Abstract
Objectives
The objectives of this study were to explore the association between complementary and alternative medicine (CAM) use as reported by youth, and parents' and children's reported quality of life in youth with diabetes.
Design
The study design was a cross-sectional survey.
Setting
Youth in Washington State participated in the SEARCH for Diabetes in Youth study, a national, multisite epidemiological study designed to assess the prevalence and incidence of diabetes in U.S. youth. Surveys assessing CAM utilization were mailed in January and April 2006.
Participants
One thousand four hundred and thirty-nine (1439) youth were mailed a CAM survey. The final sample consisted of 467 youth with both CAM survey results and quality-of-life data.
Outcome measures
Difference in mean scores on Pediatric Quality of Life Inventory (PedsQL) between CAM users and nonusers overall, and specific CAM therapies were the outcome measures.
Results
Of the 1439 participants approached, 587 (40.8%) returned the CAM survey. In adjusted analyses, children reported any CAM use as associated with more barriers to treatment (difference in mean scores −3.48, 95% confidence interval [CI] −6.65, −0.31). Children following a CAM diet reported higher quality of life (PedsQL Core Total difference 4.01, 95% CI [0.10–7.91]; Core Psychosocial difference was 6.45, 95% CI [1.95 to 10.95]), but those using stress-reduction activities reported poorer quality of life (Diabetes Total difference −4.19, 95% CI [−8.35 to −0.04]). Parent-reported quality of life was lower for children who used “other supplements” (Core Total difference −6.26, 95% CI [−11.29 to −1.24]; Core Psychosocial difference was −5.92, 95% CI [−11.65 to −0.19]).
Conclusions
CAM diets were associated with increased quality of life in youth with diabetes, whereas supplement use and stress-reduction activities were associated with decreased quality of life. The temporal sequence between CAM use and quality of life requires further study.
Introduction
Little has been reported on the use of complementary and alternative medicine (CAM) therapies as adjuncts to conventional care by people with diabetes. In data from the 2002 National Health Interview Survey's CAM supplement section, the prevalence of CAM use among adults with diabetes was estimated at 72.8%, versus 61.2% of adults without diabetes (p < 0.0001).1 Omitting prayer from the definition of CAM, the prevalence of CAM use among adults with diabetes in this study was 33.7%, versus 37.4% among adults without diabetes (p = 0.0016). In a 2002 national sample of adolescents from the general population, 79% reported having used some form of CAM in their lifetimes, and 48.5% reported use in the past month.2 CAM utilization has been found to be common in children with chronic diseases such as cystic fibrosis, inflammatory bowel disease, human immunodeficiency virus infection, asthma, cancer, and arthritis.3–9 However, limited information on CAM use in youth with diabetes is available. One study of chronically ill children reported that 60% of those with type 1 diabetes (n = 50) had used a dietary supplement, including vitamins (56%), minerals (14%), and botanicals (18%) in the previous year.10 Thirty-one percent (31%) of this use was “unprescribed,” and only 20% of those reporting unprescribed supplement use revealed this to the child's health care provider.10 In Germany, 18.4% of children with diabetes were reported as using one or more types of CAM11; however, the use of CAM within the conventional medical model varies between Germany and the United States.
Patients with chronic illness are reported to use CAM to improve psychosocial well-being and quality of life (QOL) and to increase their sense of control and responsibility for self-care.12,13 In diabetes, intensive management of diet, exercise, and pharmacological treatment is required to achieve the tight control of glucose levels necessary to prevent serious long-term health complications. In the literature, individual reactivity to stress has also been suggested to affect blood glucose readings and insulin needs, either by direct physiologic action or by a disruption of self-care.14,15 In childhood and adolescence, diabetes management can be particularly difficult due to social, environmental, physiologic, and psychologic factors. Implementing intensive management can lead to challenges in establishing autonomy, difficulty in maintaining a desired weight, and added complexity with strenuous physical activity.16 Child and parent-proxy reports using the Pediatric Quality of Life Inventory found lower QOL for all scales except physical functioning (child report only) in children aged 8–18 years with type 1 or 2 diabetes compared to healthy children.17
To explore the association between the use of CAM and QOL in youth with diabetes, we surveyed children with diabetes and their parents about the child's CAM use and linked results to Pediatric Quality of Life (PedsQL) Core Scale scores and Diabetes Module scores (parent and child report).
Methods
Study setting
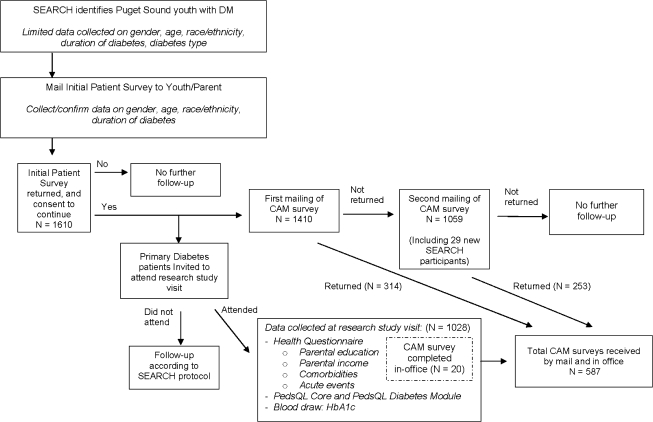
The CAM survey was conducted as an ancillary study to the SEARCH for Diabetes in Youth study (SEARCH), a detailed description of which has been published previously.18 Briefly, SEARCH is a national, multisite epidemiological study designed to assess the prevalence and incidence of diabetes in American youth. Those eligible for SEARCH at the time of this ancillary study were youth who were diagnosed with diabetes mellitus by a health care provider either as a prevalent case in 2001 or an incident case in 2002–2005 and who were under 20 years of age at the time of enrollment. An Initial Patient Survey (IPS) was distributed to collect demographic information (age, age at diagnosis, gender, and race/ethnicity). Those who returned the IPS were invited to attend a SEARCH study visit, at which time additional demographic, clinical, and QOL data were collected by interview, questionnaire administration, blood draw, and physical examination. Those eligible for the CAM survey included participants from one of the six SEARCH sites, Washington State, which enrolled children and adolescents residing in five counties in the Puget Sound region (King, Pierce, Snohomish, Kitsap, or Thurston Counties) (Fig. 1).
FIG. 1.
Flowchart of data collected from SEARCH for Diabetes in Youth and by complementary and alternative medicine (CAM) survey. Total numbers for SEARCH populations based on reference date of June 15, 2006. DM, diabetes mellitus; PedsQL, Pediatric Quality of Life Inventory; HbA1c, glycosylated hemoglobin.
The CAM survey was administered via mail or in-person at SEARCH study visits. The survey used numerical identification numbers to link the CAM survey to SEARCH outcome data. The first mailing was sent to 1410 SEARCH participants in January 2006. The second mailing was sent in April 2006 to 1030 nonrespondents and 29 individuals newly enrolled in SEARCH; newly enrolled participants received only one survey. Surveys were collected through August 2006.
Institutional review board approval for this project was issued by Bastyr University in Kenmore, WA, and Seattle Children's Hospital, Seattle, WA, and all relevant institutional review boards associated with SEARCH recruiting sites.
Data collection instruments
CAM survey
The CAM survey was developed by a team including an adolescent medicine physician, pediatric endocrinologist, pediatric naturopathic physician, and a health educator. The survey was further refined through repeated administration to pediatric nurses, pediatric researchers, naturopathic medical students, and parents. The final survey instrument comprised a 31 page self-administered questionnaire containing 27 multipart questions and requiring 15–30 minutes to complete. The survey instructions were written to the child and requested that the child complete the survey alone or with the assistance of a parent/guardian, with responses reflecting the viewpoint of the child. The readability calculation of our CAM survey yielded a Flesch-Kincaid grade reading level of 5.7 (Microsoft Office Professional Edition 2003, Word program; Microsoft Inc., Redmond, WA).
CAM modalities assessed in the survey included the following: nutrition choices, vitamin/mineral use, herb use, other supplement use, and stress reduction activities. To evaluate the use of CAM in relation to a diagnosis of diabetes, the question for each modality was phrased “Since you were diagnosed with diabetes, have you …” to exclude use of CAM prior to the diagnosis. Write-in spaces for other CAM types were included for many questions, and the investigators manually coded the participants' written answers with the intent of capturing CAM use.
Table 1 outlines the specific inclusions used to define CAM users. Briefly, children who reported seeing a CAM provider in the past 6 months or having seen a CAM provider specifically for the treatment of diabetes were classified as a CAM user. Survey respondents were also considered CAM users if they endorsed use of an alternative diet practice; took herbs, supplements, vitamins, or minerals (other than standard daily multivitamins/multiminerals); or participated in stress reduction activities (Table 1). “Stress reduction activities” were defined as affirmative responses to the question “Since you were diagnosed with diabetes, have you participated in any activities to reduce your stress or help control your diabetes?” Use of spiritual or religious practices was not considered CAM use.
Table 1.
Specific Questions and Endorsements That Defined a Complementary and Alternative Medicine (CAM) User
| CAM definition |
|---|
| Questions: |
| Since you were diagnosed with diabetes, have you … |
| i. made any changes in the food you eat? |
| ii. taken any vitamins and minerals? |
| iii. taken any herbs? |
| iv. taken any supplements? |
| v. taken any combination supplements that contain vitamins, minerals, and herbs together? |
| vi. participated in any activities to reduce your stress or help control your diabetes? |
| Have you seen an Acupuncturist/Chiropractor/Curandero(a)/Herbalist/ Homeopath/Massage Therapist/Naturopathic Doctor/Osteopathic Doctor/Sobadero(a)/Spiritualist/other CAM providers? |
| i. Were your visits to the [provider] for treatment of diabetes? |
| ii. Have you seen the [provider] in the last 6 months? |
| Endorsements (by checkbox, or fill in) | |
|---|---|
| Diet | Stress reduction activities |
| Avoid wheata | Biofeedback |
| Avoid dairya | Breathing exercises |
| Organic/antibiotic-free/hormone-freea | Guided imagery |
| Blood type diet | Hypnosis |
| Massageb | |
| Meditation | |
| Supplements | Self-hypnosis |
| 5-HTP | Reflexology |
| α-Lipoic acid | Yogab |
| Bach Flower remedies | Acupunctureb |
| Brewer's yeast | Chiropracticb |
| Essential fatty acids | Massage with acupunctureb |
| Garlic | Reiki |
| Ginger root | |
| Glucose balance | Saw CAM provider |
| Green tea/theanine | For treatment of diabetes |
| Homeopathic remedies | In past 6 monthsc |
| l-Carnitine | |
| l-Lysine | Vitamins/minerals |
| l-Tyrosine | Used any vitamin or mineral |
| Melatonin | other than a multivitamin/multimineral |
| Onion | |
| Probiotics | Herbs |
| Any combination supplement | Used any herbs other than cooking |
If endorsed that this activity was used to help control diabetes.
If endorsed/wrote in this activity under “to reduce your stress or help control your diabetes”.
If duration of diabetes was greater than 6 months.
5-HTP, 5-hydroxytryptophan.
Pediatric Quality of Life Inventory
The SEARCH study uses the Pediatric Quality of Life Inventory (PedsQL) child version and parent proxy version to assess health-related QOL, and scores of both versions were examined in our analysis. The PedsQL has been empirically validated for the pediatric age range in both parent proxy and child report formats.19 A diabetes module (PedsQL Diabetes Module) has been designed and validated for youth with type 1 and type 2 diabetes.17 The PedsQL yields six scores including total, physical health, psychosocial health, emotional functioning, social functioning, and school functioning. The diabetes module yields six scores including symptoms, treatment barriers, treatment adherence, worry, communication, and diabetes module total. All scales are scored from 0 to 100, with higher scores reflecting higher QOL and fewer problems.
SEARCH data
CAM survey results were linked to the respondents' previously collected data in the SEARCH data set, including the following: sociodemographic characteristics (age, gender, ethnicity, parental income, parental education), duration of diabetes, type of diabetes, QOL scores, and other medical conditions. Age was defined as the age of the child on June 15, 2006, the reference date for linking SEARCH and CAM data. All other variables are reported from the baseline SEARCH data, which were collected between 2002 and the reference date in 2006. Diabetes type was categorized as type 1 (including type 1 and type 1A), type 2, or other (hybrid, other, or unknown) by the diabetes medical provider. For the subset of youth who participated in a SEARCH study visit in addition to completing an IPS, data on comorbidities (coded as present or not) and quality of life were also available.
Statistical analysis
For these cross-sectional study analyses, the sample was restricted to those participants for whom CAM survey results and SEARCH PedsQL data were both available. Descriptive statistics were used to summarize the characteristics of those who returned the survey. Subgroups were compared using Pearson's χ2 test. The mean scores of the PedsQL Core scales and the PedsQL Diabetes Module scales were compared in CAM users and non-CAM users using a t test for independent samples. Multivariate linear regression modeling with robust standard errors was used to determine the difference in QOL between those who used CAM and those who did not, controlling for potential confounders such as age, race/ethnicity, parental education, diabetes type, and presence of comorbidities.20–23 Two-sample t tests and multivariate linear regression modeling were performed to assess the association of QOL and specific CAM therapies use versus no CAM use for the selected scales. Results for all analyses were considered significant at two-sided p < 0.05.
Results
Study population
Of the 1439 SEARCH participants approached to complete the CAM survey, 587 (40.8%) completed the survey. Among these same 1439 SEARCH participants, 1028 completed the SEARCH initial study visit, and PedsQL results were collected for 995 participants (96.8%) at the time of that visit. Of those who provided QOL data at a SEARCH study visit, 46.9% also returned a CAM survey. The final sample consisted of 467 participants who completed both the CAM survey and the PedsQL.
Characteristics associated with CAM use
Of the CAM survey respondents, 170 (36.4%) were defined as CAM users. Compared to CAM nonusers, CAM users were significantly older, and had a longer duration of diabetes, higher parental education, and more comorbidities (Table 2).
Table 2.
Characteristics of Complementary and Alternative Medicine (CAM) Users/Nonusers for Whom Quality of Life Data Were Available
| Characteristics | CAM User (n = 170) | CAM Nonuser (n = 297) | Mean difference 95% CI |
|---|---|---|---|
| Male | 75 (44.1) | 147 (49.5) | |
| Age at reference datea | 13.8 (±3.9) | 12.5 (±4.1) | −1.3 (−2.10, −0.60) |
| >Race/ethnicity | |||
| Asian/Pacific Islander | 3 (1.8) | 4 (1.4) | |
| Black | 1 (0.6) | 4 (1.4) | |
| Hispanic | 10 (5.9) | 16 (5.4) | |
| Multiple | 7 (4.1) | 9 (3.0) | |
| Other or missing | 2 (1.2) | 1 (0.3) | |
| White | 147 (86.5) | 263 (88.6) | |
| Parental education | |||
| HS grad or less | 13 (7.8) | 36 (12.2) | |
| Some college to Associate's degree | 45 (27.0) | 113 (38.2) | |
| Bachelor's degree or more | 109 (65.3) | 147 (49.7) | |
| Parental income | |||
| <$50,000 | 42 (27.3) | 80 (28.4) | |
| $50,000+ | 112 (72.7) | 202 (71.6) | |
| Duration of diabetes (months)a | 49.3 (±46.9) | 34.7 (±37.0) | −14.6 (−22.79, −6.32) |
| Diabetes type 1 or 1A | 163 (95.9) | 294 (99.0) | |
| Comorbidities present | 38 (22.4) | 33 (11.1) | |
Numbers are count (%) unless designated mean (±standard deviation). % may not equal 100% due to rounding or missing data.
CI, confidence interval.
CAM use overall and QOL
In unadjusted analysis and in multivariate linear regression analyses adjusting for age, parental education, duration of diabetes, type of diabetes, and comorbidities, QOL and CAM use were not associated with the exception of treatment barriers as reported by the child (Table 3). Children who used CAM reported significantly lower QOL scores on the treatment barriers scale of the PedsQL diabetes module (unadjusted difference in means = −3.80, 95% confidence interval [CI] [−7.00, −0.60]; adjusted difference in means = −3.48, 95% CI [−6.65, −0.31], p = 0.03). This was not the case with respect to treatment barriers as reported by parents, which, although somewhat lower, were not significantly so.
Table 3.
Mean Quality of Life Scores (±Standard Deviation) of Complementary and Alternative Medicine (CAM) Users/Nonusers
| Scalesa | CAM user (n = 170) | CAM nonuser (n = 297) | Model estimatedbdifference in means (95% CI) |
|---|---|---|---|
| PedsQL Core (Parent) | |||
| Emotional | 68.5 (±18.0) | 70.3 (±16.6) | −1.17 (−4.59, 2.26) |
| Physical | 85.6 (±15.2) | 87.2 (±13.5) | −0.91 (−3.58, 1.77) |
| School | 73.0 (±19.0) | 72.8 (±17.5) | 1.02 (−2.61, 4.65) |
| Social | 82.0 (±18.0) | 84.9 (±15.6) | −2.24 (−5.64, 1.15) |
| Psychosocial | 74.6 (±15.6) | 76.3 (±13.1) | −0.81 (−3.67, 2.04) |
| Total | 78.4 (±13.9) | 80.1 (±11.8) | −0.83 (−3.31, 1.65) |
| PedsQL Diabetes Module (parent) | |||
| Symptoms | 65.7 (±14.7) | 66.8 (±13.6) | −1.21 (−3.90, 1.49) |
| Treatment Barriers | 70.2 (±19.0) | 71.5 (±17.1) | −0.35 (−3.96, 3.27) |
| Treatment Adherence | 77.4 (±15.6) | 78.3 (±15.0) | 0.51 (−2.35, 3.38) |
| Worry | 73.0 (±20.6) | 75.5 (±19.8) | −1.86 (−5.77, 2.05) |
| Communication | 73.1 (±24.5) | 75.0 (±23.3) | −0.32 (−4.92, 4.28) |
| Diabetes total | 70.8 (±12.8) | 72.1 (±11.9) | −0.65 (−3.03, 1.73) |
| PedsQL Core (Child) | |||
| Emotional | 77.1 (±17.6) | 77.0 (±18.0) | 0.30 (−3.15, 3.75) |
| Physical | 85.2 (±13.2) | 86.5 (±12.8) | −1.71 (−4.29, 0.87) |
| School | 76.1 (±17.6) | 76.0 (±17.2) | 0.20 (−3.19, 3.59) |
| Social | 85.5 (±16.3) | 84.4 (±18.5) | −0.55 (−3.80, 2.70) |
| Psychosocial | 79.5 (±14.0) | 79.2 (±14.3) | −0.10 (−2.80, 2.60) |
| Total | 81.5 (±12.4) | 81.7 (±12.5) | −0.64 (−3.03, 1.74) |
| PedsQL Diabetes Module (child) | |||
| Symptoms | 67.7 (±15.3) | 67.3 (±15.6) | −0.51 (−3.62, 2.61) |
| Treatment Barriers | 79.7 (±16.7) | 83.5 (±15.2) | −3.48 (−6.65, −0.31)* |
| Treatment Adherence | 84.5 (±13.3) | 84.7 (±13.6) | −0.56 (−3.20, 2.07) |
| Worry | 78.4 (±21.1) | 79.0 (±22.4) | −0.67 (−4.86, 3.51) |
| Communication | 80.7 (±22.1) | 82.3 (±20.0) | −2.39 (−6.66, 1.87) |
| Diabetes total | 76.1 (±12.3) | 76.8 (±12.1) | −1.19 (−3.65, 1.27) |
Scores are out of 100, with 100 being highest quality of life/fewest problems.
Adjusting for age, parental education, duration of diabetes, type of diabetes, and comorbidities.
p < 0.05.
PedsQL, Pediatric Quality of Life Inventory; CI, confidence interval.
Specific CAM therapies and QOL
In adjusted analyses of child reports (Table 4), adopters of CAM-defined dietary changes reported significantly higher QOL scores than CAM nonusers (Core Total mean difference 4.01, 95% CI [0.10–7.91]; Core Psychosocial mean difference 6.45, 95% CI [1.95–10.95]). In contrast, children participating in stress reduction activities reported poorer diabetes-related QOL (Diabetes Total mean difference −4.19, 95% CI [−8.35 to −0.04]).
Table 4.
Child Reported Quality of Life and Use of Specific Complementary and Alternative Medicine (CAM) Therapies
| Scales | No. CAM users | CAM user mean (±SD) | CAM nonuser mean (±SD)a | Unadjusted difference in means (95% CI) | Model estimatedbdifference in means (95% CI) |
|---|---|---|---|---|---|
| PedsQL Core Scales | |||||
| Psychosocial | 416 | n = 257 | |||
| Any | 159 | 79.5 (±14.0) | 79.2 (±14.3) | 0.38 (−2.42, 3.18) | −0.10 (−2.80, 2.60) |
| Diet | 30 | 83.3 (±14.6) | 4.11 (−1.58, 9.80) | 6.45 (1.95, 10.95)* | |
| Vitamins | 75 | 80.5 (±13.2) | 1.35 (−2.14, 4.83) | −0.92 (−4.45, 2.62) | |
| Herbs | 46 | 79.4 (±14.0) | 1.45 (−2.70, 5.60) | 1.18 (−3.02, 5.38) | |
| Supplements | 46 | 77.3 (±15.7) | −1.83 (−6.78, 3.13) | −2.13 (−6.82, 2.56) | |
| Stress reduction | 44 | 77.9 (±13.8) | −1.29 (−5.81, 3.24) | −1.98 (−6.24, 2.28) | |
| Total | 416 | n = 257 | |||
| Any | 159 | 81.5 (±12.4) | 81.7 (±12.5) | −0.18 (−2.65, 2.29) | −0.64 (−3.03, 1.74) |
| Diet | 30 | 83.5 (±12.5) | 1.76 (−3.11, 6.64) | 4.01 (0.10, 7.91)** | |
| Vitamins | 75 | 82.6 (±12.0) | 0.85 (−2.29, 3.99) | −1.20 (−4.39, 2.00) | |
| Herbs | 46 | 82.6 (±10.2) | 0.84 (−2.53, 4.21) | 0.35 (−3.11, 3.82) | |
| Supplements | 46 | 79.3 (±13.8) | −2.42 (−6.78, 1.93) | −2.90 (−7.13, 1.33) | |
| Stress reduction | 44 | 79.9 (±11.8) | −1.83 (−5.71, 2.06) | −2.47 (−6.00, 1.07) | |
| PedsQL Diabetes Module Scales | |||||
| Diabetes total | 417 | n = 258 | |||
| Any | 159 | 76.1 (±12.3) | 76.8 (±12.1) | −0.70 (−3.13, 1.73) | −1.19 (−3.65, 1.27) |
| Diet | 30 | 78.4 (±12.8) | 1.62 (−3.37, 6.61) | 2.31 (−2.29, 6.91) | |
| Vitamins | 75 | 76.3 (±14.5) | −0.55 (−3.78, 2.67) | −1.86 (−5.26, 1.54) | |
| Herbs | 46 | 75.7 (±12.3) | −1.09 (−5.03, 2.85) | −1.31 (−5.44, 2.81) | |
| Supplements | 46 | 73.2 (±13.4) | −3.58 (−7.81, 0.65) | −3.60 (−7.82, 0.63) | |
| Stress reduction | 44 | 72.9 (±12.8) | −3.88 (−8.02, 0.27) | −4.19 (−8.35, −0.04)** | |
Comparison group of CAM non-users defined by no use of any CAM therapies.
Adjusting for age, parental education, duration of diabetes, type of diabetes, and comorbidities.
p < 0.01, **p < 0.05.
PedsQL, Pediatric Quality of Life Inventory; SD, standard deviation; CI, confidence interval.
Mean QOL scores among those using a particular CAM therapy compared to those who did not use any CAM at all as reported by parents were significantly lower in the unadjusted analyses for those children using other supplements or stress reduction activities (Table 5). In contrast, there was no association between use of other supplements or stress reduction activities and QOL as reported by children.
Table 5.
Parent-as-Proxy Reported Quality of Life and Use of Specific Complementary and Alternative Medicine (CAM) Therapies
| Scales | No. CAM users | CAM user mean (±SD) | CAM nonuser mean (±SD)a | Unadjusted difference in means (95% CI) | Model estimatedbdifference in means (95% CI) |
|---|---|---|---|---|---|
| PedsQL Core Scales | |||||
| Psychosocial | 447 | n = 289 | |||
| Any | 158 | 74.6 (±15.6) | 76.3 (±13.1) | −1.69 (−4.57, 1.19) | −0.81 (−3.67, 2.04) |
| Diet | 34 | 73.8 (±16.0) | −2.45 (−8.23, 3.34) | −2.23 (−7.73, 3.26) | |
| Vitamins | 69 | 75.3 (±15.8) | −0.96 (−5.05, 3.13) | 0.14 (−4.05, 4.33) | |
| Herbs | 47 | 73.3 (±15.7) | −2.99 (−7.84, 1.86) | −1.97 (−6.89, 2.94) | |
| Supplements | 44 | 68.7 (±17.2) | −7.55 (−12.98, −2.13)* | −5.92 (−11.65, −0.19)** | |
| Stress reduction | 38 | 70.0 (±16.7) | −6.24 (−11.92, −0.56)** | −4.14 (−10.15, 1.88) | |
| Total | 447 | n = 289 | |||
| Any | 158 | 78.4 (±13.9) | 80.1 (±11.8) | −1.68 (−4.26, 0.89) | −0.83 (−3.31, 1.65) |
| Diet | 34 | 77.5 (±14.3) | −2.67 (−7.84, 2.50) | −2.45 (−7.26, 2.36) | |
| Vitamins | 69 | 79.3 (±14.0) | −0.85 (−4.47, 2.78) | −0.16 (−3.68, 3.35) | |
| Herbs | 47 | 77.0 (±14.6) | −3.12 (−7.60, 1.35) | −2.16 (−6.61, 2.29) | |
| Supplements | 44 | 72.5 (±16.0) | −7.62 (−12.65, −2.60)* | −6.26 (−11.29, −1.24)** | |
| Stress reduction | 38 | 74.2 (±13.2) | −5.94 (−10.48, −1.40)** | −4.15 (−8.95, 0.66) | |
| PedsQL Diabetes Module Scales | |||||
| Diabetes total | 448 | n = 290 | |||
| Any | 158 | 70.8 (±12.8) | 72.1 (±11.9) | −1.30 (−3.73, 1.12) | −0.65 (−3.03, 1.73) |
| Diet | 34 | 68.4 (±13.3) | −3.67 (−8.51, 1.17) | −3.98 (−8.63, 0.68) | |
| Vitamins | 69 | 72.1 (±12.8) | −0.01 (−3.34, 3.36) | 0.39 (−2.96, 3.74) | |
| Herbs | 47 | 70.4 (±14.5) | −1.70 (−6.16, 2.75) | −1.61 (−6.23, 3.02) | |
| Supplements | 44 | 67.5 (±14.8) | −4.61 (−9.30, 0.07) | −3.73 (−8.53, 1.07) | |
| Stress reduction | 38 | 67.4 (±13.5) | −4.68 (−9.32, −0.05)** | −3.43 (−8.17, 1.30) | |
Comparison group of CAM nonusers defined by no use of any CAM therapies.
Adjusting for age, parental education, duration of diabetes, type of diabetes, and comorbidities.
p < 0.01, **p < 0.05.
SD, standard deviation; CI, confidence interval; PedsQL, Pediatric Quality of Life Inventory.
After adjusting for age, parental education, duration of diabetes, type of diabetes, and comorbidities in multivariate linear regression, QOL as reported by parents was no longer significantly associated with stress reduction therapy use, although the sample size of this group was small (n = 38) and the trend was still in the same direction (Table 4). Use of other supplements remained significantly associated with lower QOL scores in CAM users than CAM nonusers after adjustment (difference in mean scores −5.92, 95% CI [−11.65, −0.19]).
Discussion
These cross-sectional analyses represent the first assessment of the association between QOL and CAM use in a large sample of American children with diabetes. Among over 400 youths with diabetes for whom CAM use and QOL data were available, 36.4% of the children used some form of CAM therapy, excluding multivitamin use alone or use of spiritual or religious practices alone. Overall, CAM use was not associated with significantly higher or lower QOL. Children who were CAM nonusers reported fewer treatment barriers, but parental reports did not match this finding. Use of “other supplements” was associated with a lower QOL as reported by the parents of these youths, but not by the children themselves. Children using CAM diets had a better QOL, and those using stress reduction activities had an associated poorer QOL.
Our observation of no association between CAM use in aggregate and QOL but a trend toward an association with stress management therapies and other supplement use may suggest that associations between CAM use and QOL are masked when CAM is examined as one whole construct instead of in specific modalities. This is not surprising, given that modalities included in CAM definitions vary greatly and may be used for different reasons.
To date there is limited information available on the association between QOL and CAM use, particularly in a pediatric population. Generally, it appears that larger studies report that a poorer QOL is associated with CAM use, and smaller studies have found no clear association.4,7,24–30 While not statistically significant, we observed a similar pattern among youth with diabetes; CAM users reported lower mean QOL than did CAM nonusers. However, it is difficult to compare the findings of the current study with previously conducted survey studies. CAM was defined differently across studies (i.e., with or without megavitamin therapy or spiritual practices), different QOL assessment tools were used (i.e., European Organization for Research on the Treatment of Cancer, PedsQL, Child Health Questionnaire [CHQ]), and patient populations vary by multiple characteristics and health status. The QOL challenges experienced by children living with diabetes may be different from those for children with other chronic conditions. For instance, in our study youth reported that adhering to diets that are likely viewed as “restrictive” were actually associated with enhanced general and psychosocial QOL. This could be because their dietary actions had a direct positive effect on their diabetes control and therefore improved their sense of self-efficacy.
Our study findings also suggest that parent and child QOL reporting differ. Based on preliminary reviews of parent-proxy reporting in pediatric QOL, the discrepancy in viewpoints of the parent and child appears to be a real difference in perception,31,32 suggesting that both child and parent-proxy QOL data be collected where possible. The parents' own beliefs concerning health and well-being may influence both their decision to promote CAM use in their children and their perception of their child's QOL. CAM use in primary care pediatric patients and in special-needs children has been shown to be best predicted by use of CAM by the parent or caregiver.33,34 This survey did not collect information on parental utilization of CAM or on parental QOL, so these associations could not be explored. It is possible that parents may be more conservative in exposing their ill children to CAM therapies than they might be in trying a CAM therapy themselves. Research shows that CAM use is less prevalent among adults with diabetes than the general population, and even when it is used, it is seldom used directly for treating diabetes.1,35 In our analyses, we did not limit CAM use to therapies specifically for the treatment of diabetes. Given the limited data on this topic, we felt it was important to assess all CAM use, along with contraindications, adverse events, and costs. Future analyses of these data will report on these issues specifically.
There are limitations inherent in this study. First, the study used a cross-sectional design, and thus temporal changes in QOL associated with CAM use could not be assessed. Possible explanations for cross-sectional findings include selection factors (children with more or less serious illness are more likely to be exposed to CAM therapies), information bias (people who invest in a therapy may perceive positive effects), or effects of CAM treatment (use of CAM therapies actually has a positive or negative impact on a child's health status). Response bias is always a possibility with survey methodology, and though our overall sample was large for this type of study in this population, our survey response rate is a limiting factor in the interpretation of results. Because parents were allowed to assist their children in completing the CAM survey, responses may not purely reflect the children's views. In subanalyses, small numbers of specific CAM therapy users likely reduced our statistical power to assess associations and potential confounders adequately. Finally, data concerning CAM use in the Puget Sound region may not be generalizable to the nation at large because the legal and insurance coverage for CAM providers in Washington State is more favorable than in most other states in the United States.36 This may lead to greater CAM utilization than in other areas of the country.
In our study sample, 36.4% of the children for whom QOL and CAM survey data were available used some form of CAM therapy. To better understand the experiences of these patients, their parents, and their medical care choices, factors that relate to the use of CAM in youth with diabetes should be investigated further. For future research, development of reliable, validated, widely available CAM use assessment tools for adults and children would facilitate comparison between CAM studies, as would standardizing CAM definitions. To expand on the findings of this study, longitudinal research with a larger sample size is needed to determine whether altered QOL results in a choice to seek certain CAM therapies, whether use of CAM therapies is associated with a subsequent reduction or improvement in QOL, whether these results are confounded by factors potentially related to both QOL in pediatric diabetes and CAM use, and whether different types of CAM therapies have different effects on children with diabetes.
Acknowledgments
Funding for this ancillary study was provided by the CAM Fund, Seattle Children's Hospital, Seattle, WA; Faculty Seed Grant Fund, Bastyr University, Kenmore, WA; and K23 Career Training Award #5K23-AT000929 and T32 Institutional Training Grant #T32-AT00815 at Bastyr University, from the National Center for Complementary and Alternative Medicine (NCCAM), National Institutes of Health (NIH).
SEARCH grant support: SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA #00097 and DP-05-069) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Site contract numbers: California (U01 DP000246); Colorado (U01 DP000247); Hawaii (U01 DP000245); Ohio (U01 DP000248); South Carolina (U01 DP000254); Washington (U01 DP000244); Coordinating Center (U01 DP000250).
The authors wish to acknowledge the involvement of General Clinical Research Centers (GCRC) at the following institutions in the SEARCH for Diabetes in Youth Study: Medical University of South Carolina (grant #M01 RR01070); Cincinnati Children's Hospital (grant #M01 RR08084); Seattle Children's Hospital and the University of Washington School of Medicine (grant #M01RR00037 and M01RR001271); and Colorado Pediatric General Clinical Research Center (grant #M01 RR00069).
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention, NCCAM, NIDDK, or NIH.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bell RA. Suerken CK. Grzywacz JG, et al. Complementary and alternative medicine use among adults with diabetes in the United States. Altern Ther Health Med. 2006;12:16–22. [PubMed] [Google Scholar]
- 2.Wilson KM. Klein JD. Sesselberg TS, et al. Use of complementary medicine and dietary supplements among U.S. adolescents. J Adolesc Health. 2006;38:385–394. doi: 10.1016/j.jadohealth.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Stern RC. Canda ER. Doershuk CF. Use of nonmedical treatment by cystic fibrosis patients. J Adolesc Health. 1992;13:612–615. doi: 10.1016/1054-139x(92)90376-m. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz JE. Mamula P. delRosario JF, et al. Patterns of complementary and alternative medicine use in a population of pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:599–605. doi: 10.1097/00054725-200409000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Day AS. Whitten KE. Bohane TD. Use of complementary and alternative medicines by children and adolescents with inflammatory bowel disease. J Paediatr Child Health. 2004;40:681–684. doi: 10.1111/j.1440-1754.2004.00510.x. [DOI] [PubMed] [Google Scholar]
- 6.Ang JY. Ray-Mazumder S. Nachman SA, et al. Use of complementary and alternative medicine by parents of children with HIV infection and asthma and well children. South Med J. 2005;98:869–875. doi: 10.1097/01.smj.0000173089.51284.69. [DOI] [PubMed] [Google Scholar]
- 7.Martel D. Bussieres JF. Theoret Y, et al. Use of alternative and complementary therapies in children with cancer. Pediatr Blood Cancer. 2005;44:660–668. doi: 10.1002/pbc.20205. [DOI] [PubMed] [Google Scholar]
- 8.Sawyer MG. Gannoni AF. Toogood IR, et al. The use of alternative therapies by children with cancer. Med J Aust. 1994;160:320–322. [PubMed] [Google Scholar]
- 9.Southwood TR. Malleson PN. Roberts-Thomson PJ. Mahy M. Unconventional remedies used for patients with juvenile arthritis. Pediatrics. 1990;85:150–154. [PubMed] [Google Scholar]
- 10.Ball SD. Kertesz D. Moyer-Mileur LJ. Dietary supplement use is prevalent among children with a chronic illness. J Am Diet Assoc. 2005;105:78–84. doi: 10.1016/j.jada.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Dannemann K. Hecker W. Haberland H, et al. Use of complementary and alternative medicine in children with type 1 diabetes mellitus: Prevalence, patterns of use, and costs. Pediatr Diabetes. 2008;9(3 pt 1):228–235. doi: 10.1111/j.1399-5448.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- 12.Boon H. Stewart M. Kennard MA, et al. Use of complementary/alternative medicine by breast cancer survivors in Ontario: Prevalence and perceptions. J Clin Oncol. 2000;18:2515–2521. doi: 10.1200/JCO.2000.18.13.2515. [DOI] [PubMed] [Google Scholar]
- 13.Cassileth BR. Deng G. Complementary and alternative therapies for cancer. Oncologist. 2004;9:80–89. doi: 10.1634/theoncologist.9-1-80. [DOI] [PubMed] [Google Scholar]
- 14.Kramer JR. Ledolter J. Manos GN. Bayless ML. Stress and metabolic control in diabetes mellitus: Methodological issues and an illustrative analysis. Ann Behav Med. 2000;22:17–28. doi: 10.1007/BF02895164. [DOI] [PubMed] [Google Scholar]
- 15.Riazi A. Pickup J. Bradley C. Daily stress and glycaemic control in type 1 diabetes: Individual differences in magnitude, direction, and timing of stress-reactivity. Diabetes Res Clin Pract. 2004;66:237–244. doi: 10.1016/j.diabres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 16.O'Neil KJ. Jonnalagadda SS. Hopkins BL. Kicklighter JR. Quality of life and diabetes knowledge of young persons with type 1 diabetes: Influence of treatment modalities and demographics. J Am Diet Assoc. 2005;105:85–91. doi: 10.1016/j.jada.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Varni JW. Burwinkle TM. Jacobs JR, et al. The PedsQL in type 1 and type 2 diabetes: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. 2003;26:631–637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- 18.The SEARCH for Diabetes in Youth Study Group. SEARCH for Diabetes in Youth: A multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–471. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Varni JW. Seid M. Kurtin PS. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Keles H. Ekici A. Ekici M, et al. Effect of chronic diseases and associated psychological distress on health-related quality of life. Intern Med J. 2007;37:6–11. doi: 10.1111/j.1445-5994.2006.01215.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams J. Wake M. Hesketh K, et al. Health-related quality of life of overweight and obese children. JAMA. 2005;293:70–76. doi: 10.1001/jama.293.1.70. [DOI] [PubMed] [Google Scholar]
- 22.Meuleners LB. Lee AH. Quality of life profile—adolescent version: Assessing the relationship of covariates to scale scores using structural equation modeling. Qual Life Res. 2005;14:1057–1063. doi: 10.1007/s11136-004-2573-1. [DOI] [PubMed] [Google Scholar]
- 23.Hassan K. Loar R. Anderson BJ. Heptulla RA. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. J Pediatr. 2006;149:526–531. doi: 10.1016/j.jpeds.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 24.Soo I. Mah JK. Barlow K, et al. Use of complementary and alternative medical therapies in a pediatric neurology clinic. Can J Neurol Sci. 2005;32:524–528. doi: 10.1017/s0317167100004558. [DOI] [PubMed] [Google Scholar]
- 25.Burstein HJ. Gelber S. Guadagnoli E. Weeks JC. Use of alternative medicine by women with early-stage breast cancer [see comments] NEJM. 1999;340:1733–1739. doi: 10.1056/NEJM199906033402206. [DOI] [PubMed] [Google Scholar]
- 26.Paltiel O. Avitzour M. Peretz T, et al. Determinants of the use of complementary therapies by patients with cancer. J Clin Oncol. 2001;19:2439–2448. doi: 10.1200/JCO.2001.19.9.2439. [DOI] [PubMed] [Google Scholar]
- 27.MacLennan AH. Myers SP. Taylor AW. The continuing use of complementary and alternative medicine in South Australia: Costs and beliefs in 2004. Med J Aust. 2006;184:27–31. doi: 10.5694/j.1326-5377.2006.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 28.Sollner W. Maislinger S. DeVries A, et al. Use of complementary and alternative medicine by cancer patients is not associated with perceived distress or poor compliance with standard treatment but with active coping behavior: A survey. Cancer. 2000;89:873–880. doi: 10.1002/1097-0142(20000815)89:4<873::aid-cncr21>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 29.Nagel G. Hoyer H. Katenkamp D. Use of complementary and alternative medicine by patients with breast cancer: Observations from a health-care survey. Support Care Cancer. 2004;12:789–796. doi: 10.1007/s00520-004-0675-5. [DOI] [PubMed] [Google Scholar]
- 30.Montazeri A. Sajadian A. Ebrahimi M. Akbari ME. Depression and the use of complementary medicine among breast cancer patients. Support Care Cancer. 2005;13:339–342. doi: 10.1007/s00520-004-0709-z. [DOI] [PubMed] [Google Scholar]
- 31.Sherifali D. Pinelli J. Parent as proxy reporting: Implications and recommendations for quality of life research. J Fam Nurs. 2007;13:83–98. doi: 10.1177/1074840706297789. [DOI] [PubMed] [Google Scholar]
- 32.Eiser C. Morse R. Can parents rate their child's health-related quality of life? Results of a systematic review. Qual Life Res. 2001;10:347–357. doi: 10.1023/a:1012253723272. [DOI] [PubMed] [Google Scholar]
- 33.Sanders H. Davis MF. Duncan B, et al. Use of complementary and alternative medical therapies among children with special health care needs in southern Arizona. Pediatrics. 2003;111:584–587. doi: 10.1542/peds.111.3.584. [DOI] [PubMed] [Google Scholar]
- 34.Sawni-Sikand A. Schubiner H. Thomas RL. Use of complementary/alternative therapies among children in primary care pediatrics. Ambul Pediatr. 2002;2:99–103. doi: 10.1367/1539-4409(2002)002<0099:uocata>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Lind BK. Lafferty WE. Grembowski DE. Diehr PK. Complementary and alternative provider use by insured patients with diabetes in Washington State. J Altern Complement Med. 2006;12:71–77. doi: 10.1089/acm.2006.12.71. [DOI] [PubMed] [Google Scholar]
- 36.Bellas A. Lafferty WE. Lind B. Tyree PT. Frequency, predictors, and expenditures for pediatric insurance claims for complementary and alternative medical professionals in Washington State. Arch Pediatr Adolesc Med. 2005;159:367–372. doi: 10.1001/archpedi.159.4.367. [DOI] [PubMed] [Google Scholar]