Abstract
The Akt signaling pathway plays a key role in promoting the survival of various types of cells from stress-induced apoptosis, and different members of the Akt family display distinct physiological roles. Previous studies have shown that in response to UV irradiation, Akt2 is sensitized to counteract the induced apoptosis. However, in response to oxidative stress such as hydrogen peroxide, it remains to be elucidated what member of the Akt family would be activated to initiate the signaling cascades leading to resistance of the induced apoptosis. In the present study, we present the first evidence that knockdown of Akt1 enhances cell survival under exposure to 50 μM H2O2. This survival is derived from selective upregulation and activation of Akt2 but not Akt3, which initiates 3 major signaling cascades. First, murine double minute 2 (MDM2) is hyperphosphorylated, which promotes p53 degradation and attenuates its Ser-15 phosphorylation, significantly attenuating Bcl-2 homologous antagonist killer (Bak) upregulation. Second, Akt2 activation inactivates glycogen synthase kinase 3 beta (GSK-3β) to promote stability of myeloid leukemia cell differentiation protein 1 (MCL-1). Finally, Akt2 activation promotes phosphorylation of FOXO3A toward cytosolic export and thus downregulates Bim expression. Overexpression of Bim enhances H2O2-induced apoptosis. Together, our results demonstrate that among the Akt family members, Akt2 is an essential kinase in counteracting oxidative-stress-induced apoptosis through multiple signaling pathways. Antioxid. Redox Signal. 15, 1–17.
Introduction
The c-Akt is a cellular homolog of the transforming oncogene of the AKT retrovirus (44). Simultaneous with the identification of c-Akt, the protein kinase B (PKB) and a kinase related to A- and C-protein kinases were cloned (12, 24). c-Akt1, PKB, and Rac-activated protein kinase (RAC-PK) were found to be encoded by the same gene (herein referred to as Akt). Two additional Akt isoforms have also been identified, c-Akt2 and c-Akt3 (35, 44). Akt1, Akt2, and Akt3 are encoded by three independent genes located on different chromosomes, and share more than 80% amino acid identity (6). The Akt family proteins contain a central kinase domain with specificity for serine or threonine residues in substrate proteins. In addition, the amino terminus of Akt includes a pleckstrin homology (PH) domain, which mediates lipid–protein and/or protein–protein interactions (13).
In response to extracellular growth or survival factors, the Akt signaling pathway becomes activated to coordinate various cellular events and eventually controls physiological processes such as cell proliferation, cell growth, cell migration, and cell survival (14). The activation of Akt kinase is an intricately regulated process. First, the binding of growth factors to receptor protein tyrosine kinase leads to membrane recruitment and activation of phosphatidylinositide 3′-OH kinase (PI3K). Next, The activated PI3K converts phosphatidylinositol 3, 4 biphosphate (PIP2) into phosphatidylinositol 3,4,5 triphosphate (PIP3) (14); the binding of PIP3 to the PH domain of the primed Akt (after phosphorylation of Thr-450 by JNK1/2 (42)) leads to Akt translocation from the cytoplasm to the plasma membrane, where Akt becomes activated as a result of phosphorylation of Thr-308 within the T loop of the catalytic domain by phosphoinositide-dependent kinase 1 (PDK1) and Ser-473 in the hydrophobic motif located in C-terminal noncatalytic region by the mammalian target of rapamycin complexes (1, 16). On the other hand, the lipid protein phosphatase and tensin homolog (PTEN), the PH domain leucine-rich repeat protein phosphatase (PHLPP), and the protein serine/threonine phosphatase-1 and-2A exercise functions of terminating Akt signaling through different mechanisms (7, 17, 26, 45, 47). Besides that, the ubiquitin-dependent degradation has been reported to negatively regulate the protein stability of Akt (48).
Oxidative stress is implicated in aging and many human diseases such as macular degeneration, cataractogenesis, and cardiovascular disorders and cancer (2, 5, 9, 21, 23, 30, 43). Recent studies indicate that short-term oxidative stress may be important in the prevention of aging through the induction of mitohormesis (3). The enzymes against oxidative damage to cellular components are superoxide dismutases, glutathione metabolizing enzymes (peroxidases, reductases, and transferase), and catalase (19). In addition to these major antioxidative enzymes, Han et al. found that upregulation of p53 target genes is a conserved response to oxidative stress (22). Finally, PTEN inactivation by oxidative insult is a physiological mechanism by which Akt becomes activated (6). Cellular reactive oxygen species (ROS) can also activate Akt in a PI3K-dependent manner (9, 10). Therefore, when a cell is challenged by oxidative stress, the cell fate is at least determined by regulation of expression of antioxidant enzymes and the count balance between two antagonizing pathways: the pro-apoptotic p53 pathway and antiapoptotic Akt signaling pathway.
Previous studies reveal the presence of diverse phenotypes in Akt knockout mice. These results suggest distinct physiological roles for each Akt isoform in regulating different biological processes. PKB1/Akt1 determines animal size, and modulates neonatal mortality and adipogenesis in mice (49), whereas PKB2/Akt2 has a critical role in glucose metabolism and contributes to organismal growth (10). A recent study revealed that Akt2 is also critical for UV response (25). On the other hand, knockout of both Akt1 and Akt2 seems to enhance the ability of cells to resist oxidative stress damage (37). However, the specific function of each isoform in response to oxidative stress has not been established. In the present study, we present the first evidence that distinct resistance against oxidative stress appears when Akt1 is knocked down in human lens epithelial cells (HLECs). This resistance is derived from specific induction of Akt2 expression and its activation. As a result of Akt2 upregulation and activation, three downstream signaling pathways are modulated. First, Akt activation enhances the phosphorylation of murine double minute 2 (MDM2) and its ability to negatively regulate p53 stability and activity, thereby attenuating oxidative-stress-induced upregulation of the proapoptotic gene Bcl-2 homologous antagonist killer (Bak) expression. Second, Akt activation leads to increased stabilization of myeloid leukemia cell differentiation protein 1 (MCL-1) through the inhibition of glycogen synthase kinase 3 beta (GSK-3β) activity. Finally, Akt activation promotes phosphorylation and degradation of FOXO3A, downregulating expression of the proapoptotic regulator, Bim. Thus, in responding to oxidative insult, Akt2 in HLECs becomes induced and activated, which regulates multiple downstream signaling transduction pathways to antagonize the induced apoptosis. Our results lead to the conclusion that Akt2 is an essential kinase that antagonizes oxidative stress damage.
Materials and Methods
Animals
Mice used in this study were handled in compliance with the Guide for the Care and Use of Laboratory Animals (National Academy Press). Four-week-old mice and 14.5-, 17.5-, and 19.5-day-old embryonic mice were obtained from UNMC and Hunan Normal University animal facilities. A total of 36 four-week mice were used for collection of the corneal, retinal, lens epithelium, and lens fiber cells. These samples were used for extraction of total RNA and proteins.
Antibodies
All primary and secondary antibodies for Western blotting were used at a concentration of 1:1000 unless otherwise stated. The following antibodies were used: phospho-Akt (9272 & 4691), Akt2 (2964), Akt3 (4059), phospho-Akt at Ser-473 (9271 & 4060), phospho-MDM2 at Ser-166 (3521), phospho-p53 at Ser15 (9286), total p53 (2524), phospho-GSK-3β at Ser-9 (9336), total GSK-3β (9315), FOXO1 (9462), FOXO3A (9467), phospho-FOXO1/phospho-FOXO3A at Thr-24/Thr-32 (9464), Mcl-1 (4572), and Bim (2819) from Cell Signaling Inc.; Akt1 (sc-5298) from Santa Cruz Biotech.; MDM2 (M4308) from Sigma; and Bak (06-536) from Upstate. The HRP-conjugated secondary antibodies were purchased from Amersham.
Cell culture
HLECs were cultured in monolayer at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Sigma) supplemented with 10% FBS, 2 mM L-glutamine, and 1% penicillin and streptomycin as previously described (46, 47).
Silencing of Akt1, Akt2, and Bak
Stable knockdown of Akt1, Akt2, Akt1/2, and Bak was conducted as previously described (41, 47). Human Akt1 small interference RNA (shRNA) plasmid (sc-29195-SH), Akt2 shRNA plasmid (sc-29197-SH) and Bak shRNA (sc-29786-SH) were purchased from Santa Cruz Biotechnology. The HLECs stably transfected with Akt1, Akt2, or Akt1/2 or mock shRNA plasmids were screened under 0.25 μg/ml puromycin (Sigma) for 4 weeks. After screening, individual stable clones were grown in the same medium containing 0.375 μg/ml puromycin. For experiments described in Figure 7, the mock shRNA plasmid knockdown clone was further transfected with either mock shRNA plasmid or Bak shRNA plasmid to transiently knock down Bak. The knockdown of Akt1, Akt2, Akt1/2, or Bak was confirmed by Western blot analysis.
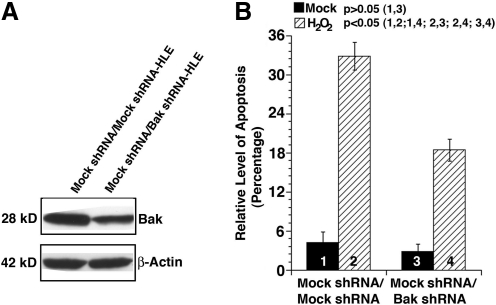
FIG. 7.
Knockdown of Bak significantly attenuates oxidative-stress-induced apoptosis. (A) Western blot analysis to confirm the knockdown of Bak in mock shRNA-transfected HLECs. The mock shRNA-transfected HLEC stable clone was transfected again with either mock shRNA plasmid or Bak shRNA plasmid for 24 h. Afterward, these cells were harvested for determination of the endogenous Bak level or used for analysis of oxidative-stress-induced apoptosis (B). Note that the Bak shRNA plasmid-transfected cells displayed more than 60% knockdown in Bak level. (B) Knockdown of Bak significantly attenuates oxidative-stress-induced apoptosis. Apoptosis was analyzed using cell flow cytometry as described in Figure 3. Data were analyzed with the SNK test.
Preparation of expression constructs
The human Bim and MCL-1 cDNAs were obtained through polymerase chain reaction (PCR) cloning. The total RNAs isolated from HLECs were used for reverse transcription (RT) as described before (31). The cDNA was then used for amplification of the Bim and MCL-1 cDNAs using the following oligos: For Bim, 5′-GCGAATTCATGGCAAA-GCAACCTT3′ (forward, F) and 5′-CCGTCGACTCAATGCATTCTCCACCTGGC-3′ (reverse, R). For MCL-1, 5′-GCGAATTCATGTTTGGCCTCAAA-3′ (F) and 5′-GCGTCGACCTATCTTAT-TAGATA-3′ (R). Both Bim and MCL-1 fragments were cloned into pCI-neo vector at EcoRI (5′) and SalI (3′) sites. The dominant negative mutants for MDM2, GSK-3β and FOXO3A, the expression constructs for Akt1 and Akt2 were purchased from Addgene, Inc.
Stable and transient transfection
The pCI-neo, pCI-Bim, pCI-MCL-1, pCDNA3-neo, pCDNA3-Akt1, and pcDNA3-Akt2 were amplified in DH-5α and purified by QIAGEN kits as described before (31). After treatment with RNase and extracted by phenol/chloroform, the pCI-neo and pCI-MCL-1 were separately transfected into the mock shRNA knockdown stable clone and the pCI-neo and pCI-Bim were separately transfected into the Akt1 shRNA knockdown stable clone. The pcDNA3 and pcDNA3-Akt1 were transfected into Akt1 shRNA knockdown stable clone. The pcDNA3 and pcDNA3-Akt2 were transfected into HLE parent cells. The transfected cells were either used for experimentation or subjected to G418 (400 μg/ml) selection for 4–6 weeks. In the latter case, the individual clones for the stably transfected cell lines were established and confirmed with RT-PCR and western blot analysis.
Oxidative stress treatment of HLECs
HLECs grown to 100% confluence were treated with a stable concentration of 50 μM H2O2 generated from glucose and glucose oxidase as described before (28).
Cell flow cytometry analysis
The apoptosis rate of the pCI-neo, pCI-Bim, pCI-MCL-1- pcDNA3-neo, pcDNA3-Akt1, and pcDNA3-Akt2-transfected cells or stable clones treated with mock or 50 μM H2O2 was determined using an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit as previously described (41). Briefly, the treated cells were trypsinized, washed twice with ice-cold Dulbecco's phosphate-buffered saline, and incubated at room temperature for 15 min in the dark with Annexin V-conjugated FITC and propidium iodide in the binding buffer provided. Stained cells were analyzed by flow cytometry using the FACSCalibur (BD Biosciences).
MTT assay for cell viability
The transiently transfected cells or stable clones were grown to 95% confluence and then exposed to 50 μM H2O2 generated from a stable system containing 50 μU glucose oxidase and 9.5 μM glucose within 2 h of treatment. After treatment, the medium was removed, and 100 μl MTT [3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazo-lium bromide)] solution (100 μg/ml, diluted in PBS) was added into each cell culture well and incubated for 3 h. At the end of incubation, the cell suspension was centrifuged to collect the pellet and 500 μl DMSO was added to dissolve the crystal with a 5 min gentle shake. After the crystal was dissolved, the absorbance of each well at OD570nm was determined in a Beckman Spectrophotometer. The relative cell death was compared between the stably transfected mock and Akt knockdown clones.
Preparation of total RNAs from mouse eye tissues
Mice were euthanatized by CO2 inhalation and the intact eyeballs were removed. Afterward, four different eye tissues, retina, lens fiber, lens epithelium, and cornea, were carefully dissected by a posterior approach (28), and transferred into a prechilled Eppendorf tube containing 500 μl RNA extraction buffer (Trizol #15596-026; Gibco). The dissected tissues were homogenized on ice with an Eppendorf tube micropestle (Brinkmann Instruments, Inc.). The subsequent procedures of RNA extraction were the same as previously described (46).
RT-linked PCR
RT was performed using a kit from Invitrogen (#18085-019; Invitrogen) as previously described (46). Briefly, 2 μg of total RNA were used in a 25 μl reaction. The primers and PCR conditions are listed in Table 1. PCR products were separated by agarose gel (2%) electrophoresis and photographed under UV illumination. The following oligos were used: for Akt1 (607 bp), 5′-ACTCATTCCAGACCCACGAC-3′ (forward, F) and 5′-ATACACATCCTGCCA-CACGA-3′ (reverse, R); for Akt2 (249 bp), 5′-GAGCATAGATTCTTCCTCAGCATC-3′ (F) and 5′-GTGGTGGCAGAGGGCTGCTCACTC-3′ (R); for Akt3 (388 bp), 5′-GACTGGTGGGGCTTAGGTGTTG-3′ (F) and 5′-TGCCGTCGTCGTCATACTTTTC-3′ (R); and for β-actin (242 bp), 5′-CACTGCCGCATCCTCTTCCT-3′ (F) and 5′-ATGCCTGGGTACATGGTGGT-3′ (R).
Table 1.
Reverse Transcription–Polymerase Chain Reaction Conditions and Primers
| Oligo primer | Oligo primer sequence | Cycle no. | Ta (°C) | PCR product (bp) |
|---|---|---|---|---|
| AKT1 (+) | 5′-ACTCATTCCAGACCCACGAC-3′ | 32 | 59 | 607 |
| AKT1 (−) | 5′-ATACACATCCTGCCACACGA-3′ | |||
| AKT2 (+) | 5′-GAGCATAGATTCTTCCTCAGCATC-3′ | 5 | 64 | 249 |
| AKT2 (−) | 5′-GTGGTGGCAGAGGGCTGCTCACTC-3′ | 27 | 55 | |
| AKT3 (+) | 5′-GACTGGTGGGGCTTAGGTGTTG-3′ | 32 | 55 | 388 |
| AKT3 (−) | 5′-TGCCGTCGTCGTCATACTTTTC-3′ | |||
| β-actin (+) | 5′-CACTGCCGCATCCTCTTCCT-3′ | 25 | 57 | 242 |
| β-actin (−) | 5′-ATGCCTGGGTACATGGTGGT-3′ |
Preparation of total proteins from mouse eye tissues
The dissected mouse eye tissues, including retina, lens fiber, lens epithelium, and cornea, were separately transferred into prechilled Eppendorf tubes containing 200 μl of protein extraction buffer (50 mM Tris-HCl, pH 7.0; 0.1% β-mercaptoethanol; 0.1 mM EDTA, 0.1 mM EGTA, 2 mM leupeptin, 1 mM PMSF, 1 mM benzamidine-HCl, 2 mM DTT, 0.5% Triton X-100), and homogenized on ice with Eppendorf tube micropestles as described (28).
Western blot analysis
Western blot analysis was conducted as previously described (31, 47). Briefly, 100 μg of total proteins from four ocular tissues was separated by 10% sodium dodecylsulfate–polyacrylamide gel electrophoresis and then transferred into supported nitrocellulose membranes (Gibco BRL). Membranes were blocked with 5% nonfat dried milk in TBST (10 mM Tris, pH 8.0; 150 mM NaCl, 0.1% Tween-20) at room temperature then incubated with appropriate antibody in 5% nonfat dried milk or BSA prepared in TBST over night at 4°C with mild shaking. After three 15 min washes with TBST, immunoblots were incubated with the relevant HRP-conjugated secondary antibody at a dilution of 1:1000 for 45 min at room temperature followed by two washes with TBS-T and then another two washes with TBS (10 mM Tris, pH 8.0; 150 mM NaCl) (15 min each). Proteins were observed with an enhanced chemiluminescence detection kit from Amersham.
Quantitation and statistical analysis
After exposure, the X-ray films were analyzed with the Automated Digitizing System from the Silk Scientific Corporation as previously described (41). The relative expression levels (fold) were calculated by dividing the averaged total pixels (from three experiments) for each band under investigation by the averaged total pixels for the corresponding β-actin band.
Statistical analysis
The statistical significance (p<0.05) of the comparison among multiple samples was determined by a post hoc analysis of variance (Post hoc ANOVA), the Student-Newman-Keuls test, by SPSS version 17.0 (SPSS, Inc.).
Results
Members of the Akt family are differentially expressed in the ocular tissues
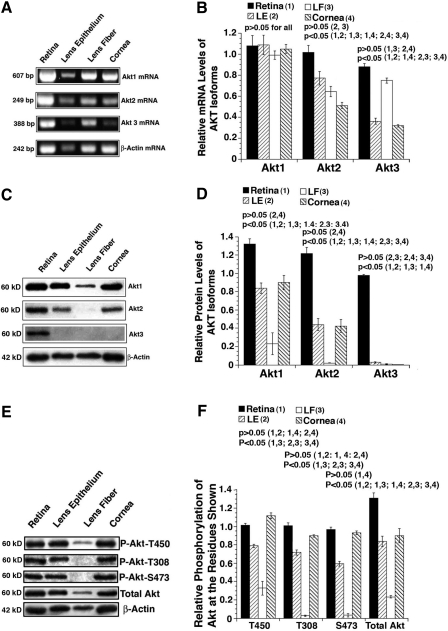
To explore the possibly differential functions of Akt1, Akt2, and Akt3 in the ocular tissues, we first analyzed their expression patterns in retina, lens epithelia, lens fiber, and cornea. As shown in Figure 1A and B, RT-PCR analysis revealed that the mRNA for Akt1 was strongly expressed in all four tissues of the mouse eye. For Akt2 mRNA, the retina again displayed the strongest level of expression, a level similar to that for Akt1. A gradually reduced level of Akt2 mRNA was observed from the lens epithelia, to the lens fiber, to the cornea (Fig. 1A, B). The Akt3 mRNA level in both the retina and lens fiber was slightly decreased compared with Akt1 mRNA levels in these tissues. In contrast, both lens epithelia and cornea had more than 50% decrease in Akt3 mRNA level in comparison to Akt1 mRNA level (Fig. 1A, B). At the protein level, the retina showed a similar expression pattern of Akt1, Akt2, and Akt3 as the mRNAs (Fig. 1C, D). In contrast, both the lens epithelia and cornea expressed Akt and Akt2 but not Akt3, and the lens fiber only expressed Akt1 (Fig. 1C, D). While Akt1 levels are dominant over Akt2 levels in both lens epithelia and cornea, the levels of the two Akt isoforms were significantly lower in lens epithelia and cornea than in the retina (Fig. 1C, D). Akt1 in the lens fiber was distinctly lower than that in other 3 ocular tissues (Fig. 1C, D).
FIG. 1.
Expression and activity of members of the Akt kinase family. (A) Reverse transcription–polymerase chain reaction to detect the mRNA levels for Akt1, Akt2, and Akt3 in the retina, lens epithelia, lens fiber, and cornea of the mouse eye. As an internal control, a β-actin DNA band of 242-bp was also amplified. The primers used in the present studies are listed in Table 1. The primers for Akt1, Akt2, Akt3, and β-actin were added to the reactions at the same time. (B) Quantitative results of the mRNA expression levels for Akt1, Akt2, and Akt3 in the retina, lens epithelia, lens fiber, and cornea of the mouse eye. Note that Akt1 is highly expressed in all four ocular tissues. (C) Western blot analysis of the protein levels of Akt1, Akt2, and Akt3 in the retina, lens epithelia, lens fiber, and cornea of the mouse eye (see Materials and Methods section for the details). (D) Quantitative results of the protein expression levels of Akt1, Akt2, and Akt3 in the retina, lens epithelia, lens fiber, and cornea of the mouse eye. (E) Western blot analysis of total Akt1 and Akt1 phosphorylation status at T450, T308, and S473 in the retina, lens epithelia, lens fiber, and cornea of the mouse eye. (F) Quantitative results of total Akt1 and Akt1 phosphorylation status at T450, T308, and S473 in the retina, lens epithelia, lens fiber, and cornea of the mouse eye. Data were analyzed with the Student-Newman-Keuls (SNK) test.
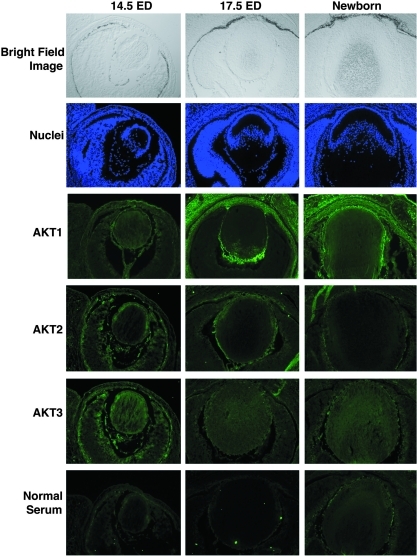
Next, we examined the activity of Akt kinase in the adult ocular tissues. As shown in Figure 1E and F, both the retina and cornea display similar levels of phosphorylation at T450, T308, and S473. The lens epithelia had a reduction in total Akt activity of about 40%. In contrast, the fiber cells had little Akt activity, though the residue at T450 of Akt was phosphorylated to some degree (Fig. 1E, F). To explore the possible functions of the Akt family members during mouse eye development, we conducted immunohistochemistry analysis. As shown in Figure 2, from 14.5 ED to 19.5 ED (newborn mouse), expression of Akt1 was gradually increased in both lens epithelium and cornea but decreased in retina and lens fiber cells. In contrast, both Akt2 and Akt3 were gradually decreased in all the ocular tissues. Thus, during mouse development, all three isoforms of Akt are actively expressed. In contrast, in the adult mouse, whereas the lens and cornea have Akt1 and Akt2 as the major Akt family members, the retina has all three isoforms of Akt expressed.
FIG. 2.
Expression of members of the Akt kinase family during mouse eye development. The mouse eye tissues were fixed and sectioned as described in the Materials and Methods section. The sections were washed and blocked with 500 μl of 5% normal goat serum (Sigma) in PBS for 1 h at room temperature and then incubated overnight in 400 μl of diluted antibodies (1:100) in a humidified chamber at 4°C. The sections were then washed followed by an incubation in 400 μl of secondary antibodies linked to fluorescein isothiocyanate from Vector Laboratories at 1:1000 dilution in blocking solution for 1 h in the absence of visible light. After incubation, sections were washed and then observed under a Zeiss confocal fluorescence microscope. For negative controls, the sections were treated in the same way except that the primary antibody was replaced by normal serum bright field image: morphology of the eye section at 14.5 ED, 17.5 ED and newborn; nuclei: Hoechst staining of the nuclei in the eye section at 14.5 ED, 17.5 ED and newborn. Akt1: the signal for total Akt1 in the eye section at 14.5 ED, 17.5 ED and newborn. Akt2: the signal for total Akt2 in the eye section at 14.5 ED, 17.5 ED and newborn. Akt3: the signal for total Akt3 in the eye section at 14.5 ED, 17.5 ED and newborn. Normal serum: negative controls. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Knockdown of Akt1 attenuates oxidative-stress-induced apoptosis of HLECs
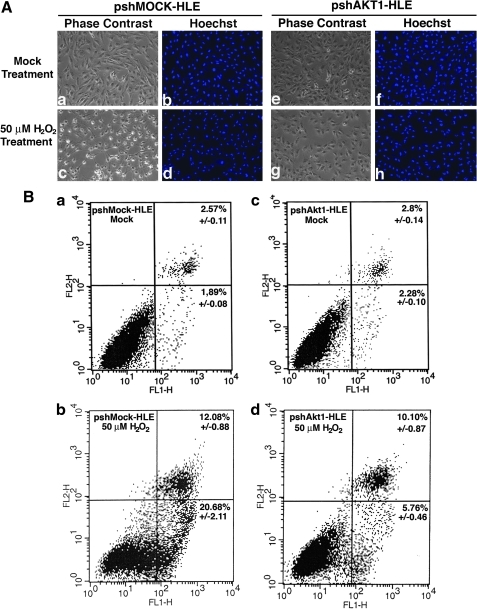
Since Akt1 is the predominant isoform in all three ocular tissues, to evaluate the function of Akt1 in the cellular response to oxidative stress, we have transfected HLECs with Akt1 shRNA expression vector or mock shRNA vector. The stable clones were screened in the medium containing 0.25 μg/ml puromycin, and then cultured in the medium containing 0.375 μg/ml puromycin. The knockdown of Akt1 was confirmed with western blot analysis (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertonline.com/ars). When the stable Akt1 knockdown or mock knockdown clones of HLECs were treated with a stable level of 50 μM H2O2 generated from glucose oxidation (28) for 2 h, both morphological examination (Fig. 3A-c and 3A-g) and cell flow cytometry analysis (Fig. 3B-b and 3B-d) revealed that over 50% more apoptotic cells were observed in mock knockdown cells than in Akt1 knockdown cells (also see Supplementary Fig. S1B). To confirm that the observed differential apoptosis is derived from Akt1 knockdown, we overexpressed Akt1 in Akt1 knockdown cells, as shown in Supplementary Fig. S1C and S1D, overexpression of Akt1 significantly restored the sensitivity to oxidative-stress-induced apoptosis. The apoptotic nature was further confirmed by Hoechst staining of the condensed nuclei (Figs. 3A-d and 3A-h). Thus, to our surprise, knockdown of Akt1 led to enhanced resistance to oxidative-stress-induced apoptosis.
FIG. 3.
Analysis of oxidative-stress-induced apoptosis in human lens epithelial cells (HLECs). The mock (A-a, b, c, d and B-a, b) and Akt1 (A-e, f, g, h; B-c, d) small interference RNA (shRNA) plasmid-transfected cells were treated under mock condition (A-a, b, e, f, and B-a, b) or with 50 μM H2O2 (A-c, d, g, h, and B-c, d) for 2 h and then used for analysis of apoptosis by Hoechst staining (A) or cell flow cytometry (B). (A) Morphological changes recorded by phase-contrast microscopy (A-a, c, e and g) or fluorescence microscopy (A-b, d, f, and h). (B) Cell flow cytometry results as described before (41). Note that in Akt1 knockdown cells, H2O2-induced apoptosis was significantly attenuated in comparison with that in the mock shRNA plasmid-transfected cells. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Akt1 knockdown does not change the expression levels of the antioxidative enzymes
A recent study (37) reported that knockout of Akt1 and Akt2 led to upregulation of the antioxidative stress enzymes in mouse embryonic fibroblast cells, and thus protected them from oxidative-stress-induced apoptosis. To determine if the Akt1 knockdown also enhances the expression levels of the antioxidative enzymes, we conducted Western blot analysis of the MnSOD, catalase, glutathione peroxidase, glutathione reductase, and glutathione transferase levels in mock and Akt1 knockdown cells. As shown in Supplementary Figure S2, knockdown of Akt1 did not significantly change the expression levels of these enzymes. Thus, different mechanism exists to explain how knockdown of Akt1 lead to enhanced resistance against oxidative-stress-induced apoptosis.
Knockdown of Akt1 led to enhanced total Akt activity in response to oxidative stress
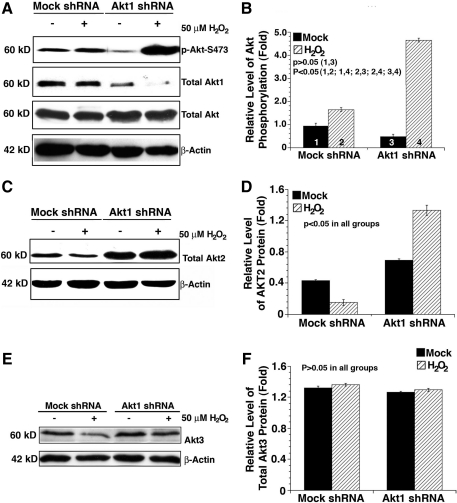
To explore the possible mechanisms mediating the enhancement of the antiapoptotic ability in the Akt1 knockdown cells under oxidative stress insult, we examined the expression levels of total Akt and Akt1, and also the phosphorylation level of Akt at S473. As shown in Figure 4A, in the Akt1 knockdown cells, where Akt1 is knocked down more than 80%, the total Akt remained unchanged and the Akt activity (S473 phosphorylation [Supplementary Fig. S3]) was significantly upregulated under oxidative stress insult. In contrast, in the mock knockdown cells, hydrogen peroxide caused much less upregulation in Akt activity though the total Akt and Akt1 displayed little change (Fig. 4A, B). Thus, oxidative stress led to differential Akt activity in mock and Akt1 knockdown cells.
FIG. 4.
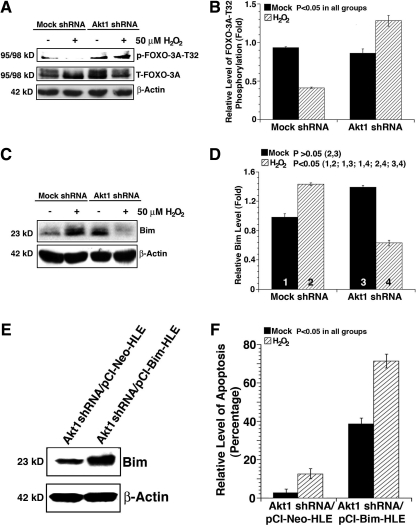
Oxidative-stress-induced changes in the total akt activity and also expression levels of Akt1, Akt2, and Akt3. (A) Oxidative stress differentially regulates Akt activity in the mock and Akt1 shRNA plasmid-transfected HLECs. Both mock and Akt1 shRNA plasmid-transfected HLECs were either mocked treated (by H2O) or treated with 50 μM H2O2 for 2 h. After treatment, the cells were harvested for analysis of total Akt activity, total Akt protein, total Akt1 protein, and β-actin (as a loading reference) using the method as described in the Materials and Methods section. Note that in Akt1 knockdown cells, Akt activity was significantly upregulated. (B) Quantitative results of the Akt activity as reflected by S-473 phosphorylation against total Akt. (C) Oxidative stress differentially regulates the Akt2 level in the mock and Akt1 shRNA plasmid-transfected HLECs. Both mock and Akt1 shRNA plasmid-transfected HLECs were treated as described in (A). Note that in Akt1 knockdown cells, the Akt2 protein level was significantly upregulated in both mock- and H2O2-treated HLECs. (D) Quantitative results of the Akt2 level. (E) Oxidative stress does not significantly change the Akt3 level in the mock and Akt1 shRNA plasmid-transfected HLECs. Both mock and Akt1 shRNA plasmid-transfected HLECs were treated as described in (A). (F) Quantitative results of the Akt3 level. Data were analyzed with analyzed with the SNK test.
Knockdown of Akt1 promotes upregulation of Akt2 but not Akt3 that accounts for enhanced total Akt activity in response to oxidative stress
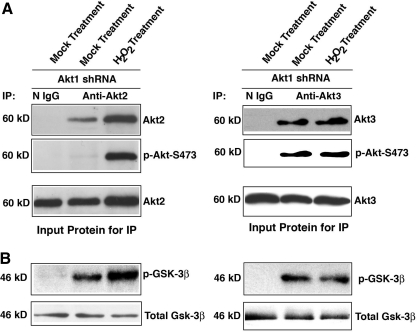
To understand why the Akt activity became increased in the Akt1 knockdown cells in response to oxidative stress, we examined the expression level of Akt2 and Akt3. As shown in Figure 4C and D, whereas Akt2 became downregulated in mock knockdown cells responding to oxidative stress, it was significantly upregulated in Akt1 knockdown cells. Compared with Akt2, Akt3 was slightly upregulated in both mock and Akt1 knockdown cells (Fig. 4E, F). To further confirm that the upregulated Akt activity was derived from Akt2 but not Akt3, we conducted immunoprecipitation-linked kinase assays using purified GSK-3β as substrate. As shown in Figure 5, oxidative stress induced significant increase in Akt2 activity (Fig. 5A) but very little in Akt3 activity (Fig. 5B) as reflected by the differential levels of the phosphorylation in GSK-3β. Thus, knockdown of Akt1 lead to a positive feedback regulation of Akt2 (Fig. 4).
FIG. 5.
Immunoprecipitation-linked kinase assays to demonstrate that knockdown of Akt1 leads to upregulation of Akt2 expression and activity but not Akt3. (A) Both mock- and H2O2-treated Akt1 knockdown cells were harvested for extraction of total proteins, which were used for immunoprecipitation with anti-Akt2 or Akt3 specific antibodies (Cell Signaling, Inc.). (B) The precipitated Akt2 and Akt3 are then used for kinase assays with purified glycogen synthase kinase 3 beta (GSK-3β) as a substrate. After the kinase reaction, the phosphorylated GSK-3β was analyzed by Western blot analysis. Note that under H2O2 treatment, Akt2 was significantly upregulated, but Akt3 was not changed as reflected by the level of phosphorylation in GSK-3β.
Enhanced Akt activity upregulates MDM2 phosphorylation and attenuates p53 phosphorylation at S15 and Bak expression
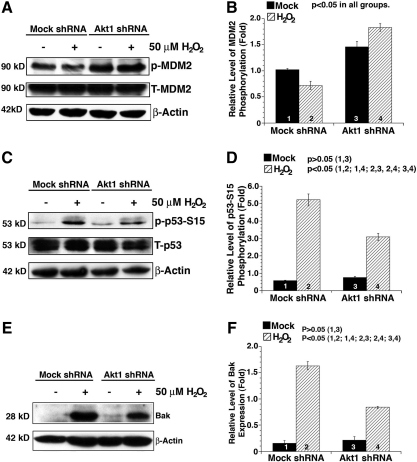
Since distinct differential Akt activity and apoptosis were observed in Akt1 and mock knockdown cells in response to oxidative stress, we speculated that the enhanced Akt activity attenuates p53-dependent apoptosis through regulation of the MDM2 phosphorylation in HLECs. As expected, in the mock knockdown cells, the phosphorylation of MDM2 at S166 was downregulated by H2O2 treatment; in contrast, Akt1 knockdown alone caused significant upregulation of the MDM2 phosphorylation at Ser166 likely due to upregulation of Akt2 (Fig. 6A, B). Moreover, hydrogen peroxide treatment further enhanced this phosphorylation. Phosphorylation of S166 in MDM2 enhances its interaction with p53 and thus promotes degradation of the later (33, 50). As a result of changed MDM2 phosphorylation, we observed significant difference in the p53 phosphorylation at S15 after H2O2 treatment (Fig. 6C, D), which leads to differential upregulation in the expression of the recently identified p53 target gene, Bak (41), but not Bid and Bad (Supplementary Fig. S4). To demonstrate that MDM2 directly regulates p53 in this cell line, we expressed the dominant negative mutant MDM2 in Akt1 knockdown cells, as shown in Supplementary Figure S5, mutant MDM2 interferes the interaction between MDM2 and p53, leading to enhanced p53 phosphorylation at S15. To demonstrate that Bak plays a key role in oxidative-stress-induced apoptosis, we knocked down Bak in the mock shRNA plasmid-transfected HLEC clone. As shown in Figure 7, cell flow cytometry assay revealed that knockdown of Bak attenuated about 40% of the induced apoptosis by H2O2. Thus, knockdown of Akt1 leads to Akt2 upregulation, which promotes survival through negative regulation of the MDM2-p53-Bak pathway.
FIG. 6.
Oxidative stress differentially regulates the murine double minute 2 (MDM2)-P53-Bcl-2 homologous antagonist killer (Bak) pathway in mock and Akt1 knockdown HLECs. (A) Oxidative stress differentially regulates MDM2 phosphorylation in the mock and Akt1 shRNA plasmid-transfected HLECs. Both mock and Akt1 shRNA plasmid-transfected HLECs were either mocked treated (by H2O) or treated with 50 μM H2O2 for 2 h. After treatment, the cells were harvested for analysis of total MDM2 and MDM2 phosphorylation at S166 and β-actin (as a loading reference) using the method as described in the Materials and Methods section. Note that in Akt1 knockdown cells, MDM2 phosphorylation at S166 was significantly upregulated. (B) Quantitative results of the MDM2 phosphorylation at S166. (C) Oxidative stress differentially regulates p53 phosphorylation at S15 in the mock and Akt1 shRNA plasmid-transfected HLECs. Both mock and Akt1 shRNA plasmid-transfected HLECs were treated as described in (A). Note that in Akt1 knockdown cells, p53 phosphorylation level at S15 was much less upregulated in responding H2O2 treatment. (D) Quantitative results of the p53 phosphorylation at S15. (E) Oxidative stress differentially regulates Bak level in the mock and Akt1 shRNA plasmid-transfected HLECs. Both mock and Akt1 shRNA plasmid-transfected HLECs were treated as described in (A). Note that in Akt1 knockdown cells, Bak expression was much less upregulated under H2O2 treatment. Both mock and Akt1 shRNA plasmid-transfected HLECs were treated as described in (A). (F) Quantitative results of the Bak level. Data were analyzed with the SNK test.
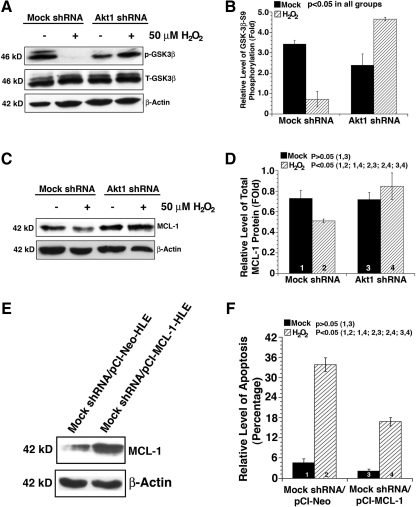
Enhanced Akt activity downregulates GSK-3β activity to augment MCL-1 stability
Because Akt regulates multiple targets, we next examined the phosphorylation (activity) status of GSK-3β, an important apoptosis regulator through modulation of MCL-1 function. As shown in Figure 8A and B, in mock knockdown cells, hydrogen peroxide treatment caused the disappearance of GSK-3β phosphorylation at S9, and thus an increase in its enzyme activity. In contrast, in Akt1 knockdown cells, GSK-3β phosphorylation was upregulated about 50% compared with mock-treated Akt1 knockdown cells. We next examined the expression level of the MCL-1, a GSK-3β substrate. As shown in Figure 8C and D, in mock knockdown cells, MCL-1 was downregulated by hydrogen peroxide. In contrast, in Akt1 knockdown cells, MCL-1 stability was obviously increased. To confirm that GSK-3β directly regulates MCL-1 in this cell line, we introduced either the vector or the GSK kinase dead expression construct into Akt1 knockdown HLECs. As shown in Supplementary Figure S6, expression of the kinase dead GSK-3β promotes MCL-1 stability. To demonstrate that upregulation of MCL-1 could attenuate oxidative-stress-induced apoptosis, we have prepared the MCL-1 expression construct and overexpressed MCL-1 in mock shRNA plasmid-transfected HLEC clone (Fig. 8E). As shown in Figure 8F, overexpression of MCL-1 significantly attenuated oxidative-stress-induced apoptosis in mock shRNA-transfected cells. Together, these results demonstrate that knockdown of Akt1 causes upregulated Akt activity, which promotes survival also via regulation GSK-3β-MCL-1 pathway.
FIG. 8.
Oxidative-stress differentially regulates the GSK-3β-myeloid leukemia cell differentiation protein 1 (MCL-1) pathway in mocked and Akt1 knockdown HLECs. (A) Oxidative stress differentially regulates GSK-3β phosphorylation in the mock and Akt1 shRNA plasmid-transfected HLECs. Both mock and Akt1 shRNA plasmid-transfected HLECs were either mocked treated (by H2O) or treated with 50 μM H2O2 for 2 h. After treatment, the cells were harvested for analysis of total GSK-3β and GSK-3β phosphorylation at S9, and β-actin (as a loading reference) using the method as described in the Materials and Methods section. Note that in Akt1 knockdown cells, GSK-3β phosphorylation at S9 was significantly upregulated. (B) Quantitative results of the GSK-3β phosphorylation at S9. (C) Oxidative stress differentially regulates the MCL-1 level in the mock and Akt1 shRNA plasmid-transfected HLECs. Both mock and Akt1 shRNA plasmid-transfected HLECs were treated as described in (A). Note that in Akt1 knockdown cells, MCL-1 level was significantly upregulated in responding H2O2 treatment. (D) Quantitative results of the MCL-1 level. Overexpression of MCL-1 (E) attenuates oxidative-stress-induced apoptosis in the mock shRNA plasmid-transfected HLECs (F). Data were analyzed with the SNK test.
Enhanced Akt activity upregulates the phosphorylation status of FOXO3A to downregulate Bim
FOXO3A and FOXO1 transcriptional factors are Akt targets activating many proapoptotic genes such as puma and bim. The phosphorylation of these transcription factors by Akt induces their nuclear export and transactivity suppression. As shown in Figure 9A and B, hydrogen peroxide significantly downregulates FOXO3A phosphorylation at T32 in mock knockdown cells but significantly upregulates its phosphorylation in Akt1 knockdown cells. In comparison, the phosphorylation status of FOXO1A at T24 did not show such pattern (Supplementary Fig. S7). As a result of this distinctly differential phosphorylation of FOXO3A, expression of Bim, a proapoptotic member of the Bcl-2 family, displayed a completely opposite pattern. In the mock knockdown cells, H2O2 induced significant upregulation of Bim (Fig. 9C). In contrast, in Akt1 knockdown cells, Bim was significantly downregulated by H2O2 treatment (Fig. 9D). Knockdown of Akt1 led to upregulation of the Bim basic level, which was consistent with the slightly increased background apoptosis in the Akt1 knockdown cells (Fig. 3B-c vs. Fig. 3B-a). To demonstrate that FOXO3A directly controls Bim in HLE, we expressed either a vector or a FOXO3A T32A mutant in HLECs. As shown in Supplementary Figure S8, expression of the FOXO3A T32A (which will stay in nucleus constantly) mutant significantly enhanced the expression of Bim. To confirm that Bim is implicated in oxidative-stress-induced apoptosis, we overexpressed Bim in the Akt1 knockdown HLECs. As shown in Figure 9E and F, overexpression of Bim dramatically enhances basic and oxidative-stress-induced apoptosis. Together, these results demonstrate that knockdown of Akt1 leads to upregulated Akt activity, which promotes survival via regulation FOXO3A-Bim pathway.
FIG. 9.
Oxidative-stress differentially regulates the FOXO3A-Bim pathway in mocked and Akt1 knockdown HLECs. (A) Oxidative stress differentially regulates FOXO3A phosphorylation in the mock and Akt1 shRNA plasmid-transfected HLECs. Both mock and Akt1 shRNA plasmid-transfected HLECs were either mocked treated (by H2O) or treated with 50 μM H2O2 for 2 h. After treatment, the cells were harvested for analysis of total FOXO3A and FOXO3A phosphorylation at T32, and β-actin (as a loading reference) using the method as described in the Materials and Methods section. Note that in Akt1 knockdown cells, FOXO3A phosphorylation at T32 was significantly upregulated. (B) Quantitative results of the FOXO3A phosphorylation at T32. (C) Oxidative stress differentially regulates Bim level in the mock and Akt1 shRNA plasmid-transfected HLECs. Both mock and Akt1 shRNA plasmid-transfected HLECs were treated as described in (A). Note that in Akt1 knockdown cells, the Bim level was significantly downregulated in responding to H2O2 treatment. (D) Quantitative results of the Bim level. Overexpression of Bim (E) significantly enhances oxidative-stress-induced apoptosis in the Akt1 shRNA plasmid-transfected HLECs (F). Data were analyzed the SNK test.
Knockdown of Akt2 enhances oxidative-stress-induced apoptosis
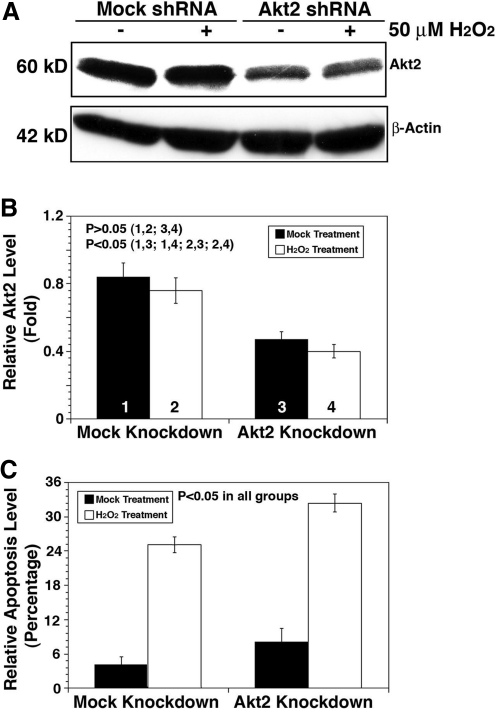
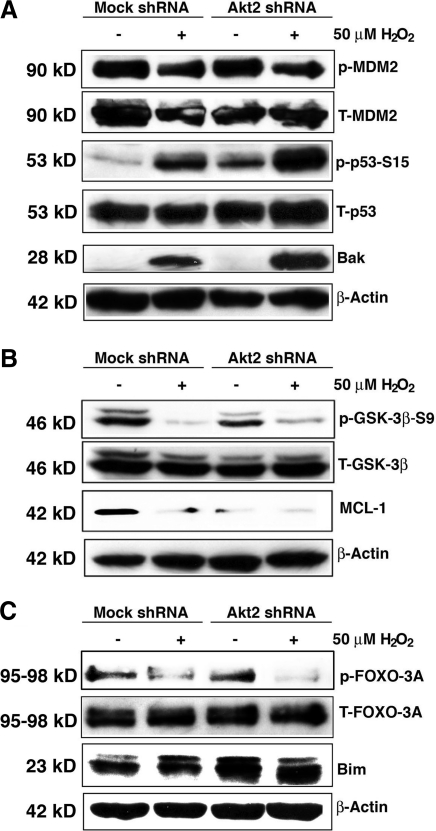
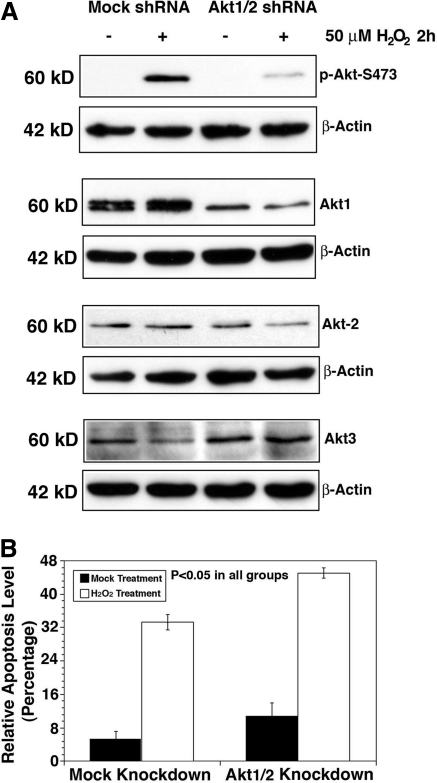
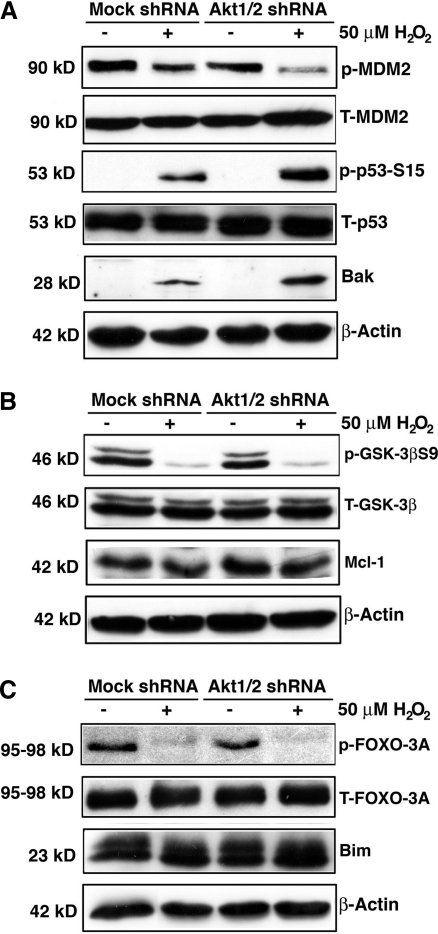
To further confirm that it is Akt2 upregulation that plays a key role in resisting oxidative-stress-induced apoptosis under Akt1 knockdown, we conducted direct knockdown of Akt2 in HLECs. As shown in Figure 10, knockdown of Akt2 lead to significant increase in oxidative-stress-induced apoptosis compared with mock knockdown cells. Under this condition, both Akt1 and Akt3 are not significantly upregulated (Supplementary Fig. S9). When Akt2 is knocked down, phosphorylation of MDM2 is attenuated, leading to p53 hyperphosphorylation and enhanced Bak expression (Fig. 11A). Phosphorylation of GSK-3β is also attenuated, which lead to enhanced degradation of MCL-1 (Fig. 11B); moreover, phosphorylation of FOXO3A is attenuated and thus expression of Bim is upregulated (Fig. 11C). When both Akt1 and Akt2 were knocked down, enhanced apoptosis compared to mock knockdown cells was observed (Fig. 12), and similar changes in the above-mentioned molecular cascades were also observed (Fig. 13). To further confirm the function of Akt2 in resisting oxidative-stress-induced apoptosis, we finally overexpressed Akt2 in wild-type HLECs. As shown in Supplementary Fig. S10, overexpression of Akt2 significantly attenuated oxidative-stress-induced apoptosis. Thus, our results confirm that through regulation of the three pathways, Akt2 mediates resistance against oxidative-stress-induced apoptosis.
FIG. 10.
Knockdown of Akt2 leads to enhanced apoptosis induced by hydrogen peroxide. (A) Western blot analysis to confirm the knockdown of Akt2 in HLECs. The HLECs were transfected with either mock or Akt2 shRNA plasmids for 24 h. Afterward, these cells were subjected to selection by puromycin for the establishment of the stable clones, which were harvested for Western blot analysis using anti-Akt2 (A). The quantitative data were shown in (B). The identified mock and Akt2 knockdown clones were used for analysis of the ability against oxidative-stress-induced apoptosis (C). Note that the Akt2 shRNA plasmid-transfected cells displayed significantly more apoptosis. Data were analyzed with the SNK test.
FIG. 11.
Analysis of the MDM-p53-Bak, GSK-3β-MCL-1, and FOXO3A-Bim pathways in mock and Akt2 knockdown cells. (A) Oxidative stress differentially regulates MDM2 phosphorylation in the mock and Akt2 shRNA plasmid-transfected HLECs. Both mock and Akt2 shRNA plasmid-transfected HLECs were either mocked treated (by H2O) or treated with 50 μM H2O2 for 2 h. After treatment, the cells were harvested for analysis of total MDM2 and MDM2 phosphorylation at S166, total p53 and p53 phosphorylation at Ser-15, and β-actin (as a loading reference) using the method as described in the Materials and Methods section. Note that in Akt2 knockdown cells, MDM2 phosphorylation at S166 was significantly downregulated. (B) Oxidative stress differentially regulates GSK-3β phosphorylation in the mock and Akt2 shRNA plasmid-transfected HLECs. Both mock and Akt2 shRNA plasmid-transfected HLECs were either mocked treated (by H2O) or treated with 50 μM H2O2 for 2 h. After treatment, the cells were harvested for analysis of total GSK-3β and GSK-3β phosphorylation at S9, MCL-1 level and β-actin (as a loading reference) using the method as described in the Materials and Methods section. Note that in Akt2 knockdown cells, GSK-3β phosphorylation at S9 was significantly downregulated. (C) Oxidative stress differentially regulates FOXO3A phosphorylation at T32 in the mock and Akt2 shRNA plasmid-transfected HLECs. Both mock and Akt2 shRNA plasmid-transfected HLECs were either mocked treated (by H2O) or treated with 50 μM H2O2 for 2 h. After treatment, the cells were harvested for analysis of total FOXO3A and FOXO3A phosphorylation at T32, Bim level and β-actin (as a loading reference) using the method as described in the Materials and Methods section. Note that in Akt2 knockdown cells, FOXO3A phosphorylation at T32 was significantly downregulated.
FIG. 12.
Knockdown of Akt1/2 leads to enhanced apoptosis induced by hydrogen peroxide. (A) Western blot analysis to confirm the knockdown of Akt1/2 in HLECs. The HLECs were transfected with either mock or Akt1/2 shRNA plasmids for 24 h. Afterward, these cells were subjected to selection by puromycin for the establishment of the stable clones, which were harvested for Western blot analysis using antiphospho-Akt at S473 or total Akt1, 2, or 3 (A). The identified mock and Akt2 knockdown clones were used for analysis of the ability against oxidative-stress-induced apoptosis (B). Note that the Akt1/2 shRNA plasmid-transfected cells displayed significantly more apoptosis. Data were analyzed with the SNK test.
FIG. 13.
Analysis of the MDM-p53-Bak, GSK-3β-MCL-1, and FOXO3A-Bim pathways in mock and Akt1/2 knockdown cells. (A) Oxidative stress differentially regulates MDM2 phosphorylation in the mock and Akt1/2 shRNA plasmid-transfected HLECs. Both mock and Akt1/2 shRNA plasmid-transfected HLECs were either mocked treated (by H2O) or treated with 50 μM H2O2 for 2 h. After treatment, the cells were harvested for analysis of total MDM2 and MDM2 phosphorylation at S166, total p53 and p53 phosphorylation at Ser-15, and β-actin (as a loading reference) using the method as described in the Materials and Methods section. Note that in Akt1/2 knockdown cells, MDM2 phosphorylation at S166 was significantly downregulated. (B) Oxidative stress differentially regulates GSK-3β phosphorylation in the mock and Akt1/2 shRNA plasmid-transfected HLECs. Both mock and Akt1/2 shRNA plasmid-transfected HLECs were either mocked treated (by H2O) or treated with 50 μM H2O2 for 2 h. After treatment, the cells were harvested for analysis of total GSK-3β and GSK-3β phosphorylation at S9, MCL-1 level and β-actin (as a loading reference) using the method as described in the Materials and Methods section. Note that in Akt1/2 knockdown cells, GSK-3β phosphorylation at S9 was significantly downregulated. (C) Oxidative stress differentially regulates FOXO3A phosphorylation in the mock and Akt1/2 shRNA plasmid-transfected HLECs. Both mock and Akt1/2 shRNA plasmid-transfected HLECs were either mocked treated (by H2O) or treated with 50 μM H2O2 for 2 h. After treatment, the cells were harvested for analysis of total FOXO3A and FOXO3A phosphorylation at T32, Bim level and β-actin (as a loading reference) using the method as described in the Materials and Methods section. Note that in Akt1/2 knockdown cells, FOXO3A phosphorylation at T32 was significantly downregulated.
Discussion
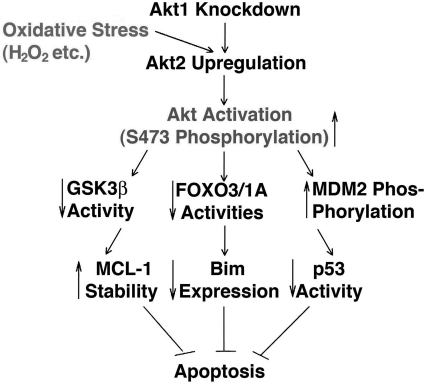
In the present study, we have demonstrated the following: (i) Three members (Akt1, Akt2, and Akt3) of the Akt family are differentially expressed during mouse eye development. In the adult mouse eye, where the retina expresses all three members of the Akt family, both the lens and cornea have Akt1 as the dominant isoform and Akt2 as a minor form but lack Akt3. (ii) When Akt1 is knocked down, the lens epithelial cells display enhanced resistance against H2O2-induced apoptosis; in contrast, when Akt2 or Akt1/2 is knocked down, apoptosis is enhanced. (iii) The enhanced resistance of the HLECs is derived from upregulated expression of Akt2 and also enhanced Akt activity. (iv) The enhanced Akt activity upregulated MDM2 activity to negatively regulate p53-S15 phosphorylation and Bak expression, and knockdown of Bak attenuates oxidative-stress-induced apoptosis. (v) The enhanced Akt activity negatively regulates GSK-3β to promote MCL-1 stability, and overexpression of MCL-1 prevents oxidative-stress-induced apoptosis to some degree. (vi) The enhanced Akt activity negatively regulates the transactivity of the FOXO3A transcription factor to downregulate Bim expression and thus abrogate oxidative-stress-induced apoptosis. Overexpression of Bim in Akt1 knockdown cells significantly enhanced H2O2-induced apoptosis. Together, our results demonstrate that when Akt1, a major isoform of the Akt family is knocked down, Akt2 is upregulated as a positive feedback. Moreover, oxidative stress also enhances Akt2 upregulation and Akt activity which does not change the expression levels of the antioxidative stress enzymes but prevents the induced apoptosis through negative regulation of the three major signaling pathways: MDM2-P53-Bak, GSK-3β-MCL-1, and FOXO3A-Bim (Fig. 14).
FIG. 14.
Schematic diagram to show that knockdown of Akt1 leads to upregulation of Akt2 in response to oxidative stress and its regulation of oxidative-stress-induced apoptosis through three pathways. Under Akt1 knockdown, oxidative stress induces upregulation of Akt2, which contributes to upregulation of the total Akt activity. As a result of the increased Akt activity, the MDM2-p53-Bak pathway was attenuated, the GSK-3β-MCL-1 pathway was enhanced, and the FOXO3A-Bim pathway was almost blocked. Together, these lead to enhanced resistance against oxidative-stress-induced apoptosis.
Akt2 is an important kinase mediating resistance against oxidative-induced apoptosis
In the present study, we have demonstrated that in the mouse eye, where the retina expresses all three isoforms of the Akt family, the ocular lens and cornea express only Akt1 and Akt2 with Akt1 as the dominant isoform. This expression pattern is generally consistent with the previous conclusion that Akt1 and Akt2 are ubiquitously expressed in all tissues, whereas Akt3 is expressed in the testis and neuronal tissues [(6) and references therein].
Recent studies have revealed that the different isoforms of the Akt family display differential functions. First, knockout of individual isoforms has provided critical evidence. Knockout of Akt1 leads to growth retardation and increased apoptosis (11). Akt2 knockout mice show diabetes-mellitus-like syndrome due to an impaired insulin response (18). Akt3 knockout mice show reduced brain size and attenuated neuronal mTOR signaling (6). Our present study provides additional novel evidence.
Oxidative stress is implicated in many human diseases, including both ocular (5, 9, 21, 23, 43) and nonocular diseases (2, 3, 30). In the ocular tissues, oxidative stress is one of the initiating factors causing cataractogenesis (9, 21, 43). It is also actively involved in macular degeneration (5) and other retinal degenerative diseases. Oxidative stress is also a major factor for cardiovascular and neurological diseases (30). It is also implicated in carcinogenesis (2). One of the major mechanisms for oxidative-stress-induced pathology is the induction of apoptosis (3, 19). Under oxidative stress conditions, one of the major responses is the activation of the Akt pathway, which helps to resist the effects of dementia. Although it is well established that oxidative stress activates the Akt pathway in most tissues studied, it remains largely unknown which isoform is mainly involved in this process. In the present study, through knockdown of Akt1 and Akt2, we demonstrate that Akt2 is a major kinase that counteracts oxidative damage. When Akt1 is knocked down, Akt2 is upregulated through positive feedback. Moreover, when Akt1 is knocked down, oxidative stress significantly upregulates Akt2 expression and the total Akt activity [S473 phosphorylation]. At present, we do not know the underlying regulating mechanism; one could speculate that oxidative stress may act two aspects to activate and upregulate Akt2. First, oxidative stress such as hydrogen peroxide may activate other phosphatases or kinases which can directly phosphorylate Akt2 to enhance its activity. It has been shown that oxidative stress leads to the activation of Akt either through PTEN inactivation (27) or p66shc activation (36). In addition, cellular ROS generated by H2O2 can also activate Akt in a PI3K-dependent manner (9, 10). Second, oxidative stress may activate key transcription factors such as hypoxia inducible factor-1 (HIF-1) to upregulate Akt2. In any case, upregulation of Akt2 expression and phosphorylation contributes to enhanced Akt signaling, which through modulation of the three downstream targets, significantly attenuates apoptosis. So, for the first time ever, our results reveal that Akt2 is the major isoform of the Akt kinase family that resists oxidative damage in the ocular lens and that Akt2 is a major player to counteract stress-induced apoptosis. Our results are consistent with a recent study where Akt2 was found to be a major kinase resisting UV-induced apoptosis (25).
AKT regulates different signaling pathways to counteract stress-induced apoptosis
Although our results that knockdown of Akt1 enhances resistance to oxidative-stress-induced apoptosis are similar to those of Nogueira et al. (37), who demonstrated that Akt1 and Akt2 double knockout in mouse cells causes resistance to ROS-mediated apoptosis, the underlying molecular mechanisms are different. In mice, the enhanced antiapoptotic ability is derived from upregulated expression of the two antioxidative stress enzymes: MnSOD and catalase through enhanced transactivity of FOXO3A (37). In our study, we did not observe significant changes in these antioxidative stress enzymes (Supplementary Fig. S2) even in double knockdown of Akt1 and Akt2 (data not shown). In contrast, we found that the Akt1 knockdown cells exhibited enhanced Akt activity due to Akt2 upregulation and activation. The enhanced Akt activity counteracts apoptotic pathways through at least three major pathways.
First, the enhanced Akt activity negatively regulates the MDM2-p53-Bak pathway. The tumor suppressor, p53, regulates transcriptional activation of genes involved in cell cycle arrest, DNA repair, and apoptosis in response to cellular stresses (39). The stability and activity of p53 is negatively controlled through its interaction with MDM2, an E3 ubiquitin ligase, which mediates ubiquitin-mediated degradation of p53 (34). p53 is stabilized by p19ARF, another tumor suppressor that permits the transcriptional activity of p53 by blocking shuttles of Mdm2 from the nucleus to the cytoplasm (40). Since Akt phosphorylates Mdm2 at Serine 186 and Serine 166 (33, 50), Akt-mediated phosphorylation of MDM2 at these residues promotes its translocation from the cytoplasm to the nucleus and its interaction with p300 (20), which abolishes p19ARF antagonism against MDM2, thus triggering p53 nuclear exportation and promoting ubiquitin-mediated degradation of p53. In our results, we show that the enhanced Akt activity upregulates MDM2 phosphorylation at S166, which enhances its interaction with p53 and target the latter into cytoplasm for degradation, leading to downregulated p53 phosphorylation and activity, which attenuates Bak expression. In this way, the upregulated Akt activity negatively regulates apoptosis induced by oxidative stress.
Second, the enhanced Akt activity regulates the GSK-3β-MCL-1 pathway. GSK-3β is a substrate for Akt kinase and its activity can be inhibited by Akt phosphorylation at Ser-9. Recently, accumulating evidence shows that GSK-3β is involved in regulation of apoptosis. GSK-3β was first found to exert pro-apoptotic effects by regulating mitochondrial localization of Bax (29), a key component of the intrinsic apoptotic pathway. More recently, Green and coworkers (32) demonstrated that direct phosphorylation by GSK-3β promotes MCL-1 degradation via the ubiquitin-proteasome pathway and triggers cytochrome c release and activation of the intrinsic death pathway. In our results, we observed that the enhanced Akt activity under Akt1 knockdown increases phosphorylation of GSK-3β. As a result, GSK-3β activity is downregulated and the stability of MCL-1 is enhanced. Thus, the enhanced Akt activity also regulates the GSK-3β-MCL-1 pathway to resist oxidative-stress-induced apoptosis.
Finally, the enhanced Akt phosphorylation modulates the FOXO3A-Bim pathway. FOXO3A and FOXO1 transcriptional factors are Akt targets activating proapoptotic genes such as the pro-apoptotic Bcl-2 family member, Bim (15), which plays a critical role in regulating apoptosis. The upregulation of Bim expression directly correlated with initiation of the apoptotic program (4). Phosphorylation of FOXO3A and FOXO1 by Akt facilitates their association with 14-3-3 binding proteins and favors their cytoplasmic retention, which eventually suppresses their transcriptional activities for proapoptotic gene expression (8). In addition, Akt was recently found to be capable of controlling cell growth and survival through proteasome-dependent degradation of FOXO1, FOXO3A (38). Hence, Akt exerts dual regulation of FOXO transcriptional factors to antagonize apoptosis. In the present study, we observed that the enhanced Akt activity strongly regulates FOXO3A but much less to FOXO1. As a result, Bim expression and the apoptosis induced by oxidative stress were significantly attenuated. In addition, since p53 regulates Bim at the transcription level, the negative regulation of p53 by Akt via MDM2 also contributes to the downregulation of Bim.
In summary, as a result of Akt1 knockdown, Akt2 activation controls at least these three pathways to maintain the mitochondrial integrity in the HLECs and suppresses the induced apoptosis under oxidative stress. In addition, Akt2 is the key isoform of the Akt family kinases to counteract stress response.
Supplementary Material
Abbreviations Used
- Bak
Bcl-2 homologous antagonist killer
- FITC
fluorescein isothiocyanate
- FOXO
the O subclass of the forkhead family of transcription factors
- GSK-3β
glycogen synthase kinase 3 beta
- HIF-1
hypoxia inducible factor-1
- HLEC
human lens epithelial cells
- JNK
c-Jun terminal kinase
- MCL-1
myeloid leukemia cell differentiation protein 1
- MDM
murine double minute
- mTORC
mammalian target of rapamycin complexes
- MTT
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazo-lium bromide
- PCR
polymerase chain reaction
- PDK1
phosphoinositide-dependent kinase 1
- PH
pleckstrin homology
- PHLPP
pleckstrin homology domain leucine-rich repeat protein phosphatase
- PI3K
phosphatidylinositide 3′-OH kinase
- PIP2
phosphatidylinositol 3, 4 biphosphate
- PIP3
phosphatidylinositol 3,4,5 triphosphate
- PKB
protein kinase B
- PTEN
phosphatase and tensin homolog
- RAC-PK
Rac-activated protein kinase
- ROS
reactive oxygen species
- RT
reverse transcription
- shRNA
small interference RNA
- SNK
Student-Newman-Keuls
Acknowledgments
This study was supported in part by the NIH/NEI grants 1R01EY18380 & 1R01EY015765, the Lotus Scholar Professorship Funds from Hunan Province Government and Hunan Normal University, and the Changjiang Scholar Team Award from National Education Ministry of China and Hunan Provincial Innovation Grant for graduate students.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alessi DR. Andjelkovic M. Caudwell B. Cron P. Morrice N. Cohen P. Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Arrigo AP. The cellular “networking” of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol. 2007;594:14–26. doi: 10.1007/978-0-387-39975-1_2. [DOI] [PubMed] [Google Scholar]
- 3.Balaban RS. Nemoto S. Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Bouillet P. Metcalf D. Huang DC. Tarlinton DM. Kay TW. Kontgen F. Adams JM. Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 5.Bramall AN. Wright AF. Jacobson SG. McInnes RR. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev Neurosci. 2010;33:441–472. doi: 10.1146/annurev-neuro-060909-153227. [DOI] [PubMed] [Google Scholar]
- 6.Brazil DP. Yang ZZ. Hemmings BA. Advances in protein kinase B signaling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Brognard J. Sierecki E. Gao T. Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Brunet A. Bonni A. Zigmond MJ. Lin MZ. Juo P. Hu LS. Anderson MJ. Arden KC. Blenis J. Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen KC. Zhou Y. Zhang W. Lou MF. Control of PDGF-induced reactive oxygen species (ROS) generation and signal transduction in human lens epithelial cells. Mol Vis. 2007;13:374–387. [PMC free article] [PubMed] [Google Scholar]
- 10.Cho H. Mu J. Kim JK. Thorvaldsen JL. Chu Q. Crenshaw EB., 3rd Kaestner KH. Bartolomei MS. Shulman GI. Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 11.Cho H. Thorvaldsen JL. Chu Q. Feng F. Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 12.Coffer PJ. Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 13.Datta K. Franke TF. Chan TO. Makris A. Yang SI. Kaplan DR. Morrison DK. Golemis EA. Tsichlis PN. AH/PH domain-mediated interaction between Akt molecules and its potential role in Akt regulation. Mol Cell Biol. 1995;15:2304–2310. doi: 10.1128/mcb.15.4.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta SR. Brunet A. Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 15.Dijkers PF. Medema RH. Lammers JW. Koenderman L. Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 16.Franke TF. Kaplan DR. Cantley LC. Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 17.Gao T. Furnari F. Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 18.George S. Rochford JJ. Wolfrum C. Gray SL. Schinner S. Wilson JC. Soos MA. Murgatroyd PR. Williams RM. Acerini CL. Dunger DB. Barford D. Umpleby AM. Wareham NJ. Davies HA. Schafer AJ. Stoffel M. O'Rahilly S. Barroso I. A family with severe insulin resistance, diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb TM. Leal JF. Seger R. Taya Y. Oren M. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene. 2002;21:1299–1303. doi: 10.1038/sj.onc.1205181. [DOI] [PubMed] [Google Scholar]
- 20.Grossman SR. Perez M. Kung AL. Joseph M. Mansur C. Xiao ZX. Kumar S. Howley PM. Livingston DM. p300/MDM2 complexes participate in MDM2-mediated p53 degradation. Mol Cell. 1998;2:405–415. doi: 10.1016/s1097-2765(00)80140-9. [DOI] [PubMed] [Google Scholar]
- 21.Gupta V. Awasthi N. Wagner BJ. Specific activation of the glucocorticoid receptor and modulation of signal transduction pathways in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:1724–1734. doi: 10.1167/iovs.06-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han ES. Muller FL. Pérez VI. Qi W. Liang H. Xi L. Fu C. Doyle E. Hickey M. Cornell J. Epstein CJ. Roberts LJ. Van Remmen H. Richardson A. The in vivo gene expression signature of oxidative stress. Physiol Genomics. 2008;34:112–126. doi: 10.1152/physiolgenomics.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halliwell B. Gutteridge J. Free Radicals in Biology and Medicine. New York: Oxford University Press; 1999. [Google Scholar]
- 24.Jones PF. Jakubowicz T. Pitossi FJ. Maurer F. Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MA. Kim HJ. Jee HJ. Kim AJ. Bae YS. Bae SS. Yun J. Akt2, but not Akt1, is required for cell survival by inhibiting activation of JNK and p38 after UV irradiation. Oncogene. 2009;28:1241–1247. doi: 10.1038/onc.2008.487. [DOI] [PubMed] [Google Scholar]
- 26.Kuo YC. Huang KY. Yang CH. Yang YS. Lee WY. Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 27.Leslie NR. Bennett D. Lindsay YE. Stewart H. Gray A. Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li WC. Kuszak JR. Dunn K. Wang RR. Ma W. Wang GM. Spector A. Leib M. Cotliar AM. Weiss M, et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995;130:169–181. doi: 10.1083/jcb.130.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linseman DA. Butts BD. Precht TA. Phelps RA. Le SS. Laessig TA. Bouchard RJ. Florez-McClure ML. Heidenreich KA. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24:9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Z. Xu X. Hu X. Lee S. Traverse JH. Zhu G. Fassett J. Tao Y. Zhang P. dos Remedios C. Pritzker M. Hall JL. Garry DJ. Chen Y. Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao YW. Liu JP. Xiang H. Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 32.Maurer U. Charvet C. Wagman AS. Dejardin E. Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Mayo LD. Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michael D. Oren M. The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol. 2003;13:49–58. doi: 10.1016/s1044-579x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 35.Nakatani K. Sakaue H. Thompson DA. Weigel RJ. Roth RA. Identification of a human Akt3 (protein kinase B gamma) which contains the regulatory serine phosphorylation site. Biochem Biophys Res Commun. 1999;257:906–910. doi: 10.1006/bbrc.1999.0559. [DOI] [PubMed] [Google Scholar]
- 36.Nemoto S. Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 37.Nogueira V. Park Y. Chen CC. Xu PZ. Chen ML. Tonic I. Unterman T. Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plas DR. Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278:12361–12366. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 39.Polyak K. Xia Y. Zweier JL. Kinzler KW. Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 40.Pomerantz J. Schreiber-Agus N. Liegeois NJ. Silverman A. Alland L. Chin L. Potes J. Chen K. Orlow I. Lee HW. Cordon-Cardo C. DePinho RA. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 41.Qin J. Chen HG. Yan Q. Deng M. Liu J. Doerge S. Ma W. Dong Z. Li DW. Protein phosphatase-2A is a target of epigallocatechin-3-gallate and modulates p53-Bak apoptotic pathway. Cancer Res. 2008;68:4150–4162. doi: 10.1158/0008-5472.CAN-08-0839. [DOI] [PubMed] [Google Scholar]
- 42.Shao Z. Bhattacharya K. Hsich E. Park L. Walters B. Germann U. Wang YM. Kyriakis J. Mohanlal R. Kuida K. Namchuk M. Salituro F. Yao YM. Hou WM. Chen X. Aronovitz M. Tsichlis PN. Bhattacharya S. Force T. Kilter H. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res. 2006;98:111–118. doi: 10.1161/01.RES.0000197781.20524.b9. [DOI] [PubMed] [Google Scholar]
- 43.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–1182. [PubMed] [Google Scholar]
- 44.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stambolic V. Suzuki A. de la Pompa JL. Brothers GM. Mirtsos C. Sasaki T. Ruland J. Penninger JM. Siderovski DP. Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 46.Xiang H. Wang J. Mao Y. Liu M. Reddy VN. Li DW. Human telomerase accelerates growth of lens epithelial cells through regulation of the genes mediating RB/E2F pathway. Oncogene. 2002;21:3784–3791. doi: 10.1038/sj.onc.1205455. [DOI] [PubMed] [Google Scholar]
- 47.Xiao L. Gong LL. Yuan D. Deng M. Zeng XM. Chen LL. Zhang L. Yan Q. Liu JP. Hu XH. Sun SM. Liu J. Ma HL. Zheng CB. Fu H. Chen PC. Zhao JQ. Xie SS. Zou LJ. Xiao YM. Liu WB. Zhang J. Liu Y. Li DW. Protein phosphatase-1 regulates Akt1 signal transduction pathway to control gene expression, cell survival and differentiation. Cell Death Differ. 2010;17:1448–1462. doi: 10.1038/cdd.2010.16. [DOI] [PubMed] [Google Scholar]
- 48.Yan D. Guo L. Wang Y. Requirement of dendritic Akt degradation by the ubiquitin-proteasome system for neuronal polarity. J Cell Biol. 2006;174:415–424. doi: 10.1083/jcb.200511028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang ZZ. Tschopp O. Hemmings-Mieszczak M. Feng J. Brodbeck D. Perentes E. Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 50.Zhou BP. Liao Y. Xia W. Zou Y. Spohn B. Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.