Abstract
Through employment of deuterium-labelled substrates, the triflic acid-catalyzed intramolecular exo-addition of the X–H(D) (X = N, O) bond of a sulfonamide, alcohol, or carboxylic acid across the C=C bond of a pendant cyclohexene moiety was found to occur, in each case, with exclusive formation (≥90%) of the anti-addition product without loss or scrambling of deuterium as determined by 1H and 2H NMR spectroscopy and MS analysis. Kinetic analysis of triflic acid-catalyzed intramolecular hydroamination of N-(2-cyclohex-2'-enyl-2,2-diphenylethyl)-p-toluenesulfonamide (1a) established the second-order rate law: rate = k2[HOTf][1a] and the activation parameters: ΔH‡ = 9.7 ± 0.5 kcal mol−1 and ΔS‡ = −35 ± 5 cal K−1 mol−1. An inverse α-secondary kinetic isotope effect of kD/kH = 1.15 ± 0.03 was observed upon deuteration of the C2' position of 1a, consistent with partial C–N bond formation in the highest energy transition state of catalytic hydroamination. The results of these studies were consistent with a mechanism for the intramolecular hydroamination of 1a involving concerted, intermolecular proton transfer from an N-protonated sulfonamide to the alkenyl C3' position of 1a coupled with intramolecular anti-addition of the pendant sulfonamide nitrogen atom to the alkenyl C2' position.
Introduction
The catalytic addition of the X–H (X = O, N) bond of a nitrogen or oxygen nucleophile across the C=C bond of an electronically unactivated alkene (hydrofunctionalization) represents an attractive and atom economical approach to the formation of C–X bonds.[1] Intramolecular processes are particularly attractive as expedient routes to the synthesis of oxygen and nitrogen heterocycles. Although much of the effort in the area of catalytic alkene hydrofunctionalization has focused on transition metal-based processes, Brønsted acids also catalyze the hydrofunctionalization of C=C bonds, oftentimes with rates and selectivities comparable to transition metal-catalyzed methods.[2–5] For this reason, there is growing concern that a number of metal-based hydrofunctionalization processes, particularly those that employ electrophilic metal complexes or metal triflates in combination with modestly basic nucleophiles, may be catalyzed by Brønsted acid generated under reaction conditions.[5,6]
Distinguishing between transition metal- and Brønsted acid-catalyzed pathways for intramolecular alkene hydrofunctionalization is complicated by a conspicuous gap in our understanding of the mechanisms of Brønsted acid-catalyzed alkene hydrofunctionalization. Whereas the mechanisms of Brønsted acid-mediated intermolecular alkene hydrofunctionalization have been studied for decades,[7–33] mechanistic information regarding the corresponding intramolecular processes is scarce, and none of the available data pertains to electronically unactivated alkenes. Hosomi has reported that the intramolecular hydroalkoxylation and hydroamination of vinylsilanes with alcohols and sulfonamides occurs with ~85% syn-stereoselectivity, which was attributed to intramolecular proton transfer from a protonated nucleophile to the C=C bond of the alkene followed by stereoselective trapping of the more stable β-silylcarbenium rotamer.[34] Hartwig and Schlummer proposed a similar mechanism for the triflic acid-catalyzed intramolecular hydroamination of vinylarenes with sulfonamides involving intramolecular proton transfer from the protonated sulfonamide to the alkene followed by trapping of the resulting benzylic carbenium ion. However, this latter study included neither stereochemical nor kinetic data.[4]
Owing to the considerable current interest in the catalytic intramolecular hydrofunctionalization of electronically unactivated alkenes,[1] we sought to gain information regarding the mechanisms of the Brønsted acid-catalyzed intramolecular hydrofunctionalization of unactivated alkenes. Here we report the stereochemical analysis of the Brønsted acid-catalyzed intramolecular hydrofunctionalization of a cyclohexene moiety with a sulfonamide, alcohol, and carboxylic acid supported by the kinetic analysis of Brønsted acid-catalyzed intramolecular hydroamination. The results of these studies, particularly in the case of intramolecular hydroamination, support a mechanism involving concerted, intermolecular protonation of the alkene coupled with intramolecular anti-addition of the pendant nucleophile.
Results
Stereochemistry of Hydroamination
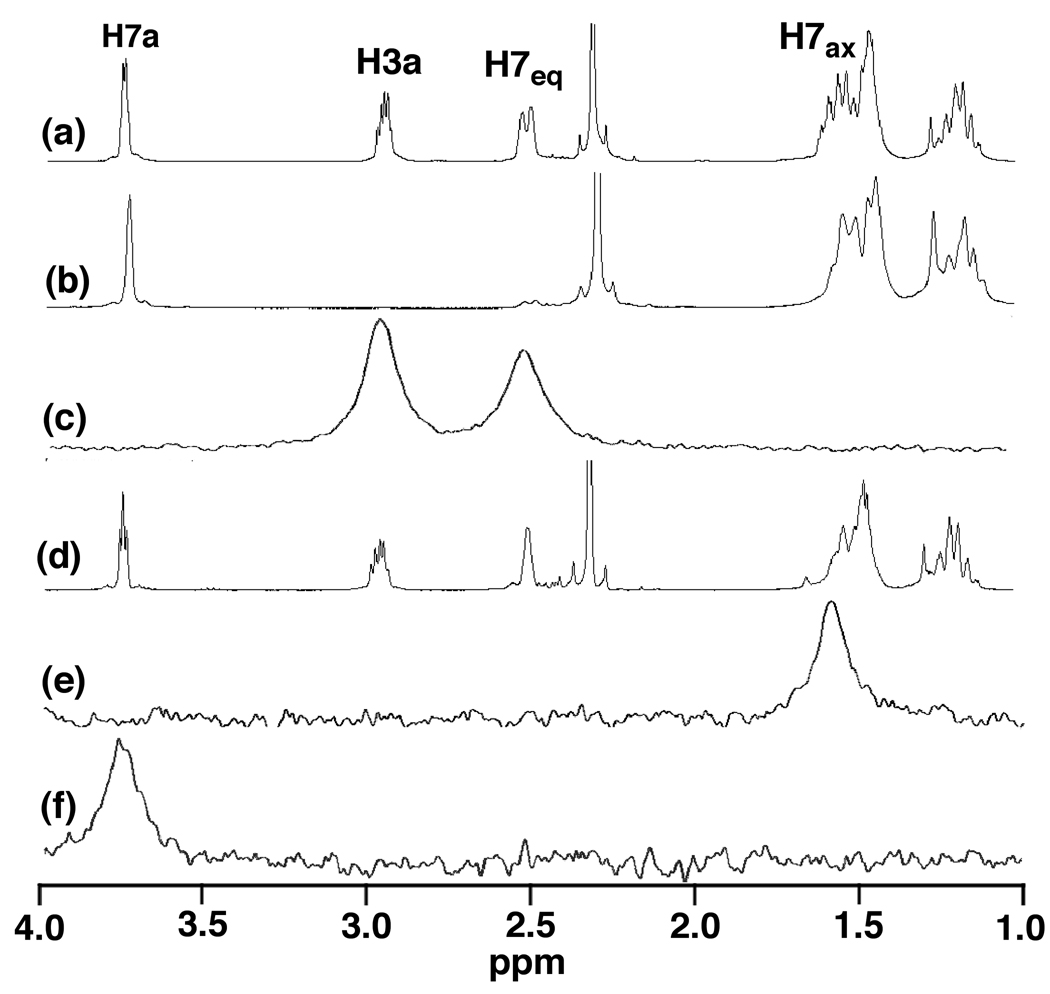
To evaluate the stereoselectivity of Brønsted acid-catalyzed intramolecular alkene hydroamination, we targeted the doubly deuterium-labelled γ-alkenyl sulfonamide N-(2-1',3'-dideuteriocyclohex-2'-enyl-2,2-diphenylethyl)-p-toluenesulfonamide (1a-1’,3’-d2) that has been previously employed to evaluate the stereoselectivity of gold(I)-catalyzed alkene hydroamination.[35] Treatment of 1a-1’3’-d2 (83 ± 1% d2, 17% d1 by MS, ~85% deuterated at C3' by 1H NMR) with a catalytic amount of triflic acid (5 mol %) in toluene at 85 °C for 48 h led to 5-exo hydroamination with isolation of 2a-3a,7eq-d2 (83 ± 1% d2, 16% d1 by MS) as the exclusive dideuterated isotopomer in 96% yield without loss of deuterium (eq 1).[36] Single crystal X-ray analysis of protio isotopomer 2a revealed a cis-fused chair cyclohexane with an equatorial diphenylalkyl substituent and an axial sulfonamide substituent.[37] High-field 1H NMR (Figure 1, spectrum a), 1H-1H COSY, and 1H-1H NOESY analysis confirmed that the solid-state conformation of 2a was preserved in solution and allowed unambiguous assignment of all aliphatic proton resonances.[38] Integration of the H7eq resonance at δ = 2.49 in the 1H NMR spectrum of 2a-3a,7eq-d2 revealed ~85% deuteration at this position (Figure 1, spectrum b). More importantly, 2H NMR analysis of 2a-3a,7eq-d2 displayed a ~1:1 ratio of resonances at δ = 2.95 (C3a) and δ = 2.49 (C7eq) with no detectable deuteration at either the C7ax (δ ≈ 1.58) or C7a (δ 3.73) positions (Figure 1, spectrum c). Together, these observations established the net anti-addition of the N–H bond across the pendant C=C bond of 1a-1’,3’-d2 without loss or scrambling of deuterium.
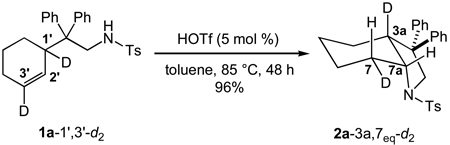 |
eq 1 |
Figure 1.
Partial 1H NMR Spectrum of 2a (a), Partial 1H and 2H NMR Spectra of 2a-3a,7eq-d2 (b and c), Partial 1H and 2H NMR Spectra of 2a-7ax-d1 (d and e), and Partial 2H NMR Spectra of 2a-7a-d1 (f)
To corroborate the findings outlined in the preceding paragraph, we evaluated the stereoselectivity of the acid-catalyzed intramolecular deuterioamination of N-deuterated isotopomer 1a-N-d.[39] Treatment of 1a-N-d (~90% d by 1H NMR) with a catalytic amount of DOTf (5 mol %) at 85 °C in toluene for 48 h led to isolation of 2a-7ax-d1 (90 ± 1% d by MS) as the exclusive deuterated isotopomer in 77% yield (eq 2). Integration of the H7eq resonance at δ = 2.49 in the 1H NMR spectrum of 2a-7ax-d1 revealed no significant deuteration at this position (Figure 1, spectrum d), while 2H NMR analysis displayed a single resonance at δ = 1.58 corresponding to deuteration of the C7ax position with no detectable deuteration at either the C7eq (δ = 2.49) or C7a (δ = 3.73) positions (Figure 1, spectrum e).
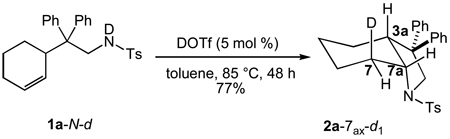 |
eq 2 |
The absence of deuterium incorporation into the C7a and C7eq positions of 2a-7ax-d1 in the DOTf-catalyzed cyclization of 1a-N-d argues against reversible deuteronation of the C2' or C3' carbon atoms of the cyclohexenyl moiety prior to cyclization. To further probe for reversible protonation/deuteronation of the alkene prior to cyclization, the reaction of 1a-N-d (~90% d) and a catalytic amount of DOTf (5 mol %) at 60 °C in toluene was monitored periodically by 2H NMR spectroscopy and quenched at ~50% conversion by addition of triethylamine. A similar experiment utilizing 1H NMR analysis of a mixture of 1a-N-d (~90% d) and DOTf (5 mol %) in toluene-d8 was run concurrently. 1H and 2H NMR analysis of the respective solutions revealed no positional isomerization and no detectable incorporation of deuterium into the C2' or C3' positions (δ = 5.68 and 5.58) of 1a-N-d. These observations, together with those outlined above, argue strongly against reversible deuteronation of either the cyclohexene C2' or C3' carbon atom prior to cyclization.
Stereoselectivity of Hydroalkoxylation and Hydroacyloxylation
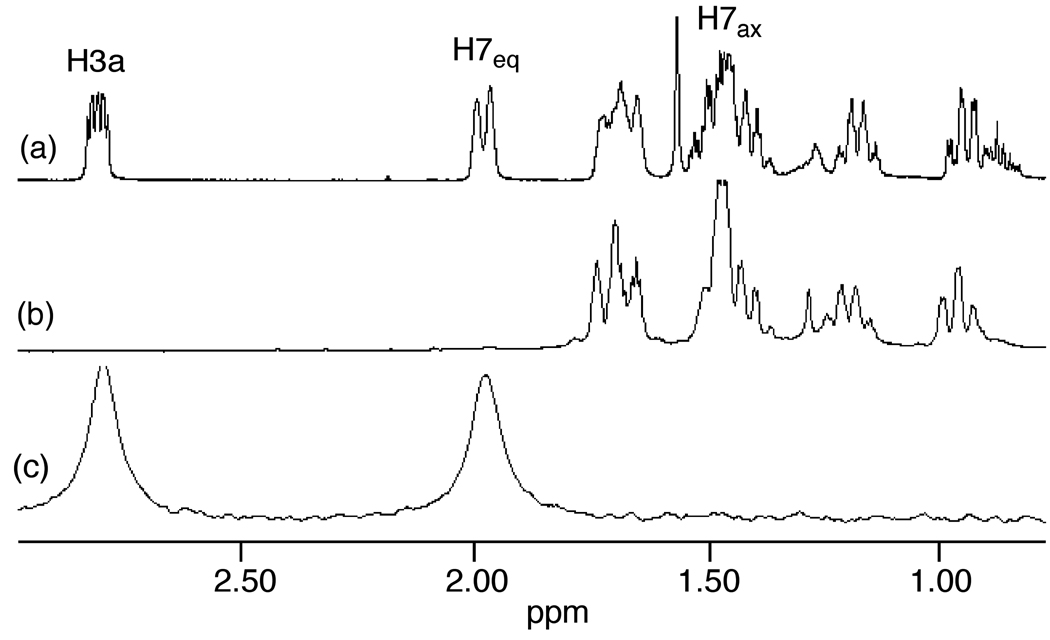
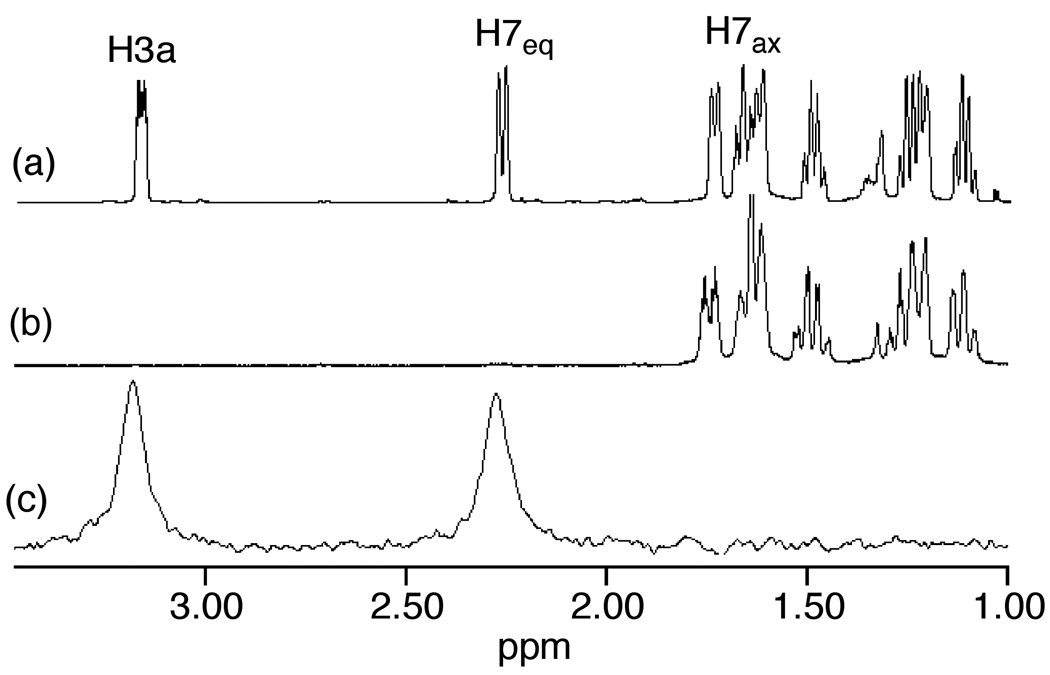
We extended our stereochemical analysis of acid-catalyzed alkene hydrofunctionalization to include catalytic hydroalkoxylation and hydroacyloxylation employing an approach similar to that employed for intramolecular hydroamination. In one experiment, treatment of the γ-alkenyl alcohol 1b-1’,3’-d2 (96% d2 by MS) with a catalytic amount of triflic acid (5 mol %) led to 5-exo hydroalkoxylation to form 2b-3a,7eq-d2 in 96% isolated yield as the exclusive dideuterated isotopomer with no loss or scrambling of deuterium (95% d2 by MS) (eq 3). 1H and 2H NMR analysis of 2b-3a,7eq-d2 revealed ≥95% deuteration of the C7eq (δ = 1.89) and C3a (δ = 2.71) positions with no detectable deuteration at the C7ax (δ = 1.34) or C7a (δ = 4.12) positions (Figure 2). Similarly, treatment of β-alkenyl carboxylic acid 1c-1’,3’-d2 (>99% d2 by MS) with a catalytic amount of triflic acid (5 mol %) led to 5-exo hydroacyloxylation to form 2c-3a,7eq-d2 as the exclusive dideuterated isotopomer (>98% d2 by MS) in quantitative yield (eq 4). 1H and 2H NMR analysis of 2c-3a,7eq-d2 revealed ≥95% deuteration of the C7eq (δ = 2.16) and C3a (δ = 3.07) positions with no detectable deuteration at the C7ax (δ = 1.55) or C7a (δ = 4.61) positions (Figure 3). In both cases, these results established the net anti-addition of the O–H bond across the C=C bond of the cyclohexene moiety.
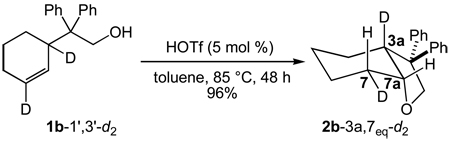 |
eq 3 |
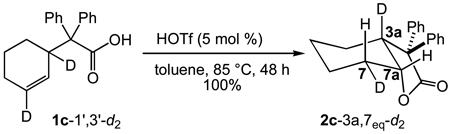 |
eq 4 |
Figure 2.
Partial 1H NMR Spectrum of 2b (a) and Partial 1H and 2H NMR Spectra of 2b-3a,7eq-d2 (b and c) and 2H NMR Spectra of 2a-7ax-d1 (d and e), and Partial 2H NMR Spectra of 2a-7a-d1 (f)
Figure 3.
Partial 1H NMR Spectrum of 2c (a) and Partial 1H and 2H NMR Spectra of 2c-3a,7eq-d2 (b and c).
Effect of Solvent and Acid on Hydrofunctionalization
The efficiency and stereoselectivity of Brønsted acid-catalyzed intramolecular hydrofunctionalization was evaluated as a function of acid and solvent at 85 °C (Table 1).Treatment of substrates 1-1’,3’-d2 with a catalytic amount of triflic acid (5 mol %) in diglyme at 85 °C for 48 h led, in each case, to complete consumption of starting material to form 5-exo hydrofunctionalization products 2-3a,7eq-d2 as the exclusive dideuterated isotopomers (Table 1, entries 1 – 3). In comparison, reaction of substrates 1-1’,3’-d2 with triflic acid in acetonitrile effected a significant decrease in reaction rate but led, in each case, to formation of 5-exo hydrofunctionalization products 2-3a,7eq-d2 as the exclusive isotopomers (Table 1, entries 4 – 6). Attempts to cyclize substrates 1-1’,3’-d2 with weaker acids such HCl or trifluoroacetic acid (TFA) in toluene at 85 °C for 48 h gave low conversions (Table 1, entries 7 – 12).
Table 1.
Brønsted Acid-Catalyzed Intramolecular Hydrofunctionalization of Substrates 1-d2 at 85 °C for 48 h as a Function of Solvent and Acid (5 mol %).
| entry | substrate | solvent | acid | product[a,b] | convn(%)[b] |
|---|---|---|---|---|---|
| 1 | 1a-1’3’-d2 | diglyme | HOTf | 2a-3a,7eq-d2 | ≥95 |
| 2 | 1b-1’3’-d2 | diglyme | HOTf | 2b-3a,7eq-d2 | ≥95 |
| 3 | 1c-1’3’-d2 | diglyme | HOTf | 2c-3a,7eq-d2 | ≥95 |
| 4 | 1a-1’3’-d2 | CH3CN | HOTf | 2a-3a,7eq-d2 | 54 |
| 5 | 1b-1’3’-d2 | CH3CN | HOTf | 2b-3a,7eq-d2 | 36 |
| 6 | 1c-1’3’-d2 | CH3CN | HOTf | 2c-3a,7eq-d2 | 43 |
| 7 | 1a-1’3’-d2 | toluene | HCl | 2a-3a,7eq-d2 | 11 |
| 8 | 1b-1’3’-d2 | toluene | HCl | 2b-3a,7eq-d2 | ≤5 |
| 9 | 1c-1’3’-d2 | toluene | HCl | 2c-3a,7eq-d2 | ≤5 |
| 10 | 1a-1’3’-d2 | toluene | TFA | 2a-3a,7eq-d2 | 12 |
| 11 | 1b-1’3’-d2 | toluene | TFA | 2b-3a,7eq-d2 | 22 |
| 12 | 1c-1’3’-d2 | toluene | TFA | 2c-3a,7eq-d2 | ≤5 |
Stereoselectivity was ≥90% in all cases.
Conversion and stereoselectivity were determined by 1H NMR of the purified reaction mixture.
Kinetics of Hydroamination
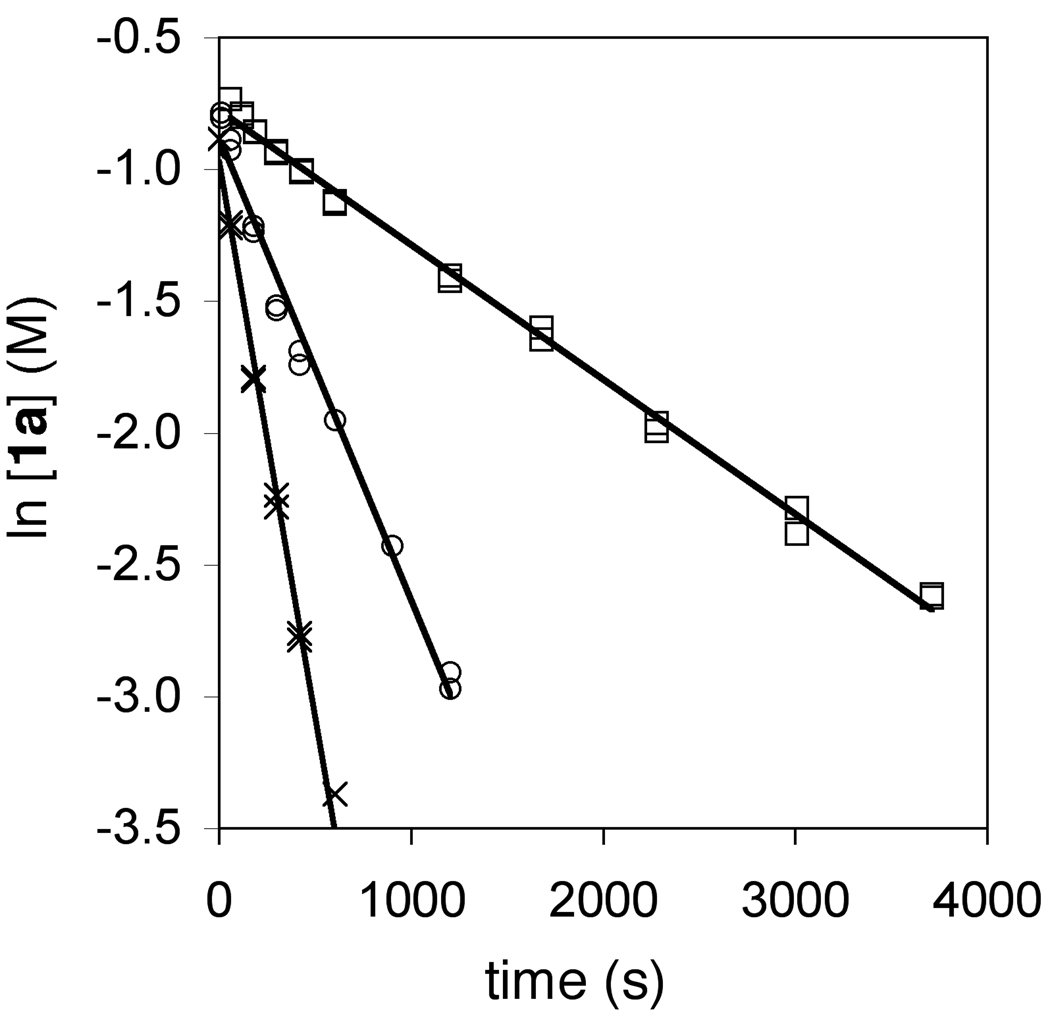
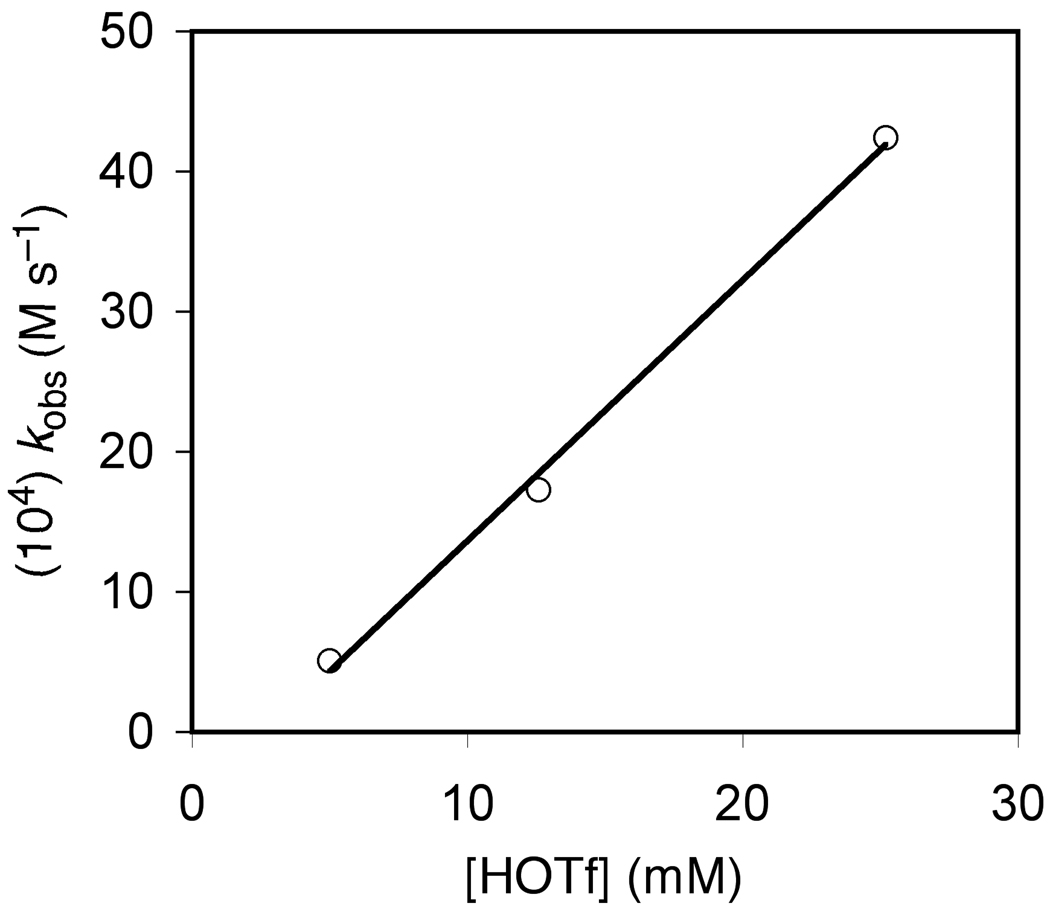
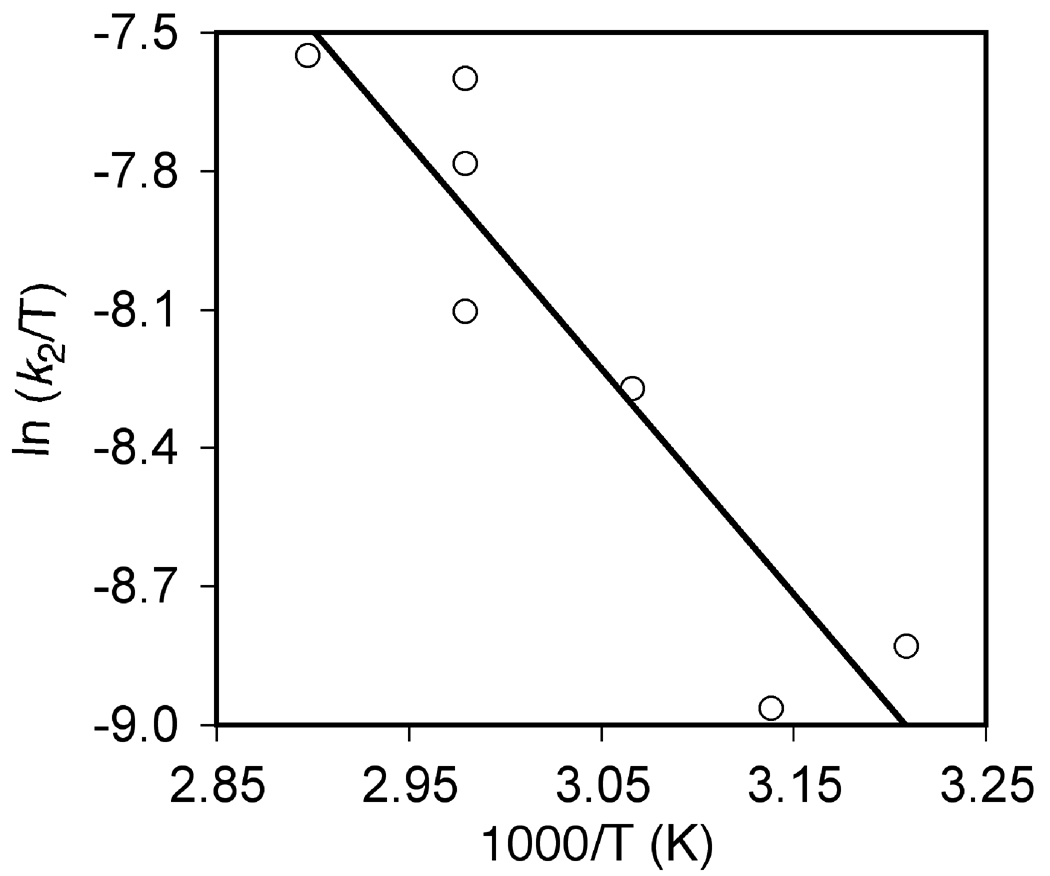
We sought to gain additional information regarding the mechanism of Brønsted acid-catalyzed hydroamination through kinetic analysis of the triflic acid-catalyzed conversion of 1a to 2a. To this end, reaction of 1a (0.50 M) with a catalytic amount of HOTf (25 mM) in toluene at 62 °C was analyzed periodically by liquid chromatography. A plot of ln[1a] versus time was linear to ~3 half lives with an observed rate constant of kobs = 4.2 ± 0.1 × 10−3 s−1, which established the first-order dependence of the rate on [1a] (Figure 4; Table 2, entry 1). To determine the dependence of the rate on triflic acid concentration, observed rate constants for the conversion of 1a to 2a were determined at [HOTf] = 5.0 and 12.6 mM (Figure 4; Table 2, entries 2 and 3). A plot of kobs versus [HOTf] was linear (Figure 5), which established the first-order dependence of the rate on [HOTf] and the second-order rate law: rate = k2[1a][HOTf] where k2 = 1.37 ± 0.04 × 10−1 M−1 s−1 (ΔG‡336K = 21.2 ± 0.1 kcal mol−1). To determine the activation parameters for the triflic acid-catalyzed conversion of 1a to 2a, second-order rate constants for the conversion of 1a to 2a were determined as a function of temperature from 39 to 72 °C (Table 2, entries 4–7). An Eyring plot of these data provided the activation parameters: ΔH‡ = 9.7 ± 0.5 kcal mol−1 and ΔS‡ = −34 ± 5 cal K−1 mol−1 (Figure 6).
Figure 4.
First-Order Plots for the Conversion of 1a ([1a]0 = 0.50 M) to 2a Catalyzed by Triflic Acid {[HOTf] = 5.0 (□), 12.6 (○), and 25.2 (×) mM} in Toluene at 62 °C
Table 2.
Observed Rate Constants for the Conversion of 1a ([1a]0 = 0.5 M) to 2a Catalyzed by HOTf in Toluene as a Function of Temperature and [HOTf]
| entry | [HOTf] (mM) | temp (°C) | (103) kobs (M s-1) |
|---|---|---|---|
| 1 | 25 | 62.5 | 4.2 ± 0.1 |
| 2 | 12.6 | 62.5 | 1.54 ± 0.07 |
| 3 | 5.0 | 62.5 | 0.511 ± 0.007 |
| 4 | 25 | 38.5 | 1.15 ± 0.04 |
| 5 | 25 | 45.5 | 1.02 ± 0.03 |
| 6 | 25 | 53.0 | 2.10 ± 0.08 |
| 7 | 25 | 72.0 | 2.29 ± 0.07 |
Figure 5.
Plot of kobs Versus Triflic Acid Concentration for the Conversion of 1a ([1a]0 = 0.5 M) to 2a in Toluene at 62 °C
Figure 6.
Eyring Plot for the Conversion of 1a ([1a]0 = 0.5 M) to 2a Catalyzed by HOTf (25 mM) in Toluene over the Temperature Range 39–72 °C
Hartwig and Schlummer have shown that N-alkyl-p-toluenesulfonamides are quantitatively protonated by triflic acid,[4] consistent with the significantly greater acidity of HOTf (pKa ≈ −15) relative to a protonated sulfonamide (pKa ≈ −5);[40–42] it is also known that sulfonamides are protonated at nitrogen rather than at oxygen.[43] Therefore, the active catalytic species in the conversion of 1a to 2a is most likely an N-protonated sulfonamide. Laughlin[41] and Olavi et al.[42] have shown that the conjugate acids of N-alkyl sulfonamides are more acidic than are the conjugate acids of N,N-dialkyl sulfonamides by ~0.5 pKa (H0) units. However, if 1a•HOTf were more acidic than 2a•HOTf, deviation from first-order behavior in the conversion of 1a to 2a would be observed due to the changing composition of the acidic species with increasing conversion. Because no significant deviation from linearity was observed in any of the pseudo first-order plots for the conversion of 1a to 2a (Figure 4), it appears that acidity and/or reactivity of 1a•HOTf and 2a•HOTf are not significantly different. For these reasons, the rate law for the conversion of 1a to 2a of k2[1a][HOTf] is more appropriately described as rate = k2[1a][R2NTs•HOTf] [R2NTs = 1a and 2a].
α-Secondary Kinetic Isotope Effect
α-Secondary kinetic isotope effects (KIEs) have been utilized to probe for transition state rehybridization in the elucidation of a range of organic reaction mechanisms.[44] As was outlined by Steitwieser,[45] α-secondary KIEs are typically attributed to changes in the stretching and bending frequencies of a C–H bond undergoing ground state → transition state rehybridization.[46] Because C–H stretching and in-plane bending frequencies differ little between sp3 and sp2 centers, α-KIEs arise primarily from the significantly higher out-of-plane bending frequency of an sp3 C–H bond (~1340 cm−1) relative to an sp2 C–H bond (~800 cm−1), which produces a normal KIE in the case of sp3 → sp2 rehybridization and an inverse KIE in the case of sp2 → sp3 rehybridization.
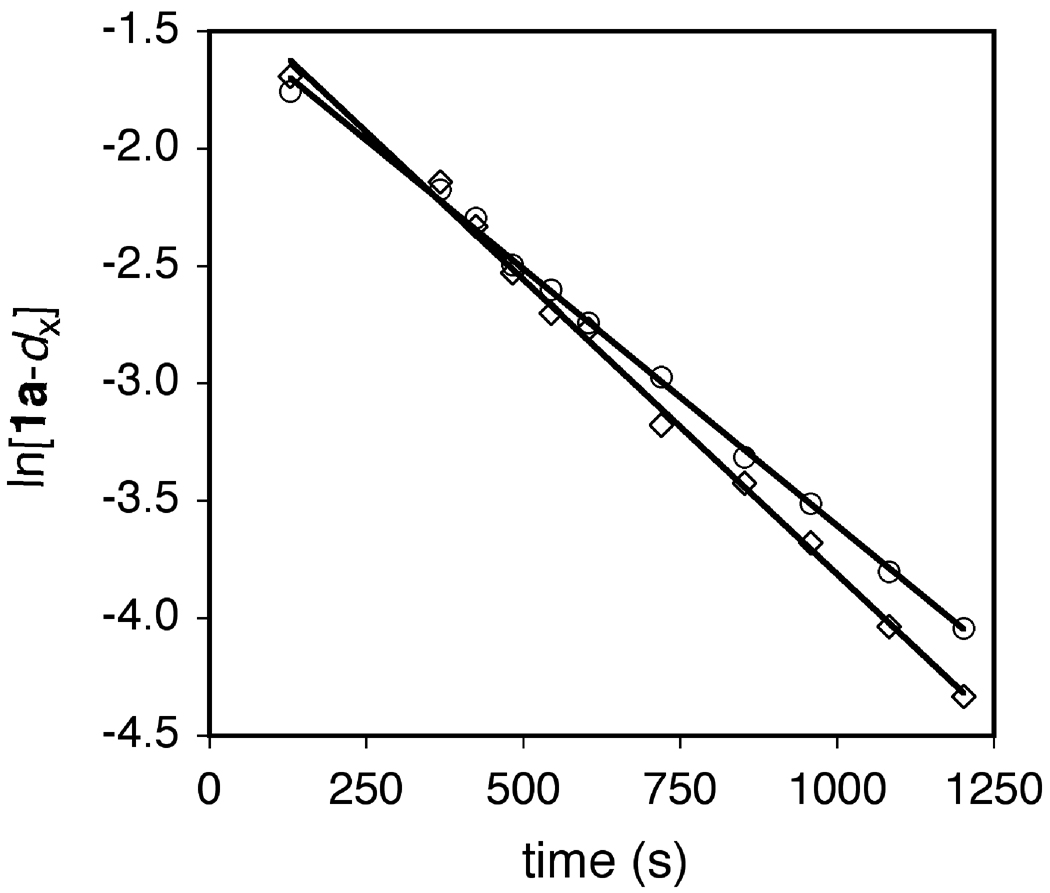
To probe for rehybridization of the C2' carbon atom of the cyclohexenyl moiety in the transition state of the turnover-limiting step of the triflic acid-catalyzed hydroamination of 1a, we determined the α-secondary KIE resulting from deuteration of the C2' carbon of the cyclohexenyl moiety employing deuterated isotopomer 1a-2’-d1. Owing to the small magnitude of secondary KIEs[44] and to avoid errors associated with variations in catalyst concentration and temperature, the α-secondary KIE was determined though a competition experiment. To this end, a ~1:1 mixture of 1a and 1a-2’-d1 and a catalytic amount of HOTf (5 mol %) in toluene was heated at 60 °C and analyzed periodically by LCMS. The concentrations of 1a and 1a-2’-d1 were determined from total conversion and from the isotopic ratios 1a:1a-2’-d1 and 2a:2a-7a-d1. Plots of ln[1a] and ln[1a-2’-d1] versus time were linear to ~3 half lives with observed rate constants of kobs = 2.19 ± 0.03 × 10−3 s−1 and 2.51 ± 0.05 × 10−3 s−1, respectively (Figure 7), which correspond to an inverse KIE of kD/kH = 1.15 ± 0.03. In a separate experiment, treatment of 1a-2’-d1 (75 ± 1 % d1 by MS) with a catalytic amount of HOTf (5 mol %) at 85 °C in toluene for 48 h led to isolation of 2a-7a-d1 (76 ± 1% d by MS) as the exclusive deuterated isotopomer in 77% yield without detectable loss or scrambling of deuterium as determined by MS and 2H NMR analysis (eq 5; Figure 1, spectrum f).
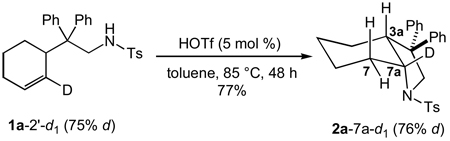 |
eq 5 |
Figure 7.
First-Order Plots for the Conversion of 1a to 2a (○) and 1a-2'-d1 to 2a-7a-d1 (□), Catalyzed by Triflic Acid (5 mol %) in Toluene at 60 °C
Discussion
Mechanisms of Intermolecular Electrophilic Alkene Hydrofunctionalization
Extensive kinetic analyses of the Brønsted acid-catalyzed hydration and intermolecular hydroalkoxylation of conjugated and non-conjugated alkenes reveals that with few exceptions, these transformations occur via irreversible, turnover-limiting protonation of the C=C bond followed by nucleophilic trapping of a solvated carbenium ion intermediate.[7] Although early work by Taft suggested that proton transfer was preceded by reversible formation of a π-protonium complex,[8] this hypothesis has been largely discounted.[7,9] Deviations from the general hydration mechanism are rare but may occur in the cases of particularly long-lived or short-lived carbenium ions. For example, mechanisms involving rapid and reversible C=C protonation followed by rate-limiting nucleophilic addition to the resulting carbenium ion have been documented in the cases of highly stabilized carbenium ions.[10]
Drawing from the analyses of Jencks,[11] Kresge posited that preassociation or concerted mechanisms for alkene hydration may be enforced by short carbenium ion lifetimes.[12] On the basis of this analysis, Kresge considered, but ultimately discounted, a preassociation pathway for the acid-catalyzed hydration of trans-cyclooctene; however, Kresge also suggested that preassociation pathways may be operative for the hydration of unstrained olefins that generate secondary carbenium ions in dilute acid conditions.[12] Similarly, consideration of carbenium ion lifetimes led both Jencks[13] and Herlihy[14] to propose concerted pathways for the hydration of monosubstituted alkenes under dilute acid conditions, although in neither case were these pathways rigorously established.
The mechanisms of the addition of hydrogen halides to alkenes and the addition of acetic acid to non-conjugated alkenes catalyzed by hydrogen halides and related Brønsted acids have also been investigated.[15–32] These transformations typically occur with ~85% anti-selectivity in the case of non-conjugated acyclic alkenes and with >95% anti-selectivity in the case of non-conjugated cyclic alkenes.[16–25,27] Hydrogen halide addition typically obeys the ternary rate law: rate = k[alkene][HX]2, whereas the acid-catalyzed hydroacetoxylation typically obeys the binary rate law: rate = k[alkene][HX].[17–19,26–29] Both AdE3 pathways involving concerted C–H and C–X (X = halide or OAc) addition across the C=C bond of the alkene[16,18,19,22,23,25,27,28] and/or stepwise AdE2 pathways involving rate-limiting, halide assisted protonation of the alkene followed by rapid trapping of a tight carbenium ion pair have been proposed to account for these observations.[17,18,22,25,26,29] Initial formation of a π-protonium complex has also been invoked[16,24,25] but, as was the case for alkene hydration, little direct evidence supports these hypotheses.[30]
In contrast to the behavior of weaker Brønsted acids, Roberts reported that the HOTf-catalyzed addition of acetic acid-O-d to cyclopentene was non-stereoselective.[31] In a separate study, Pasto reported that the HOTf-catalyzed addition of acetic acid-O-d to 2-butene occurred with modest (57–72%) anti-stereoselectivity and was accompanied by alkene isomerization and H/D exchange.[23] The latter transformation was proposed to occur through an AdE2 pathway involving reversible formation of a carbenium ion pair that was trapped by acetic acid. The slight preference for anti-addition was attributed to steric shielding of the syn face of the carbenium ion in the tight ion pair. In comparison, the addition of hydrogen halides to cyclic and acyclic vinyl arenes occurs with up to 90% syn-selectivity in low-polarity solvents such as dichloromethane.[32] This behavior is in accord with a stepwise AdE2 pathway involving turnover-limiting protonation to form a tight ion pair that undergoes rapid collapse (syn-addition) or rearrangement followed by collapse (nonselective).
Recently, the mechanisms of triflic acid-catalyzed intermolecular alkene hydrofunctionalization with phenols and protected amines has been investigated by a pair of DFT studies.[33] In both cases, calculations predict a concerted, syn-addition of H–X (X = N, O) bond of the nucleophile across the C=C bond of the alkene via an eight-membered cyclic transition state in which triflic acid interacts with both the alkene and the nucleophile.[33]
Mechanism of Acid-Catalyzed Conversion of 1a to 2a
Our experimental observations rule out several potential mechanisms for the acid-catalyzed conversion of 1a to 2a. The absence of deuterium scrambling, alkene isomerization, and/or incorporation of deuterium into unreacted starting material in the acid-catalyzed reactions of isotopomers 1a-1’,3’-d2, 1a-N-d, and 1a-2’-d1 argues strongly against mechanisms involving rapid and reversible protonation/deuteronation of the C2' or C3' alkenyl carbon atoms followed by turnover-limiting attack of the pendant sulfonamide on a C2' carbenium ion. Furthermore, the anti-stereoselectivity and second-order rate law for the conversion of 1a to 2a rule out a mechanism analogous to those proposed by Hosomi[35] and Hartwig[4] involving intramolecular proton transfer from a protonated sulfonamide to the C3' alkenyl carbon atom followed by trapping of the resulting carbenium ion with the neutral sulfonamide moiety.
We therefore considered mechanisms for the acid-catalyzed conversion of 1a to 2a initiated by turnover-limiting, irreversible intermolecular proton transfer from a protonated sulfonamide to the C3' alkenyl carbon of 1a. Of the possible mechanisims that meet this requirement, stepwise pathways involving a solvationally equilibrated carbenium ion (AdE2) or a tight ion pair are inconsistent with our experimental observations, as is a stepwise preassociation pathway. Because the regio- and stereoselectivity of C–N bond formation in the conversion of 1a to 2a is largely predetermined by substrate geometry, protonation must occur regio- and stereoselectively at the C3' position of the cyclohexene moiety on the face opposite that occupied by the diphenylethylsulfonamide group. Although delivery of a proton to the less sterically hindered face of the alkene is reasonable, regioselective protonation of the electronically unbiased C=C bond at C3' without participation of the pendant sulfonamide group appears unlikely. Preassociation of the sulfonamide nitrogen atom and alkene C2' atom prior to intermolecular proton transfer to C3' in a manner analogous to that suggested by Kresge[12] accounts for the regioselectivity of proton transfer only if the nitrogen atom is felt in the transition state for protonation, at which point, C–H and C–N bond formation become concerted.[11]
Key to distinguishing between stepwise and concerted mechanisms for the conversion of 1a to 2a is the α-secondary KIE of kD/kH = 1.15 ± 0.03 determined for the conversion of 1a-2’-d1 to 2a-7a-d1. This observation points to significant C–N bond formation in the turnover-limiting step of hydroamination and argues strongly against a stepwise pathway involving turnover-limiting carbenium ion formation. Employing Streitwieser's approximation[45] of the Bigeleisen equation[47] and representative C–H stretching and bending frequencies for the sp2 carbon of cis-2-butene as a model for 1a and the sp3 methine carbon of a secondary alcohol as a model for 2a,[48,49] we estimate a maximum α-secondary KIE for the conversion of 1a to 2a resulting from conversion of an alkene ground state to a tetrahedral transition state of kD/kH ≈ 1.20 at 333 K. Consideration of calculated fractionation factors for H/D exchange between olefinic and aliphatic positions predicts a similar value.[50] Conversely, because conversion of 1a to 2a via turnover-limiting carbenium ion formation would occur without transition state rehybridization, such a process should occur without a significant α-secondary KIE. Supporting this contention, the calculated fractionation factor of 1.179 for H/D exchange between a secondary carbenium ion and secondary alkyl moiety indicates that fractionation factors between an olefinic and carbenium hydrogen differ by only a few percent.[51]
Available experimental data regarding the α-secondary KIEs of the Brønsted acid-promoted addition of nucleophiles to alkenes are in accord with the analysis provide above. For example, the thiocyanate-catalyzed isomerization of maleic acid-d2 to fumaric acid-d2 displayed an inverse KIE of kD/kH = 1.17 (corrected for H/D exchange) at 25 °C, attributed to turnover-limiting conjugate addition of thiocyanate to an O-protonated maleic acid.[52] In contrast, acid-catalyzed hydration of α-deuteriostyrene[53] or 4,4-dideuterio-1-phenyl-1,3-butadiene[54] displayed no detectable KIE, consistent with turnover-limiting carbenium ion formation. Acid-catalyzed hydrolysis of ethyl vinyl ether produced a small inverse α-secondary KIE of kD/kH = 1.036 ± 0.004; however, this effect was attributed to an inductive KIE rather than to an α-secondary KIE.[55]
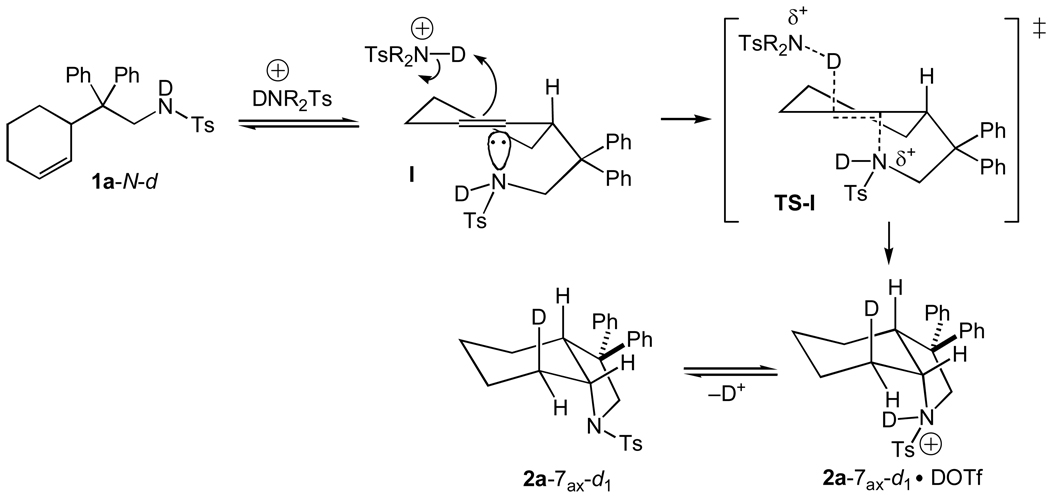
All of our experimental observations, including the second-order rate law, activation parameters, inverse α-secondary KIE, and anti-stereoselectivity are consistent with a concerted mechanism for the conversion of 1a to 2a. Our proposed mechanism is depicted in Scheme 1 for the DOTf-catalyzed conversion of 1a-N-d to 2a-7ax-d1. As has been noted by Jencks,[11] preassociation is a necessary prerequisite for concerted reaction pathways and, as such, conversion of 1a-N-d to 2a-7ax-d1 is likely initiated by formation of the alkene-sulfonamide encounter complex I. Intermolecular deuteron transfer from an N-deuterated sulfonamide to the C3' alkenyl carbon atom of 1a-N-d in concert with intramolecular anti-addition of the pendant sulfonamide nitrogen atom to the C2' alkenyl carbon atom via transition state TS-I would generate the N-deuterated sulfonamide cation 2a-7ax-d1•DOTf. Intermolecular deuteron transfer from 2a-7ax-d1•DOTf either to a second sulfonamide nitrogen atom or to the C3' carbon atom of a second molecule of 1a-N-d would release 2a-7ax-d1 and continue the catalytic cycle (Scheme 1).
Scheme 1.
Proposed Mechanism of the DOTf-Catalyzed Cyclization of 1a-N-d
Conclusion
We have shown that the Brønsted acid-catalyzed intramolecular hydrofunctionalization of a cyclohexenyl moiety with a sulfonamide, alcohol, or carboxylic acid occurs with net anti-addition of the H–X (X = N, O) bond across the pendant C=C bond of the cyclohexene moiety and without positional isomerization or H/D exchange. Kinetic analysis of the intramolecular hydroamination of cyclohexenyl sulfonamide derivative 1a established a second-order rate law and large negative entropy of activation. Kinetic analysis of the intramolecular hydroamination of deuterated isotopomer 1a-3'-d revealed an inverse α-secondary KIE of kD/kH = 1.15 ± 0.03, consistent with significant C–N bond formation in the turnover-limiting step of hydroamination. All of our experimental observations regarding the Brønsted acid-catalyzed the conversion of 1a to 2a support a mechanism involving concerted intermolecular transfer of a proton from an N-protonated sulfonamide to the C3' carbon atom of 1a coupled with intramolecular anti-addition of the pendant sulfonamide nitrogen atom to the C2' carbon atom. The high anti-stereoselectivity and absence of deuterium scrambling in the Brønsted acid-catalyzed intramolecular hydroalkoxylation of 2b-1',3'-d2 and hydroacyloxylation of 2c-1',3'-d2 suggests that these transformations occur via a similar pathway.
Perhaps the most significant implication of our study is that the stereoselectivity of these Brønsted acid-catalyzed intramolecular hydrofunctionalization processes is indistinguishable from that expected for an outer-sphere transition metal-catalyzed pathway.[56] Furthermore, in the event that Brønsted acid were generated stoichiometrically from a metal precursor, the resulting acid-catalyzed intramolecular hydrofunctionalization would appear to conform to the second-order rate law: rate = [metal][substrate], apparently consistent with a metal-catalyzed cyclization. The absence of positional isomerization or olefinic H/D exchange would also appear consistent with a metal-catalyze transformation. As a result, it appears that strong corroborating evidence and/or rigorous control experiments are required to confidently discount the presence of Brønsted acid-catalyzed reaction pathways in metal-based alkene hydrofunctionalization processes.
Unknown at this time is the effect of substrate structure on the mechanism of Brønsted acid-catalyzed intramolecular alkene hydrofunctionalization, such as in the cases of acyclic alkenes or conjugated alkenes. Further studies in this area will probe the stereochemistry and mechanisms of these permutations of acid-catalyzed alkene hydrofunctionalization.
Experimental Section
General procedure for acid-catalyzed hydrofunctionalization
Synthesis of 2a.[35] Triflic acid (0.7 µL, 7.5 × 10−3 mmol) was added via syringe to a solution of 1a (64.7 mg, 0.150 mmol) in toluene (0.3 mL). The resulting solution was heated at 60 °C for 3 h, cooled to room temperature, and filtered through a plug of silica gel. Solvent was evaporated under vacuum to give pure 2a (64.5 mg, 100 %) as a white solid. The aliphatic protons of 2a were unambiguous assigned on the basis of combined 1H–1H 800 MHz COSY and 1H–1H NOESY analysis at 45 °C in CDCl3 (See Supporting Information).[38] 1H NMR (800 MHz, CDCl3, 45 °C): δ 7.43 (d, J = 8.0 Hz, 2 H), 7.19 (t, J = 8.0 Hz, 2 H), 7.09 (t, J = 8.0 Hz, 1 H), 7.07 - 6.98 (m, 9 H), 4.48 (d, J = 11.1 Hz, 1 H; H2), 4.25 (d, J = 11.1 Hz, 1 H; H2), 3.78 (m, 1 H; H7a), 2.95 (dt, J = 10.4, 4.8 Hz, 1 H; H3a), 2.49 (br d, J = 14.4 Hz, 1 H; H7eq), 2.32 (s, 3 H), 1.58 (m, 1 H; H7ax), 1.55–1.41 (m, 4 H; H5 and H6), 1.28–1.15 (m, 2 H; H4). 13C{1H} NMR (126 MHz, CDCl3, 25 °C): δ 145.1, 143.8, 142.8, 134.2, 129.3, 128.5, 128.4, 127.6, 127.1, 126.7, 126.1, 125.7, 59.1, 58.2, 55.5, 44.3, 28.8, 25.5, 24.5, 21.5, 20.1.
All remaining acid-catalyzed hydrofunctionalization reactions were performed employing analogous procedures.
Kinetic Experiments
Triflic acid (5.00 µL, 5.6 × 10−2 mmol, 25 mM) was added via a gas-tight syringe equipped with a stainless steel needle into a solution of 1a (488 mg, 1.13 mmol, 0.50 M) in dry toluene (2.25 mL) that had been pre-equilibrated at 62.5 °C. The reaction mixture was stirred and aliquots were periodically removed via syringe, quenched with saturated aqueous NaHCO3, extracted with acetonitrile, and analyzed by liquid chromatography equipped with a UV detector. The conversion of 1a to 2a was quantitative and occurred without formation of intermediates or byproducts. Furthermore, analysis of stock solutions of 1a and 2a revealed that the UV response factors of 1a and 2a were not significantly different (≤ 0.1%) over the concentration range utilized in these experiments. For these reasons, the concentration of 1a was determined from the integration of the peaks in the LC spectrum corresponding to 1a and 2a according to the formula [1a] = 0.50 M × {[1a]/[1a] + [2a]}. A plot of ln[1a]t versus time was linear to ~3 half-lives (Figure 4, Table 2), with an observed rate constant of kobs = 4.2 ± 0.1 × 10−3 s−1. Employing a similar procedure, observed rate constants for the reaction of 1a with triflic acid were determined as a function of [HOTf] and temperature
α-Secondary KIE for the conversion of 1a to 2a
A mixture of 1a (162 mg, 0.376 mmol) and 1a-2’-d1 (74% d1, 326 mg, 0.754 mmol) was dissolved in toluene (2.25 mL). Mass spectral analysis of the resulting solution revealed a 47.6:52.4 mixture of d0:d1 isotopomers. The solution was equilibrated at 59.5 °C and triflic acid (4.0 mg, 5.7 × 10−2 mmol) was added. The resulting solution was stirred and aliquots were removed periodically via syringe, quenched with saturated aqueous NaHCO3, extracted with acetonitrile, and analyzed by LCMS for conversion and isotopic abundance. The concentrations of 1a and 1a-2’-d1 were determined from total conversion, obtained by integration of the peaks in the LC spectrum corresponding to 1a + 1a-2’-d1 and 2a + 2a-7a-d1 and from the isotopic ratios 1a:1a-2’-d1 and 2a:2a-7a-d1 determined from MS analysis of the corresponding LC peaks. Plots of ln[1a] and ln[1a-2’-d1] versus time were linear to ~3 half lives with observed rate constants of kobs = 2.19 ± 0.03 × 10−3 s−1 and kobs = 2.51 ± 0.05 × 10−3 s−1, respectively (Figure 6), which correspond to an inverse KIE of kD/kH = 1.15 ± 0.03.
Supplementary Material
Acknowledgements
Acknowledgment is made to the NIH (GM-080422) for support of this research and to the NCBC (2008-IDG-1010) for support of the Duke University NMR facility. We thank Dr. Marina G. Dickens for obtaining the X-ray crystal structure of 2a. REMB thanks Duke University of a Burroughs Wellcome fellowship.
References
- 1.(a) Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem. Rev. 2008;108:3795–3892. doi: 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]; (b) Widenhoefer RA, Han X. Eur. J. Org. Chem. 2006:4555–4563. [Google Scholar]; (c) Pohlki F, Doye S. Chem. Soc. Rev. 2003;32:104–114. doi: 10.1039/b200386b. [DOI] [PubMed] [Google Scholar]; (d) Hong S, Marks TJ. Acc. Chem. Res. 2004;37:673–686. doi: 10.1021/ar040051r. [DOI] [PubMed] [Google Scholar]
- 2.(a) Marcsekova K, Doye S. Synthesis. 2007:145–154. [Google Scholar]; (b) Anderson LL, Arnold J, Bergman RG. J. Am. Chem. Soc. 2005;127:14542–14543. doi: 10.1021/ja053700i. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Beller M, Thiel OL, Trauthwein H. Synlett. 1999:243–245. [Google Scholar]; (d) Yin Y, Zhao G. J. Fluorine Chem. 2007;128:40–45. [Google Scholar]; (e) Haskins CM, Knight DW. Chem. Commun. 2002:2724–2725. doi: 10.1039/b207755h. [DOI] [PubMed] [Google Scholar]; (f) Yin Y, Zhao GG. Heterocycles. 2006;68:23–31. [Google Scholar]; (g) Motokura K, Nakagiri N, Mori K, Mizugaki T, Ebitani K, Jitsukawa K, Kaneda K. Org. Lett. 2006;8:4617–4620. doi: 10.1021/ol0619821. [DOI] [PubMed] [Google Scholar]; (h) Yang L, Xu LW, Xia CG. Tetrahedron Lett. 2008;49:2882–2885. [Google Scholar]; (i) Jimenez O, Muller TE, Schwieger W, Lercher JA. J. Catal. 2006;239:42–50. [Google Scholar]; (j) Ackermann L, Kaspar LT, Althammer A. Org. Biomol. Chem. 2007;5:1975–1978. doi: 10.1039/b706301f. [DOI] [PubMed] [Google Scholar]; (k) Jazzar R, Dewhurst RD, Bourg JB, Donnadieu B, Canac Y, Bertrand G. Angew. Chem. Int. Ed. 2007;46:2899–2902. doi: 10.1002/anie.200605083. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Lapis AAM, DaSilveira Neto BA, Scholten JD, Nachtigall FA, Eberlin MN, Dupont J. Tetrahedron Lett. 2006;47:6775–6779. [Google Scholar]; (m) Yadav JS, Reddy BVS, Raju A, Ravindar K, Narender R. Lett. Org. Chem. 2008;5:651–654. [Google Scholar]; (n) Yang L, Xu LW, Xia CG. Synthesis. 2009:1969–1974. [Google Scholar]; (o) Griffiths-Jones CM, Knight DW. Tetrahedron. 2010;66:4150–4166. [Google Scholar]; (p) Ackermann L, Althammer A. Synlett. 2008:995–998. [Google Scholar]
- 3.(a) Lemechko P, Grau F, Antoniotti S, Duñach E. Tetrahedron Lett. 2007;48:5731–5734. [Google Scholar]; (b) Coulombela L, Duñach E. Green Chem. 2004;6:499–501. [Google Scholar]; (c) Franck X, Figadere B, Cavé A. Tetrahedron Lett. 1997;38:1413–1414. [Google Scholar]; (d) Miura K, Hondo T, Takahashi T, Hosomi A. Tetrahedron Lett. 2000;41:2129–2132. [Google Scholar]; (e) Wang B, Gu Y, Yang L, Suo J, Kenichi O. Catal. Lett. 2004;96:71–74. [Google Scholar]; (f) Linares-Palomino PJ, Salido S, Altarejos J, Sanchez A. Tetrahedron Lett. 2003;44:6651–6655. [Google Scholar]; (g) Coulombel L, Duñach E. Synth. Commun. 2005;35:153–160. [Google Scholar]; (h) Zhou Y, Woo LK, Angelici RJ. Appl. Catal., A. 2007;333:238–241. [Google Scholar]
- 4.Schlummer B, Hartwig JF. Org. Lett. 2002;4:1471–1474. doi: 10.1021/ol025659j. [DOI] [PubMed] [Google Scholar]
- 5.(a) Rosenfeld DC, Shekhar S, Takemiya A, Utsunomiya M, Hartwig JF. Org. Lett. 2006;8:4179–4182. doi: 10.1021/ol061174+. [DOI] [PubMed] [Google Scholar]; (b) Li Z, Zhang J, Brouwer C, Yang CG, Reich NW, He C. Org. Lett. 2006;8:4175–4178. doi: 10.1021/ol0610035. [DOI] [PubMed] [Google Scholar]
- 6.(a) Taylor JG, Adrio LA, Hii KK. Dalton Trans. 2010;39:1171–1175. doi: 10.1039/b918970j. [DOI] [PubMed] [Google Scholar]; (b) Adrio LA, Quek LS, Taylor JG, Hii KK. Tetrahedron. 2009;65:10334–10338. [Google Scholar]; (c) McBee JL, Bell AT, Tilley TD. J. Am. Chem. Soc. 2008;130:16562–16571. doi: 10.1021/ja8030104. [DOI] [PubMed] [Google Scholar]; (d) Cheng X, Xia Y, Wei H, Xu B, Zhang C, Li Y, Qian G, Zhang X, Li K, Li W. Eur. J. Org. Chem. 2008:1929–1936. [Google Scholar]; (e) Taylor JG, Whittall N, Hii KK. Org. Lett. 2006;8:3561–3564. doi: 10.1021/ol061355b. [DOI] [PubMed] [Google Scholar]; (f) Wei H, Qian G, Xia Y, Li K, Li Y, Li W. Eur. J. Org. Chem. 2007:4471–4474. [Google Scholar]; (g) Wabnitz TC, Yu J-Q, Spencer JB. Chem. – Eur. J. 2004;10:484–493. doi: 10.1002/chem.200305407. [DOI] [PubMed] [Google Scholar]
- 7.(a) Chwang WK, Nowlan VJ, Tidwell TT. J. Am. Chem. Soc. 1977;99:7233–7238. [Google Scholar]; (b) Nowlan VJ, Tidwell TT. Acc. Chem. Res. 1977;10:252–258. [Google Scholar]; (c) Kresge AJ, Chiang Y, Fitzgerald PH, McDonald RS, Schmid GH. J. Am. Chem. Soc. 1971;93:4907–4908. [Google Scholar]; (d) Koshy KM, Roy D, Tidwell TT. J. Am. Chem. Soc. 1979;101:357–363. [Google Scholar]; (e) Csizmadia VM, Koshy KM, Lau KCM, McClelland RA, Nowlan VJ, Tidwell TT. J. Am. Chem. Soc. 1979;101:974–979. [Google Scholar]; (f) Chwang WK, Knittel P, Koshy KM, Tidwell TT. J. Am. Chem. Soc. 1977;99:3395–3401. [Google Scholar]
- 8.(a) Taft RW. J. Am. Chem. Soc. 1952;74:5372–5376. [Google Scholar]; (b) Taft RW, Purlee EL, Riesz P, DeFazio CA. J. Am. Chem. Soc. 1955;77:1584–1587. [Google Scholar]; (c) Boyd RH, Taft RW, Wolfe AP, Christman DR. J. Am. Chem. Soc. 1960;82:4729–4736. [Google Scholar]
- 9.(a) Freeman F. Chem. Rev. 1975;75:439–490. [Google Scholar]; (b) Banthorpe DV. Chem. Rev. 1970;70:295–322. [Google Scholar]
- 10.(a) Cooper JD, Vitullo VP, Whalen DL. J. Am. Chem. Soc. 1971;93:6294–6296. [Google Scholar]; (b) Hevesi L, Piquard J-L, Wautier H. J. Am. Chem. Soc. 1981;103:870–875. [Google Scholar]; (c) Okuyama T, Fueno T. J. Am. Chem. Soc. 1980;102:6590–6591. [Google Scholar]; (d) Wautier H, Desauvage S, Hevesi L. J. Chem. Soc., Chem. Commun. 1981:738–739. [Google Scholar]
- 11.(a) Jencks WP. Chem. Rev. 1972;72:705–718. [Google Scholar]; (b) Jencks WP. Acc. Chem. Res. 1980;13:161–169. [Google Scholar]; (c) Jencks WP. Chem. Soc. Rev. 1981;10:345–375. [Google Scholar]
- 12.Chiang Y, Kresge AJ. J. Am. Chem. Soc. 1985;107:6363–6367. [Google Scholar]
- 13.Dietze PE, Jencks WP. J. Am. Chem. Soc. 1987;109:2057–2062. [Google Scholar]
- 14.Herlihy KR. Aust. J. Chem. 1989;42:1345–1350. [Google Scholar]
- 15.Fahey RC. In: Topics in Stereochemistry. Eliel EL, Allinger NL, editors. vol. 3. New York: Wiley-Interscience; 1968. pp. 237–342. [Google Scholar]
- 16.(a) Hammond GS, Nevitt TD. J. Am. Chem. Soc. 1954;76:4121–4123. [Google Scholar]; (b) Hammond GS, Collins CH. J. Am. Chem. Soc. 1960;82:4323–4327. [Google Scholar]
- 17.(a) Pocker Y, Stevens KD. J. Am. Chem. Soc. 1969;91:4205–4210. [Google Scholar]; (b) Weiss HM, Touchette KM. J. Chem. Soc., Perkin Trans. 1998;26:1517–1522. [Google Scholar]
- 18.Fahey RC, McPherson CA. J. Am. Chem. Soc. 1971;93:2445–2453. [Google Scholar]
- 19.Pasto DJ, Meyer GR, Lepeska B. J. Am. Chem. Soc. 1974;96:1858–1866. [Google Scholar]
- 20.Becker KB, Grob CA. Synthesis. 1973;12:789–790. [Google Scholar]
- 21.Fahey RC, Smith RA. J. Am. Chem. Soc. 1964;86:5035–5036. [Google Scholar]
- 22.Fahey RC, Monahan MW. J. Am. Chem. Soc. 1970;92:2816–2820. [Google Scholar]
- 23.Pasto DJ, Gadberry JF. J. Am. Chem. Soc. 1978;100:1469–1473. [Google Scholar]
- 24.Staab HA, Wittig CM, Naab P. Chem. Ber. 1978;111:2965–2981. [Google Scholar]
- 25.Naab P, Staab HA. Chem. Ber. 1978;111:2982–2996. [Google Scholar]
- 26.Pocker Y, Stevens KD, Champoux JJ. J. Am. Chem. Soc. 1969;91:4199–4205. [Google Scholar]
- 27.Fahey RC, McPherson CA, Smith RA. J. Am. Chem. Soc. 1974;96:4534–4542. [Google Scholar]
- 28.Fahey RC, Monahan MW, McPherson CA. J. Am. Chem. Soc. 1970;92:2810–2815. [Google Scholar]
- 29.(a) Corriu R, J Guenzet J. Tetrahedron. 1970;26:671–684. [Google Scholar]; (b) Fahey RC, McPherson CA. J. Am. Chem. Soc. 1969;91:3865–3869. [Google Scholar]
- 30.Allen AD, Tidwell TT. J. Am. Chem. Soc. 1982;104:3145–3149. [Google Scholar]
- 31.Roberts RMG. J. Chem. Soc., Perkin Trans. 1976;2:1183–1190. [Google Scholar]
- 32.(a) Dewar JS, Fahey RC. Angew. Chem. Internat. Edit. 1964;3:245–249. [Google Scholar]; (b) Dewar JS, Fahey RC. J. Am. Chem. Soc. 1963;85:3645–3648. [Google Scholar]; (c) Abraham RJ, Monasterios JR. J. Chem. Soc., Perkin Trans. 1975;2:574–578. [Google Scholar]; (d) Berlin KD, Lyerla RO, Gibbs DE, Devlin JP. Chem. Comm. 1970:1246–1247. [Google Scholar]; (e) Dewar JS, Fahey RC. J. Am. Chem. Soc. 1963;85:2245–2248. [Google Scholar]; (f) Dewar JS, Fahey RC. J. Am. Chem. Soc. 1963;85:2248–2252. [Google Scholar]; (g) Izawa K, Okuyama T, Fueno T. Bull. Chem. 1974;47:1477–1479. [Google Scholar]
- 33.(a) Li X, Ye S, He C, Yu Z-X. Eur. J. Org. Chem. 2008:4296–4303. [Google Scholar]; (b) Kovács G, Lledós A, Ujaque G. Organometallics. 2010;29:5919–5926. [Google Scholar]
- 34.(a) Miura K, Hondo T, Nakagawa T, Takahashi T, Hosomi A. Org. Lett. 2000;2:385–388. doi: 10.1021/ol991341o. [DOI] [PubMed] [Google Scholar]; (b) Miura K, Okajima S, Hondo T, Nakagawa T, Takahashi T, Hosomi A. J. Am. Chem. Soc. 2000;122:11348–11357. [Google Scholar]
- 35.Zhang J, Yang C-G, He C. J. Am. Chem. Soc. 2006;128:1798–1799. doi: 10.1021/ja053864z. [DOI] [PubMed] [Google Scholar]
- 36.Subsequent experimentation revealed that triflic acid-catalyzed cyclization of substrates 1 occurs at lower temperature and with shorter reaction time (60 °C, 3 h) with the same stereochemical outcome.
- 37.X-ray data for 2a: Monoclinic, P2(1)/c, T = 296 K, a = 8.8385(10) Å, b = 26.705(3) Å, c = 9.4913(11) Å, β = 94.690(8)°, V = 2232.8(5) Å3, Z = 4, R[F2 > 2σ(F2)] = 0.042, wR(F2) = 0.138. CCDC-798744 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 38.We were unable to assign the relative configuration of 2a-3a,7eq-d2 from the H7a - H7eq and H7a - H7ax three-bond coupling constant as has been previously reported.[35] The equatorial conformation of the H7a proton leads to similar dihedral angles for H7a–C7a–C7–H7eq and H7a–C7a–C7–H7ax. As a result, protons H7eq and H7ax display similar three-bond coupling constants to H7a of 4.8 Hz and 3.5 Hz, respectively, and both H7eq and H7ax display strong cross peaks to H7a in the 1H-1H NOESY spectrum. As such, the more reliable determinant to assign the H7ax and H7eq protons was the presence of a cross peak between H3 and H7ax and the absence of a cross peak between H3 and H7eq in the 1H-1H NOESY spectrum of 2a (see Supporting Information).
- 39.The isotopomer 1a-N-d was generated in situ by stirring a toluene solution of purified 1a with D2O at room temperature followed by removal of the toluene solution via syringe; attempted isolation or purification of 1a-N-d led to significant loss of deuterium. The deuterium content of 1a-N-d (~90% d) was determined by 1H NMR integration. Owing to the nominal solubility of water in toluene (0.033%), this sample also contained 7.9 µmol (~16 mM) D2O.
- 40.Howells RD, McCown JD. Chem. Rev. 1977;77:69–92. [Google Scholar]
- 41.Laughlin RG. J. Am. Chem. Soc. 1967;89:4268–4271. [Google Scholar]
- 42.Olavi P, Virtanen I, Maikkula M. Tetrahedron Lett. 1968;9:4855–4858. [Google Scholar]
- 43.(a) Birchall T, Gillespie RJ. Can. J. Chem. 1963;41:2642–2650. [Google Scholar]; (b) Menger FM, Mandell L. J. Am. Chem. Soc. 1967;89:4424–4426. [Google Scholar]
- 44.(a) Melander L, Saunders WH., Jr . Reaction Rates of Isotopic Molecules. New York: Wiley; 1980. [Google Scholar]; (b) Hengge AC. Secondary Isotope Effects. In: Kohen A, Limbach H, editors. Isotope Effects in Chemistry and Biology. Boca Raton: CRC; 2006. [Google Scholar]; (c) Buncel E, Lee CC, editors. Isotopes in Organic Chemistry, Vol. 7, Secondary and solvent isotope effects. Amsterdam: Elsevier; 1987. [Google Scholar]; (d) Matsson O, Westaway KC. Adv. Phys. Org. Chem. 1998;35:143–248. [Google Scholar]
- 45.Streitwieser A, Jagow RH, Fahey RC, Suzuki S. J. Am. Chem. Soc. 1958;80:2326–2332. [Google Scholar]
- 46.For alternative explanations see: Strauss OP, Safarik I, O'Callaghan WB, Gunning HE. J. Am. Chem. Soc. 1972;94:1828–1834. Safarik I, Strauss OP. J. Phys. Chem. 1978;76:3613–3617. Bender BS. J. Am. Chem. Soc. 1995;117:11239–11246.
- 47.(a) Bigeleisen J. J. Chem. Phys. 1949;17:675–678. [Google Scholar]; (b) Bigeleisen J, Mayer MG. J. Chem. Phys. 1947;15:261–267. [Google Scholar]; (c) Bigeleisen J, Wolfsberg M. Adv. Chem. Phys. 1958;1:15–76. [Google Scholar]
- 48.Values employed for cis-2-butene (cm−1): 2980 (stretching), 1425 (in-plane bending), 852 (out-of-plane bending).[49] Values employed for secondary alcohol (cm−1): 2900 (stretching), 1340 (in-plane binding), 1340 (out-of-plane bending).[49] See Supporting Information for additional details.
- 49.Roeges NPG. A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures. John Wiley & Sons; 1994. [Google Scholar]
- 50.(a) Hartshorn SR, Shiner VJ. J. Am. Chem. Soc. 1972;94:9002–9012. [Google Scholar]; (b) Gajewski JJ, Olson LP, Tupper KJ. J. Am. Chem. Soc. 1993;115:4548–4553. [Google Scholar]; (c) Okuyama T, Fueno T. J. Am. Chem. Soc. 1983;105:4390–4395. [Google Scholar]
- 51.Hout RF, Levi BA, Hehre WJ. J. Comp. Chem. 1983;4:499–505. [Google Scholar]
- 52.Seltzer S. J. Am. Chem. Soc. 1961;83:1861–1865. [Google Scholar]
- 53.(a) Schubert WM, Lamm B, Keeffe JR. J. Am. Chem. Soc. 1964;86:4727–4729. [Google Scholar]; (b) Schubert WM, Lamm B. J. Am. Chem. Soc. 1966;88:120–124. [Google Scholar]
- 54.Pocker Y, Hill MJ. J. Am. Chem. Soc. 1969;91:7154–7158. [Google Scholar]
- 55.Kresge AJ, Weeks DP. J. Am. Chem. Soc. 1984;106:7140–7143. [Google Scholar]
- 56.Collman JP, Hegedus LS, Norton JR, Finke RG. Principles and Applications of Organotransition Metal Chemistry. Mill Valley, CA: University Science Books; 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.