Abstract
DJ-1 is a member of the large and functionally diverse DJ-1/PfpI superfamily and has homologs in nearly all organisms. Because of its connection to parkinsonism and cancer, human DJ-1 has been intensely studied for over a decade. The current view is that DJ-1 is a multifunctional oxidative stress response protein that defends cells against reactive oxygen species and mitochondrial damage, although the details of its biochemical function remain unclear. A conserved cysteine residue in DJ-1 (Cys106) is both functionally essential and subject to oxidation to the cysteine-sulfinate and cysteine-sulfonate. Consequently, the oxidative modification of Cys106 has been proposed to allow DJ-1 to act as a sensor of cellular redox homeostasis and to participate in cytoprotective signaling pathways in the cell. This review explores the current evidence for the role of cysteine oxidation in DJ-1 function, with emphasis on emerging models for how oxidative modification may regulate DJ-1's protective function and also contribute to dysfunction and disease. Antioxid. Redox Signal. 15, 111–122.
Introduction
DJ-1 is a small protein (∼20 KDa) that is a member of the eponymous DJ-1 superfamily and has homologs distributed across all biological kingdoms (8, 53). Elucidating the function and regulation of DJ-1 has been an active field of study for over a decade. This effort intensified considerably after the discovery that the gene for human DJ-1 (PARK7) is mutated in rare forms of recessively inherited parkinsonism (13). Because DJ-1 was not obviously connected to Parkin, α-synuclein, or UCH-L1, the first three proteins implicated in heritable parkinsonism (19), these uncommon DJ-1 mutations suggested that parkinsonism may have a complex etiology that involved multiple pathways leading to disease (19). Previously, DJ-1 had been characterized as an oncogene whose transforming ability is enhanced by H-ras (67), closely followed by reports from independent groups showing that DJ-1 negatively regulates RNA binding by a large multiprotein complex (32) and that the rat DJ-1 homolog enhances male rodent fertility (84, 97, 98). Subsequent studies have proposed additional roles for DJ-1 in ischemic injury (1, 105), amyotrophic lateral sclerosis (4, 49), multiple cancers (21, 31, 40, 61, 67, 72, 78, 90, 106, 112), and androgen receptor regulation (68, 75, 85, 87, 91). The diversity of disease states in which DJ-1 is implicated reflects the current view that it is a multifunctional protein with several proposed biochemical and cellular activities. Among these proposed roles for DJ-1 are a redox-regulated chaperone (48, 80), an RNA binding protein (11, 92), a cysteine protease (16, 42, 70), a transcriptional coactivator (18, 104, 108, 109), and a protein that interacts with the apoptosis-implicated proteins Daxx (38), apoptosis signal regulating kinase 1 (ASK1) (36, 63, 95), and p53 (14, 25, 26, 40, 83). In addition, DJ-1 also binds to proteins related to androgen receptor function (81, 87, 91) and sumoylation (25, 82), although the functional role of these interactions requires clarification. As a consequence of this complexity, a clear consensus model for DJ-1's biochemical and cellular function(s) has yet to emerge. Therefore, while much has been learned about DJ-1, important questions about the function of this exciting and enigmatic protein remain to be answered.
Due to its connection to multiple diseases, much of the work on DJ-1 has focused on determining the role of the protein in the normal function of animal cells. These studies have shown that DJ-1 confers protection against oxidative stress (15, 56, 57, 73, 86, 88) and enhances cell survival when challenged with pro-apoptotic stimuli (31, 113), although the mechanisms by which DJ-1 accomplishes this are not fully understood. A promising observation that may connect the established role for DJ-1 in oxidative stress response to possible biochemical functions of the protein is that a conserved cysteine residue (Cys106) is both critical for DJ-1 function and very sensitive to oxidative modification (12, 15, 41, 57, 95). This review will focus on what has been learned about the role of cysteine oxidation in DJ-1 function, with an emphasis on the possibility that cysteine oxidation is an important post-translational modification of this protein and its homologs.
Structural Biology of DJ-1
The crystal structure of DJ-1 was solved independently by five groups and provided a wealth of information about the protein (33, 34, 48, 89, 101). DJ-1 possesses a flavodoxin-like core fold and is a homodimer both in the crystal and in solution (Fig. 1). The DJ-1 homodimer provided an explanation for the parkinsonian phenotype of the L166P missense mutation (13), as this mutation in α-helix G was predicted to disrupt the dimer interface and lead to poor folding of the protein (33, 34, 48, 89, 101). This was confirmed in cell culture, where poorly folded L166P DJ-1 is rapidly destroyed (29, 58, 64), resulting in low steady state levels of the protein (58) and rapid turnover via both proteosomal and nonproteosomal degradation pathways in HEK293 cells (29). Although L166P DJ-1 protein levels are greatly reduced, recent nuclear magnetic resonance spectroscopic work has shown that L166P DJ-1 is natively unfolded and therefore would not be functional even if it were abundant in the cell (54). Crystal structures have also been determined for the E64D (30), M26I, A104T, and E163K disease-associated DJ-1 mutants (46), and nuclear magnetic resonance spectroscopy has been used to study E64D, M26I, A104T, and D149A DJ-1 in solution (54). Surprisingly, most of the structural differences between these mutant proteins and wild-type DJ-1 are modest, and none of these mutant proteins display profound structural defects like L166P DJ-1 (46, 54). The current data suggest that even the minor structural defects introduced by some disease-related mutations can cause considerable loss of DJ-1 function in vivo, particularly for M26I DJ-1. More study is needed to determine the detailed biophysical basis of the pathogenicity of these mutants.
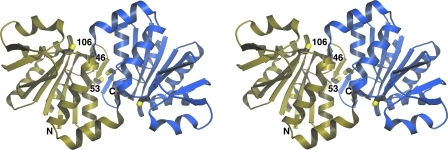
FIG. 1.
A divergent-eye stereo view of the DJ-1 dimer. A ribbon diagram of dimeric DJ-1 is shown with the molecular twofold axis perpendicular to the plane of the page. One monomer is shown in gold and the other in blue. The three cysteine residues in DJ-1 are shown in stick representation, although Cys46 is partially obscured in this view. Cys106 resides at the bottom of a prominent surface grove and is situated at the sharp turn between a strand and helix that is called the “nucleophile elbow.” Cys106 is the only functionally essential cysteine residue in DJ-1. C, carboxy terminus; N, amino terminus.
Analysis of the crystal structure of DJ-1 also led to the first speculation that Cys106 (human DJ-1 residue numbering) might be an important residue for DJ-1 function (33, 34, 48, 89, 101). Cys106 is a highly conserved residue that resides at the sharp turn between a β-strand and an α-helix called the “nucleophile elbow” in the α/β hydrolase proteins (Fig. 1). Cys106 has energetically strained backbone torsion angles and is particularly sensitive to X-ray radiation damage, suggesting enhanced reactivity (101). At the time that the DJ-1 crystal structure was solved, the archaeal cysteine protease PH1704 from Pyrococcus horikoshii was the only other member of the DJ-1 superfamily whose structure was known (23), leading to a proposal that DJ-1 was a cysteine protease employing Cys106 as the catalytic nucleophile (33, 34). Some subsequent studies have observed a weak proteolytic activity for DJ-1 (16, 42, 70), whereas others failed to detect this activity (48, 80, 101). From a structural perspective, despite having similar monomer structures, DJ-1 and PH1704 form completely different dimers and DJ-1 lacks the Glu-His-Cys catalytic triad found in PH1704 and related PfpI-like proteases. However, Cys106 is within ∼5 Å of a histidine residue (His126) that was proposed to provide the second member of a catalytic dyad like that found in the caspases (16, 34, 70). Importantly, the imidazole sidechain of this histidine residue is not oriented properly for hydrogen bonding with Cys106 (33, 34, 48, 89, 101) and is not conserved in close DJ-1 homologs from insects (including the characterized Drosophila melanogaster homolog DJ-1β) and prokaryotes (100), making an evolutionarily conserved and physiologically relevant proteolytic role for Cys106 unlikely.
Although most available evidence suggests that DJ-1 is not likely to be a physiologically relevant protease, the structural environment and ionization state of Cys106 still allow for the speculative possibility that DJ-1 may have an undiscovered enzymatic activity. Cys106 has a low thiol pKa value of ∼5 and therefore exists almost exclusively as the reactive thiolate anion at physiological pH (103). Bond length analysis using atomic resolution X-ray crystallography demonstrates that the highly conserved Glu18 residue has a protonated carboxylic acid sidechain that donates a hydrogen bond to Cys106 and facilitates ionization of the thiol, thereby depressing its pKa value (103). At present, the data suggest that the primary importance of Glu18 is stabilizing the Cys106-SO2− species that is important for DJ-1 function (12, 15) (Fig. 2; also see below). However, the presence of a reactive thiolate anion near a protonated glutamic acid in DJ-1 is intriguing, as this might be a potential nucleophile-general acid/base dyad that is sometimes associated with enzyme active sites. A computational study of DJ-1 superfamily proteins of known structure revealed that the in silico ionization profiles for residues that surround the conserved active site cysteine residue are perturbed, which is commonly observed in enzyme active sites (99). However, an elegant recent study in which an engineered C106DD mutant of DJ-1 protected cells against oxidative stressors almost as well as the wild-type protein (see below) provides a powerful argument against an enzymatic activity for DJ-1 (95). This result would seem to rule out a significant role for a putative Cys106 nucleophile for the cytoprotective aspect of DJ-1's function. These recently reported C106DD and C106EE mutants are of great importance because they are the first mutations at Cys106 that preserve DJ-1 protective function, and therefore have the potential to be powerful tools in the study of DJ-1 biochemistry.
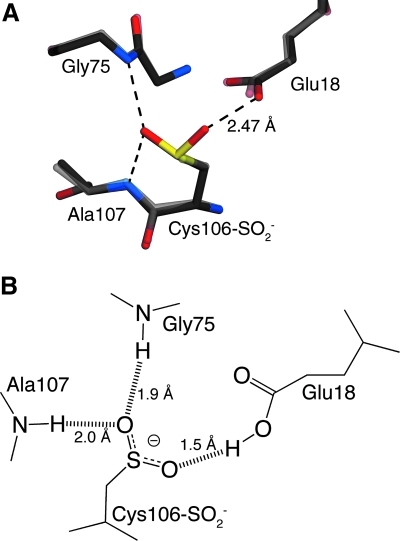
FIG. 2.
Two views of the stable Cys106-sulfinate oxidized form of DJ-1. In (A), the region around Cys106 from the crystal structure of Cys106-sulfinate DJ-1 (opaque; PDB accession code 1SOA) is superimposed over the structure of reduced DJ-1 (semitransparent; PDB accession code 1P5F). Stabilizing hydrogen bonds between the Cys106-SO2− and surrounding residues are shown in dashed lines, with the key interaction between Glu18 and Cys106-SO2− labeled. Oxidation of Cys106 occurs very readily and alters the function of the protein, but does not result in any notable structural change. In (B), the lengths of the key hydrogen bonds are shown, emphasizing the unusually short and strong hydrogen bond between Cys106-SO2− and the protonated carboxylate of Glu18. This interaction plays an important role in stabilizing the oxidized form of the protein.
Cys106 Is Essential for DJ-1 Protective Function and Is Sensitive to Oxidation
As discussed above, the crystal structure of DJ-1 provided the first experimental data indicating that Cys106 is an important residue. Phylogenetic analysis has also underscored the significance of Cys106, as human DJ-1 contains three cysteine residues (Cys46, Cys53, and Cys106) (Fig. 1) of which Cys106 is by far the best conserved (8, 53). The only organisms whose DJ-1 homologs are currently known to lack this cysteine are a small and functionally uncharacterized subset of plant DJ-1s, in which this residue is replaced by aspargine, glycine, or serine. Many studies have since shown that Cys106 is required for DJ-1 to confer cellular protection against oxidative stress (1, 3, 12, 15, 36, 40, 41, 57, 88, 95), with only one report identifying Cys53 as the functionally essential residue (80). The mutation of Cys106 to alanine, serine, or aspartic acid eliminates the ability of DJ-1 to protect against oxidative stress in several model systems, including various types of cultured mammalian cells (12, 15, 36, 95), D. melanogaster (57), and rodent models of ischemia (1). Because of its high degree of conservation and clear functional importance, Cys106 has been extensively studied as a means to understand the biochemical function of DJ-1.
The evidence that Cys106 is a critical residue for DJ-1-mediated protection against oxidative stress is further bolstered by several independent studies that have identified Cys106 as the favored target of oxidative modification. An oxidized form of Cys106 was first reported in DJ-1 from human umbilical vein endothelial cells (41). Using two-dimensional gel electrophoresis followed by mass spectrometry of trypsinized protein spots with more acidic pI values, a Cys106-sulfonate (Cys106-SO3−) species in DJ-1 was identified that increased during hydrogen peroxide stress (41). These observations both built upon and helped explain two prescient reports from the Nakagawa group in 2001 that showed that DJ-1 (as well as peroxiredoxins II and III) converted to more acidic isoforms in two-dimensional gel electrophoresis when cells were subjected to sublethal amounts of oxidative or endotoxin stress (59, 60). Remarkably, Mitsumoto et al. speculated that “oxidative conversion of a sulfhydryl group(s) at a Cys residue(s) to cysteine sulfinic acid (Cys-SO2H) is the most plausible candidate to be responsible” for their observations (60). The determination of the 1.20 Å resolution crystal structure of oxidized DJ-1 directly confirmed their prediction, showing that Cys106 was easily oxidized to Cys106-sulfinate (Cys106-SO2−) under very mild conditions, even unintentionally (15). The Cys106-SO2− moiety is specifically stabilized by three hydrogen bonds to surrounding residues, including a short, strong 2.47 Å hydrogen bond to the protonated carboxylic sidechain of Glu18 (15) (Fig. 2). A prior structural study had also observed Cys106 oxidation, although the resolution of the diffraction data (3.0 Å) was insufficient for a detailed structural analysis of the modification (48). Importantly, no evidence for a Cys106-SO3− species was found in any crystal structure of oxidized DJ-1. Subsequent X-ray crystallographic studies of both close DJ-1 structural homologs like Escherichia coli YajL (100) and more distant ones such as Saccharomyces cereviseae YDR533c (102) and D. radiodurans DR1199 (27) also show similar oxidation of the structurally equivalent cysteine residue, suggesting that susceptibility to oxidative cysteine modification may be a conserved feature of DJ-1 superfamily proteins. Whether these oxidative modifications have a functional role in other DJ-1 superfamily proteins is unknown.
As a pertinent methodological aside, the widespread use of two-dimensional gel electrophoresis for studying cysteine oxidation suffers from difficulties that deserve consideration. The standard sample preparation protocol before first dimension separation by isoelectric focusing involves reduction of the protein using dithiothreitol or a similar reductant, followed by irreversible alkylation of free thiols with iodoacetamide. This protocol will reduce and protectively alkylate cysteine residues that were initially free, participating in disulfide bonds, or oxidized to cysteine-sulfenic acid, but it will not modify cysteine residues oxidized to cysteine-sulfinate. As a consequence, cysteine-sulfinate will be vulnerable to further oxidation during isoelectric focusing and gel electrophoresis. Unintended oxidation will only be exacerbated if the cysteine alkylation step is omitted. This is especially worrisome because proteins are typically fully denatured and thus maximally susceptible to cysteine oxidation during isoelectric focusing, during the second dimension of separation using sodium dodecyl sulfate polyacrylamide gel electrophoresis (typically performed in gels that are mildly oxidizing), and during sample analysis that usually involves spot excision and trypsin digestion followed by mass spectrometry. Therefore, determining an accurate inventory of oxidized forms of cysteine in the cell using these techniques can be challenging and tends to favor more extensively oxidized species. These caveats also emphasize the dire need for better analytical tools for the study of cysteine-sulfinate and cysteine-sulfonate in vivo (74).
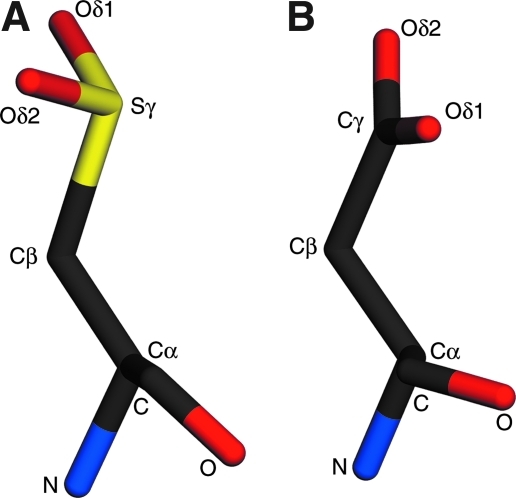
Despite the preponderance of evidence showing that Cys106 is both easily oxidized and functionally essential in DJ-1, none of these studies establish a causative relationship between oxidation of Cys106 and protection by DJ-1. Two hypotheses can be proposed: the specific oxidation of Cys106 is important for cytoprotection by DJ-1 or, alternatively, Cys106 itself is a critical residue whose oxidation is either incidental or detrimental to protein function. Cysteine-sulfinate and cysteine-sulfonate are often viewed as irreversible forms of oxidative protein damage and therefore it would be unusual if the oxidation of Cys106 to either of these species was protective in DJ-1, giving a priori weight to the second hypothesis. Here the crystal structure of Cys106-SO2− DJ-1 provides little insight, as the structures of the reduced and oxidized proteins are virtually identical (15). Thus, it is unclear how Cys106 oxidation could alter DJ-1 function from a structural perspective. In addition, a C106D mutant that was introduced to mimic the Cys106-SO2− species abolished DJ-1 protection against oxidative stress in both cultured human M17 neuroblastoma cells (15) and D. melanogaster (57), demonstrating that introduction of an anionic group at residue 106 is not sufficient to preserve DJ-1 activity. In considering this result, however, despite their similar electrostatic properties, the molecular geometries of aspartate and cysteine-sulfinate are quite different (Fig. 3). The sulfur atom of cysteine-sulfinate has a lone pair of electrons that is absent on the topologically equivalent carbon atom of aspartate. Consequently, the three hydrogen bonds formed between Cys106-SO2− and surrounding residues in the crystal structure of oxidized DJ-1 cannot be simultaneously satisfied by an aspartate due to the planarity of its carboxylate sidechain. Therefore, C106D is not a good structural mimic for Cys106-SO2− oxidized DJ-1. Because Cys106 is essential for DJ-1 function and largely intolerant of mutagenesis, it has been difficult to devise effective experiments to determine if Cys106 provides an essential thiol or if it is the oxidation of Cys106 that is the important modification.
FIG. 3.
Aspartate is not a good structural mimic for cysteine-sulfinate. In both panels, all atoms are labeled. (A) A stick representation of cysteine-sulfinate, illustrating that the lone pair of electrons on the Sγ atom results in a bent geometry for the oxygen atoms (Oδ1 and Oδ2) in relation to the sulfur atom. (B) The different electronic configuration of the Cγ atom in aspartate results in sp2 hybridization and a planar arrangement of the oxygen atoms with Cγ atom. This geometric difference prevents aspartate from satisfying the same set of hydrogen bonds that are made by Cys106-SO2− (Fig. 2), which may help explain why the C106D DJ-1 mutant is not functional.
Recent work from two independent groups tested these competing hypotheses about Cys106 in complementary ways. Their combined results support the hypothesis that oxidation of Cys106 is important for DJ-1 function. The first of these studies used targeted mutagenesis of the neighboring protonated Glu18 residue to modulate Cys106 oxidation through changes in hydrogen bonding (12). This strategy was used because it did not require mutation of Cys106 and therefore could independently address the relative importance of the thiol and the sulfinate at Cys106. Two very structurally similar DJ-1 mutants, E18N and E18D, had profoundly different effects on Cys106 oxidation, with E18N favoring oxidation and E18D strongly disfavoring it. In cell culture models using both human M17 neuroblastoma cells and DJ-1−/− mouse embryonic fibroblasts, the oxidation-competent E18N protein was cytoprotective and promoted normal mitochondrial morphology, whereas the oxidation-impaired E18D mutant did not (12). Therefore, the oxidation of Cys106 is critical for cytoprotection by DJ-1 in these model systems. In the second study, missense/insertion mutations at Cys106 (C106DD and C106EE) were designed to mimic the oxidized form(s) of the residue (95). These DJ-1 mutants (unlike C106A, C106E, or C106D) were protective during oxidative challenge of cultured DJ-1−/− mouse embryonic fibroblasts and enhanced binding of DJ-1 to ASK1, again indicating that oxidized Cys106 is more important for cytoprotection than is the thiol itself (95). As mentioned above, this later study is remarkable for having reported the first mutation at Cys106 that preserves DJ-1 function. The C106DD/EE mutants lack a nucleophilic thiol(ate) group at residue 106, and thus are incapable of performing many of the proposed enzymatic activities for DJ-1. Therefore, these mutants merit greater study as a useful tool to test hypotheses about DJ-1's biochemical function that involve a proposed catalytic role for Cys106.
Effects of Extensive Oxidation on DJ-1 Stability and Function
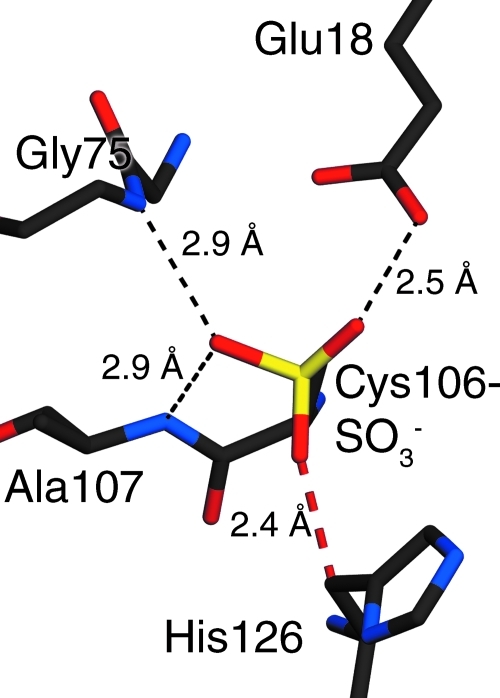
Both Cys106-SO2− and Cys106-SO3− have been observed in DJ-1, sometimes in the same study (57, 62, 111). The relative functional importance of the Cys106-SO2− and Cys106-SO3− species is unknown. Problematically, current methods of protein analysis (particularly two-dimensional gel electrophoresis) provide ample opportunity for further oxidation of cysteine-sulfinate-containing samples to the cysteine-sulfonate, thereby complicating the study of these modifications. Although mass spectrometry studies of DJ-1 by multiple groups have observed both Cys106-SO2− and Cys106-SO3− in DJ-1 (57, 62, 111), crystal structures of oxidized DJ-1 show only the Cys106-SO2− species (12, 15). Further, analysis of the crystal structure of DJ-1 suggests that formation of Cys106-SO3− is sterically disfavored by surrounding residues, particularly the Cβ atom of His126 (Fig. 4). Thus, oxidation of Cys106 to the sulfonate may require (or facilitate) structural changes in DJ-1 that oppose crystallization and could have functional importance. In solution, Cys106-SO2− DJ-1 is resistant to further controlled oxidation, with one report demonstrating that greater than a 20:1 molar ratio of hydrogen peroxide to DJ-1 was required to begin to detect Cys106-SO3− by matrix-assisted laser desorption/ionization mass spectrometry; this highly oxidized form of DJ-1 also had reduced secondary structure by CD spectroscopy and was prone to aggregation (111). In addition, the formation of Cys106-SO3− was also accompanied by the oxidation of other cysteine and methionine residues in the protein. A separate detailed in vitro biophysical study of DJ-1 showed that mutating Cys106 to alanine ameliorates oxidation-induced destabilization of DJ-1 (35). This oxidation-induced destabilization and aggregation was further exacerbated by the pathogenic M26I mutation, suggesting a connection between extensive cysteine/methionine oxidation in DJ-1 and parkinsonism. In vivo support for a potentially pathogenic role for highly oxidized DJ-1 was provided by an impressive two-dimensional gel electrophoresis/mass spectrometry study of DJ-1 in postmortem brain tissue of normal patients as well as those with either Parkinson's or Alzheimer's disease (17). DJ-1 from diseased tissue was much more extensively oxidized at cysteine and methionine residues than in healthy cells, although no peptides containing Cys106 could be isolated. In total, the current data suggests that while mild oxidation to Cys106-SO2− is closely correlated with DJ-1 cytoprotection, extensive oxidation of DJ-1 is associated with aging and neurodegenerative disease.
FIG. 4.
His126 may sterically impede oxidation of Cys106 to the sulfonate. A hypothetical model of the Cys106-sulfonate was built using the crystal structure of DJ-1 Cys106-sulfinate (PDB accession code 1SOA) as the starting model. Stabilizing hydrogen bonds between the Cys106-SO3− and surrounding residues are shown in dashed black lines, with lengths given in angstroms (Å). The 2.4 Å clash between His126 and an oxygen atom of Cys106-SO3− is shown with a red dashed line. This steric clash may disfavor formation of the Cys106-sulfonate and cause conformational changes in Cys106-SO3− DJ-1.
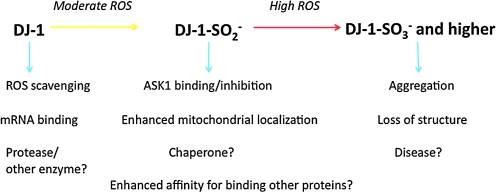
There are (at least) two major open questions about Cys106-SO3− in DJ-1: what are the relative amounts of reduced DJ-1, Cys106-SO2−, and Cys106–SO3− oxidized protein in vivo under basal and stress conditions, and does the formation of Cys106-SO3− have a physiologically relevant role in modulating the activity of DJ-1? As discussed above, addressing the first question has been hampered by the inherent limitations of current analytical tools for studying oxidized cysteine residues. For the second question, it is possible that Cys106-SO3− is the most functionally important modification and that Cys106-SO2− has been mistakenly identified as important as an accidental consequence of that fact that it is a stable obligatory intermediate in the formation of Cys106-SO3− DJ-1. Alternatively, the Cys106-SO2− and Cys106-SO3− modifications might exert opposing effects on DJ-1 stability and protective function (Fig. 5). This model would provide a mechanism to turn off DJ-1 activity by formation of the destabilizing Cys106-SO3− modification in cases of extreme cellular oxidative stress, thereby allowing the cell to commit to apoptosis. Derangements in this process and inappropriate retention of DJ-1-mediated protection in cases of severe cellular dysfunction might contribute to DJ-1's proliferative role in some cancers. An additional feature of this second model is that some parkinsonian DJ-1 missense mutations may selectively destabilize the highly oxidized protein and thus lead to loss-of-function and cell death under a moderate cellular oxidative burden where wild-type DJ-1 could still confer protection (35, 46). Both of these hypotheses are consistent with the current data, and thus additional experimental testing is required to address them.
FIG. 5.
A schema of the current evidence for various roles of the major oxidized forms of DJ-1. A question mark is used to indicate unresolved aspects of the current set of proposals for DJ-1 function.
Roles for Cysteine Oxidation in DJ-1's Cellular Function
Cysteine oxidation appears to be important for DJ-1-mediated cytoprotection, but what is the mechanism by which oxidation affects the physiological function of the protein? A completely satisfactory answer to this question is not currently available due to the complex, multifaceted function of DJ-1 in the cell. As a summary of the author's view of this difficult question, any plausible model for the oxidative regulation of DJ-1 should have the following four features: a function for DJ-1 that requires Cys106 or a modified version of this residue, a pro-survival function for some oxidized form of DJ-1, a function that would have relatively greater importance for the survival of dopaminergic neurons (as somatic DJ-1 deficiency leads to the selective death of these cells), and a function that is relevant in the large number of organisms that have a close DJ-1 homolog. There are currently several different proposals that possess some of these features and have support in the literature, which are discussed below.
Proposed DJ-1 activities that directly involve Cys106 oxidation
The oxidation of Cys106 necessarily consumes reactive oxygen species (ROS) and thus it has been proposed that DJ-1 acts a direct scavenger of ROS (3, 88). DJ-1 can remove hydrogen peroxide from solution in vitro, and DJ-1−/− mice accumulate mitochondrial hydrogen peroxide and suffer from diminished mitochondrial function, albeit without frank neurodegeneration (3). Given the established facile oxidation of Cys106, there is no question that Cys106-SO2− formation will remove ROS from the cell, but the more significant question is whether ROS scavenging by DJ-1 has a physiologically relevant role in decreasing cellular ROS burden. DJ-1 oxidized at Cys106 is not reverted to the thiol and thus accumulates as the oxidized isoforms. Turnover of the oxidized isoforms and new synthesis of the reduced protein is therefore required for the clearance of oxidized DJ-1 (24). Consequently, DJ-1 is not an ROS detoxifying enzyme like the peroxiredoxins or glutaredoxins, which are true catalysts capable of multiple turnovers. In addition, the protective C106DD DJ-1 mutant again provides valuable insight here, as a Cys106-dependent ROS scavenging role for DJ-1 would predict that this mutant should be nonfunctional, which is not the case (95). Therefore, although the oxidation of Cys106 undoubtedly results in some reduction of cellular ROS, the current data suggest that this effect is not a major contributor to the protective function of DJ-1.
DJ-1 possesses a chaperone activity against various protein substrates in vitro and possibly in vivo. The DJ-1 superfamily contains a clade of functionally validated microbial chaperones (65, 76), although they differ significantly from DJ-1 in three-dimensional structure and oligomerization (48). One of the original DJ-1 structural reports showed that the protein had the ability to suppress the heat-induced aggregation of citrate synthase and luciferase, two widely used generic protein substrates for quantifying chaperone activity in vitro (48). However, another group did not detect this activity under similar assay conditions (70). Additional support for a proposed DJ-1 chaperone function was provided by a combined in vitro and cell culture study in which DJ-1 prevented the aggregation of citrate synthase, insulin, and α-synuclein in vitro only in oxidizing conditions (80). Further, DJ-1 could suppress the cytotoxic aggregation of α-synuclein and neurofilament light subunit in both DJ-1−/− human embryonic stem cells and when overexpressed in murine Cath.a-differentiated neuroblastoma cells (80). Cys53 was identified as the key residue for DJ-1's redox-sensitive chaperone activity in this study, which conflicts with all other reports showing that Cys106 is the critical residue. To address this contradiction, a separate group used an assortment of biophysical methods to show that DJ-1 requires Cys106 for suppression of α-synuclein fibrillation in vitro and, further, that oxidation to Cys106-SO2− is required for robust activity (111). Very recently, the E. coli DJ-1 homolog YajL was also found to possess chaperone activity in vitro using citrate synthase, the ribosomal protein S1, and the ribosomal protein L3 and substrates (45). When the YajL gene is deleted in E. coli, several proteins were found to aggregate in vivo, suggesting possible physiological relevance for YajL chaperone activity in this system. Like human DJ-1, YajL is prone to oxidation at Cys106 (100) and the protein requires this residue to confer protection against hydrogen peroxide stress in E. coli (45), indicating that the prokaryotic and vertebrate DJ-1 homologs likely share some aspects of their function.
As with other proposals of a biochemical function for DJ-1, the physiological relevance of this in vitro chaperone activity is not resolved. A troubling aspect of the in vitro studies of DJ-1 chaperone activity is that they are performed in artificial solution conditions, often with a large excess of DJ-1 to substrate. In addition, although Cys106-SO2− human DJ-1 is a better chaperone than the reduced protein (111), there are no structural changes upon oxidation of DJ-1 that provide a clue as to why this form of the protein would be more active (15). Cellular and in vivo studies of DJ-1 chaperone activity have produced mixed results and, consequently, it is difficult to reach a conclusion about the relevance of this activity. A study using transgenic mice overexpressing the toxic A53T α-synuclein mutant showed no changes in viability or extent of α-synuclein aggregation when crossed into a DJ-1 null background (77). Similarly, a Saccharomyces cerevisiae model of α-synuclein toxicity also showed no change in yeast viability when human DJ-1 was coexpressed with α-synuclein (94). Therefore, DJ-1 does not ameliorate α-synuclein toxicity in either these disparate model organisms, suggesting that if DJ-1 is a chaperone, α-synuclein is not a likely substrate in vivo. In contrast, DJ-1 was protective in cooperation with heat shock protein 70 (Hsp70) in cultured human and rat cellular models of α-synuclein toxicity (9, 52), consistent with two previous reports showing that oxidative stress enhanced the interaction between DJ-1 and Hsp70, CHIP, and mitochondrial Hsp70 (mortalin) (51) and that DJ-1 could protect against A53T α-synuclein toxicity in an Hsp70-dependent manner in cultured human dopaminergic N27 cells (110). Additionally, a cellular model using a fluorescence-based foldase reporter indicated that DJ-1 possessed a cellular chaperone activity that was abrogated by the parkinsonian L166P mutation and could be restored by the action of the BAG1 cochaperone (22). In total, the current evidence for a physiological significant chaperone activity for DJ-1 is contradictory and defies straightforward summary. However, the balance of the data suggests that the proposed chaperone activity for DJ-1 is weak and requires chaperones such as Hsp70 for physiological relevance.
An early observation that DJ-1 negatively regulated RNA binding by a 400 kDa multiprotein complex in FTO-2B rat hepatoma cells (32) suggested a possible role for DJ-1 in RNA binding and regulation. A recent pair of studies in human M17 neuroblastoma cells, mouse and human brain tissue extended this association by demonstrating that DJ-1 preferentially interacts with GG/CC-rich sequences in mRNA transcripts encoding proteins involved in the phosphatase and tensin homolog (PTEN)/Akt pathway, ROS detoxification, and mitochondrial function (11, 92). Oxidation of DJ-1 eliminates mRNA binding (92). These observations are intriguing because they independently connect DJ-1 to multiple pathways that had previously been implicated in DJ-1 function using other techniques. In addition, an RNA binding activity for DJ-1 that regulates the translation of multiple protective transcripts may explain the apparent diversity of DJ-1's cellular roles in the context of a single activity. However, the molecular basis of this interaction is not obvious, as DJ-1 lacks canonical RNA interaction motifs, and the role of oxidation in regulating this activity is also not clear. Future study will hopefully clarify the basis of DJ-1's interaction with mRNA.
A promising model for DJ-1's cytoprotective function in higher organisms has emerged from the work of several groups that have recently identified ASK1 as a target of DJ-1 action. DJ-1 was first implicated in the ASK1/p38/mitogen-activated protein kinase pathway in a study showing that DJ-1 binds to the pro-apoptotic protein Daxx and prevents its translocation from the nucleus into the cytoplasm, thereby preventing Daxx from activating ASK1 and, ultimately, initiating apoptosis (38). Recently, DJ-1 has been connected more directly to ASK1 by multiple studies that demonstrate that DJ-1 can inhibit ASK1 activity in an oxidation-dependent manner that requires Cys106. Two studies have detected a direct physical interaction between DJ-1 and ASK1 (63, 95), demonstrating that DJ-1 is recruited to the ASK1 signalosome under oxidative stress conditions where it inhibits the mitogen-activated protein kinase cascade and apoptosis. In one study, this recruitment of oxidized DJ-1 to an inhibitory site near the N-terminus of ASK1 (also bound by the negative regulator thioredoxin) could be recapitulated by the C106DD and C106EE mutations, suggesting that oxidative modification of Cys106 is important (95). This study also showed that the peripheral Cys46 and Cys53 residues can modulate the redox activity of Cys106 and, consequently, DJ-1's association with ASK1. A very recent study supports the role of ASK1/thioredoxin interaction in DJ-1 function by demonstrating that DJ-1 prevents the dissociation of the negative regulator thioredoxin from ASK1 during oxidative stress, thereby inhibiting ASK1 activity and enhancing cell survival (36). Therefore, the ASK1/p38/MAP kinase signaling cascade appears to be an important target of DJ-1's protective function.
Proposed cellular roles for DJ-1 that effect cellular redox balance
This review is focused on the role of cysteine oxidation in DJ-1 function; however, a considerable body of work has implicated DJ-1 in redox-relevant processes where the role of cysteine modification in DJ-1 has either not been specifically tested or is unclear. Cellular redox homeostasis is heavily dependent on glutathione, the principal cellular small molecule thiol in eukaryotic cells. DJ-1 elevates glutathione levels (52, 110) by the increasing the transcription and enzymatic activity of glutamate cysteine ligase, which is the rate-limiting enzyme in glutathione biosynthesis (110). One attractive feature of this model for DJ-1 function is it may help explain why DJ-1 is abundantly expressed in astrocytes (7). These glial cells protect neighboring neurons through secretion of protective small molecule compounds like glutathione and its precursors and, if DJ-1 increases the amounts of the soluble protective factors in glia, it may protect neurons via this mechanism as well (5, 6, 43, 66, 96).
Mitochondria are the primary source of ROS in eukaryotes, and several groups have implicated DJ-1 in promoting proper mitochondrial function and defense against damage caused by complex I inhibition (10, 15, 50, 71, 107). The significance of DJ-1's association with mitochondria is twofold: first, mitochondrial dysfunction has long been suspected of playing an important role in the etiology of parkinsonism (20) and, second, oxidative stress can both cause and result from mitochondrial damage. Bonifati et al. made the first suggestion that a pool of DJ-1 might associate with the mitochondria, particularly the overexpressed pathogenic L166P mutant DJ-1 (13). A series of studies have shown that, although DJ-1 is predominantly a cytosolic protein with some nuclear localization, oxidative stress enhances the association of a pool of DJ-1 with the mitochondria (10, 12, 15, 50, 71). Cys106 oxidation appears to facilitate this association (12, 15), although some DJ-1 is found associated with mitochondria even when this residue is mutated to serine, indicating that oxidation is not a prerequisite for mitochondrial localization (37). DJ-1 has been found both on the outer mitochondrial membrane by subcellular fractionation of M17 neuroblastoma cells (15) and also in the intermembrane space and mitochondrial matrix by immunogold staining of DJ-1 in murine brain tissue (107). Importantly, intentionally targeting DJ-1 to the mitochondria by fusing a mitochondrial localization sequence to the N-terminus of DJ-1 enhances the protective function of the protein in SK-N-BE (2) neuroblastoma cells, indicating that the mitochondrion is an important site of DJ-1 action (37). Moreover, mitochondria in DJ-1−/− mouse embryonic fibroblasts have a shorter and more fragmented morphology (12, 44) that can only be rescued by DJ-1 that is capable of oxidizing at Cys106 (12). This phenotype is not rescued by C106A DJ-1 or by the oxidation-impaired E18D mutant protein (12). Although questions remain about the extent and mechanism of the mitochondrial localization of DJ-1, there is solid evidence that the protein protects against mitochondrial damage and can partially localize to this organelle under oxidative stress conditions.
DJ-1 also interacts with the critical pro-survival cell signaling pathways involving PTEN/phosphoinositide 3-kinase/Akt and p53. The first evidence for DJ-1 involvement in the PTEN/phosphoinositide 3-kinase/Akt pathway came from genetic studies of D. melanogaster that identified DJ-1 as a suppressor of the pro-apoptotic PTEN phosphatase (39). An association between DJ-1 and the PTEN/Akt pathway provides a tantalizing biochemical rationalization of the frequently observed correlation between elevated DJ-1 expression and aggressive cancers of multiple tissue types (2, 21, 31, 40, 61, 72, 75, 78, 79, 90, 106, 112). Recently, a direct physical association between DJ-1 and PTEN that requires the reduced form of Cys106 has been reported (40). Although Cys106 appears to have some role in regulating DJ-1 association with PTEN, a protective role for DJ-1 with reduced Cys106 contrasts with some other studies showing that the oxidized form of the protein is correlated with cytoprotection (12, 15, 95). DJ-1 also plays a role in the hypoxic response through an Akt and mammalian target of rapamycin-dependent modulation of the transcription factor hypoxia inducible factor 1, resulting in cellular protection against hypoxic stress (93). DJ-1 also negatively regulates the tumor suppressor p53, providing another biochemical connection between DJ-1 and cancer (14, 25, 26, 40, 83). In a DJ-1 knockdown zebrafish model, dopaminergic neurons were highly sensitized to cell death by peroxide and proteosomal stress. This sensitivity could be relieved by pharmacological inhibition of p53 activity (14). Like PTEN, there is evidence that DJ-1 physically interacts with p53, although the importance of Cys106 for this interaction is unknown (40). Additional evidence suggesting a role for DJ-1 in modulating the transcriptional response to oxidative stress was reported in two studies in which DJ-1 was identified as a positive regulator of the antioxidant transcription factor NF-E2-related factor 2 (Nrf2) (18, 55). The relevance of this association is not settled, however, as some evidence shows that DJ-1 stabilizes Nrf2 and enhances the cellular antioxidant response, whereas a recent study in primary neuronal cell culture and mice indicates that there is no physiological interaction between DJ-1 and Nrf2 (28). There are several members of the DJ-1 superfamily in which a DJ-1-like domain is fused to a helix-turn-helix DNA binding domain (8, 53). The best characterized of these transcription factors is AdpA, a Streptomyces griseus transcription factor that regulates secondary metabolism (69). This DJ-1/AraC-like DNA binding domain fusion, found in many other prokaryotic members of the DJ-1 superfamily, suggests a conserved connection between DJ-1 and transcriptional regulation.
Conclusions
Even a casual perusal of this article should be enough to convince the reader that a compact summative model of DJ-1 function is not possible at this time. Currently, the areas of consensus are that DJ-1 is a dimeric cytoprotective protein with homologs in many organisms, that the protein requires the conserved Cys106 for this protective function, that Cys106 is susceptible to oxidation to the cysteine-sulfinate and cysteine-sulfonate under oxidative stress conditions, and that DJ-1 participates in multiple pathways to promote cell survival under oxidative and mitochondrial stress conditions. The more contested areas of the DJ-1 field center on how DJ-1 confers protection against oxidative stress, including ongoing debates about the relevance of several proposed biochemical activities for DJ-1 and the role of cysteine oxidation in the function of the protein. An additional source of lingering confusion is how a 20 kDa single domain protein can participate in so many diverse cellular processes. For this question, protein size discrimination should not exert undue influence on the thinking of researchers, as other small proteins such as calmodulin can participate in an impressive array of signaling pathways in response to appropriate stimuli. With homologs in nearly every organism, DJ-1 appears to be even more ancient and widely distributed than calmodulin and thus is likely to have evolved elegant (and probably complex) mechanisms for regulation and cytoprotection.
There is an urgent need for more information about the nature of DJ-1 binding to other proteins. Most models for DJ-1 function include some role for protein–protein interactions, yet these interactions have not been structurally or biophysically characterized. The pocket around Cys106 is prone to interactions with other molecules (47), and it is reasonable to propose that this functionally important area is involved in protein binding. Moreover, protein binding near Cys106 would help rationalize some of the contentious proposals of a protease or chaperone function for DJ-1, as both of these weak activities may result from a basal, low affinity binding of DJ-1 to protein substrates. As described above, the physiological relevance of these activities requires additional experimental scrutiny.
The view of DJ-1 biology has clarified tremendously in the 13 years since its discovery. Many advances in the understanding of DJ-1 function have been driven by its connection to human disease; however, DJ-1 homologs are present in almost all organisms. Animal DJ-1 appears to be regulated by oxidation of the conserved cysteine residue, and current data suggest that this type of modification may be widespread in the DJ-1 superfamily, including its many prokaryotic members. It therefore is likely that the DJ-1 superfamily is one of the most ancient examples of direct cysteine oxidation being employed as a key regulatory modification. With countless uncharacterized members in all kingdoms of life that invite study, the DJ-1 superfamily is poised to provide a rich set of examples of the versatility of cysteine chemistry in catalysis and regulation.
Abbreviations Used
- ASK1
apoptosis signal regulating kinase 1
- Hsp70
heat shock protein 70
- Nrf2
NF-E2-related factor 2
- PTEN
phosphatase and tensin homolog
- ROS
reactive oxygen species
Acknowledgment
This work was supported, in whole or in part, by National Institutes of Health grants P20 RR017675-08 and R01 GM092999-01 to M.A.W.
References
- 1.Aleyasin H. Rousseaux MW. Phillips M. Kim RH. Bland RJ. Callaghan S. Slack RS. During MJ. Mak TW. Park DS. The Parkinson's disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc Natl Acad Sci U S A. 2007;104:18748–18753. doi: 10.1073/pnas.0709379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsøe L. Stacy JE. Fosså A. Funderud S. Brekke OH. Gaudernack G. Identification of prostate cancer antigens by automated high-throughput filter immunoscreening. J Immunol Methods. 2008;330:12–23. doi: 10.1016/j.jim.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Andres-Mateos E. Perier C. Zhang L. Blanchard-Fillion B. Greco TM. Thomas B. Ko HS. Sasaki M. Ischiropoulos H. Przedborski S. Dawson TM. Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annesi G. Savettieri G. Pugliese P. D'Amelio M. Tarantino P. Ragonese P. La Bella V. Piccoli T. Civitelli D. Annesi F. Fierro B. Piccoli F. Arabia G. Caracciolo M. Cirò Candiano IC. Quattrone A. DJ-1 mutations and parkinsonism-dementia-amyotrophic lateral sclerosis complex. Ann Neurol. 2005;58:803–807. doi: 10.1002/ana.20666. [DOI] [PubMed] [Google Scholar]
- 5.Ashley AK. Hanneman WH. Katoh T. Moreno JA. Pollack A. Tjalkens RB. Legare ME. Analysis of targeted mutation in DJ-1 on cellular function in primary astrocytes. Toxicol Lett. 2009;184:186–191. doi: 10.1016/j.toxlet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader V. Ran Zhu X. Lubbert H. Stichel CC. Expression of DJ-1 in the adult mouse CNS. Brain Res. 2005;1041:102–111. doi: 10.1016/j.brainres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Bandopadhyay R. Kingsbury AE. Cookson MR. Reid AR. Evans IM. Hope AD. Pittman AM. Lashley T. Canet-Aviles R. Miller DW. McLendon C. Strand C. Leonard AJ. Abou-Sleiman PM. Healy DG. Ariga H. Wood NW. de Silva R. Revesz T. Hardy JA. Lees AJ. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson's disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 8.Bandyopadhyay S. Cookson MR. Evolutionary and functional relationships within the DJ1 superfamily. BMC Evol Biol. 2004;4:6. doi: 10.1186/1471-2148-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batelli S. Albani D. Rametta R. Polito L. Prato F. Pesaresi M. Negro A. Forloni G. DJ-1 modulates alpha-synuclein aggregation state in a cellular model of oxidative stress: relevance for Parkinson's disease and involvement of HSP70. PLoS One. 2008;3:e1884. doi: 10.1371/journal.pone.0001884. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Blackinton J. Ahmad R. Miller DW. van der Brug MP. Canet-Avilés RM. Hague SM. Kaleem M. Cookson MR. Effects of DJ-1 mutations and polymorphisms on protein stability and subcellular localization. Brain Res Mol Brain Res. 2005;134:76–83. doi: 10.1016/j.molbrainres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Blackinton J. Kumaran R. van der Brug MP. Ahmad R. Olson L. Galter D. Lees A. Bandopadhyay R. Cookson MR. Post-transcriptional regulation of mRNA associated with DJ-1 in sporadic Parkinson disease. Neurosci Lett. 2009;452:8–11. doi: 10.1016/j.neulet.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackinton J. Lakshminarasimhan M. Thomas KJ. Ahmad R. Greggio E. Raza AS. Cookson MR. Wilson MA. Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J Biol Chem. 2009;284:6476–6485. doi: 10.1074/jbc.M806599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonifati V. Rizzu P. van Baren MJ. Schaap O. Breedveld GJ. Krieger E. Dekker MC. Squitieri F. Ibanez P. Joosse M. van Dongen JW. Vanacore N. van Swieten JC. Brice A. Meco G. van Duijn CM. Oostra BA. Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 14.Bretaud S. Allen C. Ingham PW. Bandmann O. p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson's disease. J Neurochem. 2007;100:1626–1635. doi: 10.1111/j.1471-4159.2006.04291.x. [DOI] [PubMed] [Google Scholar]
- 15.Canet-Avilés RM. Wilson MA. Miller DW. Ahmad R. McLendon C. Bandyopadhyay S. Baptista MJ. Ringe D. Petsko GA. Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J. Li L. Chin LS. Parkinson disease protein DJ-1 converts from a zymogen to a protease by carboxyl-terminal cleavage. Hum Mol Genet. 2010;19:2395–2408. doi: 10.1093/hmg/ddq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J. Sullards MC. Olzmann JA. Rees HD. Weintraub ST. Bostwick DE. Gearing M. Levey AI. Chin LS. Li L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clements CM. McNally RS. Conti BJ. Mak TW. Ting JP. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cookson MR. Pathways to Parkinsonism. Neuron. 2003;37:7–10. doi: 10.1016/s0896-6273(02)01166-2. [DOI] [PubMed] [Google Scholar]
- 20.Cookson MR. DJ-1, PINK1, and their effects on mitochondrial pathways. Mov Disord. 2010;25(Suppl 1):S44–S48. doi: 10.1002/mds.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson B. Hadar R. Schlossberg A. Sternlicht T. Slipicevic A. Skrede M. Risberg B. Flørenes VA. Kopolovic J. Reich R. Expression and clinical role of DJ-1, a negative regulator of PTEN, in ovarian carcinoma. Hum Pathol. 2008;39:87–95. doi: 10.1016/j.humpath.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Deeg S. Gralle M. Sroka K. Bahr M. Wouters FS. Kermer P. BAG1 restores formation of functional DJ-1 L166P dimers and DJ-1 chaperone activity. J Cell Biol. 2010;188:505–513. doi: 10.1083/jcb.200904103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du X. Choi IG. Kim R. Wang W. Jancarik J. Yokota H. Kim SH. Crystal structure of an intracellular protease from Pyrococcus horikoshii at 2-A resolution. Proc Natl Acad Sci U S A. 2000;97:14079–14084. doi: 10.1073/pnas.260503597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan X. Kelsen SG. Merali S. Proteomic analysis of oxidative stress-responsive proteins in human pneumocytes: insight into the regulation of DJ-1 expression. J Proteome Res. 2008;7:4955–4961. doi: 10.1021/pr800295j. [DOI] [PubMed] [Google Scholar]
- 25.Fan J. Ren H. Fei E. Jia N. Ying Z. Jiang P. Wu M. Wang G. Sumoylation is critical for DJ-1 to repress p53 transcriptional activity. FEBS Letters. 2008;582:1151–1156. doi: 10.1016/j.febslet.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Fan J. Ren H. Jia N. Fei E. Zhou T. Jiang P. Wu M. Wang G. DJ-1 decreases bax expression through repressing p53 transcriptional activity. J Biol Chem. 2008;283:4022–4030. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- 27.Fioravanti E. Dura MA. Lascoux D. Micossi E. Franzetti B. McSweeney S. Structure of the stress response protein DR1199 from Deinococcus radiodurans: a member of the DJ-1 superfamily. Biochemistry. 2008;47:11581–11589. doi: 10.1021/bi800882v. [DOI] [PubMed] [Google Scholar]
- 28.Gan L. Johnson DA. Johnson JA. Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J Neurosci. 2010;31:967–977. doi: 10.1111/j.1460-9568.2010.07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorner K. Holtorf E. Odoy S. Nuscher B. Yamamoto A. Regula JT. Beyer K. Haass C. Kahle PJ. Differential effects of Parkinson's disease-associated mutations on stability and folding of DJ-1. J Biol Chem. 2004;279:6943–6951. doi: 10.1074/jbc.M309204200. [DOI] [PubMed] [Google Scholar]
- 30.Hering R. Strauss KM. Tao X. Bauer A. Woitalla D. Mietz EM. Petrovic S. Bauer P. Schaible W. Muller T. Schols L. Klein C. Berg D. Meyer PT. Schulz JB. Wollnik B. Tong L. Kruger R. Riess O. Novel homozygous p.E64D mutation in DJ1 in early onset Parkinson disease (PARK7) Hum Mutat. 2004;24:321–329. doi: 10.1002/humu.20089. [DOI] [PubMed] [Google Scholar]
- 31.Hod Y. Differential control of apoptosis by DJ-1 in prostate benign and cancer cells. J Cell Biochem. 2004;92:1221–1233. doi: 10.1002/jcb.20159. [DOI] [PubMed] [Google Scholar]
- 32.Hod Y. Pentyala SN. Whyard TC. El-Maghrabi MR. Identification and characterization of a novel protein that regulates RNA-protein interaction. J Cell Biochem. 1999;72:435–444. [PubMed] [Google Scholar]
- 33.Honbou K. Suzuki NN. Horiuchi M. Niki T. Taira T. Ariga H. Inagaki F. The crystal structure of DJ-1, a protein related to male fertility and Parkinson's disease. J Biol Chem. 2003;278:31380–31384. doi: 10.1074/jbc.M305878200. [DOI] [PubMed] [Google Scholar]
- 34.Huai Q. Sun Y. Wang H. Chin LS. Li L. Robinson H. Ke H. Crystal structure of DJ-1/RS and implication on familial Parkinson's disease. FEBS Lett. 2003;549:171–175. doi: 10.1016/s0014-5793(03)00764-6. [DOI] [PubMed] [Google Scholar]
- 35.Hulleman JD. Mirzaei H. Guigard E. Taylor KL. Ray SS. Kay CM. Regnier FE. Rochet JC. Destabilization of DJ-1 by familial substitution and oxidative modifications: implications for Parkinson's disease. Biochemistry. 2007;46:5776–5789. doi: 10.1021/bi7001778. [DOI] [PubMed] [Google Scholar]
- 36.Im JY. Lee KW. Junn E. Mouradian MM. DJ-1 protects against oxidative damage by regulating the thioredoxin/ASK1 complex. Neurosci Res. 2010;67:203–208. doi: 10.1016/j.neures.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Junn E. Jang WH. Zhao X. Jeong BS. Mouradian MM. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res. 2008;87:123–129. doi: 10.1002/jnr.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junn E. Taniguchi H. Jeong BS. Zhao X. Ichijo H. Mouradian MM. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Natl Acad Sci U S A. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim RH. Peters M. Jang Y. Shi W. Pintilie M. Fletcher GC. DeLuca C. Liepa J. Zhou L. Snow B. Binari RC. Manoukian AS. Bray MR. Liu FF. Tsao MS. Mak TW. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Kim YC. Kitaura H. Taira T. Iguchi-Ariga SM. Ariga H. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int J Oncol. 2009;35:1331–1341. [PubMed] [Google Scholar]
- 41.Kinumi T. Kimata J. Taira T. Ariga H. Niki E. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- 42.Koide-Yoshida S. Niki T. Ueda M. Himeno S. Taira T. Iguchi-Ariga SM. Ando Y. Ariga H. DJ-1 degrades transthyretin and an inactive form of DJ-1 is secreted in familial amyloidotic polyneuropathy. Int J Mol Med. 2007;19:885–893. [PubMed] [Google Scholar]
- 43.Kotaria N. Hinz U. Zechel S. von Bohlen Und Halbach O. Localization of DJ-1 protein in the murine brain. Cell Tissue Res. 2005;322:503–507. doi: 10.1007/s00441-005-0023-1. [DOI] [PubMed] [Google Scholar]
- 44.Krebiehl G. Ruckerbauer S. Burbulla LF. Kieper N. Maurer B. Waak J. Wolburg H. Gizatullina Z. Gellerich FN. Woitalla D. Riess O. Kahle PJ. Proikas-Cezanne T. Kruger R. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson's disease-associated protein DJ-1. PLoS One. 2010;5:e9367. doi: 10.1371/journal.pone.0009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kthiri F. Le HT. Gautier V. Caldas T. Malki A. Landoulsi A. Bohn C. Bouloc P. Richarme G. Protein aggregation in a mutant deficient in yajL, the bacterial homolog of the Parkinsonism-associated protein DJ-1. J Biol Chem. 2010;285:10328–10336. doi: 10.1074/jbc.M109.077529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakshminarasimhan M. Maldonado MT. Zhou W. Fink AL. Wilson MA. Structural impact of three Parkinsonism-associated Missense mutations on human DJ-1. Biochemistry. 2008;47:1381–1392. doi: 10.1021/bi701189c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landon MR. Lieberman RL. Hoang QQ. Ju S. Caaveiro JM. Orwig SD. Kozakov D. Brenke R. Chuang GY. Beglov D. Vajda S. Petsko GA. Ringe D. Detection of ligand binding hot spots on protein surfaces via fragment-based methods: application to DJ-1 and glucocerebrosidase. J Comput Aided Mol Des. 2009 doi: 10.1007/s10822-009-9283-2. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SJ. Kim SJ. Kim IK. Ko J. Jeong CS. Kim GH. Park C. Kang SO. Suh PG. Lee HS. Cha SS. Crystal structures of human DJ-1 and Escherichia coli Hsp31, which share an evolutionarily conserved domain. J Biol Chem. 2003;278:44552–44559. doi: 10.1074/jbc.M304517200. [DOI] [PubMed] [Google Scholar]
- 49.Lev N. Ickowicz D. Barhum Y. Melamed E. Offen D. DJ-1 changes in G93A-SOD1 transgenic mice: implications for oxidative stress in ALS. J Mol Neurosci. 2009;38:94–102. doi: 10.1007/s12031-008-9138-7. [DOI] [PubMed] [Google Scholar]
- 50.Lev N. Ickowicz D. Melamed E. Offen D. Oxidative insults induce DJ-1 upregulation and redistribution: implications for neuroprotection. Neurotoxicology. 2008;29:397–405. doi: 10.1016/j.neuro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Li HM. Niki T. Taira T. Iguchi-Ariga SM. Ariga H. Association of DJ-1 with chaperones and enhanced association and colocalization with mitochondrial Hsp70 by oxidative stress. Free Radic Res. 2005;39:1091–1099. doi: 10.1080/10715760500260348. [DOI] [PubMed] [Google Scholar]
- 52.Liu F. Nguyen JL. Hulleman JD. Li L. Rochet JC. Mechanisms of DJ-1 neuroprotection in a cellular model of Parkinson's disease. J Neurochem. 2008;105:2435–2453. doi: 10.1111/j.1471-4159.2008.05333.x. [DOI] [PubMed] [Google Scholar]
- 53.Lucas JI. Marin I. A new evolutionary paradigm for the Parkinson disease gene DJ-1. Mol Biol Evol. 2007;24:551–561. doi: 10.1093/molbev/msl186. [DOI] [PubMed] [Google Scholar]
- 54.Malgieri G. Eliezer D. Structural effects of Parkinson's disease linked DJ-1 mutations. Protein Sci. 2008;17:855–868. doi: 10.1110/ps.073411608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malhotra D. Thimmulappa R. Navas-Acien A. Sandford A. Elliott M. Singh A. Chen L. Zhuang X. Hogg J. Pare P. Tuder RM. Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Meulener M. Whitworth AJ. Armstrong-Gold CE. Rizzu P. Heutink P. Wes PD. Pallanck LJ. Bonini NM. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 57.Meulener MC. Xu K. Thomson L. Thompson L. Ischiropoulos H. Bonini NM. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc Natl Acad Sci U S A. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller DW. Ahmad R. Hague S. Baptista MJ. Canet-Aviles R. McLendon C. Carter DM. Zhu PP. Stadler J. Chandran J. Klinefelter GR. Blackstone C. Cookson MR. L166P mutant DJ-1, causative for recessive Parkinson's disease, is degraded through the ubiquitin-proteasome system. J Biol Chem. 2003;278:36588–36595. doi: 10.1074/jbc.M304272200. [DOI] [PubMed] [Google Scholar]
- 59.Mitsumoto A. Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- 60.Mitsumoto A. Nakagawa Y. Takeuchi A. Okawa K. Iwamatsu A. Takanezawa Y. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic Res. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- 61.Miyajima Y. Sato Y. Oka H. Utsuki S. Kondo K. Tanizaki Y. Nagashio R. Tsuchiya B. Okayasu I. Fujii K. Prognostic significance of nuclear DJ-1 expression in astrocytoma. Anticancer Res. 2010;30:265–269. [PubMed] [Google Scholar]
- 62.Miyazaki S. Yanagida T. Nunome K. Ishikawa S. Inden M. Kitamura Y. Nakagawa S. Taira T. Hirota K. Niwa M. Iguchi-Ariga SM. Ariga H. DJ-1-binding compounds prevent oxidative stress-induced cell death and movement defect in Parkinson's disease model rats. J Neurochem. 2008;105:2418–2434. doi: 10.1111/j.1471-4159.2008.05327.x. [DOI] [PubMed] [Google Scholar]
- 63.Mo JS. Jung J. Yoon JH. Hong JA. Kim MY. Ann EJ. Seo MS. Choi YH. Park HS. DJ-1 modulates the p38 mitogen-activated protein kinase pathway through physical interaction with apoptosis signal-regulating kinase 1. J Cell Biochem. 2010;110:229–237. doi: 10.1002/jcb.22530. [DOI] [PubMed] [Google Scholar]
- 64.Moore DJ. Zhang L. Dawson TM. Dawson VL. A missense mutation (L166P) in DJ-1, linked to familial Parkinson's disease, confers reduced protein stability and impairs homo-oligomerization. J Neurochem. 2003;87:1558–1567. doi: 10.1111/j.1471-4159.2003.02265.x. [DOI] [PubMed] [Google Scholar]
- 65.Mujacic M. Baneyx F. Regulation of Escherichia coli hchA, a stress-inducible gene encoding molecular chaperone Hsp31. Mol Microbiol. 2006;60:1576–1589. doi: 10.1111/j.1365-2958.2006.05207.x. [DOI] [PubMed] [Google Scholar]
- 66.Mullett SJ. Hinkle DA. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol Dis. 2009;33:28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagakubo D. Taira T. Kitaura H. Ikeda M. Tamai K. Iguchi-Ariga SM. Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 68.Niki T. Takahashi-Niki K. Taira T. Iguchi-Ariga SM. Ariga H. DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol Cancer Res. 2003;1:247–261. [PubMed] [Google Scholar]
- 69.Ohnishi Y. Yamazaki H. Kato JY. Tomono A. Horinouchi S. AdpA, a central transcriptional regulator in the A-factor regulatory cascade that leads to morphological development and secondary metabolism in Streptomyces griseus. Biosci Biotechnol Biochem. 2005;69:431–439. doi: 10.1271/bbb.69.431. [DOI] [PubMed] [Google Scholar]
- 70.Olzmann JA. Brown K. Wilkinson KD. Rees HD. Huai Q. Ke H. Levey AI. Li L. Chin LS. Familial Parkinson's disease-associated L166P mutation disrupts DJ-1 protein folding and function. J Biol Chem. 2004;279:8506–8515. doi: 10.1074/jbc.M311017200. [DOI] [PubMed] [Google Scholar]
- 71.Ooe H. Taira T. Iguchi-Ariga SM. Ariga H. Induction of reactive oxygen species by bisphenol A and abrogation of bisphenol A-induced cell injury by DJ-1. Toxicol Sci. 2005;88:114–126. doi: 10.1093/toxsci/kfi278. [DOI] [PubMed] [Google Scholar]
- 72.Pardo M. Garcia A. Thomas B. Pineiro A. Akoulitchev A. Dwek RA. Zitzmann N. The characterization of the invasion phenotype of uveal melanoma tumour cells shows the presence of MUC18 and HMG-1 metastasis markers and leads to the identification of DJ-1 as a potential serum biomarker. Int J Cancer. 2006;119:1014–1022. doi: 10.1002/ijc.21942. [DOI] [PubMed] [Google Scholar]
- 73.Park J. Kim SY. Cha GH. Lee SB. Kim S. Chung J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 74.Paulsen CE. Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pitkanen-Arsiola T. Tillman JE. Gu G. Yuan J. Roberts RL. Wantroba M. Coetzee GA. Cookson MS. Kasper S. Androgen and anti-androgen treatment modulates androgen receptor activity and DJ-1 stability. Prostate. 2006;66:1177–1193. doi: 10.1002/pros.20450. [DOI] [PubMed] [Google Scholar]
- 76.Quigley PM. Korotkov K. Baneyx F. Hol WG. The 1.6-A crystal structure of the class of chaperones represented by Escherichia coli Hsp31 reveals a putative catalytic triad. Proc Natl Acad Sci U S A. 2003;100:3137–3142. doi: 10.1073/pnas.0530312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramsey CP. Tsika E. Ischiropoulos H. Giasson BI. DJ-1 deficient mice demonstrate similar vulnerability to pathogenic Ala53Thr human alpha-syn toxicity. Hum Mol Genet. 2010;19:1425–1437. doi: 10.1093/hmg/ddq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sekito A. Taira T. Niki T. Iguchi-Ariga SM. Ariga H. Stimulation of transforming activity of DJ-1 by Abstrakt, a DJ-1-binding protein. Int J Oncol. 2005;26:685–689. [PubMed] [Google Scholar]
- 79.Shen Z. Jiang Z. Ye D. Xiao B. Zhang X. Guo J. Growth inhibitory effects of DJ-1-small interfering RNA on laryngeal carcinoma Hep-2 cells. Med Oncol. 2010 doi: 10.1007/s12032-010-9474-7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 80.Shendelman S. Jonason A. Martinat C. Leete T. Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shih HM. Chang CC. Kuo HY. Lin DY. Daxx mediates SUMO-dependent transcriptional control and subnuclear compartmentalization. Biochem Soc Trans. 2007;35:1397–1400. doi: 10.1042/BST0351397. [DOI] [PubMed] [Google Scholar]
- 82.Shinbo Y. Niki T. Taira T. Ooe H. Takahashi-Niki K. Maita C. Seino C. Iguchi-Ariga SM. Ariga H. Proper SUMO-1 conjugation is essential to DJ-1 to exert its full activities. Cell Death Differ. 2006;13:96–108. doi: 10.1038/sj.cdd.4401704. [DOI] [PubMed] [Google Scholar]
- 83.Shinbo Y. Taira T. Niki T. Iguchi-Ariga SM. DJ-1 restores p53 transcription activity inhibited by Topors/p53BP3. Int J Oncol. 2005;26:641–648. [PubMed] [Google Scholar]
- 84.Siva AB. Sundareswaran VR. Yeung CH. Cooper TG. Shivaji S. Hamster contraception associated protein 1 (CAP1) Mol Reprod Dev. 2004;68:373–383. doi: 10.1002/mrd.20085. [DOI] [PubMed] [Google Scholar]
- 85.Taira T. Iguchi-Ariga SM. Ariga H. Co-localization with DJ-1 is essential for the androgen receptor to exert its transcription activity that has been impaired by androgen antagonists. Biol Pharm Bull. 2004;27:574–577. doi: 10.1248/bpb.27.574. [DOI] [PubMed] [Google Scholar]
- 86.Taira T. Saito Y. Niki T. Iguchi-Ariga SM. Takahashi K. Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–8. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takahashi K. Taira T. Niki T. Seino C. Iguchi-Ariga SM. Ariga H. DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor. J Biol Chem. 2001;276:37556–37563. doi: 10.1074/jbc.M101730200. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi-Niki K. Niki T. Taira T. Iguchi-Ariga SM. Ariga H. Reduced anti-oxidative stress activities of DJ-1 mutants found in Parkinson's disease patients. Biochem Biophys Res Commun. 2004;320:389–397. doi: 10.1016/j.bbrc.2004.05.187. [DOI] [PubMed] [Google Scholar]
- 89.Tao X. Tong L. Crystal structure of human DJ-1, a protein associated with early onset Parkinson's disease. J Biol Chem. 2003;278:31372–31379. doi: 10.1074/jbc.M304221200. [DOI] [PubMed] [Google Scholar]
- 90.Tian M. Cui YZ. Song GH. Zong MJ. Zhou XY. Chen Y. Han JX. Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer. 2008;8:241. doi: 10.1186/1471-2407-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tillman JE. Yuan J. Gu G. Fazli L. Ghosh R. Flynt AS. Gleave M. Rennie PS. Kasper S. DJ-1 binds androgen receptor directly and mediates its activity in hormonally treated prostate cancer cells. Cancer Res. 2007;67:4630–4637. doi: 10.1158/0008-5472.CAN-06-4556. [DOI] [PubMed] [Google Scholar]
- 92.van der Brug MP. Blackinton J. Chandran J. Hao LY. Lal A. Mazan-Mamczarz K. Martindale J. Xie C. Ahmad R. Thomas KJ. Beilina A. Gibbs JR. Ding J. Myers AJ. Zhan M. Cai H. Bonini NM. Gorospe M. Cookson MR. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci U S A. 2008;105:10244–10249. doi: 10.1073/pnas.0708518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vasseur S. Afzal S. Tardivel-Lacombe J. Park DS. Iovanna JL. Mak TW. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci U S A. 2009;106:1111–1116. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Volles MJ. Lansbury PT., Jr. Relationships between the sequence of alpha-synuclein and its membrane affinity, fibrillization propensity, and yeast toxicity. J Mol Biol. 2007;366:1510–1522. doi: 10.1016/j.jmb.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waak J. Weber SS. Gorner K. Schall C. Ichijo H. Stehle T. Kahle PJ. Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J Biol Chem. 2009;284:14245–14257. doi: 10.1074/jbc.M806902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Waak J. Weber SS. Waldenmaier A. Gorner K. Alunni-Fabbroni M. Schell H. Vogt-Weisenhorn D. Pham TT. Reumers V. Baekelandt V. Wurst W. Kahle PJ. Regulation of astrocyte inflammatory responses by the Parkinson's disease-associated gene DJ-1. FASEB J. 2009;23:2478–2489. doi: 10.1096/fj.08-125153. [DOI] [PubMed] [Google Scholar]
- 97.Wagenfeld A. Gromoll J. Cooper TG. Molecular cloning and expression of rat contraception associated protein 1 (CAP1), a protein putatively involved in fertilization. Biochem Biophys Res Commun. 1998;251:545–549. doi: 10.1006/bbrc.1998.9512. [DOI] [PubMed] [Google Scholar]
- 98.Wagenfeld A. Yeung CH. Shivaji S. Sundareswaran VR. Ariga H. Cooper TG. Expression and cellular localization of contraception-associated protein. J Androl. 2000;21:954–963. [PubMed] [Google Scholar]
- 99.Wei Y. Ringe D. Wilson M. Ondrechen M. Identification of functional subclasses in the DJ-1 superfamily proteins. PLoS Comput Biol. 2007;3:e10. doi: 10.1371/journal.pcbi.0030010. [DOI] [PubMed] [Google Scholar]
- 100.Wilson M. Ringe D. Petsko GA. The atomic resolution crystal structure of the YajL (ThiJ) protein from Escherichia coli: a close prokaryotic homologue of the Parkinsonism-associated protein DJ-1. J Mol Biol. 2005;353:678–691. doi: 10.1016/j.jmb.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 101.Wilson MA. Collins JL. Hod Y. Ringe D. Petsko GA. The 1.1-A resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson's disease. Proc Natl Acad Sci U S A. 2003;100:9256–9261. doi: 10.1073/pnas.1133288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilson MA. St. Amour CV. Collins JL. Ringe D. Petsko GA. The 1.8-A resolution crystal structure of YDR533Cp from Saccharomyces cerevisiae: a member of the DJ-1/ThiJ/PfpI superfamily. Proc Natl Acad Sci U S A. 2004;101:1531–1536. doi: 10.1073/pnas.0308089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Witt AC. Lakshminarasimhan M. Remington BC. Hasim S. Pozharski E. Wilson MA. Cysteine pKa depression by a protonated glutamic acid in human DJ-1. Biochemistry. 2008;47:7430–7440. doi: 10.1021/bi800282d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu J. Zhong N. Wang H. Elias JE. Kim CY. Woldman I. Pifl C. Gygi SP. Geula C. Yankner BA. The Parkinson's disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum Mol Genet. 2005;14:1231–1241. doi: 10.1093/hmg/ddi134. [DOI] [PubMed] [Google Scholar]
- 105.Yanagisawa D. Kitamura Y. Inden M. Takata K. Taniguchi T. Morikawa S. Morita M. Inubushi T. Tooyama I. Taira T. Iguchi-Ariga SM. Akaike A. Ariga H. DJ-1 protects against neurodegeneration caused by focal cerebral ischemia and reperfusion in rats. J Cereb Blood Flow Metab. 2008;28:563–578. doi: 10.1038/sj.jcbfm.9600553. [DOI] [PubMed] [Google Scholar]
- 106.Yuen HF. Chan YP. Law S. Srivastava G. El-Tanani M. Mak TW. Chan KW. DJ-1 could predict worse prognosis in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3593–3602. doi: 10.1158/1055-9965.EPI-08-0214. [DOI] [PubMed] [Google Scholar]
- 107.Zhang L. Shimoji M. Thomas B. Moore DJ. Yu SW. Marupudi NI. Torp R. Torgner IA. Ottersen OP. Dawson TM. Dawson VL. Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- 108.Zhong N. Kim CY. Rizzu P. Geula C. Porter DR. Pothos EN. Squitieri F. Heutink P. Xu J. DJ-1 transcriptionally up-regulates the human tyrosine hydroxylase by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor. J Biol Chem. 2006;281:20940–20948. doi: 10.1074/jbc.M601935200. [DOI] [PubMed] [Google Scholar]
- 109.Zhong N. Xu J. Synergistic activation of the human MnSOD promoter by DJ-1 and PGC-1alpha: regulation by SUMOylation and oxidation. Hum Mol Genet. 2008;17:3357–3367. doi: 10.1093/hmg/ddn230. [DOI] [PubMed] [Google Scholar]
- 110.Zhou W. Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 111.Zhou W. Zhu M. Wilson MA. Petsko GA. Fink AL. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J Mol Biol. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 112.Zhu XL. Wang ZF. Lei WB. Zhuang HW. Jiang HY. Wen WP. DJ-1: a novel independent prognostic marker for survival in glottic squamous cell carcinoma. Cancer Sci. 2010;101:1320–1325. doi: 10.1111/j.1349-7006.2010.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zucchelli S. Vilotti S. Calligaris R. Lavina ZS. Biagioli M. Foti R. De Maso L. Pinto M. Gorza M. Speretta E. Casseler C. Tell G. Del Sal G. Gustincich S. Aggresome-forming TTRAP mediates pro-apoptotic properties of Parkinson's disease-associated DJ-1 missense mutations. Cell Death Differ. 2009;16:428–438. doi: 10.1038/cdd.2008.169. [DOI] [PubMed] [Google Scholar]