Abstract
FADD/Mort1 is required for signaling induced by death-receptors such as Fas. In earlier studies, FADD-deficient mice died in utero and a FADD deficiency in embryonic stem cells inhibited T cell production in viable FADD-/-→RAG-1-/- chimeras. To analyze the temporal requirement of FADD in the development and function in the T lineage, it is necessary to establish viable mutant mice producing detectable FADD-deficient T cells. We generated mice that express a functional FADD:GFP fusion gene reconstituting normal embryogenesis and lymphopoiesis in the absence of the endogenous FADD. Efficient T cell-specific deletion of FADD:GFP was achieved, as indicated by the presence of a high percentage of GFP-negative thymocytes and peripheral T cells in mice expressing Lck-Cre or CD4-Cre. Sorted GFP-negative thymocytes and peripheral T cells contained undetectable levels of FADD and were resistant to apoptosis induced by Fas, TNF, and TCR restimulation. These T cell-specific FADD-deficient mice contain normal thymocyte numbers, but fewer peripheral T cells. Purified peripheral FADD-deficient T cells failed to undergo extensive homeostatic expansion after adoptive transfer into lymphocyte-deficient hosts, and responded poorly to proliferation induced by ex vivo TCR stimulation. Furthermore, deletion of FADD in pre-activated mature T cells using retrovirus-Cre resulted in no proliferation. These results demonstrate that FADD plays a dispensable role during thymocyte development, but is essential in maintaining peripheral T cell homeostasis and regulating both apoptotic and proliferation signals.
Keywords: Transgenic/knockout mice, T cells, apoptosis, cell proliferation, homeostasis
Introduction
Proliferation and apoptosis (or programmed cell death) are induced at various stages during lymphocyte development and immune responses (1-3). For example, signal transduction induced by the pre-TCR results in proliferation of CD4-CD8- double-negative (DN) T cells and subsequent differentiation into CD4+CD8+ double-positive (DP) T cells in the thymus (4). At the DP stage, autoreactive T cells are eliminated by apoptosis (5, 6), and those which evade this thymic negative selection process are deleted in the periphery primarily by activation-induced cell death (AICD) (7, 8). In normal and healthy mice, the peripheral naïve T cell pool is maintained at a steady-state by homeostatic mechanisms which in part involve TCR and self peptide/MHC interaction (9, 10). During an immune response, antigen-specific T cells are induced to proliferate and differentiate into effector cells, and AICD is required in the subsequent contraction phase to eliminate activated T cells (2, 3, 5). One of the major players involved in negative selection and AICD is Bim, the proapoptotic member of the Bcl-2 family proteins (11, 12). The death-receptor (DR) Fas (Apo-1 or CD95) also plays an important role in lymphocyte apoptosis required for maintaining homeostasis in the immune system (13, 14). Mutations in the Fas gene result in a lymphoproliferative syndrome characterized by lymphadenopathy as well as autoimmune diseases (15-17).
The Fas-associated death domain-containing protein (FADD) was initially identified as an adaptor required for cell death signal transduction initiated by Fas (18-20). Subsequent studies indicated that FADD is also involved in signaling induced by other death-receptors (DRs) such as TNF receptor I (TNFR-I), TRAIL receptors (TRAIL-Rs or DR4/5), and DR3 (21-27). FADD contains two protein-protein interaction structures: the death domain (DD) at the carboxy terminus and the death effector domain (DED) at the amino terminus. The DD of FADD binds to a similar DD located within the intracellular tail of Fas, whereas the DED of FADD associates with the DED present in pro-caspase-8 (28, 29). Cell death signaling is initiated by clustering of Fas induced by engagement of the trimeric Fas ligand (FasL). As a result, a death-inducing signaling complex (DISC) containing FasL, Fas, FADD and procaspase-8 is assembled (30), and aggregation of pro-caspase-8 in the DISC facilitates its auto-proteolysis. After assembly of the resulting subunits to become a fully active cysteine protease, caspase-8 processes and activates downstream caspases, leading to apoptotic cell death.
The in vivo function of FADD has been previously investigated by gene targeting in germ cells (31, 32). The resulting FADD-deficient (FADD-/-) mice died in utero, an unexpected phenotype given the normal development of mice lacking Fas, TNFR-I, TRAIL-R, or DR3 (33-37). In an alternative approach, homozygous FADD-/- embryonic stem (ES) cells were generated and injected into blastocysts of lymphocyte-deficient RAG-1-/- mice (31). The resulting viable FADD-/-→Rag-1-/- chimeras produced few thymocytes and an undetectable level of B cells. In other studies, a truncated FADD (FADD-DD) containing the DD but lacking the DED was shown to block ex vivo apoptotic responses (20, 22), and expression of FADD-DD specifically in T cells appears to perturb thymic development in some transgenic mouse lines (38) but not in others (39, 40). Although FADD-deficient thymocytes were resistant to Fas-induced apoptosis, there was no massive accumulation of T cells in the periphery of FADD-/-→Rag-1-/- chimeras (31), unlike that present Fas-deficient mice. The very few FADD-deficient T cells recovered from the periphery of FADD-/-→Rag-1-/- chimeras proliferated poorly in response to TCR stimulation (31, 38, 40-42), and peripheral T cells expressing FADD-DD also have abnormal proliferation responses (31, 38, 40-44).
While there is a better understanding of the molecular process in FADD-mediated apoptosis signaling induced by DRs, little is know about the nature of the potential involvement of FADD in early T cell development and peripheral T cell proliferation responses. In FADD-/-→Rag-1-/- chimeras, FADD is absent in hematopoietic stem cells and therefore the temporal requirement of FADD during T cell development could not readily be determined. In previously described tFADD mice expressing a LoxP-containing FADD transgene to reverse embryonic lethality caused by a lack of the endogenous FADD, thymic development appeared to be inhibited when the T cell-specific Lck-Cre transgene was expressed (45). Interestingly, T cell-specific deletion of caspase-8, which is known to be immediately downstream of FADD in DR signaling, did not affect thymocyte development (46). Because of the potential involvement of FADD during early hematopoiesis and/or thymic development, it becomes uncertain whether the defect detected in FADD-/-→Rag-1-/- chimeras is indicative of a role for FADD in peripheral T cell proliferation or a secondary effect resulting from abnormal development. Likewise, the FADD-/-→Rag-1-/- chimeras, which are lymphopenic, may not be a plausible system for determining the function of FADD in T cell apoptotic responses. The tFADD mice reportedly contain mostly tFADD-expressing T cells and undetectable FADD-deficient T cells since there was no obvious reduction of the FADD protein after the expression of Lck-Cre (45). The implication of this phenotype is unclear and further analysis is needed in order to determine whether FADD deficiency in the thymus completely blocks development and/or results in immediate T cell death. In order to address these issues, it is necessary to establish viable mutant mice which contains detectable FADD-deficient T cells by inducing stage-specific deletion of FADD during development, and to analyze the effect of a FADD deficiency induced in normally differentiated T cells.
In this study, we generated novel mutant mice in which efficient deletion of a GFP-tagged FADD gene was induced specifically in DN or DP thymocytes by using the Lck-Cre or CD4-Cre transgenes. In addition, deletion of the FADD:GFP fusion gene in CD4+ or CD8+ single-positive (SP) T cells was induced using retrovirus-mediated delivery of the Cre recombinase. High percentages of FADD-deficient T cells were readily detected as the GFP-negative (GFP-) population in the thymus and periphery by flow cytometric analysis. The data obtained from analysis of these T cell-specific FADD-deficient mice demonstrate that FADD deficiency has no obvious effect in thymocyte development, but blocks cell death responses, and impairs peripheral homeostasis as well as TCR-induced proliferation responses in mature T cells.
Materials and Methods
Generation of T cell-specific FADD:GFP-deficient mice
The 12-kb EcoR I genomic DNA fragment containing two coding exons of the mouse FADD gene was isolated from a cosmid clone described elsewhere (20). The GFP gene coding region was amplified by PCR from the pEGFP-C1 plasmid (Clontech) and fused to the 3’ end of the FADD coding region in the 12-kb EcoR I fragment. Two LoxP sites were inserted to flank the FADD:GFP coding region. The resulting FADD:GFP fusion construct was injected into mouse embryos to generate transgenic mice at the Kimmel Cancer Center Facilities of Thomas Jefferson University. Mouse ear tissue lysates were prepared and used in genotyping for FADD:GFP+ founders by PCR using GFP-specific primers. Tail genomic DNA was digested with EcoR I for genotyping by Southern blot analysis. A 0.4-kb DNA fragment was used as a probe to hybridize to the second exon of FADD. The FADD:GFP+ founders were crossed with wild-type C57BL/6 (B6) mice for 5 to 6 generations. Heterozygous FADD knockout (FADD+/-) mice, generated in a previous study (31), were crossed to B6 mice for more than 12 generations. The FADD:GFP transgene was then crossed into FADD+/- mice, and the resulting FADD+/- FADD:GFP+ mice were backcrossed with FADD+/- mice to produce viable FADD-/- FADD:GFP+ mice. To generate T cell-specific FADD-deficient mice, lck-Cre+ and CD4-Cre+ transgenic mice (47) were obtained from Taconic (Germantown, NY), and were crossed with FADD+/- mice. The resulting FADD+/- Lck-Cre+ or FADD+/- CD4-Cre+ mice were crossed with FADD-/- FADD:GFP+ mice to generate FADD-/- FADD:GFP+ Lck-Cre+ or FADD-/- FADD:GFP+ CD4-Cre+ mice. For genotyping, Southern blot analysis was performed using mouse tail DNA and the 0.4-kb probe which detects the endogenous and knockout alleles as well as FADD:GFP as EcoR I fragments of different sizes. The Lck-Cre and CD4-Cre transgenes were detecte by PCR-based genotyping using mouse ear tissue lysates and Cre-specific primers (5’-CCAGCTAAACATGCTTCATCGTC-3’ and 5’-CCTGATCCTGGCAATTTCGG-3’). All animal studies were approved by the Institutional Review Board at Thomas Jefferson University.
Flow cytometry
Lymphocytes were isolated from the thymus, spleen and lymph nodes of 2 to 7 month old mice. To determine GFP expression, single cell suspensions were directly subjected to flow cytometric analysis using a Coulter Epics XL analyzer (Bechman Coulter, Fullerton, CA). To detect the expression of CD4 and CD8, lymphocytes were stained on ice for 20 to 30 min with fluorochrome-conjugated antibodies (Caltag) in PBS containing FBS (1%) and sodium azide (0.05%). The WinMDI software was used for generating histograms and dot-plots. MoFlo high-speed cell sorters (DakoCytomation) at Kimmel Cancer Center at Thomas Jefferson University and the Wistar Institute were used to isolate GFP+ and GFP- T cells. Cell purity was typically more than 90%.
Western blot analysis
Total thymocytes, splenocytes, and lymph node cells were isolated from mice of various genotypes. GFP+ and GFP- cells were purified from the spleen and lymph nodes by high-speed cell sorting. Activated GFP+ and GFP- T cells were prepared by stimulation with anti-CD3 and anti-CD28 antibodies for two days and incubation for another two days in the presence of IL-2 (see below). Cells were washed with ice-cold PBS and resuspended in a lysis buffer containing Tris-HCl (50 mM, pH 8.0), NaCl (150 mM), EDTA (1 mM), NP-40 (1%, Calbiochem), PMSF (1 mM, Sigma), pepstatin (0.7 μg/ml, Roche Biochemical), and a protease inhibitor cocktail (Roche Biochemical). After incubation on ice for 30 min, cell lysates were collected after a 5 min centrifugation (14,000 g) at 4°C, and protein concentrations were determined using a Bio-Rad kit. Proteins (20 μg) were resolved by a 10% SDS-PAGE and blotted to Protran nitrocellulose membranes (Schleicher & Schuell), followed by staining with Ponceau S solution (Sigma) to assure equal loading and transfer. Rabbit polyclonal anti-FADD antibodies and reaction conditions have been described elsewhere (20). The Western Lighting Chemiluminescence Reagent Plus (PerkinElmer) was used to detect signals on X-ray films (Kodak).
Cell culture
T cells were grown in RPMI 1640 media (Mediatech, Herndon, VA) supplemented with FBS (10%), penicillin (100 U/ml), streptomycin (100 μg/lm), sodium pyruvate (1 mM), and 2-ME (100 μM). All media supplements were purchased from Mediatech. Cells were incubated at 37°C in the presence of CO2 (5%). The IL-2-secreting cells were provided by Dr. F. Melchers, and grown in RPMI 1640 media with the abovementioned supplements. After the culture reaches saturation, the IL-2-containing supernatant was collected and used at a 1:50 dilution in T cell cultures.
Cell death assay
Thymocyte killing was performed as described (48). Various amounts of anti-Fas antibodies (Jo2, Pharmingen) and cycloheximide (30 μg/ml, Sigma) were added in triplicate to wells (105 of thymocytes/well) in a 96-well plate. Sixteen hours after treatment, cell death was determined by propidium iodide (PI, Sigma) uptake assays as previously described (20). Resting GFP+ and GFP- T cells were purified from the spleen and lymph nodes by sorting and seeded to 96-well plates (105/well). sFasL (Alexis) was added at various concentrations in triplicate. After 12 h, cell death was determined by PI uptake. To induce cell death in activated T cells, GFP+ and GFP- cells were isolated from the spleen and lymph nodes by sorting, and stimulated with anti-CD3 and anti-CD28 antibodies (see below). Two days after activation, cells were washed three times with PBS and cultured for another two days in the presence of IL-2. Activated T cells were seeded to 96-well plates (2 × 104/well) and anti-Fas antibodies, TNF-α (Alexis), or anti-CD3 antibodies were added into wells in triplicate. Cell death was determined by PI uptake 16 h post stimulation.
Adoptive transfer
B6.RAG-1-/- mice were obtained from the Jackson Laboratory and were subject to a 500 RAD irradiation. GFP- donor T cells were isolated from the spleen and lymph nodes of FADD+/-FADD:GFP+ Lck-Cre+ and FADD-/- FADD:GFP+ Lck-Cre+ mice which were crossed to B6 mice for at least five generations. Cells were resuspended at a concentration of 4 × 106/ml in PBS+FBS (5%), and mixed with equal volumes of CFSE (20 μM, Molecular Probe, Eugene, OR) in PBS+FBS. After incubation at 25°C for 5 min, the labeled cells were washed three times with PBS+FBS, once with PBS, and were injected intravenously (4-10 × 107 cell/mice) into irradiated B6.RAG-1-/- and non-irradiated B6 mice. Three days after injection, cells were recovered from the spleen and lymph nodes and analyzed using a flow cytometer.
T cell proliferation assays
96-well plates were coated with anti-CD3 ascites (1:3000 dilution; clone 500A2). GFP+ and GFP- cells were purified from the spleen and lymph nodes by high-speed sorting, and 105 cells seeded into each well in 100 μl RPMI 1640 media. Anti-CD28 ascites (1:1000 dilution; clone 37.51) were then added into wells when necessary. Forty hours post stimulation, 1 μCi of [3H] thymidine (ICN Biochemicals) was added into each well, followed by additional 8-10 h incubation. Incorporated [3H] thymidine in each well was determined using a Wallac beta counter (PerkinElmer). Data were obtained from triplicate samples for each treatment. To generate growth curves, sorted GFP+ or GFP- T cells were stimulated for two days with coated anti-CD3 plus soluble anti-CD28 antibodies or with Con A (2.5 μg/ml, Sigma) in a 96-well plate. Cells were then washed three times with PBS, once with RPMI 1640 media plus IL-2, and re-suspended (2.5 × 106/ml) in fresh RPMI 1640 plus IL-2. The cell number in each culture was determined every day by counting using a hemacytometer and a light microscope. Cell density is kept at 2 × 106/ml by expanding the culture in fresh media. To determine cell division kinetics, GFP- cells were isolated from the spleen and lymph nodes of FADD+/- FADD:GFP+ CD4-Cre+ and FADD-/- FADD:GFP+ CD4-Cre+ mice, and labeled with CFSE (5 μM) as described above. After washing, these labeled cells were then stimulated with anti-CD3 (1:3000) and anti-CD28 (1:1000) ascites for various times at 37°C and analyzed by flow cytometry.
Retrovirus-mediated gene deletion
The Cre gene was amplified from the MIEG3-Cre plasmid (Provided by Dr. D. Williams). A nuclear localization signal peptide was linked to the NH2-terminus of Cre and the resulting fusion gene was then cloned into a MSCV vector (provided by Dr. W. Pear). For virus packaging, the resulting MSCV-Cre plasmid was co-transfected along with the helper plasmid pCL-Eco (Imgenex) into HEK 293T cells. Two to three days after transfection, the virus-containing supernatant was collected and frozen at -80 °C. Total splenic and lymph node cells were isolated from FADD-/- FADD:GFP+ mice and suspended in RPMI 1640 containing supplements (see above) at a concentration of 6 × 106/ml. This cell suspension was added to a 6-well plate (4 ml/well) and stimulated with soluble anti-CD3 (1:3000 dilution) and anti-CD28 (1:1000 dilution) antibodies. After 18 h incubation at 37°C, these activated cells were washed with and resuspended in RPMI 1640 (6 × 106/ml). This cell suspension (100 μl) was mixed with the MSCV-Cre-containing supernatant (500 μl) in the presence of IL-2 and Polybrene (Sigma) in a 24-well plate. After spinning for 1 h at 2500 rpm in a Beckman Allegra 6R centrifuge at 32°C, cells were incubated for another 6 h at 37°C and then resuspended in fresh RPMI 1640 media. Two days after infection, FADD-deficient T cells were isolated by sorting for the GFP- population.
Results
FADD:GFP functions indistinguishably from FADD in vivo
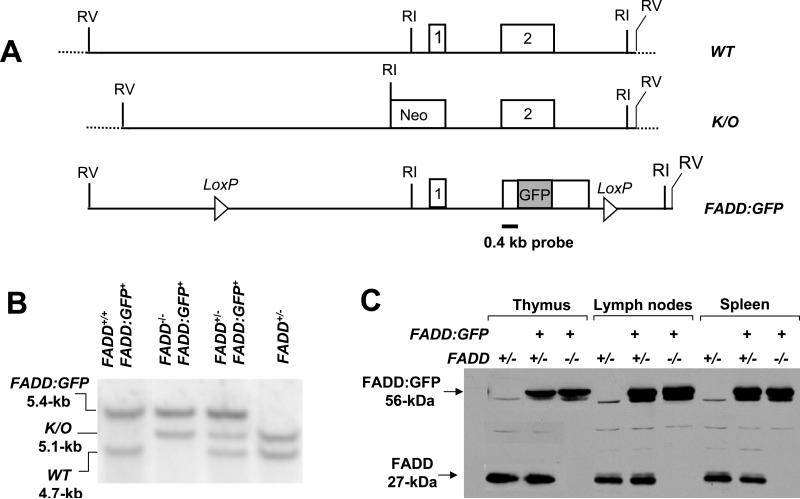
To ensure that the GFP tag would not affect the function of FADD, we performed analyses using FADD-deficient (FADD-/-) mouse embryonic fibroblasts (MEFs) (26). The GFP gene was cloned at the 3’ end of the FADD cDNA and the resulting FADD:GFP fusion cDNA was introduced into FADD-/- MEFs by retrovirus-mediated gene transfer. These FADD:GFP-reconstituted FADD-/- MEFs were killed by FasL as effectively as those reconstituted with an untagged FADD (data not shown), indicating that the FADD:GFP fusion protein functions similarly to the wild-type FADD protein in transducing the death signal initiated by Fas. To express FADD:GFP in mice, we used a 12-kb genomic DNA fragment isolated from the mouse FADD locus containing two coding exons (Fig. 1A). This FADD minigene, when present as a transgene, can restore normal development in FADD-/- mice lacking the endogenous alleles of FADD (20, 45). We cloned GFP at the 3’ end of the FADD coding region within exon 2 in the 12-kb FADD minigene and inserted two LoxP sites flanking the coding region of the FADD:GFP fusion (Fig. 1A). The resulting DNA construct was injected into mouse embryos and 15 transgenic lines were generated. Genotyping by Southern blot analysis revealed the FADD:GFP fusion gene as a 5.4-kb EcoR I fragment, distinguishable from the 4.7-kb fragment representing the endogenous FADD alleles (lane 1, Fig. 1B). Western blot analysis was performed on mice carrying FADD:GFP in order to detect expression of the FADD:GFP fusion protein. As shown in Figure 1C, anti-FADD antibodies detected a band of 56-kDa in cells isolated from the thymus, spleen, and lymph nodes, and this protein is absent in control heterozygous FADD+/- mice. This is the expected size of the full-length FADD:GFP fusion protein and is distinct from the size of the endogenous FADD (27-kDa, Fig. 1C). A monoclonal anti-GFP antibody detected only the 56-kDa protein band (data not shown). Expression of FADD:GFP was also detectable by flow cytometric analyses, as indicated by the single GFP-positive (GFP+) peak in histograms of thymocytes, spleen and lymph node cells in mice carrying FADD:GFP (Fig. 2).
FIGURE 1.
FADD:GFP reconstitutes normal development in FADD-/- mice. (A) Diagrams of the wild-type (WT) FADD gene consisting of two exons (boxes 1 and 2); the knockout allele (K/O); and the FADD:GFP construct. LoxP sites and the 0.4-kb probe used for genotyping by Southern blots are indicated. RI: EcoR I. RV: EcoR V. (B) Genotyping by Southern blot analysis using mouse tail DNA identified various genotypes present in the offspring of crosses between FADD+/- and FADD+/- FADD:GFP+ mice. Only when FADD:GFP is present, can FADD-/- mice become viable and develop normally. (C) Anti-FADD antibodies detected the FADD and FADD:GFP proteins in thymocytes, spenocytes and lymph node cells from mice of various genotypes by Western blot analyses. FADD:GFP differs in size from the endogenous FADD which is absent in FADD-/- FADD:GFP+ mice.
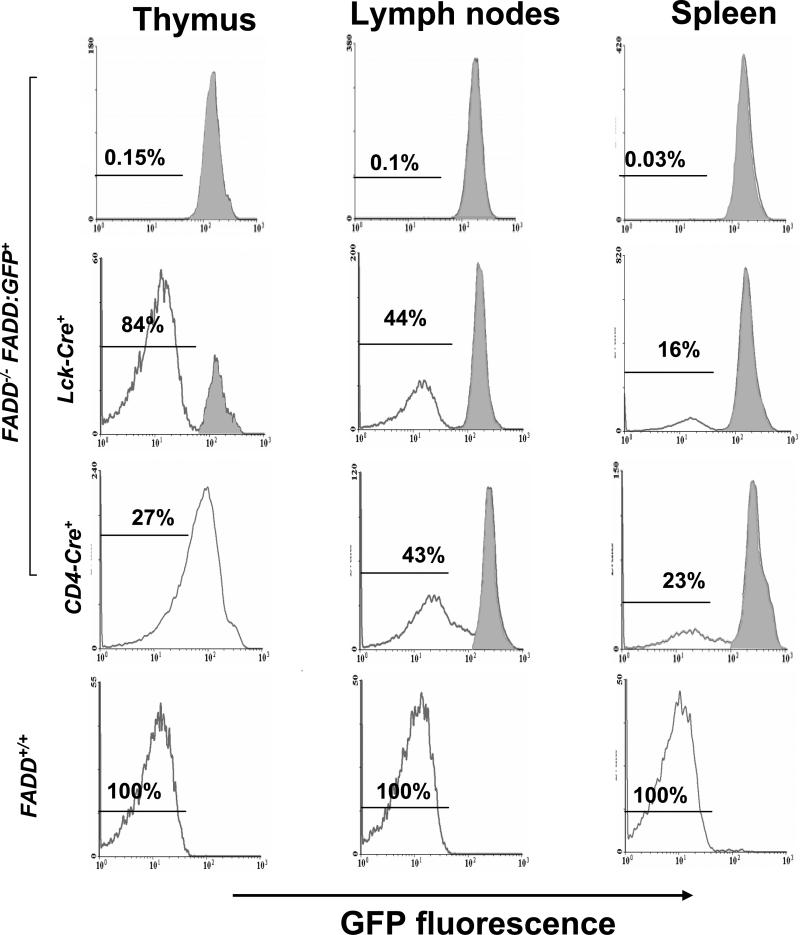
FIGURE 2.
Detection of the expression and deletion of FADD:GFP by flow cytometric analysis. Gene deletion efficiency in various lymphoid organs was indicated by the percentage of GFP- cells detected in FADD-/- FADD:GFP+ mice containing either Lck-Cre or CD4-Cre. Cells from FADD-/- FADD:GFP+ mice (top) were used as GFP+ controls, which uniformly express FADD:GFP as indicated by a single right histogram peak (shaded). Cells from wild type FADD+/+ mice were used as GFP- controls (bottom). Using these two different controls, GFP+ cells in mice expressing either Lck-Cre or CD4-Cre were determined to be those represented by areas shaded in histograms (two middle rows).
To determine whether expression of FADD:GFP would complement a FADD deficiency, we introduced FADD:GFP into FADD-/- mice in which the endogenous FADD gene was inactivated in germ cells by deletion of the promoter region and exon 1 (Fig. 1A) (31). Since FADD-/- mice die at around day 10 of gestation, we first crossed FADD:GFP into viable heterozygous FADD+/- mice. The resulting FADD+/- FADD:GFP+ mice were backcrossed with FADD+/- mice. Genotypes of the offspring were determined by Southern blotting (Fig. 1B), and viable homozygous knockout FADD-/- mice containing FADD:GFP were detected at expected Mendelian genetic frequencies. These FADD-/- FADD:GFP+ mice showed no obvious abnormalities at various stages during development, indicating that FADD:GFP functions similarly to endogenous FADD and can restore normal embryogenesis in FADD-/- mice. To analyze the immune system of FADD-/- FADD:GFP+ mice, the thymus, spleen and lymph nodes were dissected and found to be similar in size and cellularity to those of wild-type FADD+/+ and heterozygous FADD+/- FADD:GFP+ mice (data not shown). Flow cytometric analysis revealed uniform expression of FADD:GFP, indicated by a single GFP+ histogram peak in thymocytes, spleen and lymph node cells (top, Fig. 2). Additionally, Western blot analyses confirmed expression of the FADD:GFP protein (56-kDa), and absence of the endogenous FADD protein (27-kDa, Fig. 1C). Flow cytometric analysis revealed that FADD-/- FADD:GFP+ mice contain DN, DP, and SP subpopulations of thymocytes similar to those in FADD+/+ mice (data not shown). Therefore, the GFP tag does not appear to affect the function of FADD in lymphocyte development, and FADD-/- FADD:GFP+ mice were used frequently as controls in subsequent experiments.
T cell-specific deletion of FADD:GFP
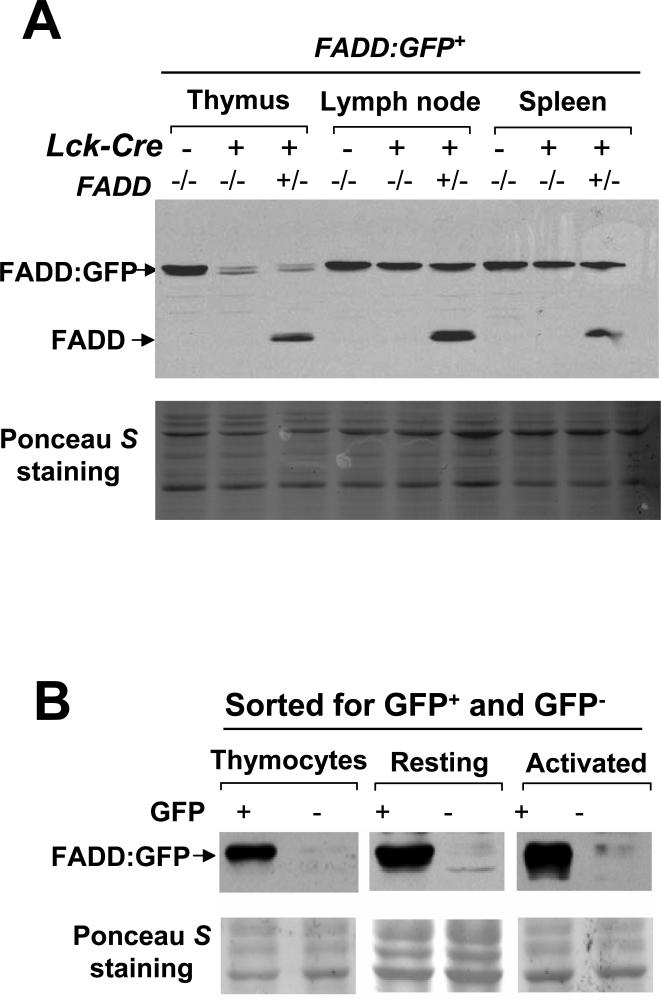
To induce deletion of the FADD:GFP fusion gene in DN immature thymocytes, we crossed the Lck-Cre transgene (47) into FADD+/- mice. The resulting FADD+/- Lck-Cre mice were then crossed with FADD-/- FADD:GFP+ mice and littermates in the offspring were analyzed by flow cytometry. A major GFP- cell population (80-95%) was detected in the thymus of FADD-/- FADD:GFP+ Lck-Cre+ mice, which was not present in mice lacking Lck-Cre (Fig. 2). Western blot analyses were then performed with total thymocytes, showing dramatically reduced levels of the FADD:GFP fusion protein in FADD-/- FADD:GFP+ Lck-Cre+ and FADD+/- FADD:GFP+ Lck-Cre+ mice in comparison with control mice lacking Lck-Cre (Fig. 3A). The remaining FADD:GFP protein may be that expressed in the 10-20% of GFP+ cells in the thymus (Fig. 2). GFP- cells were also detected in the spleen (16%) and lymph nodes (44%) in FADD-/- FADD:GFP+ Lck-Cre+ mice (Fig. 2), and these cells express the T cell marker CD3 (data not shown). When total peripheral lymphocytes were analyzed by Western blots, reduction of the FADD:GFP protein in FADD+/- FADD:GFP+ Lck-Cre+ or FADD-/- FADD:GFP+ Lck-Cre+ mice was not as obvious as that detected in thymocytes (Fig. 3A), probably due to the presence of higher percentages of GFP+ cells in the spleen (84%) and lymph nodes (56%) (Fig. 2). These peripheral GFP+ populations are mostly B220-expressing B cells (data not shown). GFP- T cells were isolated from the thymus, spleen and lymph nodes by FACS, and analyzed by Western blotting. As shown in Figure 3B, these purified GFP- T cells contain undetectable levels of the FADD:GFP protein in comparison with control GFP+ T cells isolated from FADD-/- FADD:GFP+ mice. After activation and growth in culture for several days, the FADD:GFP protein remains undetectable in mature GFP- T cells (Fig. 3B). Thus, the GFP- population in FADD-/- FADD:GFP+ Lck-Cre+ mice represents FADD-deficient (FADD-/-) T cells.
FIGURE 3.
Detection of FADD:GFP deletion by Western blots analysis using anti-FADD antibodies. (A) Efficient T cell-specific deletion of FADD:GFP in FADD+/- or FADD-/- mice was induced by the presence of Lck-Cre (+), as indicated by the dramatic reduction of the FADD:GFP protein in the thymus (top panel). FADD-/- FADD:GFP mice lacking Lck-Cre (-) were used as non-deletion controls. Deletion of FADD:GFP was not readily detectable in the spleen and lymph nodes due to the presence of the non-T cell population which express FADD:GFP in these secondary lymphoid organs. (B) Absence of FADD:GFP in purified GFP- thymocytes and mature resting and activated T cells was confirmed by anti-FADD Western blots. GFP+ T cells isolated from FADD-/- FADD:GFP+ mice were used as controls. Ponceau S staining shown at the bottom in panels (A) and (B) indicates equal loading and transfer of proteins.
We also introduced into FADD-/- FADD:GFP+ mice the CD4-Cre transgene which is expressed at the DP stage in the thymus (47). Unlike FADD-/- FADD:GFP+ Lck-Cre+ mice which contain a discrete GFP- population in the thymus, FADD-/- FADD:GFP+ CD4-Cre+ mice contained thymocytes expressing various levels of FADD:GFP, indicated by a GFP-peak shift in flow cytometric histograms (Fig. 2). In the periphery, however, GFP- cells were detected as a distinct population in the spleen (43%) and lymph nodes (23%) in FADD-/- FADD:GFP+ CD4-Cre+ mice (Fig. 2). The FADD:GFP protein was undetectable in sorted peripheral GFP- cells by Western blot analysis (data not shown). These results indicate that T cells are not completely FADD-deficient until the FADD:GFP protein, synthesized prior to CD4-Cre-mediated gene deletion at the DP stage, is degraded during the process of maturation and subsequent migration to the periphery.
FADD deficiency abrogates cell death in T cells
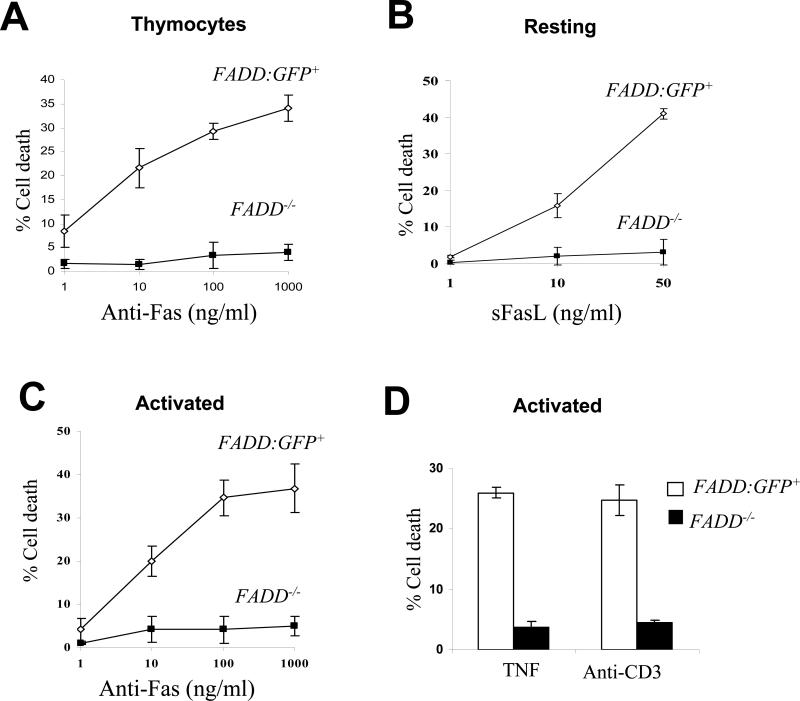
To determine Fas-induced cell death responses in FADD-deficient immature T cells, total thymocytes were prepared from FADD-/- FADD:GFP+ Lck-Cre+ mice and found to contain 80 to 97% of GFP- cells. These thymocytes were cultured in the presence of anti-Fas antibodies which induce cell death preferentially in the DP population (48). While control thymocytes from FADD-/- FADD:GFP+ mice were killed in a dose-dependent manner, FADD-deficient thymocytes are highly resistant to stimulation with various concentrations of anti-Fas antibodies (Fig. 4A). Although resting mature T cells are resistant to stimulation with anti-Fas antibodies, they are readily killed by soluble FasL (sFasL) (49). To analyze cell death response in resting mature T cells, GFP+ T cells were isolated from the spleen and lymph nodes in FADD-/- FADD:GFP+ mice, and these cells were killed in a dose-dependent manner by sFasL (FADD:GFP+, Fig. 4B). In contrast, GFP- (FADD-/-) T cells isolated from the spleen and lymph nodes in FADD-/- FADD:GFP+ Lck-Cre+ mice were resistant to stimulation by sFasL at various concentrations (Fig. 4B).
FIGURE 4.
FADD-deficient T cells are resistant to cell death induction. FADD-/- cells were the GFP- population isolated from FADD-/- FADD:GFP+ Lck-Cre+ mice. Littermate FADD-/- FADD:GFP+ mice were used to isolate the control GFP+ T cell population (FADD:GFP+). Error bars represent ± standard deviation of the means from 3-5 mice of each genotype in each treatment. (A) Thymocytes were stimulated with various concentrations of anti-Fas antibodies to induce cell death. (B) Cell death was induced in resting mature T cells by treatment with various concentration of sFasL. Proliferating T cells, activated by stimulation with anti-CD3 and anti-CD28 antibodies, were incubated with various concentrations of anti-Fas antibodies (C), TNF (50 ng/ml) or anti-CD3 antibodies (D) to induce cell death. Percent cell death is indicated on Y-axis.
Repeated stimulation of TCR results in death in activated T cells, a process called AICD involving the action of FasL and TNF (50-52). To determine cell death responses in activated T cells, GFP- and GFP+ T cells were isolated by FACS from the spleen and lymph nodes in FADD-/- FADD:GFP+ mice with or without the presence of the Lck-Cre transgene respectively. These mature T cells were activated by stimulation with anti-CD3 and anti-CD28 antibodies for two days and cultured for additional two days in the presence of IL-2 to sensitize cells for Fas-induced death (53). These activated T cells were then treated with increasing concentrations of anti-Fas antibodies. This treatment induced a dose-dependent cell death response in control FADD:GFP-expressing cells (FADD:GFP+), but not in activated GFP- FADD-deficient T cells (FADD-/-; Fig. 4C). TNF does not have much effect on resting T cells but can induce detectable death in activated T cells (50, 54). We prepared activated FADD-deficient and control mature T cells as described above by stimulation using anti-CD3 and anti-CD28 antibodies. At a concentration of 50 ng/ml, TNF induced 20% cell death in control activated T cells (FADD:GFP+) and less than 5% cell death in mutant activated T cells (FADD-/-, Fig. 4D). To examine AICD responses, activated splenic and lymph node T cells were prepared as in Fas-induced cell death assays by stimulation with anti-CD3 and anti-CD28 antibodies and a two-day incubation in the presence of IL-2. After re-stimulation with anti-CD3 antibodies, more than 40% death was induced in FADD:GFP+ control T cells, whereas less than 5% death was detected in FADD-deficient T cells (Fig. 4D).
T cell-specific FADD-deficient mice contain normal thymocyte populations but have a homeostatic defect in peripheral T cells
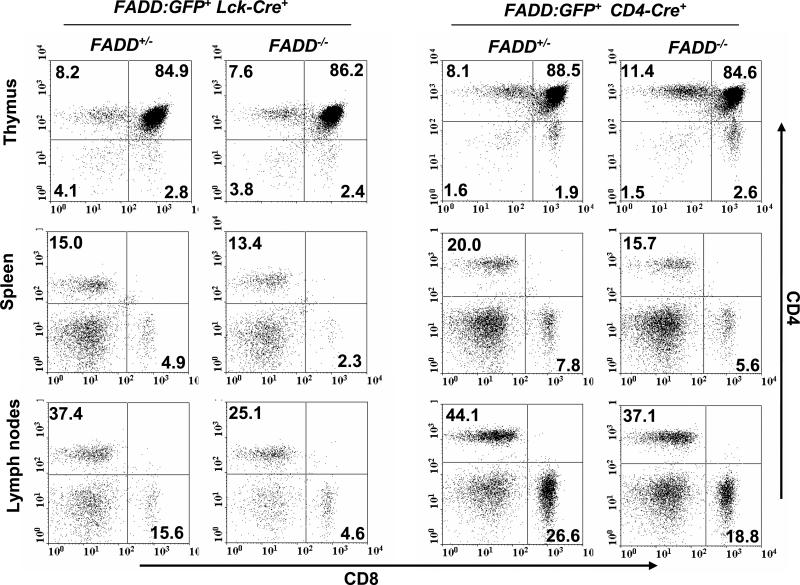
We analyzed the thymus, spleen, and lymph nodes in 1 to 7-month-old FADD-/- FADD:GFP+ mice containing either the Lck-Cre or CD4-Cre transgenes, and these lymphoid organs appear to be normal in size and total cellularity in comparison with littermate control mice containing one endogenous FADD allele (FADD+/- FADD:GFP+ Lck-Cre+ or CD4-Cre+) (data not shown). T cell sub-populations were further analyzed by flow cytometry after staining for the CD4 and CD8 co-receptors. The DN, DP, and SP thymocytes were present in FADD-/- FADD:GFP+ Lck-Cre+ mice in a pattern similar to that detected in littermate control FADD+/- FADD:GFP+ Lck-Cre+ mice (Fig. 5). Normal thymocyte subsets were also detected in FADD-/- FADD:GFP+ CD4-Cre+ mice (Fig. 5). When peripheral T cells were analyzed by flow cytometry, a reduction in the number of both CD4+ and CD8+ T cells was detected in the spleen and lymph nodes from FADD-/- FADD:GFP+ mice containing either Lck-Cre or CD4-Cre (Fig. 5). Among the FADD-/- FADD:GFP+ Lck-Cre+ mice analyzed, there is an average 46% reduction in the number of peripheral T cells in comparison to FADD+/- FADD:GFP+ Lck-Cre+ littermate controls (tables 1 and 2). In FADD-/- FADD:GFP+ CD4-Cre+ mice analyzed, the peripheral T cell number is about 70% of that in littermate control FADD+/- FADD:GFP+ CD4-Cre+ mice. Concomitant with the reduction in the peripheral T cell number, there was an increase in the number of B cells in the periphery (Fig. 5 and data not show). This is most likely a result of homeostatic proliferation of FADD-expressing B cells when there is a decrease in the T cell population.
FIGURE 5.
Flow cytometric analysis of T cell sub-populations. Cells isolated from the thymus, spleen, and lymph nodes were stained for the CD4 and CD8 co-receptors. T cell-specific gene deletion in FADD-/- FADD:GFP+ mice induced by either Lck-Cre or CD4-Cre did not alter thymocyte populations (top), but resulted in reduced T cell populations in the spleen and lymph nodes (middle and bottom). Littermate FADD+/- FADD:GFP+ mice containing Lck-Cre or CD4-Cre were used as controls.
Table 1.
Reduced peripheral T cell numbers (means of 9 mice of each genotype ± SD) in Lck-Cre-induced FADD-deficient mice.
| FADD+/- (x 10-6) | FADD-/- (x 10-6) | |||
|---|---|---|---|---|
| CD4+ | CD8+ | CD4+ | CD8+ | |
| Thymus | 4.6±1.1 | 0.9±0.4 | 4.5±2.1 | 0.9±0.2 |
| SPLN | 14.4±3.2 | 6.1±0.7 | 7.9±4.8 | 1.4±0.6 |
| LN | 7.9±1.9 | 3.1±0.9 | 4.6±0.7 | 0.9±0.3 |
Table 2.
Reduced peripheral T cell numbers (means of 3 mice of each genotype ± SD) in CD4-Cre-induced FADD-deficient mice.
| FADD+/- (x 10-6) | FADD-/- (x 10-6) | |||
|---|---|---|---|---|
| CD4+ | CD8+ | CD4+ | CD8+ | |
| Thymus | 14.1±2.1 | 3.8±1.8 | 16.0±1.1 | 4.5±1.3 |
| SPLN | 15.5±2.4 | 6.9±1.5 | 11.7±2.2 | 4.3±1.0 |
| LN | 15.3±3.8 | 9.1±2.3 | 10.9±1.4 | 5.7±2.6 |
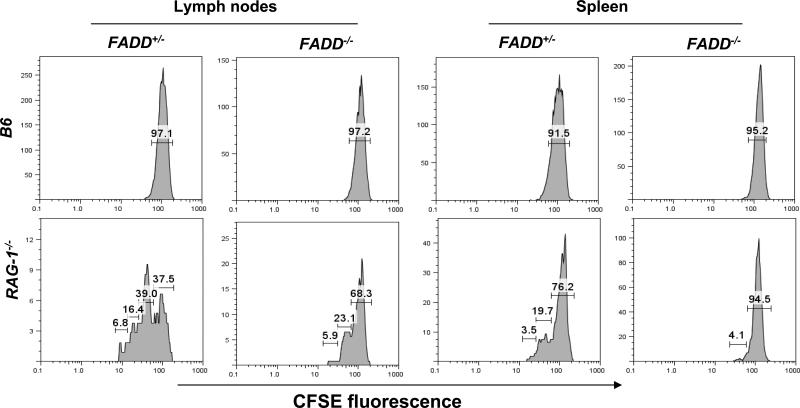
The overall size and composition of the naïve T cell pool are controlled by homeostasis mechanisms (9, 10). The reduced peripheral T cell number phenotype detected in the T cell-specific FADD-deficient mice prompted us to examine whether FADD-deficient T cells have a defect in homeostatic responses. In a lymphopenic environment, wild-type T cells tend to fill the “space” by expansion or homeostatic proliferation. We performed adoptive transfer experiments using RAG-1-/- mutant mice that lack B and T cells. Donor FADD-deficient (FADD-/-) T cells were isolated by sorting for the GFP- population from the spleen and lymph nodes of FADD-/- FADD:GFP+ Lck-Cre+ mice. For controls, FADD+/- T cells containing a single allele of the endogenous FADD gene were isolated by sorting for the GFP- population from littermate FADD+/- FADD:GFP+ Lck-Cre+ mice. Purified mature T cells were labeled with CFSE and the fluorescent intensity of CFSE is progressively reduced due to partitioning of labeled intracellular molecules from the mother cell into daughter cells during cell divisions. Three days after adoptive transfer into RAG-1-/- mice, cells were isolated from the spleen and lymph nodes and analyzed by flow cytometry. As indicated in Figure 6, 29% of FADD-deficient T cells had divided once or twice in the lymph nodes of RAG-1-/- mice, less than that detected in control FADD+/- T cells (55.4%, Fig. 6). The third cell division was detectable in FADD+/- T cells, but not in FADD-deficient T cells. There were more undivided FADD-deficient T cells (68.3%) than undivided FADD+/- T cells (37.5%) in the lymph nodes. Similar homeostatic proliferation defects were also detected in the spleen in RAG-1-/- hosts (Fig. 6). Therefore, FADD deficiency appears to inhibit homeostatic expansion of peripheral T cells. As controls, CFSE-labeled cells were also injected into non-irradiated wild-type B6 mice, in which there was an undetectable level of division of transferred FADD-/- and FADD+/- T cells due to the presence of the wild-type lymphocyte pool in the periphery (Fig. 6).
FIGURE 6.
Defective homeostatic responses in FADD-deficient T cells. Adoptively transferred FADD-deficient (FADD-/-) T cells were the GFP- population isolated from FADD-/- FADD:GFP+ Lck-Cre+ mice, which divided at largely reduced rates. Control FADD+/- T cells were isolated from FADD+/- FADD:GFP+ Lck-Cre+ littermates. The percentage of cells that underwent various divisions are indicated in brackets on the graph. CFSE-labeled T cells, adoptively transferred into wild-type B6 hosts, were used as non-proliferation controls (top).
FADD is required for ex vivo TCR-induced proliferation
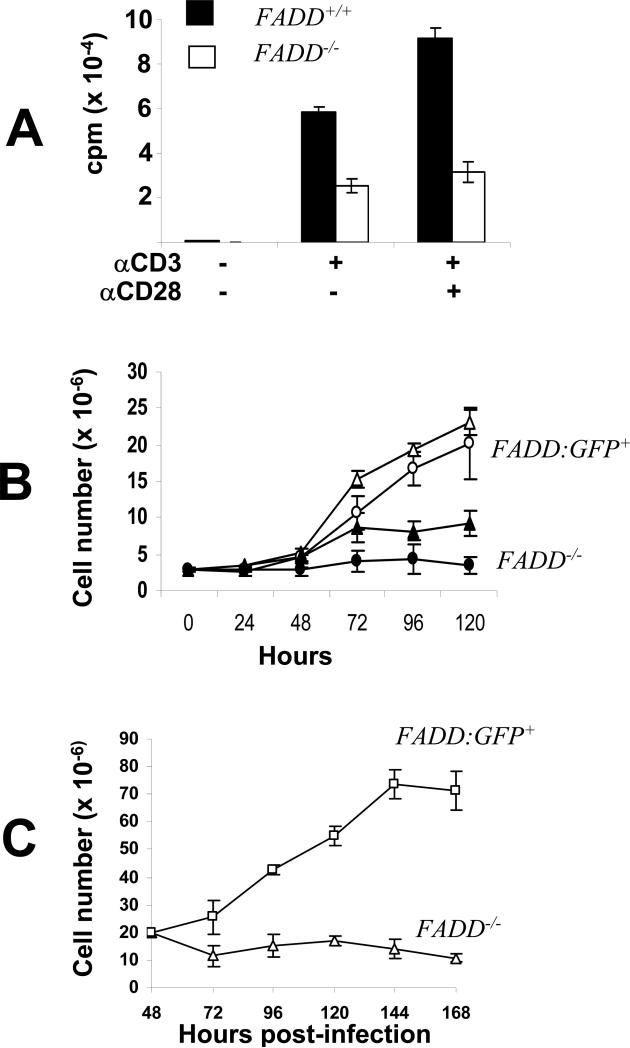
To analyze TCR-induced proliferation responses, FADD-deficient T cells were isolated by sorting for the GFP- population from the spleen and lymph nodes in FADD-/- FADD:GFP+ Lck-Cre+ mice, and stimulated with anti-CD3 antibodies. FADD+/+ T cells were isolated from age- and sex-matched wild-type mice and used as controls. Two days after stimulation, [3H] thymidine was added into each culture, followed by an additional 8 h incubation. The relative numbers of cells accumulated in each culture were indicated by the amount of [3H] thymidine incorporation. In comparison with FADD+/+ T cells, proliferation of FADD-deficient T cells was reduced by 56% (Fig. 7A). When T cells were stimulated with both anti-CD3 and anti-CD28 antibodies, FADD-deficient T cells only reached 40% of the wild type T cell proliferation capacity, indicating that co-stimulation provided by CD28 signaling could not overcome the TCR-induced proliferation defect. A direct assessment of proliferation was performed by determining the total cell number in each culture for an extended period of time. Splenic and lymph node T cells were activated for two days by stimulation with either Con A or anti-CD3 plus anti-CD28 antibodies. During an additional five-day growth in IL-2-containing media, cell numbers were counted each day and growth curves of each culture were generated. During the first 24 h, the number of Con A-stimulated FADD-deficient T cells decreased slightly, and then remained at a steady-state during the rest of culturing (Fig. 7B). In the control GFP+ T cells (FADD:GFP+) isolated from littermate FADD-/- FADD:GFP+ mice, little growth was detected in the first 24 h and an exponential growth was induced subsequently. Although treatments with anti-CD3 and anti-CD28 antibodies induced a better proliferation in FADD-deficient T cells than stimulations with Con A, a much higher growth rate was observed in control FADD:GFP+ T cells treated with anti-CD3 and CD28 antibodies (Fig. 6B).
FIGURE 7.
FADD is required for TCR-induced proliferation responses. (A) Peripheral T cells were stimulated with anti-CD3 antibodies in the presence (+) or absence (-) of anti-CD28 antibodies for two days and then pulsed with [3H] thymidine for 12 h. Levels of proliferation were indicated by the amount of radioactivity incorporated into T cells. cpm: count-per-minute. Mature FADD-/- T cells are the peripheral GFP- population isolated from FADD-/- FADD:GFP+ Lck-Cre+ mice. Peripheral T cells from wild-type mice (FADD+/+) were used as control. (B) Peripheral T cells were stimulated for two days with anti-CD3 and anti-CD28 antibodies (open and filled triangles) or with Con A (open and filled circles). The resulting activated T cells were cultured in IL-2-containing media. Growth curves were generated by counting the cell number in T cell cultures at various times after activation. Peripheral FADD-/- T cells were the GFP- population isolated from FADD-/- FADD:GFP+ Lck-Cre+ mice. The control peripheral FAGG:GFP+ T cells were isolated from FADD-/- FADD:GFP+ littermates. (C) Deletion of FADD:GFP in activated mature T cells also resulted in a reduced growth rate. FADD:GFP+ T cells were activated by stimulation with anti-CD3 and anti-CD28 antibodies for 16 h to induce cell cycle entry. The resulting activated, dividing T cells were infected with MSCV-Cre or control MSCV viruses. Two days after infection, cells were cultured in fresh IL-2-containing media and cell number is determined at indicated times. Error bars represent ± standard deviation of the arithmetic means from 3-5 mice of each genotype in each treatment.
Defective proliferation may be an indirect effect of abnormal T cell maturation and differentiation when FADD is deleted at earlier DN and DP stages using Lck-Cre and CD4-Cre. To resolve this issue, we induced deletion of FADD:GFP specifically in peripheral T cells, using a retroviral Cre delivery system. A murine stem cell virus (MSCV)-based vector was used to clone the bacterial phage Cre gene. Since MSCV only infects dividing cells, resting spleen and lymph node T cells were isolated from FADD-/- FADD:GFP+ mice and were pre-activated by stimulation with anti-CD3 and anti-CD28 antibodies. The resulting dividing T cells were infected with MSCV-Cre or control MSCV viruses. Two days after infection, GFP- T cells were isolated by sorting and confirmed to lack the FADD:GFP protein by Western blot analysis (data not shown). GFP+ T cells were isolated from control MSCV-infected cultures. These purified proliferating cells were cultured in medium containing IL-2, and growth curves were generated by determining the cell number in each culture each day. As indicated in Figure 7C, the number in the culture of activated FADD-deficient T cells decreased gradually during a five-day period, whereas FADD:GFP+ T cells infected with control viruses grew exponentially before a decline at day 4. These results indicate that the proliferation defect is not due to abnormal development, rather it is an indication of a direct involvement of FADD in peripheral T cell function.
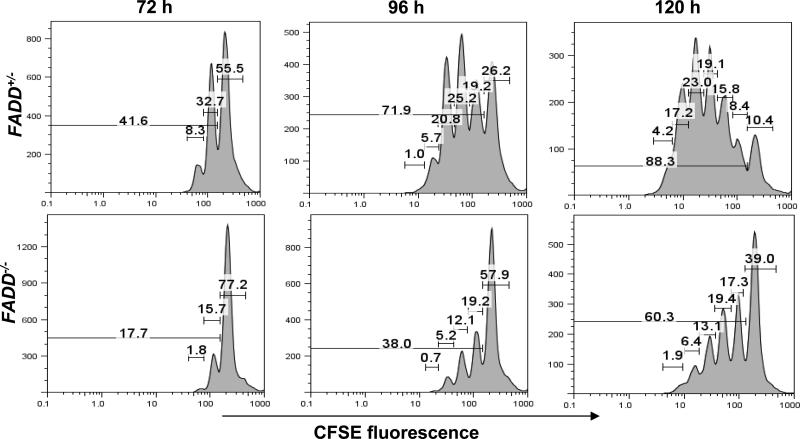
To further analyze the defect in FADD-deficient T cells, we determined cell division potential after activation is induced by TCR stimulation. FADD-deficient and FADD+/- T cells were isolated from the spleen and lymph nodes in FADD-/- FADD:GFP+ CD4-Cre+ or control littermate FADD+/- FADD:GFP+ CD4-Cre+ mice respectively, and were uniformly labeled with CFSE. To induce proliferation, CFSE-labeled T cells were stimulated with anti-CD3 and anti-CD28 antibodies and analyzed by flow cytometry. Three days after stimulation, less than 18% of FADD-deficient T cells had divided once or twice, in comparison to more than 40% in control FADD+/- T cells (left, Fig. 8). About 72% of control FADD+/- T cells and 38% of FADD-deficient T cells had divided on the fourth day after activation (center, Fig. 8). Dividing FADD+/- T cells continued to accumulate on day 5 (88.3%), whereas a high percentage of undivided cells (39%) remained in the FADD-deficient T cell culture. These results indicate that the first division in T cells is dramatically inhibited by a FADD deficiency, and the lower numbers of dividing cells is due to a reduced division potential.
FIGURE 8.
CFSE-labeling assays. FADD-deficient (FADD-/-) T cells were the GFP- population isolated from FADD-/- FADD:GFP+ CD4-Cre+ mice and the control FADD+/- T cells were the GFP- population isolated from FADD+/- FADD:GFP+ CD4-Cre+ littermates. CFSE-labeled T cells were activated by stimulation with anti-CD3 and anti-CD28 antibodies, and analyzed by flow cytometry at 72, 96 and 120 h post stimulation. The percentages of cells that underwent various divisions are indicated in brackets on the graph.
Discussion
Several reports have attempted to examine the function of FADD in the immune system. Studies using FADD-deficient ES cells in the RAG-deficient blastocyst complementation model have indicated that FADD is necessary for T cell development and apoptosis (31). However, it is not clear whether FADD is required at specific stages or throughout T cell development. There is a possibility that aberrant proliferation and apoptotic responses in T cells are indirect effects caused by abnormal development since FADD is not expressed in hematopoietic stem cells in FADD-/-→RAG-1-/- chimeras. An alternative tFADD mouse model was generated in an attempt to disrupt FADD specifically in the T cell lineage (45). In these tFADD mice, there was an accumulation of the DN population and reduction of total cellularity in the thymus. In contrast, T cell-specific deletion of caspase-8, which is immediately downstream of FADD in DR-induced signaling, had no impact on thymocyte development (46), leading to the suggestion of a caspase-8-independent function of FADD in regulating early thymocyte development. While ES cell-derived peripheral FADD-deficient T cells in FADD-/-→RAG-1-/- chimeras and FADD-DD transgenic T cells are impaired in TCR-induced proliferation, this defect was not detected in peripheral T cells in tFADD mice (45). It is possible that T cell-specific deletion of tFADD was ineffective, since a reduction of FADD protein expression in T cells was not detectable in tFADD mice expressing Lck-Cre (45). By expressing a functional GFP-tagged FADD in mice lacking the endogenous FADD and then achieving efficient stage-specific deletion of the resulting FADD:GFP fusion gene in T cells, we have been able to identify processes where FADD activity is required in T cells.
Deletion of FADD in more than 90% of the cells had no obvious effect on total thymic cellularity or subset representations (Fig. 5), indicating that FADD plays a dispensable role during development from DN to DP and SP stages in the thymus. Since a FADD deficiency in ES cells inhibits T cell production in FADD-/-→RAG-1-/- chimeras (31), FADD must play a more important role at earlier pre-thymic stages during hematopoiesis. This can be further investigated by induction of FADD:GFP deletion in hematopoietic stem cells using additional Cre-expression systems. Recent studies showed caspase-8 deletion in bone-marrow cells resulted in arrest of hemopoietic progenitor functioning (55). Although FADD-deficient thymocytes develop normally, these cells are defective in Fas-induced cells death.
In contrast to the undetectable effect in the thymus, induction of FADD:GFP deletion at the DN stage using Lck-Cre led to a reduction in the size of the peripheral T cell pool (Fig. 5). Deletion of FADD:GFP at later stages using CD4-Cre resulted in a similar defect in the periphery. These results indicate that FADD plays a more important role in mature peripheral T cells than in immature thymocytes. The nature of this FADD function is not clear. It is likely that the reduced peripheral T cell number in FADD-deficient mutant mice is a result of defective homeostatic proliferation, as indicated by results from adoptive transfer experiments using RAG-1-deficient hosts (Fig. 6). Homeostatic responses in naïve T cells is regulated in part by signals from TCR in contact with self-MHC/peptide complexes (9, 10). The homeostatic proliferation defect of FADD-deficient T cells could be an indication of abnormal signaling induced by the TCR. In ex vivo TCR stimulation assays, a FADD deficiency induced by the action of Lck-Cre or CD4-Cre dramatically inhibited proliferation responses in mature T cells (Fig. 7). Thus, FADD deficiency may block a key step shared by both the homeostatic and antigen-specific proliferation signaling processes downstream of the TCR. Deletion of FADD using retrovirus-mediated delivery of Cre into mature T cells that have been previously activated also resulted in a reduced growth rate (Fig. 7C), suggesting that proliferation defects are not due to defective development and differentiation of FADD-deficient T cell and that FADD is involved in not only initiation but also maintenance of mitogenic signals induced by TCR.
Fas-induced cell death plays a more important role in AICD in the periphery than in thymic negative selection (56, 57). The absence of lymphoproliferative diseases in FADD-deficient mice has raised the possibility that alternative Fas-induced pathways exist in peripheral T cells. This issue was not resolved previously using FADD-/-→RAG-1-/- chimeras which contains few peripheral T cells (31) or in tFADD mice which appears to contain undetectable peripheral FADD-deficient T cells (45). Using FADD:GFP mice, we demonstrated that Fas-induced death was inhibited by more than 97% in FADD-deficient mature resting and activated T cells (Fig. 4), indicating that alternative FADD-independent Fas-induced pathway is unlikely present in these cells. Although FADD-DN could inhibit cell death induced by TNF in tumor cells (22), whether FADD is involved in TNFR-I signaling in primary T cells had not been determined in previous studies. In this report, activated FADD-deficient T cells were shown to be highly resistant to TNF treatment (Fig. 4D). While TCR stimulation of resting T cells induces exit from the G0/G1 phase of the cell cycle and subsequent proliferation, the same treatment in proliferating T cells leads to apoptosis. This so-called AICD process is believed to be mediated in part by Fas, and is required in vivo to eliminate self reactive lymphocytes and antigen-specific effectors during the contraction phase following an immune response. As expected, activated FADD-deficient T cells are highly resistant to AICD induced by a second challenge with anti-CD3 antibodies (Fig. 4D). In Fas mutant mice, there is an age-dependent development of lymphadenopathy, obvious at 6 month of age. We have analyzed FADD-/- FADD:GFP+ Lck-Cre or CD4-Cre mice of 6 to 7 month of age, and none of these mice develop lymphoproliferative diseases similar to those in Fas mutant mice. It is possible that the lymphoproliferation disease, which normally develops in the absence of cell death, was inhibited by the compromised homeostatic and proliferative responses in FADD-deficient T cells. Recently, conditional Fas knockout mice were generated (58). Interestingly, inactivation of Fas in lymphocytes using the Lck-Cre and CD19-Cre transgenes did not lead to lymphoprolifartion diseases. However, deletion of Fas in not only lymphocytes but also other cell types by using the interferon-responsive MX1-Cre transgene resulted in the lpr phenotype. It remains to be determined whether deletion of FADD in both lymphocytes and non-lymphoid cells would cause similar lymphoproliferation diseases.
Fas is only one of the many proteins that participate in regulating apoptosis in lymphocytes. The Bcl-2 family members also play a critical role in either inhibiting or activating apoptosis in lymphocytes (59, 60). Bcl-2 can protect cells from apoptosis. However, previous studies have shown that Bcl-2 mediates death pathways distinct from that regulated by Fas. For example, transgenic overexpression of Bcl-2 failed to protect death of activated T cells induced by Fas signaling (61). The BH3-only member of the Bcl-2 family, Bim, has a pro-apoptotic activity. In Bim-deficient mice, thymocyte apoptosis induced by TCR stimulation is dramatically inhibited, suggesting a role for Bim in thymic negative selection (11). Other studies showed that endotoxin-induced death of activated T cells is largely dependent on the function of Bim (12). In addition, reactive oxygen species apparently are also essential for AICD in T cells (62). Therefore, multiple mechanisms are employed to ensure effective deletion of autoreactive lymphocytes and to maintain homeostasis in the immune system.
It is clear that FADD interacts with caspase-8 in signal transduction induced by death-receptors. This and previous studies showed that T cell-specific FADD-deficient mice exhibit overall phenotypic similarity to T cell-specific caspase-8-deficient mice (46), indicating that FADD and caspase-8 also interact in non-apoptotic signaling required for peripheral T cell homeostatic and proliferative responses. At least two additional proteins, FLIP and TRADD, were shown to interact with FADD. Similar to FADD or caspase-8 deficiency, inactivation of FLIP in germ cells also leads to early embryonic lethality in mice (63). Therefore FADD, caspase-8, and FLIP may be part of a novel signaling mechanism independent of DRs, which regulates embryogenesis and peripheral T cell function.
Acknowledgement
We thank Drs. Tim Manser and Bice Perussia for discussions, advice and critical reading of the manuscript; Christine Dirienzo for help in adoptive transfer experiments; Taishan Hu, Hanming Wang, and Pei-chun Tsai for technical assistance and critical reading of the manuscript.
Footnotes
This work is supported in part by grants from NCI (RO1 CA95454), W. W. Smith Charitable Trust, and CORNCERN Foundation to J.Z.
References
- 1.von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 2.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109(Suppl):S97. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 3.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 4.Haks MC, Oosterwegel MA, Blom B, Spits H, Kruisbeek AM. Cell-fate decisions in early T cell development: regulation by cytokine receptors and the pre-TCR. Seminars in Immunology. 1999;11:23. doi: 10.1006/smim.1998.0153. [DOI] [PubMed] [Google Scholar]
- 5.Strasser A, Bouillet P. The control of apoptosis in lymphocyte selection. Immunol Rev. 2003;193:82. doi: 10.1034/j.1600-065x.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 6.Nossal GJV. Negative selection of lymphocytes. Cell. 1994;76:229. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 7.Kabelitz D, Pohl T, Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993;14:338. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- 8.Budd RC. Activation-induced cell death. Curr Opin Immunol. 2001;13:356. doi: 10.1016/s0952-7915(00)00227-2. [DOI] [PubMed] [Google Scholar]
- 9.Surh CD, Sprent J. Homeostatic T Cell Proliferation: How Far Can T Cells Be Activated to Self-Ligands? J. Exp. Med. 2000;192:9F. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 11.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 12.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 13.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 14.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 15.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Ann. Rev. Immunol. 1991;9:243. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe FR, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 17.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 18.Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 1995;270:7795. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 19.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Winoto A. A mouse Fas-associated protein with homology to the human Mort1/FADD protein is essential for Fas-induced apoptosis. Mol. Cell. Biol. 1996;16:2756. doi: 10.1128/mcb.16.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu H, Shu H-B, Pan M-G, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 22.Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J. Biol. Chem. 1996;271:4961. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 23.Bodmer J-L, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, Blenis J, Tschopp J. TRAIL receptor-2 signals apoptosis through FADD and caspase 8. Nature Cell Biol. 2000;2:241. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 24.Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, Krammer PH, Walczak H. FADD/MORT1 and caspase 8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599. doi: 10.1016/s1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 25.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenouse FADD and caspase-8 to death receptor 4 and 5. Immunity. 2000;12:611. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 26.Kuang AA, Gretche DE, Zhang J, Winoto A. FADD is required for DR4- and DR5-mediated apoptosis: Lack of TRAIL-induced apoptosis in FADD-deficient mouse embryonic fibroblasts. J. Biol. Chem. 2000;275:25065. doi: 10.1074/jbc.C000284200. [DOI] [PubMed] [Google Scholar]
- 27.Chinnaiyan AM, O'Rourke K, Yu G-L, Lyons RH, Garg M, Duan DR, Xing L, Gentz R, Ni J, Dixit VM. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274:990. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 28.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Kramer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 29.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1-and TNF receptor-induced cell death. Cell. 1996;85:803. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 30.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95) associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Absence of Fas-mediated apoptosis and T cell receptor-induced proliferation in FADD-deficient mice. Nature. 1998;392:296. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 32.Yeh W-C, Pompa JL, McCurrach ME, Shu H-B, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, El-Deiry WS, Lowe SW, Goeddel DV, Mak TW. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 33.Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nature Genetics. 1995;11:294. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 34.Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 35.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 36.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Wang ECY, Thern A, Denzel A, Kitson J, Farrow SN, Owen MJ. DR3 Regulates Negative Selection during Thymocyte Development. Mol. Cell. Biol. 2001;21:3451. doi: 10.1128/MCB.21.10.3451-3461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh CM, Wen BG, Chinnaiyan AM, O'Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity. 1998;8:439. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, DeYoung A, Kasler HG, Kabra NH, Kuang AA, Diehl G, Sohn SJ, Bishop C, Winoto A. Receptor-mediated apoptosis in T lymphocytes. Cold Spring Harbor Symp. on Quant. Biol. 1999;64:363. doi: 10.1101/sqb.1999.64.363. [DOI] [PubMed] [Google Scholar]
- 40.Newton K, Harris AW, Bath ML, Smith KGC, Strasser A. A dominant interfering mutant of FADD/MORT1 enhance deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17(3):706. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beisner DR, Chu IH, Arechiga AF, Hedrick SM, Walsh CM. The Requirements for Fas-Associated Death Domain Signaling in Mature T Cell Activation and Survival. J Immunol. 2003;171:247. doi: 10.4049/jimmunol.171.1.247. [DOI] [PubMed] [Google Scholar]
- 42.Zornig M, Hueber A-O, Evan G. p53-dependent impairment of T-cell proliferation in FADD dominant-negative transgenic mice. Current Biology. 1998;8:467. doi: 10.1016/s0960-9822(98)70182-4. [DOI] [PubMed] [Google Scholar]
- 43.Newton K, Harris AW, Strasser A. FADD/MORT1 regulates the pre-TCR checkpoint and can function as a tumour suppressor. EMBO J. 2000;19:931. doi: 10.1093/emboj/19.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newton K, Kurts C, Harris AW, Strasser A. Effects of a dominant interfering mutant of FADD on signal transduction in activated T cells. Curr Biol. 2001;11:273. doi: 10.1016/s0960-9822(01)00067-7. [DOI] [PubMed] [Google Scholar]
- 45.Kabra NH, Kang C, Hsing LC, Zhang J, Winoto A. T cell-specific FADD-deficient mice: FADD is required for early T cell development. Proc Natl Acad Sci U S A. 2001;98:6307. doi: 10.1073/pnas.111158698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E, Wakeham A, Bouchard D, Yeh WC, McGlade JC, Ohashi PS, Hakem R. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 48.Ogasawara J, Suda T, Nagata S. Selective apoptosis of CD4+CD8+ thymocytes by the anti-fas antibody. J. Exp. Med. 1995;181:485. doi: 10.1084/jem.181.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suda T, Tanaka M, Miwa K, Nagata S. Apoptosis of mouse naive T cells induced by recombinant soluble Fas ligand and activation-induced resistance to Fas ligand. J. Immunol. 1996;157:3918. [PubMed] [Google Scholar]
- 50.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 51.Dhein J, Walczac H, Baumler C, Dabatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature. 1995;373:438. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 52.Brunner T, Mogil R, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Marin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 53.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 54.Sarin A, Conan-Cibotti M, Henkart PA. Cytotoxic effect of TNF and lymphotoxin on T lymphoblasts. J. Immunol. 1995;155:3716. [PubMed] [Google Scholar]
- 55.Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, Ramakrishnan P, Lapidot T, Wallach D. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 56.Singer GG, Abbas AK. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 57.Sytwu H-K, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 58.Hao Z, Hampel B, Yagita H, Rajewsky K. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J Exp Med. 2004;199:1355. doi: 10.1084/jem.20032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hildeman DA, Zhu Y, Mitchell TC, Kappler J, Marrack P. Molecular mechanisms of activated T cell death in vivo. Curr Opin Immunol. 2002;14:354. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 60.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 61.Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, Marrack PC. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 63.Yeh W-C, Ite A, Elia AJ, Ng M, Shu H-B, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, Mak TW. Requirement for Capser (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]