Abstract
Exposure to hydrogen peroxide (H2O2) and other reactive oxygen species is a universal feature of life in an aerobic environment. Bacteria express enzymes to detoxify H2O2 and to repair the resulting damage, and their synthesis is typically regulated by redox-sensing transcription factors. The best characterized bacterial peroxide-sensors are Escherichia coli OxyR and Bacillus subtilis PerR. Analysis of their regulons has revealed that, in addition to inducible detoxification enzymes, adaptation to H2O2 is mediated by modifications of metal ion homeostasis. Analogous adaptations appear to be present in other bacteria as here reviewed for Deinococcus radiodurans, Neisseria gonorrhoeae, Streptococcus pyogenes, and Bradyrhizobium japonicum. As a general theme, peroxide stress elicits changes in cytosolic metal distribution with the net effect of reducing the damage caused by reactive ferrous iron. Iron levels are reduced by repression of uptake, sequestration in storage proteins, and incorporation into metalloenzymes. In addition, peroxide-inducible transporters elevate cytosolic levels of Mn(II) and/or Zn(II) that can displace ferrous iron from sensitive targets. Although bacteria differ significantly in the detailed mechanisms employed to modulate cytosolic metal levels, a high Mn:Fe ratio has emerged as one key correlate of reactive oxygen species resistance. Antioxid. Redox Signal. 15, 175–189.
Opportunities and Challenges Afforded by Molecular Oxygen
Molecular oxygen is a defining feature of the Earth's atmosphere due to both its abundance (21% by volume at sea level) and its unique chemical reactivity. As a ground state radical, carrying two unpaired electrons, O2 reacts readily with a variety of one-electron reductants. This ability has been harnessed by both eukaryotes and many prokaryotes, and aerobic respiration is arguably the dominant form of cellular energy generation. Growth in the presence of oxygen also poses challenges for cells since partially reduced oxygen species (reactive oxygen species [ROS]) can damage many cellular constituents leading to protein and membrane damage, mutations, and ultimately cell death (39). Thus, the evolution of an oxygen-containing atmosphere, as a result of oxygenic photosynthesis by early microbes, provided both new opportunities (for energy generation) and chemical challenges for evolving life forms (40).
Hydrogen peroxide (H2O2) is among the most stable ROS and consequently can accumulate to significant levels both within cells and in the environment. H2O2 forms within cells by the auto-oxidation of flavins associated with metabolic enzymes and, as a small uncharged molecule, can also enter by diffusion from the environment (50). Auto-oxidation reactions in air-saturated environments can lead to micromolar levels of H2O2 and many organisms (animals, plants, and some bacteria) produce even higher levels as an antibacterial weapon (88).
Bacterial cells routinely express multiple enzymes dedicated to the detoxification of H2O2 and other ROS. The ubiquity of catalases, peroxidases, and superoxide dismutases (SODs)/reductases provides compelling evidence of the strong selective pressure exerted by ROS, even for those organisms that are typically found in microaerophilic or even anaerobic environments (40). Expression of these and related defensive enzymes is typically regulated by redox-sensing transcription factors. The two best characterized peroxide-sensors in the bacteria are Escherichia coli OxyR and Bacillus subtilis PerR (6, 23). Orthologs of one (and rarely both) of these H2O2-sensors are found in most bacteria and mediate the adaptive response to peroxides in which exposure to low levels of stressor enables survival when cells are subsequently faced with much higher levels of oxidant.
The ability of cells to elaborate defensive enzymes to degrade H2O2 is of paramount importance. For example, a mutant E. coli strain lacking both catalases and peroxidases (a hydroperoxidase minus or Hpx− strain) is compromised in its ability to grow under aerobic conditions even in the absence of any exogenous challenge with H2O2 (83). Increasingly, however, it is appreciated that the adaptive responses to ROS are far more nuanced than simply inducing the synthesis of defensive enzymes. Here, we focus specifically on the many ways in which bacterial cells, upon sensing H2O2, modify metal homeostasis to minimize the damage inflicted upon the cell.
Managing the Uneasy Liaison Between H2O2 and Metal Ions
Although H2O2 is quite chemically stable in simple solutions, it reacts rapidly with ferrous iron to generate highly reactive hydroxyl radical (*OH), hydroxide anion (−OH), and oxidized ferric iron (Fenton reaction). Hydroxyl radical reacts at diffusion-limited rates with many biomolecules, including DNA. Cell death due to H2O2 is primarily due to DNA damage mediated by hydroxyl radicals, likely generated by reaction with ferrous iron loosely associated with DNA (Fig. 1).
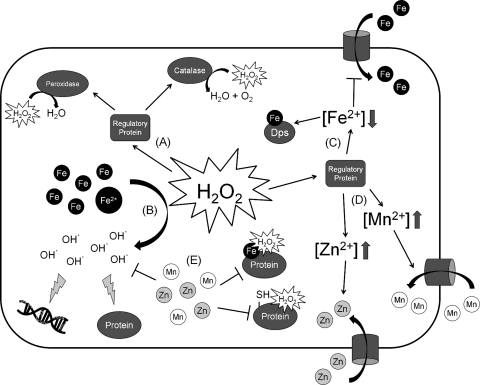
FIG. 1.
General mechanisms for protection against oxidative stress. (A) To detoxify hydrogen peroxide (H2O2), many bacteria upregulate the expression of peroxidases and catalases. (B) H2O2 reacts rapidly with ferrous iron, generating hydroxyl radical, hydroxide anion, and oxidized ferric iron (Fenton reaction). The hydroxyl radical can then subsequently damage DNA and oxidize proteins. To protect against these toxic effects, bacteria may (C) decrease intracellular iron levels by decreasing Fe import and sequestering free iron through the upregulation of Dps. (D) Bacteria may also increase intracellular levels of Mn(II) and/or Zn(II) through increased import. (E) Mn(II) and Zn(II) can competitively inhibit the damaging reactions catalyzed by Fe(II) and H2O2.
Most cells require iron as a cofactor for enzymes, including some of the very enzymes needed to help protect against ROS (e.g., heme-containing catalases and Fe-containing SOD) (40). Therefore, cells normally maintain a pool of free iron, presumably chelated by small molecules and protein chaperones, that serves as a reservoir for incorporation into newly synthesized metalloproteins. These cells face the paradox of needing to actively import an essential metal ion that can also, in the presence of ROS, catalyze destructive reactions. Moreover, ROS can further increase free iron levels by damaging metalloenzymes, including both mononuclear iron centers and Fe4S4 clusters (39). Reaction of H2O2 with iron centers can lead to protein damage via metal-catalyzed oxidation (MCO), whereas oxidation of Fe4S4 clusters can lead to disassembly and loss of iron atom(s) from the cluster (42). For select groups of bacteria, including some lactobacilli and Borellia burgdorferii, this problem is avoided altogether by dispensing with a metabolic requirement for iron (3, 75).
In addition to reduced metal centers, H2O2 can also react with the thiolate anion including the active site cysteines of some cysteine-dependent enzymes and regulators. The rate constants for oxidation of thiolates by H2O2 vary widely; many proteins are relatively resistant to oxidation, whereas some, such as E. coli OxyR and thiol-dependent peroxidases, have evolved highly reactive thiol groups (25). These proteins can function as thiol-dependent sensors (the cysteine-disulfide switch of OxyR) or as peroxidases that help detoxify H2O2.
Each of E. coli OxyR and B. subtilis PerR regulates adaptive responses to H2O2, yet they ultimately rely on distinct sensing mechanisms. OxyR lacks any bound metal centers and instead senses peroxides via thiol oxidation (51). As a result, the OxyR regulon is induced by either H2O2 or by chemicals that oxidize and thereby deplete cellular thiol reductants (disulfide stress). In contrast, PerR senses both H2O2 and cellular metal status. PerR represses transcription when associated with either Mn(II) or Fe(II), but only the Fe(II)-containing form of the repressor can sense H2O2 (33, 59). PerR bound with Fe(II) is inactivated by H2O2-dependent MCO (53). This realization provided one of the earliest hints that peroxide stress responses were intimately linked to metal ion homeostasis (17).
Work in numerous systems, several of which are reviewed here, has now established that modulation of cellular metal ion content and distribution is an important and widespread component of bacterial adaptive responses to H2O2. While the details differ between species, a number of general themes have emerged. Characterization of the OxyR and PerR regulons in a wide range of organisms suggests that peroxide stress elicits significant changes in cytosolic metal composition with the ultimate goal of reducing the levels of free, reactive ferrous iron and increasing the concentration of nonreactive divalent metals that can, by competitive binding, help prevent the adventitious association of Fe(II) with sensitive targets (Fig. 1). Iron homeostasis is modified by one or more of the following pathways: (i) repression of iron uptake (96), (ii) sequestration of cytosolic iron in ferritin and mini-ferritin (Dps family) proteins (2), and (iii) incorporation of iron back into newly synthesized or damaged Fe4S4 cluster enzymes (102). In parallel, metals such as Mn(II) and Zn(II) that can competitively inhibit the damaging reactions otherwise catalyzed by iron can be actively imported by the expression of peroxide-inducible, metal uptake systems (Fig. 1).
E. coli and the OxyR Regulon
E. coli OxyR is the prototype for a widely distributed family of redox-sensors (104). OxyR (and its orthologs) controls the adaptive response to H2O2 in many Gram-negative (and some Gram-positive) bacteria and is characteristic of the gamma and beta proteobacteria (61). OxyR is a tetrameric, DNA-binding transcriptional activator of the LysR family and binds upstream of the target genes within its regulon. Upon activation by H2O2, an intramolecular disulfide forms within each OxyR protomer, and the resulting conformational change enables productive interactions with RNA polymerase, and thereby leads to activation of the OxyR regulon (Fig. 2). In E. coli strains containing a full complement of peroxide-degrading enzymes (catalases and the alkyl hydroperoxide reductase AhpCF), activation of the OxyR regulon is commonly achieved by addition of millimolar levels of H2O2 although 5 μM H2O2 will suffice (8). Additionally, studies with strains devoid of catalases/peroxidases (Hpx−) indicate that OxyR induction commences once intracellular levels of H2O2 rise to >100 nM (83). Consistent with this, in vitro ∼50–200 nM H2O2 oxidizes 50% of the OxyR protein (8). Indeed, OxyR is one of the most sensitive peroxide sensors known that can be attributed to its highly reactive active-site thiolate (87).
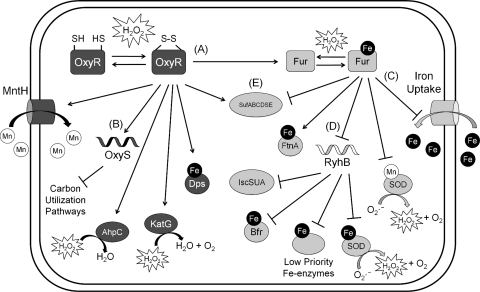
FIG. 2.
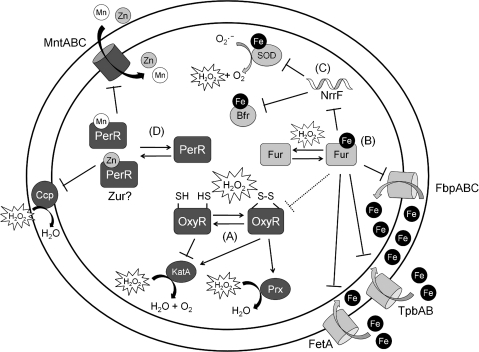
The OxyR and ferric uptake regulator (Fur) regulons in Escherichia coli. (A) Upon exposure to H2O2, an intramolecular disulfide bond forms within each OxyR protomer, resulting in the activation of OxyR. Oxidized OxyR activates the sequestration of Fe (Dps), the detoxification of H2O2 (AhpC, KatG), and the import of Mn(II) (MntH). (B) OxyR also activates the transcription of the OxyS sRNA, resulting in the reduction of carbon utilization pathways and thus reducing endogenous H2O2 production. In addition, OxyR upregulates the synthesis of Fur. In Fe-rich conditions, (C) Fur represses additional Fe uptake and (D) indirectly activates some iron-containing proteins (including Fe-superoxide dismutase [SOD] and bacterioferritin [Bfr]) via the RyhB sRNA or, in the case of the FtnA iron storage protein, by reversing H-NS-mediated silencing (63). In these conditions, the Isc Fe-S cluster biogenesis machinery is upregulated. Fur:Fe is sensitive to H2O2 and can be inactivated upon exposure. (E) However, in Fe-limited and/or oxidative stress conditions, transcription of the second Fe-S cluster assembly machinery, Suf, is favored.
The OxyR regulon has been extensively documented and includes the major vegetative catalase, AhpCF, Dps, MntH, ferric uptake regulator (Fur), and the OxyS regulatory sRNA. When initially described in 1985, OxyR and its regulon was thought to be largely unrelated to metal homeostasis. The first hint that OxyR might affect iron homeostasis emerged with the realization that Dps, a known OxyR target gene (1), is related in both sequence (73) and structure to ferritin iron storage proteins (30). Although E. coli Dps can also bind and coat DNA, recent evidence suggests that it is the iron sequestration activity that is most important for protecting cells against peroxide-mediated damage (72). The induction of one or more iron sequestration proteins is one of the most widely distributed adaptations in response to peroxide stress, although the relevant regulatory pathways are varied.
In addition to iron sequestration, peroxide stress also increases the expression of the E. coli Fur protein (103). Fur is the master regulator of iron homeostasis and binds reversibly to Fe(II) to repress the expression of iron uptake functions (54). Fur also acts indirectly, via the RyhB sRNA (58) or by antagonizing H-NS mediated repression (63), to positively regulate the expression of iron-containing enzymes and storage functions. The ability of Fur to repress iron uptake when cytosolic levels of iron are elevated is of course adaptive and helps protect against H2O2-catalyzed Fenton chemistry. A mutant strain lacking Fur has elevated intracellular levels of “free” (chelatable) Fe(II) [e.g., an increase from ∼10 to ∼70 μM in (47)] and a corresponding increase in H2O2 sensitivity. This H2O2 sensitivity is largely iron dependent and can be reversed by a cell-permeable iron chelator. It has also been noted that H2O2 may inactivate E. coli Fur, possibly by oxidation of the bound Fe(II) and/or by MCO of the protein (96). This may be mechanistically similar to the pathway of MCO of B. subtilis PerR (53). In contrast, B. subtilis Fur is relatively insensitive to MCO (53, 54). In light of the studies cited above, however, the inactivation of E. coli Fur by MCO is unlikely to be adaptive and, in fact, may even sensitize cells to peroxides.
The physiological relevance of Fur induction by OxyR has several possible components. Fur itself is quite abundant for a regulatory protein with ∼5000 molecules per cell. This doubles under oxidative stress to ∼10,000 molecules per cell (103). Presumably, this serves to increase repression of the Fur regulon when sufficient iron is available. Alternatively, Fur may sequester iron by direct binding. Finally, induction of Fur may also serve to replace Fur proteins damaged by MCO. Support for this latter model has been provided by analysis of Fur function in strains lacking Hpx− (96). In this background, the Fur regulon is constitutively expressed during aerobic growth in minimal medium, presumably due to MCO of Fur by endogenously produced H2O2, which accumulates to 0.5–1.0 μM under these conditions. In rich medium, there is sufficient iron available that Fur repression can be restored, but only if OxyR can upregulate Fur synthesis (96).
In addition to regulating iron sequestration (Dps) and synthesis of the Fur metalloregulator, H2O2 also regulates the metallation of Fe-S cluster containing proteins. E. coli contains two parallel systems, encoding by the isc and suf operons, for the assembly and insertion of Fe-S clusters into proteins. In general, the Isc system serves a housekeeping role and is feedback regulated by an Fe-S containing repressor, IscR. IscR is a transcription factor encoded as part of the iscRSUA operon where IscS, IscU, and IscA are involved directly in the biogenesis and insertion of Fe-S clusters. When the activity of the Fe-S cluster machinery is sufficient, IscR contains an Fe2S2 cluster and represses the isc promoter (82).
Under conditions of iron limitation (67) or H2O2 stress (43), the Suf system can functionally substitute for the Isc system. The Isc system in disabled by low levels of H2O2, apparently due to oxidative inactivation of nascent Fe-S clusters (43). Under these conditions, activation of the sufABCDSE operon by OxyR is required for the efficient synthesis of Fe-S containing enzymes (43). Regulation of the suf operon by Fur, and activation by apo-IscR, contributes to the ability of this system to substitute for the Isc when iron is limited (55, 64, 67).
The RyhB sRNA also contributes to the regulation of Fe-S cluster assembly. In Fe-limited conditions, the Fur-regulated sRNA RyhB triggers the processing of the iscRSUA mRNA and subsequent degradation of the downstream portion of the mRNA (iscSUA). The result is that, in response to Fe-limitation, the Isc machinery is downregulated, but the IscR protein, required for the full activation of the suf operon, is still produced (21).
In addition to Fe(II), OxyR also has a significant impact on Mn(II) homeostasis (Fig. 2). OxyR activates the expression of mntH encoding a proton-dependent Mn(II) import channel (46). The resulting increase in intracellular Mn(II) is clearly adaptive since a strain defective in this Mn(II) uptake pathway has an increased sensitivity to H2O2. At least two mechanisms for this effect can be entertained. First, Mn(II) may function by virtue of its chemical activity since Mn(II), particularly when complexed with bicarbonate, has been shown to have catalase-like activity and Mn(II) has been postulated to act as a quencher of damaging free radicals (57). Second, Mn(II) may act via chemical competition and simply displace Fe(II) bound to sites (e.g., DNA and Fe-metalloproteins) where, if present, reaction with peroxides would lead to damaging MCO reactions. To distinguish between these models, Anjem et al. measured the rates for Mn(II)-dependent detoxification and concluded that, even with generous assumptions, the chemical activity of Mn(II) would pale besides the cell's enzymatic activity and would therefore be unlikely to contribute to adaptation (4). Conversely, they show that increased levels of intracellular Mn(II) decrease the chemical oxidation of many different cytosolic proteins and enzymes. These results provide strong evidence that Mn(II) plays a role as an antioxidant largely via chemical competition rather than direct chemical activity (Fig. 1). In E. coli, Mn(II) import is conditionally evoked in response to peroxide stress (4), whereas in other bacteria relatively high ambient Mn(II) levels are maintained even in the absence of overt stress (18, 65, 71).
B. subtilis and the PerR Regulon
The adaptive response to H2O2 and metal ion homeostasis are also intricately linked in B. subtilis (Fig. 3). Indeed, the discovery of PerR, and the molecular genetic analysis of peroxide adaptation in this system evolved from studies designed to monitor changes in gene expression in response to metal deprivation (16). In these early studies, gene fusions were sought that were derepressed under conditions of iron starvation using the chelator EDDHA. Characterization of the resulting metal-regulated genes led to the identification of mrgA, encoding a Dps family protein. Like Dps, MrgA is a bacterial mini-ferritin that can both sequester iron and bind nonspecifically to DNA (15). Characterization of mrgA led to the realization that this gene was regulated both by metal ions (induced by metal starvation) and by H2O2. Moreover, both types of regulation required the same cis-acting sequence subsequently shown to be the binding site of the PerR repressor (and therefore designated as a Per box) (17). PerR, a Fur family member, is a metal-dependent DNA-binding protein (11, 33, 53). These same studies also led to the identification of two other paralogs: Fur (the bona fide iron uptake regulator) and Zur (a zinc uptake regulator) (54).
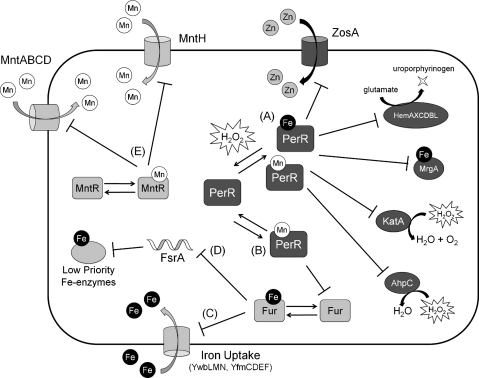
FIG. 3.
The PerR, Fur, and MntR regulons in Bacillus subtilis. (A) H2O2 oxidizes PerR:Fe, resulting in the derepression of peroxide detoxifying enzymes (KatA and AhpCF), an Fe-sequestration protein (MrgA), heme biosynthesis enzymes, and Zn(II) uptake. Although PerR:Mn can also repress these same genes, this form of PerR is relatively insensitive to oxidation by peroxide. Therefore, in low Fe but high Mn conditions, the PerR regulon is not derepressed upon exposure to peroxides. (B) Only PerR:Mn, not PerR:Fe, appears to be responsible for the repression of Fur. (C) In Fe-limited conditions, iron uptake is derepressed by Fur. (D) Production of low priority Fe-enzymes is decreased by the FsrA sRNA when Fe is limiting. (E) Independent of Fur and PerR activity, MntR represses Mn(II) uptake when Mn(II) is sufficient.
B. subtilis PerR is the prototype for a widely distributed family of H2O2-sensing metalloproteins. PerR homologs are found in many Gram-positive bacteria, particularly within the Firmicutes, but are also found in Campylobacter spp. (95) and the cyanobacteria (61). PerR is a dimeric, DNA-binding protein containing, within each monomer, a tightly held structural Zn(II) (52) and a loosely associated regulatory metal ion (53). Under conditions of metal deficiency, such as growth in minimal medium with very low levels of iron and manganese, PerR is inactive as a repressor (this inactive metalloprotein is designated PerR:Zn). In most rich medium conditions, PerR binds Fe(II) as corepressor (PerR:Zn,Fe) and this form of the protein represses the PerR regulon. H2O2 leads to MCO of the protein in which the bound Fe(II) reduces H2O2 to generate a hydroxyl radical (Fenton reaction), which, in turn, modifies either one of two specific histidines coordinated to the iron atom (53). The resulting inactivated protein, containing a single 2-oxo-histidine residue, dissociates from DNA leading to derepression of the regulon (53, 90). This modification has been observed, and the regiospecificity rationalized, by analysis of the structure of PerR in both its oxidized and reduced states by X-ray crystallography (23).
PerR can also repress gene expression when associated with other regulatory metal ions: a phenomenon studied in most detail for Mn(II) (16). The PerR:Zn,Mn form of the repressor, however, is refractory to H2O2 and, under these conditions, the PerR regulon is largely repressed and noninducible (26). Thus, PerR can be thought of as a signal integrator that simultaneously monitors both H2O2 levels and the ratio of cytosolic Mn(II) to Fe(II). Under conditions of relatively high Mn(II), and low Fe(II), many cytosolic proteins and enzymes may be relatively protected against protein-damaging MCO reactions, as noted above for E. coli, and thus induction of the PerR regulon may not be necessary. Indeed, genetic studies suggest that full induction of the PerR regulon is deleterious to the cell; a perR null mutant strain grows very slowly and has defects that can be, at least partially, explained by aberrant regulation of iron homeostasis (our unpublished results).
B. subtilis contains both PerR and Fur proteins (∼30% identical), but the two differ dramatically in their sensing properties. PerR exists largely in the PerR:Zn,Fe form in cells grown in Fe-sufficient medium, but in medium limited for iron and with sufficient Mn(II) PerR exists instead in the PerR:Zn,Mn form. Fur binds Fe(II) with somewhat higher affinity than PerR and is poised to sense severe drops in cytosolic iron levels and to respond by derepression of iron uptake pathways (and translational repression of many iron-utilizing enzymes through the FsrA sRNA) (27, 66). PerR:Zn,Fe reacts rapidly with H2O2 by MCO and is thus poised to sense submicromolar levels of H2O2 in the cell. In contrast, Fur:Zn,Fe is comparatively H2O2 resistant and does not readily undergo MCO (53). This contrasts with the situation in E. coli that has a single protein (Fur) that senses Fe(II) but can also be inactivated by H2O2, in an apparently maladaptive process (96). Defining how Fur family proteins can tune their selectivity for metal ions, and their reactivity with peroxides when bound with Fe(II) remains a challenging problem for further study (54).
Derepression of the PerR regulon leads to a large increase in peroxide resistance (11). This can be attributed, in part, to derepression of enzymatic detoxification systems, including the major vegetative catalase (KatA) and alkylhydroperoxide reductase. Coincident with derepression of KatA, which becomes one of the most abundant cellular proteins in a perR null mutant strain, there is derepression of heme biosynthesis genes, presumably to support the high level of catalase activity. Derepression of MrgA is hypothesized to decrease the levels of free Fe(II) within the cell by sequestration within the dodecameric (mini-ferritin) protein shell. As a class, Dps family proteins often help protect cells against ROS and increase survival in stationary phase (2). Stationary phase is not always well defined, but anytime cells must maintain genome integrity over extended periods of time, often under conditions of energy depletion, it is especially important to minimize DNA damage. Dps family proteins appear to protect DNA primarily by their ability to sequester Fe(II), but the ability of many family members to also bind directly to DNA (to form intracellular nanocrystals) (30) may also play a role.
Many bacteria contain multiple Dps orthologs, although the reason is not always clear. In B. subtilis, one Dps ortholog (encoded by dps) is regulated by the σB general stress response σ factor, induced upon entry into stationary phase, and plays a major role in the high intrinsic resistance of stationary phase cells to H2O2 (5). The second, MrgA, is induced as part of the PerR-regulated peroxide-stress response and also serves to protect cells against ROS-mediated damage (15). The biochemical and functional differences between these two paralogs are not yet well defined. B. anthracis also encodes two paralogs that differ in their preferred oxidant for iron mineralization. Both Dps1 and Dps2 mineralize Fe with molecular oxygen as the oxidant, whereas only Dps2 functions efficiently with H2O2. Dps2, considered the MrgA ortholog, may therefore function primarily to reduce cytosolic iron levels in response to H2O2 stress, whereas Dps1 may sequester iron when in excess. Indeed, it has been suggested that, as a class, Dps proteins may serve primarily in preventing iron-mediated Fenton chemistry; it is not clear if the mineralized iron core can be mobilized when iron is needed. In contrast, ferritins and bacterioferritins are thought to play a dominant role for iron storage (2).
In addition to its regulation of mrgA, PerR may affect metal ion homeostasis in other ways. The fur gene itself is a member of the PerR regulon although, intriguingly, the repression of Fur by PerR is mediated only by the PerR:Zn,Mn form of the repressor. As a result, and in contrast to E. coli, transcription of fur seems not to be inducible by H2O2 (26). Instead, PerR may regulate Fur expression in response to the relative levels of Fe(II) and Mn(II) within the cell (26).
PerR also represses a metal-transporting P-type ATPase known as ZosA (zinc uptake under oxidative stress) (28). Physiological studies have led to a model in which ZosA imports Zn(II) in response to oxidative stress (Fig. 3), although the actual specificity of this transporter has not yet been rigorously investigated. The proposed function of imported Zn(II) is to displace Fe(II) from sensitive sites in proteins or on the surface of DNA and thereby to function as an antioxidant via a chemical competition mechanism analogous to that discussed above for Mn(II) (28). Interestingly, and unlike the situation in E. coli, there is no evidence for derepression of mntH by ROS in B. subtilis. It is unclear why some bacteria import Mn(II) and others choose Zn(II) for the purposes of protecting against the deleterious actions of cytosolic Fe(II).
The regulatory protein responsible for maintaining Mn homeostasis in B. subtilis is MntR, which belongs to the diphtheria toxin repressor (DtxR) family of metalloproteins (60). DtxR was originally discovered as an iron-dependent repressor of toxin production. However, it now appears that DtxR homologs have a broader role and may regulate iron and/or manganese homeostasis. An mntR mutant strain is more sensitive to Mn(II) relative to wildtype, suggesting a role for MntR in controlling Mn transport. Consistent with this, in the presence of Mn(II), MntR represses expression of MntH and an Mn(II) ABC transporter encoded by mntABCD. Mutation of mntH in a mntR mutant strain restores partial resistance to Mn(II), suggesting that MntH is the major pathway of Mn(II) uptake when Mn(II) levels are high (77). An mntR mutant strain has an increased sensitivity to H2O2. This sensitivity is not due to the derepression of mntH since an mntR mntH double mutant is also H2O2 sensitive. One possibility is that derepression of the mntABCD metal uptake system leads to an increase in iron uptake. Indeed, orthologous ABC transporters import both Mn(II) and Fe(II) (20, 49, 79, 80).
The PerR-regulated response to oxidative stress described for B. subtilis and its connection to metal ion homeostasis provide a model for understanding the complex responses employed by several other bacteria. One such example is found in Staphylococcus aureus. The PerR-regulated response for S. aureus resembles closely that of B. subtilis except in the interplay between PerR activity and iron levels (36). As in B. subtilis, PerR functions as a repressor for genes of the peroxide stress response and this activity appears to require a bound metal cofactor. In both systems, Mn(II) is an efficient corepressor, but, unlike the situation in B. subtilis, high iron levels lead to derepression of the PerR regulon in S. aureus. Since the PerR regulon genes are inducible by peroxide, it seems most parsimonious to suggest that high iron leads to ROS production and inactivation of the PerR:Zn,Fe form of the repressor, thereby leading to apparent induction by iron. Alternatively, PerR in this system may function as an Mn(II)-specific repressor (analogous or equivalent to Mur) (54) and iron may function as an antagonist of its DNA-binding activity. In this scenario, peroxide stress would be sensed indirectly as an increase in free iron levels. To complicate matters further, there is an incompletely understood interplay between the PerR and Fur regulons in S. aureus (37). For example, catalase in this system is repressed by PerR in response to Mn(II) and activated by Fe(II), directly or indirectly, by Fur. These results are generally consistent with the theme that high Mn(II) levels are protective against ROS and that induction of catalase and other defensive enzymes may therefore be less important under these conditions.
Although best characterized in members of the low GC Gram-positive bacteria (Firmicutes), PerR has also been described in more distantly related bacteria. One notable example is the Gram-negative, foodborne pathogen Campylobacter jejuni (95). This organism contains both Fur and PerR orthologs that together coordinate the regulation of iron homeostasis functions and peroxide stress responsive genes. As in B. subtilis, PerR mediates an iron-dependent repression of catalase and AhpCF in this organism. Recent transcriptome analyses have investigated these responses in some detail (68), but some of the findings seem to contradict prior work, suggesting that additional studies will be required to better understand the complexities of regulation in this system.
The E. coli OxyR (Fig. 2) and B. subtilis PerR regulons (Fig. 3) provide important touchstones for thinking about the complex relationships between peroxide stress responses and metal ion homeostasis. The general themes developed from analysis of these systems inform ongoing studies of stress responses in numerous other systems. Here, we focus specifically on four diverse examples: Deinococcus radiodurans, Neisseria gonorrhoeae, Streptococcus pyogenes, and Bradyrhizobium japonicum.
Deinococcus radiodurans
D. radiodurans is known for its extreme resistance to ionizing and ultraviolet radiation. The resistance of D. radiodurans to radiation correlates with the ability of this organism to combat oxidative stress, since the lethal effects of radiation can be largely attributed to radiolysis of water leading to hydroxyl radical and H2O2, which, in turn, drives MCO of proteins and nucleic acids (18). Initially, it was suspected that D. radiodurans might possess unique DNA repair mechanisms or pathways that could account for its remarkable ability to re-assemble its chromosome and thereby recover from conditions leading to multiple double-stranded breaks. More recent results have highlighted instead a key role for metal ion homeostasis, and in particular, a high Mn:Fe ratio in protecting proteins (including the DNA repair machinery) against ROS-mediated inactivation (18).
Multiple factors regulate the expression of the genes integral to the D. radiodurans oxidative stress response (Fig. 4), including OxyR, a PerR/Fur homolog, and DtxR. OxyR is unique in that it has only one cysteine residue (C210) in contrast to the two redox-active cysteine residues present in the prototypical E. coli OxyR (14). Upon exposure to peroxides, C210 becomes oxidized to a sulfenic acid. This cysteine residue does not appear to form an intermolecular disulfide bond with another OxyR subunit, and it is not known if it becomes further oxidized. In addition to OxyR, D. radiodurans has a homolog belonging to the PerR/Fur family of proteins; however, little is currently known on its sensing mechanism and regulon. A third regulator is DtxR, which is important for the regulation of both iron and manganese homeostasis (13). Collectively, these three proteins coregulate oxidative stress responses and metal homeostasis in D. radiodurans. The regulation of catalase (katE) highlights the interplay between the regulation by these three proteins. The expression of katE is activated by oxidized OxyR (14). Additionally, its expression may be repressed by both DtxR and the PerR/Fur homolog; katE is derepressed in both a dtxR mutant strain and a perR/fur mutant strain (13, 14). Interestingly, D. radiodurans has one of the highest catalase activities reported for any bacterium (97), which may account for the complex regulation of the katE gene.
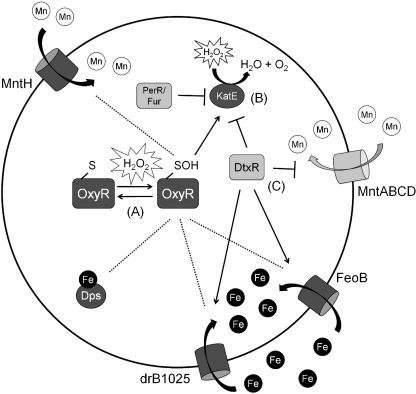
FIG. 4.
The OxyR and DtxR regulons in Deinococcus radiodurans. (A) D. radiodurans OxyR has only one redox active cysteine residue. This cysteine residue is oxidized upon exposure to H2O2. OxyR may regulate Mn and Fe homeostasis; however, the details of this regulation are not yet clearly defined. (B) Catalase activity is regulated by three regulatory proteins: OxyR, DtxR, and a PerR/Fur homolog. This tight regulation may relate to the high level of catalase activity present in this bacterium. (C) In addition to OxyR, DtxR also regulates Mn and Fe import. Mn transport is repressed under conditions where Fe transport is induced. Direct effects are shown as solid lines and potentially indirect effects are shown as dotted lines.
One of the most striking features of D. radiodurans is its very high intracellular manganese to iron ratio (0.24 for D. radiodurans compared to 0.0072 for E. coli). A high Mn:Fe ratio correlates with a high resistance to ionizing radiation-induced protein oxidation in bacteria. An ionizing radiation dose of 12,000 gray (absorbed radiation dose, Gy) decreases the number of viable D. radiodurans cells by 90% (D10). In comparison, the D10 for E. coli is 700 Gy (18), indicative of a strong correlation between intracellular Mn:Fe ratio and resistance to radiation and ROS. For example, Enterococcus faecium has a high intracellular Mn:Fe ratio (0.17) and, accordingly, has a high resistance to ionizing radiation (D10 = 2000 Gy), whereas Shewanella oneidensis and N. gonorrhoeae have low Mn:Fe ratios (0.0005 and 0.0004, respectively) and are not very resistant to ionizing radiation (D10 = 70 and 125 Gy) (19).
Consistent with the proposed importance of the Mn:Fe ratio, import of Mn appears to be inversely correlated with the import of Fe and both are under complex regulation. Expression of the D. radiodurans Mn and Fe transport proteins are coregulated by OxyR and DtxR (13, 14). D. radiodurans has two Mn(II) transporters (MntH and MntABCD) and at least two Fe transporters: an Fe(II) transport protein B (feoB) and an Fe(III) dicitrate-binding protein (drB1025). DtxR is proposed to activate transcription of the two iron transport genes while repressing the expression of the MntABCD Mn(II) transporter (13). In addition, OxyR may regulate the expression of the Fe transport proteins (14) and also activate expression of MntH, as observed for E. coli. A strain mutated in either oxyR or dtxR has an increased Mn:Fe ratio (0.321 for wildtype, 0.498 for the oxyR mutant, and 0.445 for the dtxR mutant) (13, 14). In addition to the iron transport proteins, expression of one of two dps genes in D. radiodurans is in part regulated by OxyR. However, this does not appear to be the dominant form of regulation since exposure of an oxyR mutant strain to H2O2 results in a very large upregulation of dps (about 120 times that of wildtype without H2O2), indicating that additional factors, yet unknown, likely regulate expression of dps (14). The studies reported to date in D. radiodurans indicate that this organism relies heavily on a well-coordinated modulation of metal ion homeostasis, ultimately affecting the Mn:Fe ratio within the cell, as a primary mode of defense against conditions leading to reactive radicals (oxidative stress and ionizing radiation) (18). However, additional work is needed to decipher the precise manner in which these regulators (and those yet to be defined) coordinate metal ion homeostasis with oxidative stress responses.
Neisseria gonorrhoeae
Most bacteria have either OxyR or PerR to sense and respond to H2O2 (61). Thus, it was something of a surprise when N. gonorrhoeae was proposed to have both an OxyR and a PerR regulatory system (84, 98). PerR was originally defined in this system as an Mn(II)-sensing repressor and mutants lacking PerR were highly resistant to H2O2 (98). While these properties are reminiscent of B. subtilis PerR, the N. gonorrhoeae homolog differs in other ways. Specifically, it does not appear to sense peroxide nor does it regulate classic peroxide stress response genes such as catalase.
It seems likely that OxyR is the key regulator of the adaptive response to low level peroxide stress in N. gonorrhoeae. OxyR controls a small regulon of three genes encoding catalase (katA), peroxidase (prx), and a glutathione oxidoreductase (gor) (84). N. gonorrhoeae has ∼100-fold higher catalase activity than E. coli (7). An oxyR mutant strain of N. gonorrhoeae has elevated catalase activity and is more resistant than wildtype to H2O2 stress, which suggests that OxyR acts as a repressor of katA (91). More recent results indicate that OxyR likely functions as both a repressor (in the absence of stress) and peroxide-responsive activator of catalase (38). In addition, OxyR activates the expression of prx, encoding a peroxiredoxin, and a glutathione oxidoreductase (84).
The PerR regulon, as defined by transcriptomics, bears a remarkable similarity to the Zur regulon in other bacteria. Specifically, PerR represses an ABC transporter for metal ion import and two ribosomal protein paralogs (L31 and L36). Duplicated genes encoding ribosomal proteins are found in numerous bacteria, and, in all cases examined to date, one paralog is induced in response to zinc starvation (70). Induction serves, in most cases (e.g., L31, L34, and L36), to mobilize zinc by displacement of the corresponding Zn-containing paralog from the surface of the ribosome. Alternatively, synthesis of a Zur-regulated, zinc-independent ribosomal protein (S14 paralog) enables continued ribosome synthesis in the absence of readily available zinc to metallate S14 (29, 62, 70). The ABC transporter regulated by PerR is annotated as MntABC and is involved in Mn(II) import, which plays an integral role in the oxidative stress resistance of N. gonorrhoeae (62). However, this transporter also mediates import of Zn(II), consistent with PerR functioning as a Zur ortholog (56). Further studies are needed to determine whether this PerR is formally analogous to Zur [a Zn(II)-sensing repressor], Mur [an Mn(II)-sensing repressor], or perhaps both.
The role of metal ions, and in particular Mn(II), in the oxidative stress resistance of N. gonorrhoeae is well documented. When grown in medium supplemented with 100 μM Mn(II), cells are more resistant to oxidative stress than when grown in unsupplemented media. Addition of other cations, including Zn(II), had no protective effect (92). A strain mutant in mntAB, encoding the membrane-associated components of the ABC transporter, is severely compromised in its growth under nonstressed conditions (98). Addition of either Mn(II) or Zn(II) to the growth media restored good growth of an mntC mutant and MntC was found to bind both Mn(II) and Zn(II) with similar affinity (56). Collectively, these results suggest that the ability of Neisseria to maintain cytosolic pools of Zn(II) and/or Mn(II) is critical for resistance to ROS, although there is little evidence to date to suggest that uptake is induced by oxidative stress.
In addition to PerR, N. gonorrhoeae also contains a bona fide Fur that functions specifically in iron homeostasis (Fig. 5). Fur mediates the iron-dependent repression of iron acquisition genes, including transferrin-binding proteins (TbpAB), ferric enterobactin transport protein (FetA, formerly FrpB), and ferric binding protein system (FpbABC) (41). Additionally, N. gonorrhoeae activates a bacterioferritin (bfrAB) and an Fe-containing SOD (sodB) in Fe-rich conditions. A Fur-regulated small RNA named NrrF (Neisserial regulatory RNA responsive to iron) likely mediates the indirect activation of bfrAB and sodB by Fur (24, 41). Although the upregulation of Bfr in Fe-rich conditions suggests that it serves primarily as an Fe-storage protein, a bfrB mutant strain is more sensitive to both H2O2 and paraquat highlighting again the connection between iron homeostasis and oxidative stress resistance (12).
FIG. 5.
The OxyR, PerR, and Fur regulons in Neisseria gonorrhoeae. (A) Oxidization of OxyR results in the upregulation of two H2O2 detoxifying enzymes, KatA and Prx. (B) Regulation of the oxidative stress response and Fe homeostasis are linked by the (potentially indirect) repression of OxyR by Fur:Fe. In addition, Fur may be inactivated by H2O2. In Fe-limited conditions, Fe uptake is derepressed and (C) Fe storage is repressed by the NrrF sRNA. NrrF is also responsible for the repression of an Fe-SOD. (D) In addition to OxyR, N. gonorrhoeae contains a PerR homolog that likely functions as a Zur (or possibly a Mur) regulatory protein. PerR is responsible for the repression of Mn and Zn uptake and a periplasmic peroxidase in Mn-replete (and possibly Zn-replete) conditions. Direct effects are shown as solid lines and potentially indirect effects are shown as dotted lines.
A complex picture of the adaptive response to H2O2 has emerged from a comparison of the peroxide stimulon (those genes induced by peroxide) and the regulons controlled by PerR, Fur, and OxyR (86). After 15 min of exposure to 5 mM H2O2, 75 genes were significantly upregulated and many of these were members of the Fur regulon (41). This suggests that, at least with these high levels of exogenous H2O2, the Fur repressor is inactivated. Unexpectedly, there appeared to be little induction of OxyR-regulated genes in this study. In contrast, exposure to 1 mM H2O2 did significantly upregulate the OxyR regulon (84). Moreover, H2O2-mediated induction of N. meningitidis catalase was found to be maximal with ∼135 μM H2O2, indicative of a sensitive and specific response as mediated by OxyR (38). These studies are consistent with the notion that OxyR serves as a first line of defense against low level H2O2 stress, whereas additional regulons are engaged at much higher levels of ROS.
Although the connections between the OxyR, PerR, and Fur regulons are poorly understood, there are some intriguing correlations. It has been noted, for example, that growth in iron-limiting conditions results in an increased expression of oxyR (41). OxyR regulates the expression of two peroxide detoxifying enzyme (KatA and Prx), only one of which uses iron. Whereas KatA uses a heme cofactor to reduce H2O2, Prx uses thiol-redox chemistry. One can speculate that increased expression of OxyR under iron-limiting conditions might serve to increase repression of the iron-requiring KatA while still allowing upregulation of Prx in the event of peroxide stress.
Streptococcus pyogenes
S. pyogenes, an important Gram-positive pathogen, lacks catalase and does not synthesize heme. In addition, the two known peroxidases in this organism, alkylhydroperoxide reductase (ahpCF) and glutathione peroxidase (gpoA), do not appear integral for protection against oxidative stress; a strain mutant in either ahpC or gpoA is not more sensitive to peroxide than wildtype nor is the expression of either ahpC or gpoA induced in conditions of oxidative stress (48). An ahpC mutant strain accumulates more endogenously produced peroxide than wildtype, suggesting a role for AhpC in the protection against low levels of H2O2, similar to that observed for E. coli (83). Although S. pyogenes expresses PerR, this regulatory protein does not control the expression of alkylhydroperoxide reductase or glutathione peroxidase (48). Due to this apparent lack of a strong direct defense against peroxides, it is of interest to explore the interplay between the oxidative stress response and metal homeostasis in S. pyogenes (Fig. 6).
FIG. 6.
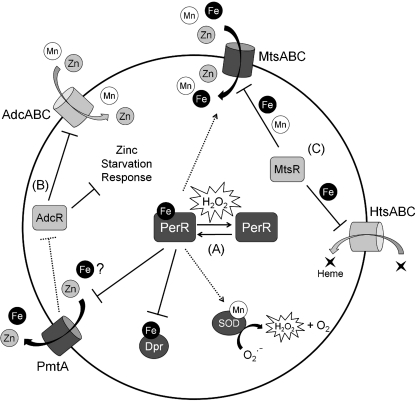
The PerR regulon in Streptococcus pyogenes. (A) Upon exposure to peroxides, oxidized PerR derepresses iron sequestration (Dps) and cation export (Zn and possibly Fe) via PmtA. Import of Fe, Zn, and Mn may also be decreased upon exposure to H2O2 via the downregulation of MtsABC. (B) The combined effect of these alterations of cation import is the activation of the zinc starvation response through AdcR. (C) Mn, Fe, and heme homeostasis are additionally controlled by MtsR in a metal-specific manner; either Fe- or Mn-bound MtsR can repress MtsABC, but only MtsR:Fe can repress heme import. Direct effects are shown as solid lines and potentially indirect effects are shown as dotted lines.
As observed for B. subtilis PerR, PerR from S. pyogenes responds to H2O2 by the derepression of oxidative stress genes (94). Among the genes repressed by PerR, one encodes a ferritin-like protein belonging to the MrgA/Dps family and a second encodes PmtA, a CPx-type heavy metal transporter homologous to ZosA (10, 94). The MrgA ortholog in this genus was originally described in Streptococcus mutans and named Dpr (a Dps homolog) (100), although the S. pyogenes ortholog has been referred to as both MrgA (9) and Dpr (93, 94). Recall that both mrgA and zosA are repressed by B. subtilis PerR. Regulation by PerR has been studied in most detail for dpr, which is induced by elevated levels of H2O2 (0.5–1.0 mM), but constitutively expressed in a perR mutant (94). Expression of dpr was also found to be elevated when cells were exposed to millimolar levels of metal ions, including Fe(II), Zn(II), and Ni(II). If PerR functions as described for B. subtilis, iron is the presumed PerR corepressor, which seems contrary to the observed induction by added iron. However, addition of high levels of Fe(II) to culture medium will likely lead to the formation of H2O2 via the reaction of Fe(II) with oxygen through Fenton chemistry, which may account for the observed derepression. The effects of Zn(II) and Ni(II) may reflect the ability of these metal ions to act as antagonists of metal-dependent DNA-binding by PerR.
Consistent with its regulation by PerR, Dpr likely confers protection against oxidative stress and this reflects its ability to chelate iron and thereby prevent Fenton chemistry (93). Indeed, addition of an iron chelator can rescue the peroxide sensitivity of a dpr mutant. The dpr mutant strain also does not grow as well in iron-limiting conditions and has a lower rate of survival in excess iron (30 mM ferrous sulfate) compared to wildtype. These results suggest that Dpr may also have physiologically significant roles in iron storage and sequestration (Fig. 6).
Although PmtA is homologous to ZosA from B. subtilis, a proposed Zn(II) uptake system (28), PmtA appears to be a metal efflux pump. A perR mutant strain, which has pmtA derepressed, has increased resistance to zinc toxicity (10). In contrast, a perR mutant strain of B. subtilis is more sensitive to zinc toxicity due to derepression of zosA (28). In support of the putative Zn(II) efflux activity of PmtA, derepression of PmtA is linked to the elevated expression of some genes regulated by AdcR, known to be induced by zinc starvation. AdcR also regulates the ABC transporter AdcABC implicated in the import of Zn(II), and possibly Mn(II), in S. pneumonia and S. mutans (22), but this operon was not induced in a perR mutant. Therefore, the link between derepression of pmtA and increased resistance to H2O2 remains unclear. Since other organisms increase the import of Mn(II) or Zn(II) in response to H2O2 stress, it seems counterintuitive that S. pyogenes would efflux Zn(II). Moreover, it is unclear how this might correlate with peroxide resistance. One possibility is that PmtA may also efflux Fe(II), and thereby reduce the incidence of Fenton chemistry within the cell. Indeed, accumulation of Fe, Mn, and Zn is decreased in the perR mutant strain of S. pyogenes (44, 78). The molecular basis for reduction in intracellular metal ion levels is presently unclear. In one study, decreased iron levels were correlated with reduced transcription of the ABC transporter MtsABC in the perR mutant (78). In addition to Fe(II), some studies suggest that MtsABC may import Mn(II) and/or Zn(II), but there are varying viewpoints on the physiological relevance of these observations (32, 44, 78, 89). Conversely, levels of mtsA were not reduced in the perR mutant in a second study (32). MtsR, an MntR ortholog, also regulates the expression of mtsABC together with the HtsABC heme importer (32).
PerR may also regulate, potentially indirectly, the expression of the single SOD (which is Mn-dependent) in S. pyogenes; a perR mutant has reduced transcription of sodA and is more sensitive to superoxide (O2∙−) (78). Although mutation of mtsABC does not affect transcription of sodA, the mtsABC mutant strain has decreased SOD activity and an increased sensitivity to O2∙−. Resistance to O2∙− can be restored to the mtsABC strain by supplementing the medium with Mn(II), suggesting that the reduced SOD activity is due to the lack of the Mn(II) cofactor (44).
The role of metal ion homeostasis systems in protection against ROS has also been studied in related Streptococci, including Streptococcus pneumoniae and S. mutans. S. pneumoniae is notable for its ability to produce millimolar levels of H2O2 during aerobic growth. In contrast with E. coli and most other systems studied to date, H2O2-mediated killing is unaffected by iron chelators and appears to be independent of Fenton chemistry. This is despite the fact that intracellular levels of chelatable iron and rates of hydroxyl radical production are comparable between E. coli and S. pneumoniae (74). It is suggested that perhaps Dpr prevents the association of redox active Fe(II) with DNA. In S. mutans, Dpr has also been described as a key factor in tolerance to oxygen due to its ability to sequester iron and prevent Fenton chemistry (99, 100). Studies in the Streptococci are generally consistent with a model in which iron homeostasis is critical to avoid ROS toxicity, Mn(II) import is important for maintaining SOD in an active form, and metal ion efflux (mediated by PmtA) contributes to resistance. However, the precise details and the apparent differences between these systems are not yet well understood.
Bradyrhizobium japonicum
B. japonicum is a soil bacterium that establishes a symbiotic relationship with host plants such as soybean in which it forms nitrogen fixing nodules associated with the plant roots. B. japonicum has a robust defense against ROS, but this differs in several ways from E. coli. Specifically, B. japonicum has only one primary catalase, KatG, and a katG mutant grows very poorly in aerobic conditions (69). The genome of B. japonicum encodes additional catalases/peroxidases, including ahpC, but they do not appear to have significant activity when cells are grown in nonstressed conditions (69). In contrast, in E. coli AhpCF is considered the primary enzyme responsible for protection against endogenously produced peroxide (83). Although B. japonicum encodes an OxyR homolog, the role of this regulator is not yet clear and an oxyR mutant has a similar resistance to H2O2 as wildtype (69).
The primary regulator responsible for controlling the response to oxidative stress and metal homeostasis in B. japonicum is a unique member of the Fur family, the iron response regulator (Irr) (85). Irr regulates intracellular heme levels through partially controlling heme biosynthesis and the import of heme from the extracellular environment (Fig. 7). In addition, Irr regulates iron uptake and storage. For example, under iron-limiting conditions Irr represses the transcription of a putative bacterioferritin (81). Unlike other members of the Fur family, Irr is active in its un-metallated form. Indeed, Irr is degraded in high iron conditions in response to direct binding of heme to Irr mediated by ferrochelatase, the enzyme responsible for the insertion of iron into protoporphyrin. In conditions of low iron, protoporphyrin binds to ferrochelatase, Irr and ferrochelatase do not form a complex, and Irr is free and active. Thus, Irr responds to heme levels directly at the site of heme biosynthesis. The heme-dependent degradation of Irr requires oxygen and thus also serves to sense ROS; mutation of katG or exposure of the cells to exogenous H2O2 promotes Irr degradation. In vitro, Irr becomes oxidized (carbonylated) in a heme-dependent manner (101). Irr thus coordinates iron homeostasis and heme biosynthesis in response to both iron availability (as sensed through heme) and oxidative stress. Phylogenomic studies indicate that this type of regulatory circuitry is likely to be widespread within the alpha-proteobacteria (45).
FIG. 7.
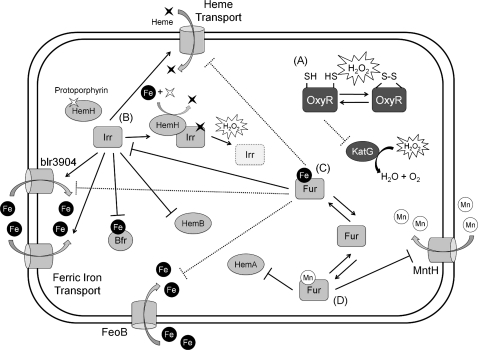
The Irr and Fur regulons of Bradyrhizobium japonicum. (A) OxyR does not appear to play a large role in the regulation of gene expression in B. japonicum. The oxidation of OxyR appears to upregulate the expression of catalase; however, this effect may be indirect, and appears to result from the derepression of katG by reduced OxyR. (B) Irr appears to play an important role in the coordination of the oxidative stress response with iron and heme homeostasis. In Fe-limited conditions, Irr is active as a regulatory protein. However, when Fe and heme are plentiful, Irr is degraded in a peroxide dependent manner. Irr is responsible for the regulation of heme transport and heme biosynthesis, inversely regulating import with synthesis. Irr also regulates Fe import and Fe storage. (C) In addition to heme directly regulating Irr activity, Fur:Fe represses the transcription of irr, preventing its production in conditions where Irr would be degraded. Fur may also regulate heme and iron homeostasis; however, these effects by Fur:Fe have not yet been shown to be direct (D). In addition to Fe, Fur also binds Mn. Fur:Mn appears to regulate Mn import and possibly heme biosynthesis. Direct effects are shown as solid lines and potentially indirect effects are shown as dotted lines.
In addition to Irr, B. japonicum expresses Fur, but this protein plays a relatively minor role in iron homeostasis. In fact, this protein responds to both Fe(II) and Mn(II), whereas, in related members of the Rhizobia, the Fur homolog functions as an Mn(II) uptake regulator designated Mur. B. japonicum Fur is similar to PerR in that it appears to integrate information about the relative levels of Fe(II) and Mn(II). For example, Fur regulates hemA, encoding ALA synthase responsible for the first step of heme biosynthesis. Unlike most Fur-regulated genes, hemA is expressed in Fe-replete conditions in wildtype, but is constitutive and unresponsive to iron in a fur mutant (31). One possible explanation for this Fe-dependent regulation of hemA is that Mn-bound Fur, but not Fe-bound Fur, can repress hemA (Fig. 7). Fur can thereby regulate heme biosynthesis and uptake in response to the Mn:Fe ratio. In contrast with hemA, hemB is regulated instead by Irr. Fur also regulates, in response to Mn(II), expression of mntH encoding the major Mn transport protein in B. japonicum (34).
Fur and Irr together regulate the transcription of irr in a manner responsive to the Mn:Fe ratio (76). In low iron, Irr acts as an anti-repressor to Fur (presumably the Mn-bound form) on the irr promoter. This antirepression is particularly noticeable in low iron, but high manganese, conditions (35). This dual regulation serves to coordinate Mn and Fe levels, reducing Fe levels when Mn levels are low (76). Since the only genes shown thus far to be directly regulated by Fur are irr and mntH, it remains possible that Fur is functioning primarily as an Mn(II) sensor (Mur) and the effects on iron and heme transport in a fur mutant strain result from the regulation of Irr by Fur (Fig. 7).
Conclusions and Perspective
Each of the six bacterial systems reviewed here provides a unique perspective on how cells adapt to peroxide stress by modulation of metal ion homeostasis. E. coli and the OxyR inducible stress response has provided a useful model for many Gram-negative bacteria, whereas B. subtilis and the PerR regulon has served this purpose for many Gram-positive organisms. These regulators are distinct as OxyR senses H2O2 via cysteine oxidation, whereas PerR relies on iron-catalyzed protein oxidation.
One of the more ubiquitous adaptations and one shared by both of these model systems is the peroxide-induction of an Fe(II) sequestering protein of the Dps family. A key role for Dps (and related proteins) in mediating peroxide resistance has now been documented in many systems, but important questions remain. It is not clear why many bacteria encode multiple Dps paralogs, and the division of labor between Dps mini-ferritins and the large ferritins is not always clear. Moreover, the fate of iron, once mineralized within Dps proteins, is not known. This iron may be irreversibly deposited as a mineral and thereby rendered inert, or it may represent a storage form that can, by processes yet to be described, be mobilized to provide iron for cellular needs. Oxidative stress may also decrease the import of iron by, for example, an OxyR-mediated increase in fur transcription. While oxidative stress induced efflux of iron has not been documented, this might be one function of the PmtA metal exporter described in S. pyogenes.
In addition to reducing the pools of reactive iron in the cell, cells also decrease the harm caused by Fenton chemistry by import of competing metals such as Mn(II) and Zn(II). Manganese, in particular, has been increasingly appreciated as a central player in the resistance to ROS and the Mn:Fe ratio within cells has emerged as a key parameter for protecting proteins against radical mediated damage. The basal levels of Mn(II) within cells varies widely. There are high Mn(II) levels in many Gram-positive bacteria and in these systems sequestration of Fe(II) may be sufficient to elevate the Mn:Fe ratio. In contrast, there is little Mn(II) in nonstressed E. coli, and in this case inducible import is additionally required for peroxide resistance. Numerous challenges remain, however, and the hard work of accurately measuring the physiologically relevant metal ions in these systems is in its early stages. It is very difficult to predict metal selectivity from sequence alone, and this has plagued efforts to assign function to transporters that import and export metals.
It has also proven challenging to define the precise functions of metalloregulators, and, in several cases, their perceived roles continue to evolve. For example, a putative Neisserial peroxide sensor (PerR) may in fact be more appropriately described as a Zur, and the Fur proteins of the Rhizobia have, in several cases, been re-defined as Mn(II) sensors and re-named as Mur. Indeed, in many organisms the Fur protein may be more properly considered a divalent ion sensor since both Fe(II) and Mn(II) can act as agonists to enable DNA binding. In at least some cases, the regulatory action of Fur family proteins is metal ion selective; examples include B. subtilis PerR and B. japonicum Fur, both of which appear to regulate distinct sets of genes depending on their associated regulatory metal ion. Alternatively, iron-specific sensing may be achieved by monitoring an iron-specific product such as heme (B. japonicum Irr) or assembly of Fe2S2 clusters (E. coli IscR). Often, the greatest insight into the regulatory logic of these and related systems emerges not from consideration of protein structure, but from a careful functional analysis of the regulated target genes. Ultimately, direct measurements of metal-protein binding interactions will be required to disentangle the complexities of the often overlapping regulatory and transport systems that control metal ion homeostasis. This work is now underway in these and related systems.
Abbreviations Used
- Bfr
bacterioferritin
- Dpr
a Dps homolog
- Dps
DNA-binding protein, stationary phase (mini-ferritin)
- DtxR
diphtheria toxin repressor
- Fur
ferric uptake regulator
- Gy
absorbed radiation dose
- H2O2
hydrogen peroxide
- Hpx
hydroperoxidases
- Irr
iron responsive regulator
- Isc
Fe-S cluster assembly machinery (housekeeping role)
- MCO
metal-catalyzed oxidation
- MntH
proton-dependent Mn(II) import
- MrgA
a Dps homolog
- Mur
an Mn(II)-sensing Fur homolog
- OxyR
oxidative stress regulator
- PerR
peroxide stress response regulator
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- Suf
a peroxide-inducible Fe-S cluster assembly system
- Zur
a Zn(II)-sensing Fur homolog
Acknowledgments
We would like to thank Dr. James Imlay for helpful comments and insightful suggestions. Work in our laboratory related to this review was supported by grants from the National Institutes of Health on metal ion homeostasis (GM059323) and from the National Science Foundation on oxidative stress responses (MCB-1020481).
References
- 1.Altuvia S. Almiron M. Huisman G. Kolter R. Storz G. The dps promoter is activated by OxyR during growth and by IHF and sigma S in stationary phase. Mol Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 2.Andrews SC. The Ferritin-like superfamily: evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim Biophys Acta. 2010;1800:691–705. doi: 10.1016/j.bbagen.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Andrews SC. Robinson AK. Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 4.Anjem A. Varghese S. Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antelmann H. Engelmann S. Schmid R. Sorokin A. Lapidus A. Hecker M. Expression of a stress- and starvation-induced dps/pexB-homologous gene is controlled by the alternative sigma factor σB in Bacillus subtilis. J Bacteriol. 1997;179:7251–7256. doi: 10.1128/jb.179.23.7251-7256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antelmann H. Helmann JD. Thiol-based redox switches and gene regulation. Antioxid Redox Signal. 2011;14:1049–1063. doi: 10.1089/ars.2010.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archibald FS. Duong MN. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect Immun. 1986;51:631–641. doi: 10.1128/iai.51.2.631-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslund F. Zheng M. Beckwith J. Storz G. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci U S A. 1999;96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenot A. King KY. Caparon MG. The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Mol Microbiol. 2005;55:221–234. doi: 10.1111/j.1365-2958.2004.04370.x. [DOI] [PubMed] [Google Scholar]
- 10.Brenot A. Weston BF. Caparon MG. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Mol Microbiol. 2007;63:1185–1196. doi: 10.1111/j.1365-2958.2006.05577.x. [DOI] [PubMed] [Google Scholar]
- 11.Bsat N. Herbig A. Casillas-Martinez L. Setlow P. Helmann JD. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen CY. Morse SA. Neisseria gonorrhoeae bacterioferritin: structural heterogeneity, involvement in iron storage and protection against oxidative stress. Microbiology. 1999;145(Pt 10):2967–2975. doi: 10.1099/00221287-145-10-2967. [DOI] [PubMed] [Google Scholar]
- 13.Chen H. Wu R. Xu G. Fang X. Qiu X. Guo H. Tian B. Hua Y. DR2539 is a novel DtxR-like regulator of Mn/Fe ion homeostasis and antioxidant enzyme in Deinococcus radiodurans. Biochem Biophys Res Commun. 2010;396:413–418. doi: 10.1016/j.bbrc.2010.04.106. [DOI] [PubMed] [Google Scholar]
- 14.Chen H. Xu G. Zhao Y. Tian B. Lu H. Yu X. Xu Z. Ying N. Hu S. Hua Y. A novel OxyR sensor and regulator of hydrogen peroxide stress with one cysteine residue in Deinococcus radiodurans. PLoS ONE. 2008;3:e1602. doi: 10.1371/journal.pone.0001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L. Helmann JD. Bacillus subtilis MrgA is a Dps(PexB) homologue: evidence for metalloregulation of an oxidative-stress gene. Mol Microbiol. 1995;18:295–300. doi: 10.1111/j.1365-2958.1995.mmi_18020295.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen L. James LP. Helmann JD. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol. 1993;175:5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L. Keramati L. Helmann JD. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc Natl Acad Sci U S A. 1995;92:8190–8194. doi: 10.1073/pnas.92.18.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daly MJ. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol. 2009;7:237–245. doi: 10.1038/nrmicro2073. [DOI] [PubMed] [Google Scholar]
- 19.Daly MJ. Gaidamakova EK. Matrosova VY. Vasilenko A. Zhai M. Venkateswaran A. Hess M. Omelchenko MV. Kostandarithes HM. Makarova KS. Wackett LP. Fredrickson JK. Ghosal D. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 20.Davies BW. Walker GC. Disruption of sitA compromises Sinorhizobium meliloti for manganese uptake required for protection against oxidative stress. J Bacteriol. 2007;189:2101–2109. doi: 10.1128/JB.01377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desnoyers G. Morissette A. Prevost K. Masse E. Small RNA-induced differential degradation of the polycistronic mRNA iscRSUA. EMBO J. 2009;28:1551–1561. doi: 10.1038/emboj.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dintilhac A. Alloing G. Granadel C. Claverys JP. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 23.Duarte V. Latour JM. PerR vs OhrR: selective peroxide sensing in Bacillus subtilis. Mol Biosyst. 2010;6:316–323. doi: 10.1039/b915042k. [DOI] [PubMed] [Google Scholar]
- 24.Ducey TF. Jackson L. Orvis J. Dyer DW. Transcript analysis of nrrF, a Fur repressed sRNA of Neisseria gonorrhoeae. Microb Pathog. 2009;46:166–170. doi: 10.1016/j.micpath.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forman HJ. Maiorino M. Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuangthong M. Herbig AF. Bsat N. Helmann JD. Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible. J Bacteriol. 2002;184:3276–3286. doi: 10.1128/JB.184.12.3276-3286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaballa A. Antelmann H. Aguilar C. Khakh SK. Song KB. Smaldone GT. Helmann JD. The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins. Proc Natl Acad Sci U S A. 2008;105:11927–11932. doi: 10.1073/pnas.0711752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaballa A. Helmann JD. A peroxide-induced zinc uptake system plays an important role in protection against oxidative stress in Bacillus subtilis. Mol Microbiol. 2002;45:997–1005. doi: 10.1046/j.1365-2958.2002.03068.x. [DOI] [PubMed] [Google Scholar]
- 29.Gabriel SE. Helmann JD. Contributions of Zur-controlled ribosomal proteins to growth under zinc starvation conditions. J Bacteriol. 2009;191:6116–6122. doi: 10.1128/JB.00802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant RA. Filman DJ. Finkel SE. Kolter R. Hogle JM. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat Struct Biol. 1998;5:294–303. doi: 10.1038/nsb0498-294. [DOI] [PubMed] [Google Scholar]
- 31.Hamza I. Qi Z. King ND. O'Brian MR. Fur-independent regulation of iron metabolism by Irr in Bradyrhizobium japonicum. Microbiology. 2000;146(Pt 3):669–676. doi: 10.1099/00221287-146-3-669. [DOI] [PubMed] [Google Scholar]
- 32.Hanks TS. Liu M. McClure MJ. Fukumura M. Duffy A. Lei B. Differential regulation of iron- and manganese-specific MtsABC and heme-specific HtsABC transporters by the metalloregulator MtsR of group A Streptococcus. Infect Immun. 2006;74:5132–5139. doi: 10.1128/IAI.00176-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbig AF. Helmann JD. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol Microbiol. 2001;41:849–859. doi: 10.1046/j.1365-2958.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 34.Hohle TH. O'Brian MR. The mntH gene encodes the major Mn2+ transporter in Bradyrhizobium japonicum and is regulated by manganese via the Fur protein. Mol Microbiol. 2009;72:399–409. doi: 10.1111/j.1365-2958.2009.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hohle TH. O'Brian MR. Transcriptional control of the Bradyrhizobium japonicum irr gene requires repression by Fur and antirepression by Irr. J Biol Chem. 2010;285:26074–26080. doi: 10.1074/jbc.M110.145979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horsburgh MJ. Clements MO. Crossley H. Ingham E. Foster SJ. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect Immun. 2001;69:3744–3754. doi: 10.1128/IAI.69.6.3744-3754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horsburgh MJ. Ingham E. Foster SJ. In Staphylococcus aureus, fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J Bacteriol. 2001;183:468–475. doi: 10.1128/JB.183.2.468-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ieva R. Roncarati D. Metruccio MM. Seib KL. Scarlato V. Delany I. OxyR tightly regulates catalase expression in Neisseria meningitidis through both repression and activation mechanisms. Mol Microbiol. 2008;70:1152–1165. doi: 10.1111/j.1365-2958.2008.06468.x. [DOI] [PubMed] [Google Scholar]
- 39.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 40.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson LA. Ducey TF. Day MW. Zaitshik JB. Orvis J. Dyer DW. Transcriptional and functional analysis of the Neisseria gonorrhoeae Fur regulon. J Bacteriol. 2010;192:77–85. doi: 10.1128/JB.00741-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jang S. Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang S. Imlay JA. Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulfur assembly system, and OxyR induces the Suf system to compensate. Molecular Microbiology. 2010;78:1448–1467. doi: 10.1111/j.1365-2958.2010.07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janulczyk R. Ricci S. Bjorck L. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect Immun. 2003;71:2656–2664. doi: 10.1128/IAI.71.5.2656-2664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston AW. Todd JD. Curson AR. Lei S. Nikolaidou-Katsaridou N. Gelfand MS. Rodionov DA. Living without Fur: the subtlety and complexity of iron-responsive gene regulation in the symbiotic bacterium Rhizobium and other alpha-proteobacteria. Biometals. 2007;20:501–511. doi: 10.1007/s10534-007-9085-8. [DOI] [PubMed] [Google Scholar]
- 46.Kehres DG. Janakiraman A. Slauch JM. Maguire ME. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+ J Bacteriol. 2002;184:3151–3158. doi: 10.1128/JB.184.12.3151-3158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keyer K. Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci U S A. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King KY. Horenstein JA. Caparon MG. Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes. J Bacteriol. 2000;182:5290–5299. doi: 10.1128/jb.182.19.5290-5299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitphati W. Ngok-Ngam P. Suwanmaneerat S. Sukchawalit R. Mongkolsuk S. Agrobacterium tumefaciens fur has important physiological roles in iron and manganese homeostasis, the oxidative stress response, and full virulence. Appl Environ Microbiol. 2007;73:4760–4768. doi: 10.1128/AEM.00531-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korshunov S. Imlay JA. Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol Microbiol. 2010;75:1389–1401. doi: 10.1111/j.1365-2958.2010.07059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee C. Lee SM. Mukhopadhyay P. Kim SJ. Lee SC. Ahn WS. Yu MH. Storz G. Ryu SE. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol. 2004;11:1179–1185. doi: 10.1038/nsmb856. [DOI] [PubMed] [Google Scholar]
- 52.Lee JW. Helmann JD. Biochemical characterization of the structural Zn2+ site in the Bacillus subtilis peroxide sensor PerR. J Biol Chem. 2006;281:23567–23578. doi: 10.1074/jbc.M603968200. [DOI] [PubMed] [Google Scholar]
- 53.Lee JW. Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 54.Lee JW. Helmann JD. Functional specialization within the Fur family of metalloregulators. Biometals. 2007;20:485–499. doi: 10.1007/s10534-006-9070-7. [DOI] [PubMed] [Google Scholar]
- 55.Lee KC. Yeo WS. Roe JH. Oxidant-responsive induction of the suf operon, encoding a Fe-S assembly system, through Fur and IscR in Escherichia coli. J Bacteriol. 2008;190:8244–8247. doi: 10.1128/JB.01161-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim KH. Jones CE. vanden Hoven RN. Edwards JL. Falsetta ML. Apicella MA. Jennings MP. McEwan AG. Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect Immun. 2008;76:3569–3576. doi: 10.1128/IAI.01725-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liochev SI. Fridovich I. Carbon dioxide mediates Mn(II)-catalyzed decomposition of hydrogen peroxide and peroxidation reactions. Proc Natl Acad Sci U S A. 2004;101:12485–12490. doi: 10.1073/pnas.0404911101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masse E. Salvail H. Desnoyers G. Arguin M. Small RNAs controlling iron metabolism. Curr Opin Microbiol. 2007;10:140–145. doi: 10.1016/j.mib.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Mongkolsuk S. Helmann JD. Regulation of inducible peroxide stress responses. Mol Microbiol. 2002;45:9–15. doi: 10.1046/j.1365-2958.2002.03015.x. [DOI] [PubMed] [Google Scholar]
- 60.Moore CM. Helmann JD. Metal ion homeostasis in Bacillus subtilis. Curr Opin Microbiol. 2005;8:188–195. doi: 10.1016/j.mib.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Morikawa K. Ohniwa RL. Kim J. Maruyama A. Ohta T. Takeyasu K. Bacterial nucleoid dynamics: oxidative stress response in Staphylococcus aureus. Genes Cells. 2006;11:409–423. doi: 10.1111/j.1365-2443.2006.00949.x. [DOI] [PubMed] [Google Scholar]
- 62.Nanamiya H. Kawamura F. Towards an elucidation of the roles of the ribosome during different growth phases in Bacillus subtilis. Biosci Biotechnol Biochem. 2010;74:451–461. doi: 10.1271/bbb.90859. [DOI] [PubMed] [Google Scholar]
- 63.Nandal A. Huggins CC. Woodhall MR. McHugh J. Rodriguez-Quinones F. Quail MA. Guest JR. Andrews SC. Induction of the ferritin gene (ftnA) of Escherichia coli by Fe2+-Fur is mediated by reversal of H-NS silencing and is RyhB independent. Mol Microbiol. 2010;75:637–657. doi: 10.1111/j.1365-2958.2009.06977.x. [DOI] [PubMed] [Google Scholar]
- 64.Nesbit AD. Giel JL. Rose JC. Kiley PJ. Sequence-specific binding to a subset of IscR-regulated promoters does not require IscR Fe-S cluster ligation. J Mol Biol. 2009;387:28–41. doi: 10.1016/j.jmb.2009.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogunniyi AD. Mahdi LK. Jennings MP. McEwan AG. McDevitt CA. Van der Hoek MB. Bagley CJ. Hoffmann P. Gould KA. Paton JC. Central role of manganese in regulation of stress responses, physiology, and metabolism in Streptococcus pneumoniae. J Bacteriol. 2010;192:4489–4497. doi: 10.1128/JB.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ollinger J. Song KB. Antelmann H. Hecker M. Helmann JD. Role of the Fur regulon in iron transport in Bacillus subtilis. J Bacteriol. 2006;188:3664–3673. doi: 10.1128/JB.188.10.3664-3673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Outten FW. Djaman O. Storz G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- 68.Palyada K. Sun YQ. Flint A. Butcher J. Naikare H. Stintzi A. Characterization of the oxidative stress stimulon and PerR regulon of Campylobacter jejuni. BMC Genomics. 2009;10:481. doi: 10.1186/1471-2164-10-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panek HR. O'Brian MR. KatG is the primary detoxifier of hydrogen peroxide produced by aerobic metabolism in Bradyrhizobium japonicum. J Bacteriol. 2004;186:7874–7880. doi: 10.1128/JB.186.23.7874-7880.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panina EM. Mironov AA. Gelfand MS. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci U S A. 2003;100:9912–9917. doi: 10.1073/pnas.1733691100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Papp-Wallace KM. Maguire ME. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- 72.Park S. You X. Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx-mutants of Escherichia coli. Proc Natl Acad Sci U S A. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pena MM. Bullerjahn GS. The DpsA protein of Synechococcus sp. Strain PCC7942 is a DNA-binding hemoprotein. Linkage of the Dps and bacterioferritin protein families. J Biol Chem. 1995;270:22478–22482. doi: 10.1074/jbc.270.38.22478. [DOI] [PubMed] [Google Scholar]
- 74.Pericone CD. Park S. Imlay JA. Weiser JN. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J Bacteriol. 2003;185:6815–6825. doi: 10.1128/JB.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Posey JE. Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- 76.Puri S. Hohle TH. O'Brian MR. Control of bacterial iron homeostasis by manganese. Proc Natl Acad Sci U S A. 2010;107:10691–10695. doi: 10.1073/pnas.1002342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Que Q. Helmann JD. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol. 2000;35:1454–68. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 78.Ricci S. Janulczyk R. Bjorck L. The regulator PerR is involved in oxidative stress response and iron homeostasis and is necessary for full virulence of Streptococcus pyogenes. Infect Immun. 2002;70:4968–4976. doi: 10.1128/IAI.70.9.4968-4976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Runyen-Janecky L. Dazenski E. Hawkins S. Warner L. Role and regulation of the Shigella flexneri sit and MntH systems. Infect Immun. 2006;74:4666–4672. doi: 10.1128/IAI.00562-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sabri M. Leveille S. Dozois CM. A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology. 2006;152:745–758. doi: 10.1099/mic.0.28682-0. [DOI] [PubMed] [Google Scholar]
- 81.Sangwan I. Small SK. O'Brian MR. The Bradyrhizobium japonicum Irr protein is a transcriptional repressor with high-affinity DNA-binding activity. J Bacteriol. 2008;190:5172–5177. doi: 10.1128/JB.00495-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwartz CJ. Giel JL. Patschkowski T. Luther C. Ruzicka FJ. Beinert H. Kiley PJ. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc Natl Acad Sci U S A. 2001;98:14895–14900. doi: 10.1073/pnas.251550898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seaver LC. Imlay JA. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol. 2001;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seib KL. Wu HJ. Srikhanta YN. Edwards JL. Falsetta ML. Hamilton AJ. Maguire TL. Grimmond SM. Apicella MA. McEwan AG. Jennings MP. Characterization of the OxyR regulon of Neisseria gonorrhoeae. Mol Microbiol. 2007;63:54–68. doi: 10.1111/j.1365-2958.2006.05478.x. [DOI] [PubMed] [Google Scholar]
- 85.Small SK. Puri S. O'Brian MR. Heme-dependent metalloregulation by the iron response regulator (Irr) protein in Rhizobium and other Alpha-proteobacteria. Biometals. 2009;22:89–97. doi: 10.1007/s10534-008-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stohl EA. Criss AK. Seifert HS. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol Microbiol. 2005;58:520–532. doi: 10.1111/j.1365-2958.2005.04839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stone JR. An assessment of proposed mechanisms for sensing hydrogen peroxide in mammalian systems. Arch Biochem Biophys. 2004;422:119–124. doi: 10.1016/j.abb.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 88.Stone JR. Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 89.Sun X. Ge R. Chiu JF. Sun H. He QY. Lipoprotein MtsA of MtsABC in Streptococcus pyogenes primarily binds ferrous ion with bicarbonate as a synergistic anion. FEBS Lett. 2008;582:1351–1354. doi: 10.1016/j.febslet.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 90.Traore DA. El Ghazouani A. Jacquamet L. Borel F. Ferrer JL. Lascoux D. Ravanat JL. Jaquinod M. Blondin G. Caux-Thang C. Duarte V. Latour JM. Structural and functional characterization of 2-oxo-histidine in oxidized PerR protein. Nat Chem Biol. 2009;5:53–59. doi: 10.1038/nchembio.133. [DOI] [PubMed] [Google Scholar]
- 91.Tseng HJ. McEwan AG. Apicella MA. Jennings MP. OxyR acts as a repressor of catalase expression in Neisseria gonorrhoeae. Infect Immun. 2003;71:550–556. doi: 10.1128/IAI.71.1.550-556.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tseng HJ. Srikhanta Y. McEwan AG. Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol. 2001;40:1175–1186. doi: 10.1046/j.1365-2958.2001.02460.x. [DOI] [PubMed] [Google Scholar]
- 93.Tsou CC. Chiang-Ni C. Lin YS. Chuang WJ. Lin MT. Liu CC. Wu JJ. An iron-binding protein, Dpr, decreases hydrogen peroxide stress and protects Streptococcus pyogenes against multiple stresses. Infect Immun. 2008;76:4038–4045. doi: 10.1128/IAI.00477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsou CC. Chiang-Ni C. Lin YS. Chuang WJ. Lin MT. Liu CC. Wu JJ. Oxidative stress and metal ions regulate a ferritin-like gene, dpr, in Streptococcus pyogenes. Int J Med Microbiol. 2010;300:259–264. doi: 10.1016/j.ijmm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 95.van Vliet AH. Ketley JM. Park SF. Penn CW. The role of iron in Campylobacter gene regulation, metabolism and oxidative stress defense. FEMS Microbiol Rev. 2002;26:173–186. doi: 10.1016/s0168-6445(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 96.Varghese S. Wu A. Park S. Imlay KR. Imlay JA. Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol Microbiol. 2007;64:822–830. doi: 10.1111/j.1365-2958.2007.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang P. Schellhorn HE. Induction of resistance to hydrogen peroxide and radiation in Deinococcus radiodurans. Can J Microbiol. 1995;41:170–176. doi: 10.1139/m95-023. [DOI] [PubMed] [Google Scholar]
- 98.Wu HJ. Seib KL. Srikhanta YN. Kidd SP. Edwards JL. Maguire TL. Grimmond SM. Apicella MA. McEwan AG. Jennings MP. PerR controls Mn-dependent resistance to oxidative stress in Neisseria gonorrhoeae. Mol Microbiol. 2006;60:401–416. doi: 10.1111/j.1365-2958.2006.05079.x. [DOI] [PubMed] [Google Scholar]
- 99.Yamamoto Y. Fukui K. Koujin N. Ohya H. Kimura K. Kamio Y. Regulation of the intracellular free iron pool by Dpr provides oxygen tolerance to Streptococcus mutans. J Bacteriol. 2004;186:5997–6002. doi: 10.1128/JB.186.18.5997-6002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamamoto Y. Higuchi M. Poole LB. Kamio Y. Role of the dpr product in oxygen tolerance in Streptococcus mutans. J Bacteriol. 2000;182:3740–3747. doi: 10.1128/jb.182.13.3740-3747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang J. Panek HR. O'Brian MR. Oxidative stress promotes degradation of the Irr protein to regulate haem biosynthesis in Bradyrhizobium japonicum. Mol Microbiol. 2006;60:209–218. doi: 10.1111/j.1365-2958.2006.05087.x. [DOI] [PubMed] [Google Scholar]
- 102.Yeo WS. Lee JH. Lee KC. Roe JH. IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol Microbiol. 2006;61:206–218. doi: 10.1111/j.1365-2958.2006.05220.x. [DOI] [PubMed] [Google Scholar]
- 103.Zheng M. Doan B. Schneider TD. Storz G. OxyR and SoxRS regulation of fur. J Bacteriol. 1999;181:4639–4643. doi: 10.1128/jb.181.15.4639-4643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng M. Storz G. Redox sensing by prokaryotic transcription factors. Biochem Pharmacol. 2000;59:1–6. doi: 10.1016/s0006-2952(99)00289-0. [DOI] [PubMed] [Google Scholar]