexities of future trial designs as we strive to improve and build on these early successes. The feasibility of evaluating new prevention modalities in large-scale efficacy trials where the control arm is a partially effective strategy will become formidable. Thus, establishing surrogate biomarkers of PK/PD, safety, and efficacy will be critical to the future of prevention research.
Acknowledgments
This work was supported by grants from the National Institutes of Health (U01 AI069551, UL1 RR025750, U19 076980, and T32 AI070117). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Natasha A. Verma, Email: nverma@montefiore.org, Department of Pediatrics, Division of Infectious Diseases, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Forchheimer Building, Room 702, Bronx, NY 10461, USA
Anna C. Lee, Email: anna.lee@einstein.yu.edu, Department of Medicine, Division of Infectious Diseases, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Mazer Building, Room 512, Bronx, NY 10461, USA
Betsy C. Herold, Email: betsy.herold@einstein.yu.edu, Department of Pediatrics, Division of Infectious Diseases, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Forchheimer Building, Room 702, Bronx, NY 10461, USA. Department of Microbiology and Immunology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Forchheimer Building, Room 702, Bronx, NY 10461, USA
Marla J. Keller, Email: marla.keller@einstein.yu.edu, Department of Medicine, Division of Infectious Diseases, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Mazer Building, Room 512, Bronx, NY 10461, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1. [Accessed December 2010];Joint United Nations Programme on HIV/AIDS: UNAIDS Report on the Global AIDS Epidemic. 2010 In Edition Available at http://www.unaids.org/documents/20101123_GlobalReport_em.pdf.
- 2••.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. This article provides the findings of the CAPRISA 004 study, which established proof of concept that a vaginal microbicide containing an antiretroviral can protect women from HIV. In this study, 1% tenofovir gel used before and after sex reduced HIV acquisition by 39%. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.Crosby RA, DiClemente RJ, Wingood GM, et al. Sexual agency versus relational factors: a study of condom use antecedents among high-risk young African American women. Sex Health. 2008;5:41–7. doi: 10.1071/sh07046. [DOI] [PubMed] [Google Scholar]
- 5.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemopro-phylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashuba A, Abdool Karim SS, Kraft E, et al. Do systemic and genital tract tenofovir concentrations predict HIV seroconversion in the CAPRISA 004 tenofovir gel trial? [abstract TUSS0503]. Presented at the XVIII International AIDS Conference; Vienna, Austria. July 18–23, 2010. [Google Scholar]
- 7.Patel S, Hazrati E, Cheshenko N, et al. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J Infect Dis. 2007;196:1394–402. doi: 10.1086/522606. [DOI] [PubMed] [Google Scholar]
- 8.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 9.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 10.Mesquita PM, Cheshenko N, Wilson SS, et al. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J Infect Dis. 2009;200:599–608. doi: 10.1086/600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson SS, Cheshenko N, Fakioglu E, et al. Susceptibility to genital herpes as a biomarker predictive of increased HIV risk: expansion of a murine model of microbicide safety. Antivir Ther. 2009;14:1113–24. doi: 10.3851/IMP1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS ONE. 2010;5:e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83:10358–65. doi: 10.1128/JVI.01073-09. This study used a repeated low-dose challenge model of simian-human immunodeficiency infection and showed that preexposure vaginal application of gel with 1% tenofovir alone or in combination with 5% emtricitabine fully protected macaques. This study provides a rationale for including antiretroviral combinations in HIV prevention trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher P, Harman S, Azijn H, et al. Inhibition of human immunodeficiency virus type 1 infection by the candidate microbicide dapivirine, a nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2009;53:487–95. doi: 10.1128/AAC.01156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nel AM, Coplan P, van de Wijgert JH, et al. Safety, tolerability, and systemic absorption of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS. 2009;23:1531–8. doi: 10.1097/QAD.0b013e32832c413d. [DOI] [PubMed] [Google Scholar]
- 17.Nel AM, Smythe SC, Habibi S, et al. Pharmacokinetics of 2 dapivirine vaginal microbicide gels and their safety vs. hydroxyethyl cellulose-based universal placebo gel. J Acquir Immune Defic Syndr. 2010;55:161–9. doi: 10.1097/QAI.0b013e3181e3293a. [DOI] [PubMed] [Google Scholar]
- 18.Romano J, Variano B, Coplan P, et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses. 2009;25:483–8. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 19.Nel A, Smythe S, Young K, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:416–23. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 20.Geonnotti AR, Katz DF. Compartmental transport model of microbicide delivery by an intravaginal ring. J Pharm Sci. 2010;99:3514–21. doi: 10.1002/jps.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borkow G, Barnard J, Nguyen TM, et al. Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J Virol. 1997;71:3023–30. doi: 10.1128/jvi.71.4.3023-3030.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz JL, Kovalevsky G, Lai JJ, et al. A randomized six-day safety study of an antiretroviral microbicide candidate UC781, a non-nucleoside reverse transcriptase inhibitor. Sex Transm Dis. 2008;35:414–9. doi: 10.1097/OLQ.0b013e318162c4d8. [DOI] [PubMed] [Google Scholar]
- 23.Buckheit RW, Jr, Hartman TL, Watson KM, et al. Comparative evaluation of the inhibitory activities of a series of pyrimidinedione congeners that inhibit human immunodeficiency virus types 1 and 2. Antimicrob Agents Chemother. 2008;52:225–36. doi: 10.1128/AAC.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Romero JA, Thorn M, Turville SG, et al. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex Transm Dis. 2007;34:9–14. doi: 10.1097/01.olq.0000223287.46097.4b. [DOI] [PubMed] [Google Scholar]
- 25.Turville SG, Aravantinou M, Miller T, et al. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS ONE. 2008;3:e3162. doi: 10.1371/journal.pone.0003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumond JB, Patterson KB, Pecha AL, et al. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:546–53. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veazey RS, Ketas TJ, Dufour J, et al. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–44. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Q, Mahmood N, Shattock RJ. High-mannose-specific deglyco-sylation of HIV-1 gp120 induced by resistance to cyanovirin-N and the impact on antibody neutralization. Virology. 2007;368:145–54. doi: 10.1016/j.virol.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buffa V, Stieh D, Mamhood N, et al. Cyanovirin-N potently inhibits human immunodeficiency virus type 1 infection in cellular and cervical explant models. J Gen Virol. 2009;90:234–43. doi: 10.1099/vir.0.004358-0. [DOI] [PubMed] [Google Scholar]
- 30.Tsai CC, Emau P, Jiang Y, et al. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20:11–8. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- 31.O’Keefe BR, Vojdani F, Buffa V, et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci USA. 2009;106:6099–104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartley O, Gaertner H, Wilken J, et al. Medicinal chemistry applied to a synthetic protein: development of highly potent HIV entry inhibitors. Proc Natl Acad Sci USA. 2004;101:16460–5. doi: 10.1073/pnas.0404802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lederman MM, Veazey RS, Offord R, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–7. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 34.Cerini F, Landay A, Gichinga C, et al. Chemokine analogues show suitable stability for development as microbicides. J Acquir Immune Defic Syndr. 2008;49:472–6. doi: 10.1097/QAI.0b013e31818c953f. [DOI] [PubMed] [Google Scholar]
- 35.Veazey RS, Ling B, Green LC, et al. Topically applied recombinant chemokine analogues fully protect macaques from vaginal simian-human immunodeficiency virus challenge. J Infect Dis. 2009;199:1525–7. doi: 10.1086/598685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009;11:78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardy E, Hebling EM, Sousa MH, et al. Delivery of microbicides to the vagina: difficulties reported with the use of three devices, adherence to use and preferences. Contraception. 2007;76:126–31. doi: 10.1016/j.contraception.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 38•.Gupta KM, Pearce SM, Poursaid AE, et al. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J Pharm Sci. 2008;97:4228–39. doi: 10.1002/jps.21331. This article describes the novel use of polyether urethane in the design of intravaginal rings to provide sustained release of antiretroviral microbicides. [DOI] [PubMed] [Google Scholar]
- 39.Johnson TJ, Gupta KM, Fabian J, et al. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur J Pharm Sci. 2010;39:203–12. doi: 10.1016/j.ejps.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Feldblum PJ, Adeiga A, Bakare R, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS ONE. 2008;3:e1474. doi: 10.1371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller MJ, Madan RP, Torres NM, et al. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One. 2011;6:e16475. doi: 10.1371/journal.pone.0016475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller MJ, Guzman E, Hazrati E, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–76. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 43.McCormack S, Ramjee G, Kamali A, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376:1329–37. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poynten IM, Millwood IY, Falster MO, et al. The safety of candidate vaginal microbicides since nonoxynol-9: a systematic review of published studies. AIDS. 2009;23:1245–54. doi: 10.1097/QAD.0b013e32832b4271. [DOI] [PubMed] [Google Scholar]
- 45•.Hendrix CW, Cao YJ, Fuchs EJ. Topical microbicides to prevent HIV: clinical drug development challenges. Annu Rev Pharmacol Toxicol. 2009;49:349–75. doi: 10.1146/annurev.pharmtox.48.113006.094906. Fundamental information regarding virus and drug distribution over time in the genital tract is needed to design a safe and effective microbicide. This article thoroughly reviews a conceptual framework for obtaining the knowledge that is likely to inform the construction of a mechanistic pharmacokinetic/pharmacodynamic model to better inform rational microbicide development. [DOI] [PubMed] [Google Scholar]
- 46•.Keller MJ, Mesquita PM, Torres NM, et al. Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials. PLoS ONE. 2010;5:e8781. doi: 10.1371/journal.pone.0008781. This is the first study to evaluate the pharmacokinetics and pharmacodynamics of a candidate microbicide following sex and demonstrated that both differ significantly from results obtained in the absence of sex. This article provides an important rationale for the inclusion of postcoital sampling in future microbicide trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keller MJ, Zerhouni-Layachi B, Cheshenko N, et al. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J Infect Dis. 2006;193:27–35. doi: 10.1086/498533. [DOI] [PubMed] [Google Scholar]
- 48. [Accessed December 2010.];World Health Organization and Joint United Nations Programme on HIV/AIDS: Next Steps with 1% Tenofovir Gel Meeting Report. Available at http://www.who.int/reproductivehealth/topics/rtis/WHO_UNAIDS_Next_steps_tenofovir_gel_Ex_report.pdf.
- 49.Welch BD, Francis JN, Redman JS, et al. Design of a potent D-peptide HIV-1 entry inhibitor with a strong barrier to resistance. J Virol. 2010;84:11235–44. doi: 10.1128/JVI.01339-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole AM, Patton DL, Rohan LC, et al. The formulated microbicide RC-101 was safe and antivirally active following intravaginal application in pigtailed macaques. PLoS ONE. 2010;5:e15111. doi: 10.1371/journal.pone.0015111. [DOI] [PMC free article] [PubMed] [Google Scholar]