Abstract
Objectives
To assess the effectiveness of β blockers in short term treatment for acute myocardial infarction and in longer term secondary prevention; to examine predictive factors that may influence outcome and therefore choice of drug; and to examine the clinical importance of the results in the light of current treatment.
Design
Systematic review of randomised controlled trials.
Setting
Randomised controlled trials.
Subjects
Patients with acute or past myocardial infarction.
Intervention
β Blockers compared with control.
Main
outcome measures All cause mortality and non-fatal reinfarction.
Results
Overall, 5477 of 54 234 patients (10.1%) randomised to β blockers or control died. We identified a 23% reduction in the odds of death in long term trials (95% confidence interval 15% to 31%), but only a 4% reduction in the odds of death in short term trials (−8% to 15%). Meta regression in long term trials did not identify a significant reduction in effectiveness in drugs with cardioselectivity but did identify a near significant trend towards decreased benefit in drugs with intrinsic sympathomimetic activity. Most evidence is available for propranolol, timolol, and metoprolol. In long term trials, the number needed to treat for 2 years to avoid a death is 42, which compares favourably with other treatments for patients with acute or past myocardial infarction.
Conclusions
β Blockers are effective in long term secondary prevention after myocardial infarction, but they are underused in such cases and lead to avoidable mortality and morbidity.
Key messages
The first randomised trials of β blockade in secondary prevention after myocardial infarction were published in the 1960s
β blockers were once heralded as a major advance, but their use for secondary prevention has declined in recent years
Firm evidence shows that long term β blockade remains an effective and well tolerated treatment that reduces mortality and morbidity in unselected patients after myocardial infarction
The benefits from β blockade compare favourably with other drug treatments for this patient group
Most evidence is for propranolol, timolol, and metoprolol, whereas atenolol, which is commonly used, is inadequately evaluated for long term use
Introduction
β Blockade was once heralded as a major advance in the treatment of patients with myocardial infarction, but current evidence suggests that less than half of eligible patients receive it.1–3 The effectiveness of β blockers was appraised by Yusuf et al in 1985,4 but since then there have been nearly 3000 deaths among 23 000 patients randomised in new trials. Trials of β blockers now include a broader group of patients such as those at high risk or with accompanying heart failure, enabling the benefits identified by Yusuf et al4 in a restricted group of trials to be extended to such patients.
Methods used in systematic reviews have also advanced. The development of regression techniques within meta analysis enables a more robust examination of the importance of factors that may mediate upon the effectiveness of specific drugs.5 Two such factors, intrinsic sympathomimetic activity and cardioselectivity, were identified as potentially important,4 and intrinsic sympathomimetic activity in particular seemed to be related to reduced therapeutic action. Given the changing use of drugs after myocardial infarction, the early promise of β blockade in these patients, and the continuing high rates of mortality associated with myocardial infarction, a new overview of these drugs is timely.
Methods
Objective
We reappraised the effectiveness of β blockers for secondary prevention after myocardial infarction. Our main outcome was all cause mortality and the secondary outcomes were non-fatal reinfarction and withdrawal from treatment. We examined the effectiveness of β blockers in the acute phase immediately after myocardial infarction; their role in longer term secondary prevention; the importance of early initiation after the onset of symptoms; the extent to which specific pharmacological features of different β blockers may affect their performance; the magnitude of benefits achieved by β blockers; and the clinical importance of β blockers.
Inclusion criteria
We included randomised trials without crossover, with treatment lasting more than one day, and with follow up that examined the clinical effectiveness of β blockers versus placebo or alternative treatment in patients who had had a myocardial infarction. Treatment may have begun at any stage before or after myocardial infarction and may have been commenced intravenously.
Search strategy
We conducted sensitive electronic searches of Medline (1966-97 through Ovid), Embase (1974-97 through Dialog), Biosis (1985-97 through Edina), Healthstar (1975-97 through Ovid), Sigle (1980-97 through Blaise-line), IHTA (1990-97 through ECRInet), conference papers index (1984-97 through Dialog), Derwent drug file (1992-97 through Dialog), dissertation abstracts (1992-97 through Dialog), Pascal (1992-97 through Dialog), international pharmaceutical abstracts (1992-97 through Dialog), and science citation index (1981-97 through BIDS).
We reviewed the reference list of each identified study. We also examined existing bibliographies and reviews for relevant studies.
Data abstraction and appraisal of study quality
From each study we abstracted data on the total number of patients randomised to active treatment or control, β blocker, route and dose of drug, duration of treatment, loss to follow up, level of blinding, concealment of allocation,6 specific study inclusion and exclusion criteria, duration of follow up, deaths, reinfarctions, and withdrawals. Data were checked by a second researcher.
Statistical analysis
We estimated pooled odds ratios for short and long term treatment trials separately using the fixed effects approach of Mantel Haenszel.7,8 As we anticipated systematic differences between the results of studies (heterogeneity), we also routinely estimated random effects pooled odds ratios. Standard random effects methods for meta-analysis (pooling the results of studies)9,10 may provide unduly precise estimates of effect, as they assume that the observed distribution of effects is the true treatment distribution—an assumption that may not be valid in sparse data.5,11,12 Therefore, we used the full random effects approach on the basis of the numerical integration techniques using Markov chain Monte Carlo simulation,with appropriate uninformative priors and the “Bugs” software described by Smith et al.5 This provides a more robust estimate of the precision of random effects estimates and can account for trial groups that experience no events without resorting to crude fixes such as adding a value to each cell to estimate an individual odds ratio. A further advantage of this approach is that the effects of predictive factors may be examined. Our main treatment related covariates were cardioselectivity and intrinsic sympathomimetic activity, which were examined in the long term trials using a nested random effects logistic regression model (see Appendix).
We also made a separate examination of the effects of initial intravenous treatment in long term trials, and the effect of additional treatment options through the proxy variable of publication date before or after the median year (1982). We assessed convergence using the methods described by Geweke13 and visual inspection of convergence plots.
We calculated risk differences using standard random effects methods,11 and because comparison of risk differences between trials may be affected by different lengths of follow up, we also estimated a pooled incidence risk difference using the approach described by Ioannidis et al.14 This is less robust than the pooled odds ratio but provides a practically interpretable estimate of absolute treatment effect derived directly from the trials.15 For the long term trials, we also calculated pooled estimates of effect for each β blocker using the fixed effects model.7,8
Results
We identified 82 randomised trials that examined the effects of β blockers compared with control and that had data on all cause mortality. Overall, 5477 of 54 234 patients (10.1%) randomised died. Fifty one trials examined acute treatment with β blockers—up to 6 weeks after onset of pain (table 1, and 31 trials examined long term treatment with β blockers—6 to 48 months (table 2).
Table 1.
Characteristics of short term trials comparing β blockers with control (see website for references)
| Trial | Average follow up | Drug* | Blinding | Concealment of allocation | Loss to follow up (%) | Outcome or endpoint | Heart failure (%) | Mortality (No/total No)
|
|
|---|---|---|---|---|---|---|---|---|---|
| β Blockers | Controls | ||||||||
| Azancot 1982w1 | 1 month | Acebutolol* | No | Unclear | 0 | Mortality | 0 | 0/14 | 0/12 |
| Balcon 1966w2 | 28 days | Propranolol | Double | Unclear | 0 | Mortality | 55 | 14/56 | 15/58 |
| Barber 1976w3 | 4 weeks | Propranolol | No | Unclear | Unclear | Mortality, reinfarction | Unclear | 10/52 | 12/47 |
| Campbell 1984w4 | In hospital | Timolol* | Unclear | Unclear | 0 | Mortality | Unclear | 1/20 | 2/19 |
| Clausen 1966w5 | 14 days | Propranolol | Unclear | Unclear | 0 | Mortality | Unclear | 18/66 | 19/64 |
| CPRG 1981w6 | 8 weeks | Oxprenolol | Double | Unclear | 0 | Mortality, reinfarction | 0 | 9/177 | 5/136 |
| Curtis 1991w7 | 3.4 days | Propranolol | Double | Unclear | 0 | Mortality | Unclear | 0/18 | 0/12 |
| Dotremont 1968w8 | 3-6 weeks | Propranolol | No | No | Unclear | Mortality | 68.6 | 4/36 | 5/36 |
| Evemy 1978w9 | 7 months | Practolol* | No | No | Unclear | Mortality | Unclear | 9/46 | 6/48 |
| Federman 1984w10 | 28 days | Timolol* | Unclear | Unclear | 0 | mortality | 0 | 1/50 | 0/50 |
| Fuccella 1968w11 | 21 days | Oxprenolol | Unclear | Unclear | 14 | Mortality | Unclear | 15/106 | 9/114 |
| Gupta 1982w12 | Unclear | Propranolol | Unclear | Unclear | 0 | Mortality | Unclear | 0/25 | 3/25 |
| Gupta 1984w13 | 72 hours | Propranolol* | No | Unclear | Unclear | Mortality | Unclear | 0/15 | 0/15 |
| Heber 1987w14 | 1 year | Labetalol* | No | Unclear | Unclear | Mortality | Unclear | 12/83 | 7/83 |
| Hutton 1979w15 | 2 days | Propranolol | Unclear | Unclear | 0 | Mortality | Unclear | 0/16 | 0/13 |
| ICSG 1984w16 | To discharge | Timolol | Double | Unclear | 0 | Mortality | 57 being treated for heart failure | 3/73 | 4/71 |
| ISIS-1 Collaborative Group 1986w17 | 1 year | Atenolol* | No | NA | Unclear | Mortality | Unclear | 1071/8037 | 1120/7990 |
| Johansson 1980w18 | 6 months | Practolol* then atenolol | Single | No | Unclear | Mortality | Unclear | 7/25 | 7/29 |
| Kahler 1968w19 | Up to 35 days | Propranolol | Double | Unclear | Unclear | Mortality, reinfarction | 11 | 3/38 | 6/31 |
| Ledwich 1968w20 | 7 days | Propranolol | Double | Unclear | Unclear | Mortality | Unclear | 2/40 | 3/40 |
| Lloyd 1988w21 | 72 hours | Sotalol* | No | Unclear | 0 | Mortality | Unclear | 0/15 | 0/15 |
| Lombardo 1979w22 | 20 days | Oxprenolol | Double | Unclear | Unclear | Mortality | 0 | 8/133 | 11/127 |
| Macleod 1980w23 | 1 week | Practolol* | Unclear | Unclear | 0 | Mortality | Unclear | 1/26 | 0/26 |
| McMurray 1991w24 | 7 days | Xamoterol | Double | Unclear | 0 | Mortality | 31 | 0/25 | 0/26 |
| MIAMI Trial Research Group 1985w25 | 15 days | Metoprolol* | Double | Yes | 0.04 | Mortality | 23.5 | 123/2877 | 142/2901 |
| Mueller 1980w26 | To discharge | Propranolol* | Double | Unclear | Unclear | Mortality | Unclear | 2/35 | 1/35 |
| Multicentre 1966w27 | 28 days | Propranolol | Double | Unclear | 1 | Mortality | 11 | 15/100 | 12/95 |
| Nigam 1983w28 | 1 week | Propranolol* | Unclear | Unclear | 0 | Mortality | Unclear | 0/20 | 0/20 |
| Norris 1968w80 | 3 weeks | Propranolol | Double | Yes | 0 | Mortality | Unclear | 31/226 | 24/228 |
| Norris 1978w29 | To discharge | Propranolol* | No | No | Unclear | Mortality | Unclear | 0/20 | 0/23 |
| Norris 1984w30 | In hospital | Propranolol* | No | NA | 0 | Mortality | Unclear | 15/364 | 14/371 |
| Owensby 1984w31 | 3 days | Pindolol* | No | NA | Unclear | Mortality | Unclear | 1/50 | 1/50 |
| Peter 1978w32 | To discharge | Propranolol* | No | Unclear | 0 | Mortality | 0 | 1/47 | 2/48 |
| Pitt 1976w33 | 14 days | Propranolol | Double | Unclear | 0 | Mortality | Unclear | 0/9 | 0/8 |
| Ranganathan 1988w34 | 28 days | Timolol* | Double intravenously then by open label orally | Unclear | 2 | Mortality | Unclear | 1/45 | 3/49 |
| Roberts 1984w35 | 36 months | Propranolol* | Single | Unclear | 0.2 | Mortality | 4.9 | 24/134 | 20/135 |
| Singh 1985w36 | 60 hours | Propranolol* | No | Unclear | 0 | Mortality | Unclear | 0/8 | 0/7 |
| Sloman 1967w37 | To discharge | Propranolol* | No | Unclear | Unclear | Mortality | Unclear | 3/26 | 4/23 |
| Snow 1980w38 | Short term | Practolol | Unclear | Unclear | 0 | Mortality | Unclear | 19/76 | 15/67 |
| Thompson 1979w39 | 1 year | Practolol | Double | Unclear | Unclear | Mortality | Unclear | 5/72 | 6/71 |
| TIMI IIB Study Group 1989w40 | 5 days | Metoprolol* (15 mg) | No | Unclear | 3.5 | Mortality, reinfarction | 1.1 | 17/696 | 17/694 |
| Tonkin 1981w41 | 1 year | Timolol | Double | Unclear | Unclear | Mortality, reinfarction | Unclear | 1/42 | 1/46 |
| UKCSG 1983w42 | To discharge | Timolol | Double | Unclear | Unclear | Mortality | Unclear | 4/56 | 5/55 |
| Van de Werf 1993w43 | 10-14 days | Atenolol | Double | Unclear | 0 | Mortality, reinfarction | Unclear | 1/100 | 4/94 |
| Von Essen 1982w44 | 14 days | Metoprolol* | Double | Unclear | 0 | Mortality | Unclear | 1/25 | 1/26 |
| Waagstein 1975w45 | 1 week | Practolol,* H87/07, or metoprolol | Double | Unclear | 0 | Mortality | Unclear | 0/38 | 0/45 |
| Wilcox 1980bw46 | 6 weeks | Oxprenolol | Double | Yes | 0 | Mortality | 28 withdrawn owing to severe heart failure | 14/157 | 10/158 |
| Yang 1987w47 | 14 days | Betaxolol | Double | Unclear | 0 | Mortality | 9.4 | 0/16 | 0/15 |
| Yusuf 1980w48 | 10 days for infarction, 1-4 years for mortality | Atenolol* | No | Unclear | Unclear | Mortality, morbidity | 6.5 | 36/244 | 44/233 |
CPRG=Coronary Prevention Research Group; ICSG=International Collaborative Study Group; ISIS-1=first international study of infarct survival; MIAMI=metoprolol in acute myocardial infarction; TIMI IIB=thrombolysis in myocardial infarction phase II trial; UKCSG=UK Collaborative Study Group. *Initial dose intravenously.
Table 2.
Characteristics of long term trials comparing β blockers with control (see website for references)
| Trial | Average follow up | Drug* | Blinding | Concealment of allocation | Loss to follow up (%) | Outcome or endpoint | Heart failure (%) | Mortality (No/total No)
|
No of reinfarctions
|
No of withdrawals
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β blockers | Controls | β blockers | Controls | β blockers | Controls | ||||||||||
| Ahlmark 1974w49 | 2 years | Alprenolol | Unclear | Unclear | Unclear | Mortality, reinfarction | Unclear | 5/69 | 11/93 | 4 | 15 | 4 | 6 | ||
| Andersen 1979w50 | About 1 year | Alprenolol | Double | Unclear | 0 | Mortality | Unclear | 61/238 | 64/242 | — | — | 59 | 49 | ||
| Boissel 1990w51 | 318 days | Acebutolol | Double | Yes | 0 | Mortality | 49.5 | 17/298 | 34/309 | — | — | 102 | 109 | ||
| Aronow 1997w52 | 1 year | Propranolol | Unclear | Unclear | Unclear | Mortality, reinfarction | 100 | 44/79 | 60/79 | 3 | 5 | — | — | ||
| Australian and Swedish study 1983w53 | 2 years | Pindolol | Double | Unclear | Unclear | Mortality, reinfarction | 61 left ventricular dysfunction | 45/263 | 47/266 | 12 | 13 | 76 | 50 | ||
| Baber 1980w54 | 9 months | Propranolol | Double | Unclear | Unclear | Mortality, reinfarction | Unclear | 28/355 | 27/365 | 17 | 27 | 82 | 88 | ||
| Barber 1967w55 | 2 years | Practolol | Unclear | Unclear | Unclear | Mortality, reinfarction | 26 | 33/207 | 38/213 | — | — | — | — | ||
| Basu 1997w56 | 6 months | Carvedilol | Double | Unclear | 0 | Mortality, reinfarction | 45 | 2/75 | 3/71 | 4 | 8 | — | — | ||
| BHAT 1982w57 | 25 months | Propranolol | Double | Yes | 0.3 | Mortality | 9.2 | 138/1916 | 188/1921 | 103 | 121 | 243 | 179 | ||
| Darasz 1995w58 | 6 months | Xamoterol | Double | Unclear | 19 | Mortality, reinfarction | 3/23 | 1/24 | — | — | 3 | 6 | |||
| EIS 1984w59 | 1 year | Oxprenolol | Double | Unclear | Unclear | Mortality, Reinfarction | 7.7 | 57/853 | 45/883 | 36 | 38 | 275 | 275 | ||
| Hansteen 1982w60 | 1 year | Propranolol | Double | Unclear | 0 | Mortality, reinfarction | 5.9 (taking digitalis) | 25/278 | 37/282 | 16 | 21 | 70 | 72 | ||
| Hjalmarson 1981w61 | 2 years | Metoprolol* | Double (3 months) then open treatment (to 2 years) | Unclear | 1.6 | Mortality at 2 years; reinfarction at 3 months | 10 | 40/698 | 62/697 | 35 | 54 | 131 | 131 | ||
| Julian 1982w62 | 12 months | Sotalol | Double | Yes | 0 | Mortality, reinfarction | 0 | 64/873 | 52/583 | 37 | 38 | 218 | 121 | ||
| Kaul 1988w63 | 6 months | Propranolol (iv) | Double | Unclear | 0 | Mortality, reinfarction | Unclear | 3/25 | 3/25 | 0 | 4 | 0 | 0 | ||
| LIT Research Group 1987w64 | 18 months | Metoprolol | Double | Unclear | 0.2 | Mortality | 2.1 | 86/1195 | 93/1200 | — | — | 381 | 355 | ||
| Manger Cats 1983w65 | 1 year | Metoprolol | Double | Unclear | 0 | Mortality | Unclear | 9/273 | 16/280 | — | — | — | — | ||
| Mazur 1984w66 | 1.5 years | Propranolol | No | Unclear | Unclear | Mortality, reinfarction | Unclear | 5/101 | 11/103 | 5 | 7 | — | — | ||
| Multicentre international 1975w67 | Up to 24 months | Practolol | Double | Unclear | 3.4 | Mortality, reinfarction | 0 | 102/1533 | 127/1520 | 69 | 89 | 389 | 382 | ||
| Norwegian Multicentre Study Group 1981w68 | 17 months | Timolol | Double | Unclear | Unclear | Mortality | 33 | 98/945 | 152/939 | 88 | 141 | 275 | 219 | ||
| Rehnqvist 1980w69 | 1 year | Metroprolol | Unclear | Unclear | 0 | Mortality | Unclear | 4/59 | 6/52 | — | — | 12 | 5 | ||
| Rehnqvist 1983w70 | 36 months | Metoprolol | Double | Unclear | 0 | Mortality, reinfarction | 24 (taking digitalis) | 25/154 | 31/147 | 18 | 31 | 38 | 35 | ||
| Reynolds 1972w71 | 1 year | Alprenolol | Double | Yes | Unclear | Mortality, reinfarction | Unclear | 3/38 | 3/39 | 3 | 2 | 4 | 3 | ||
| Roqué 1987w72 | 24 months | Timolol* | Double | Unclear | Unclear | Mortality | Unclear | 7/102 | 12/98 | — | — | — | — | ||
| Salathia 1985w73 | 1 year | Metoprolol* | Double | Unclear | 0.5 | Mortality, | 10 | 49/416 | 52/348 | — | — | 95 | 66 | ||
| Schwartz 1992 (high risk and low risk)w74 | 22 months | Oxprenolol | High risk† and low risk‡ groups | Unclear | 0 | Mortality, reinfarction | 2 in high risk group; unclear for low risk group | 2/48 15/437 | 12/56 27/432 | 0 | 2 | 11 | 9 | ||
| SSSD 1993w75 | 3 years | Metoprolol | No | Unclear | 1.9 | Mortality, reinfarction | 100 | 17/130 | 9/123 | 5 | 6 | — | — | ||
| Taylor 1982w76 | 48 months | Oxprenolol | Double | Done | Unclear | Mortality, reinfarction | 0 | 60/632 | 48/471 | 67 | 58 | 185 | 141 | ||
| Wilcox 1980aw77 | 1 year | Propranolol* or atenolol | Double | Done | 0 | Death | Unclear | 19/127 17/132 | 19/129 | — | — | 44 51 | 40 40 | ||
| Wilhelmsson 1974w78 | 2 years | Alprenolol | Double | Unclear | 7 | Mortality | Unclear | 7/114 | 14/116 | 16 | 18 | 8 | 8 | ||
| Yusuf 1979w79 | 12 months | Atenolol | Double | Unclear | 23 | Death; electrocardiogr aphic signs | Unclear | 1/11 | 1/11 | — | — | 2 | 1 | ||
BHAT=β-blocker heart attack trial; LIT=lopressor intervention. *Initial dose intravenously. †Single blind. ‡Double blind.
Short term trials
Overall, 3062 of 29 260 patients (10.5%) randomised in short term trials died. Although 51 trials were identified that examined the effects of short term treatment, only 45 of these had observed deaths in either the intervention or control groups. The major challenge to the quality of this group of trials was that small numbers of patients randomised to treatment or control led to many trials with either no, or only a small number of, deaths.
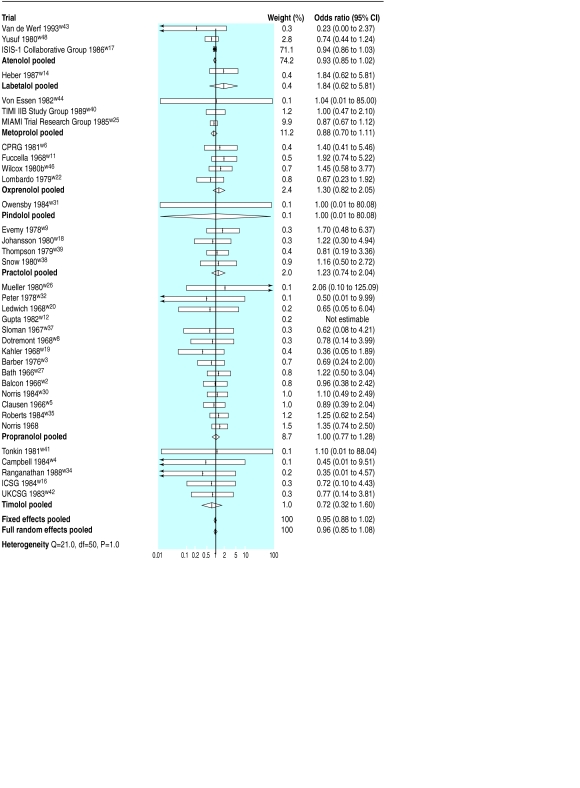
The pooled random effects odds ratio for the short term trials was 0.96 (95% confidence interval 0.85 to 1.08); that is, a small and non-significant reduction in the odds of death (fig 1). Even if this result is correct it would represent a reduction of only 0.4 deaths in 100 patients, which does not achieve conventional levels of significance (−0.2 to 1) as 250 patients would require treatment to avoid one death (100 to ∞). Analysis of predicted benefit by drug identified no individual drug that differed significantly in effect from the pooled result.
Figure 1.
Odds of death and pooled odds ratios in short term trials (arrows indicate 95% confidence intervals exceeding range of plot). ISIS-1=first international study of infarct survival; TIMI IIB=thrombolysis in myocardial infarction phase II trial; MIAMI=metoprolol in acute myocardial infarction; CPRG=Coronary Prevention Research Group; ICSG=International Collaborative Study Group; UKCSG=UK Collaborative Study Group
Although most trials were undertaken before the second international study of infarct survival in 198816 firmly established the importance of thrombolysis, a large trial of thrombolysis in myocardial infarction in 198917 randomised patients who had received recombinant tissue plasminogen activator within 4 hours of the onset of pain to early metoprolol or control. During 5 days of follow up, there was no difference in mortality between the two groups. Two subsequent myocardial infarctions were, however, avoided for every 100 patients treated (0.2 to 4).
Long term trials
Overall effects
Overall, 2415 of 24 974 patients (9.7%) randomised in the 31 long term trials died. In general, the quality of studies was reasonably high, with adequate follow up achieved in many trials (table 2), though the proxy quality variable, concealment of allocation, was seldom adequately reported.
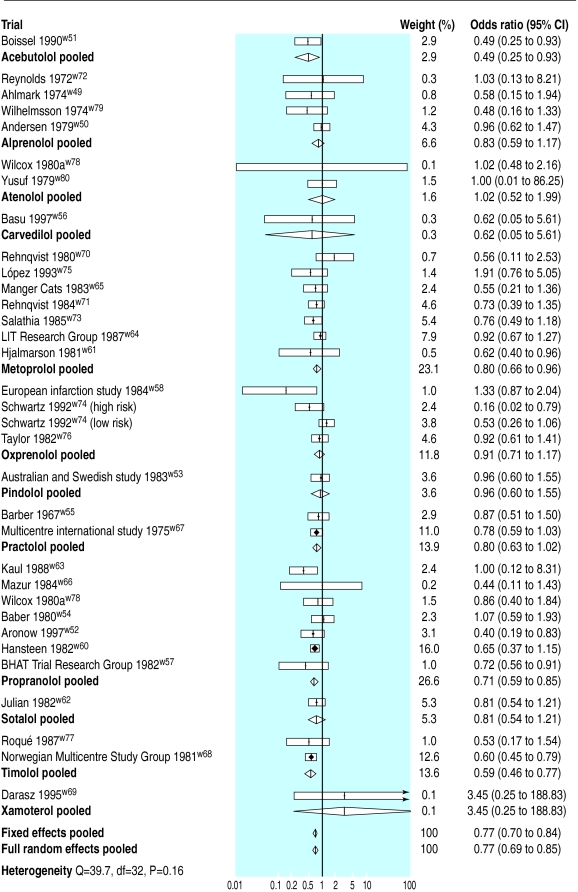
Overall, the pooled odds ratio from the full random effects model was 0.77 (0.69 to 0.85). Results from the standard fixed effects model were similar (fig 2).
Figure 2.
Odds of death and pooled odds ratios in long term trials. LIT=lopressor intervention; BHAT=β-blocker heart attack trial
Because of potential confounding due to the differences in length of study follow up, we used the random effects approach for incidence of risk difference to estimate the normalised annual reduction in mortality across the trials. This approach suggests an annual reduction of 1.2 deaths in 100 patients treated with β blockers after myocardial infarction (0.6 to 1.7); that is, about 84 patients will require treatment for 1 year to avoid one death. A similar approach was used to estimate the effects of treatment on reinfarction, although only 21 of the 34 comparisons provided data on reinfarction, resulting in wider confidence intervals and the potential for reporting bias. This analysis suggests an annual reduction in reinfarction of 0.9 events in every 100 (0.3 to 1.6); that is, about 107 patients would require treatment for 1 year to avoid one non-fatal reinfarction.
Predictors of benefit
Initial intravenous dose—
We investigated the extent to which initiation of treatment with an intravenous dose of β blockers predicted mortality in the long term trials. Applying initial intravenous dose as a covariate term in the analysis suggested no additional benefit among patients treated in this manner (odds ratio 0.87, 0.61 to 1.22). Equally, this analysis indicates that there is no reason to delay treatment with a β blocker and that early initiation will lead to a greater period when benefits may be accrued from treatment.
Presence of cardioselectivity or intrinsic sympathomimetic activity—We anticipated that the presence of cardioselectivity and intrinsic sympathomimetic activity would be important predictors of benefit in the trials, a hypothesis examined by Yusuf et al.4
Classification of β blockers into those with or without important cardioselective activity or intrinsic sympathomimetic effect is not clear cut, and there is some debate in the literature on the attributes of acebutolol in particular.18–20 Table 3 describes the attributes of β blockers used in the trials.
Table 3.
Classification of attributes of different β blocker drugs
| β Blocker | Cardioselectivity | Intrinsic sympathomimetic activity |
|---|---|---|
| Acebutolol | – | – |
| Alprenolol | – | + |
| Atenolol | + | – |
| Betaxolol | + | – |
| Carvedilol | – | – |
| Labetalol | – | – |
| Metoprolol | + | – |
| Oxprenolol | – | + |
| Pindolol | – | + |
| Practolol | + | + |
| Propranolol | – | – |
| Sotalol | – | – |
| Timolol | – | – |
| Xamoterol | + | + |
+=Significant activity; –=no significant activity.
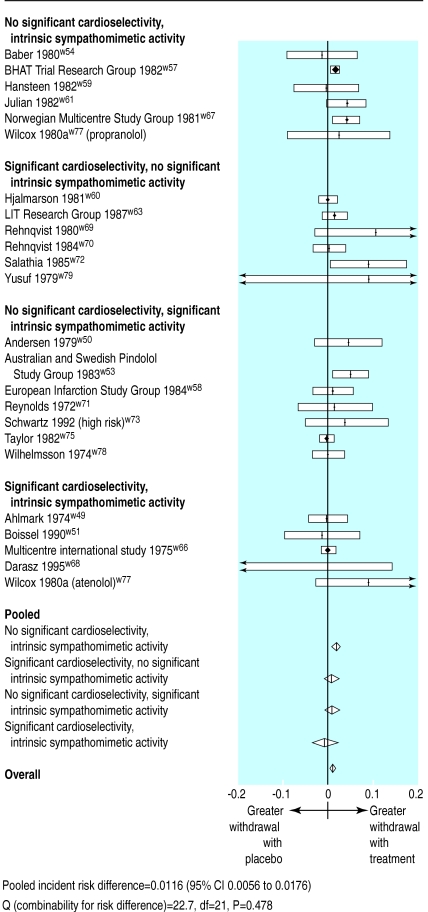
The odds ratio for the predictive effect of cardioselectivity on mortality was 1.10 (0.89 to 1.39), showing a non-significant trend towards reduced benefits. The odds ratio for the predictive effect of the presence of intrinsic sympathomimetic activity was 1.19 (0.96 to 1.47), which approaches statistical significance. The results were not sensitive to the classification of acebutolol.
Reduction of benefits over time—We investigated whether benefits were reduced in the trials with additional therapeutic options for treatment introduced, in particular the increasing use of thrombolytic treatment, and aspirin. There is no evidence that treatment in trials after 1982 (the median trial) led to differences in benefit (odds ratio 1.04, 0.82 to 1.28).
Choice of drug—Individually, only four drugs achieved a statistically significant reduction in the odds of death: propranolol (0.71, 0.59 to 0.85); timolol (0.59, 0.46 to 0.77); metoprolol (0.80, 0.66 to 0.96); and acebutolol (0.49, 0.25 to 0.93). The effectiveness of acebutolol is supported by a single moderately sized study, which is open to considerable measurement error. However, trials including propranolol, timolol, and metoprolol include 63% of the available evidence on the effects of long term β blockade in patients who have had a myocardial infarction.
Withdrawal from treatment
Different definitions and reporting made comparison of withdrawal of treatment withdrawal between trials problematic. Similar withdrawal rates between active treatment and placebo groups concealed two opposing effects: more patients are withdrawn from treatment groups because of suspected adverse cardiovascular reactions (most commonly brachycardia and hypotension), whereas in the placebo group withdrawal is more common because of the need for β blockade for hypertension and angina. Trials reports of dizziness, depression, cold extremities, and fatigue were only marginally more common in the treatment than control groups.
Withdrawal in trials from both treatment and control groups varied from 10% to 30%. No adequate studies have been retrieved to compare directly the comparative tolerability of β blockers with different cardioselectivity or intrinsic sympathomimetic activity.
Overall, 5151 of 21 954 patients (23.5%) withdrew from treatment (table 2). Overall, withdrawal was slightly more common in patients taking β blockers—the difference in the annualised rate of withdrawal compared with placebo being 1.16 in 100 patients treated (0.56 to 1.76, random effects; fig 3). No clinically important differences in withdrawal were observed between β blockers of differing cardioselectivity and intrinsic sympathomimeticity.
Figure 3.
Incidence (yearly) of withdrawal from trials
Discussion
Considerable evidence supports the routine long term use of β blockers in patients who have had a myocardial infarction, with substantial benefits in terms of reduced mortality and morbidity. Short term β blockade immediately after acute myocardial infarction seems unlikely to be of major benefit unless treatment is continued long term. This finding contradicts recent suggestions that β blockers should be more commonly used intravenously in acute myocardial infarction.21 In fact, evidence strongly indicates that unless β blockers are continued long term, the benefits suggested by Owen21 will not be observed.
The benefits of β blockers on all cause mortality are impressive when compared with other frequently used long term treatments for the same patient group. Table 4 shows the effects of different drugs on the number of patients that would need to be treated for 2 years to avoid one death—for example, after a myocardial infarction 42 patients would need to be treated with β blockers whereas 292 patients would need to be treated with antiplatelets.22 The number and length of long term trials showing a consistent benefit for β blockers in unselected patients after myocardial infarction suggest lasting benefits in this comparatively high risk group, and suggest that β blockers should be continued indefinitely.
Table 4.
Comparison of effect on mortality of different drugs
| Drug | Number needed to treat* |
|---|---|
| β Blockers | 42 |
| Angiotensin converting enzyme inhibitors | No long term trials in unselected patients |
| Antiplatelet agent22 | 153 |
| Statin29 | 94 |
| Calcium channel blockers (diltiazem)30 | ∞ |
| Thrombolysis and aspirin for 4 weeks16 | 24 |
| Warfarin31 32 | 63 |
Number needed to avoid death in 2 years of treatment in unselected patients after myocardial infarction.
Have benefits from β blockade declined with availability of new treatments?
Our finding that β blockers benefit a broader group of patients after myocardial infarction supports the findings of the β blocker pooling project.23 Our finding also agrees with those of the cooperative cardiovascular project, which examined the medical records of 201 752 patients who had had a myocardial infarction.24 In that study, mortality was lower in every subgroup of patients treated with β blockade than in untreated patients. The findings of the cooperative cardiovascular project agree with our meta regression analysis, which found no evidence of a reduction in benefits from β blockade in more recent randomised trials. Indeed, rather than being overtaken by newer treatments, β blockers have a comparatively large effect in reducing mortality (table 4).
Which β blocker?
Cardioselectivity was associated with a non-significant trend towards reduced benefit. The presence of an intrinsic sympathomimetic effect predicted a near significant reduction in benefits and thus drugs with this characteristic should be avoided. We found evidence to support the long term use of propranolol and timolol, the only two drugs indicated for prophylaxis after myocardial infarction in the British National Formulary. The use of either drug led to a substantial reduction in the odds of death, with narrow confidence intervals (fig 2). In contrast, atenolol, which is commonly prescribed in secondary prevention, has been inadequately evaluated in this setting. Although similar efficacy may be achieved—we found no evidence that all β blockers are not equal—it cannot be presumed that the benefits from propranolol, timolol, and metoprolol will be achieved with other drugs.
Have benefits from intravenous β blockers declined over time?
It may be hypothesised that intervention with thrombolytic drugs and antiplatelets reduces the potential for patients to benefit from intravenous β blockade. The first international study of infarct survival25 was completed before the results of the second international study16 became available, and before the use of thrombolytic and antiplatelet treatment was established. In contrast, the comparison of early versus delayed β blockade in a large trial of thrombolysis in myocardial infarction was undertaken in patients who all received thrombolytic and antiplatelet treatment.17 Although the much larger first international study of infarct survival trial25 achieved a slightly larger reduction in the odds of death with β blockers, measurement error could not be excluded as an explanation for this difference, as indicated by the test for heterogeneity between the trials (Q=0.025, df=1, P=0.87). The thrombolysis in myocardial infarction trial did suggest that early use of intravenous β blockers could reduce the early risk of serious arrhythmias.
Are β blockers underused?
Concern has been voiced that β blockers are used in less than half of eligible patients after myocardial infarction,1–3 despite substantial benefits and generally low treatment costs. Concern that side effects affect the usefulness of β blockers must be tempered by the low yearly withdrawal from β blockers in the long term trials we reviewed. The clinical implications of our results are clear. New is not necessarily better, especially if the aim is to reduce mortality in patients after myocardial infarction. Furthermore, the underuse of β blockers in this group leads to a rate of avoidable death that should not be considered acceptable among those keen to practice evidence based medicine.
Renewed interest in β blockers, particularly in patients with heart failure,26–28 may lead to substantial benefits for a broad range of patients.
Supplementary Material
Acknowledgments
We thank Andrew Herxheimer, who assisted in the categorisation of included compounds, and Anne Burton for her diligent help in locating studies and in the preparation of the manuscript.
Appendix
Statistical model for random effects regression analysis
Where pt is the probability of an event in the intervention group, pc is the probability of an event in the control group, I is the presence or absence of significant intrinsic sympathomimetic activity, and S is the presence or absence of significant cardioselectivity. Similarly, α is a constant, δ describes the overall treatment effect, β describes the effect of intrinsic sympathomimetic activity, and γ describes the effect of cardioselectivity.
Footnotes
Funding: SmithKline Beecham Pharmaceuticals UK. The views expressed are those of the authors and not necessarily those of the sponsor.
Competing interests: This study was funded through an unrestricted educational grant by SmithKline Beecham, who supply carvedilol in the United States. JGFC has spoken at many meetings and educational programmes on drugs in heart failure, organised by pharmaceutical and device companies, and received fees and expenses. He has also received research funding from industry as well as the NHS, British Heart Foundation, and US Veterans Administration.
website extra: References for the trials appear on the BMJ’s website www.bmj.com
References
- 1.Smith J, Channer KS. Increasing prescription of drugs for secondary prevention after myocardial infarction. BMJ. 1995;311:917–918. doi: 10.1136/bmj.311.7010.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eccles M, Bradshaw C. Use of secondary prophylaxis against myocardial infarction in the north of England. BMJ. 1991;302:91–92. doi: 10.1136/bmj.302.6768.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viskin S, Barron HV. β-Blockers prevent cardiac death following myocardial infarction: so why are so many infarct survivors discharge without β-blockers? Am J Cardiol. 1996;78:821–822. doi: 10.1016/s0002-9149(96)00428-6. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. β-Blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 5.Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta analysis: a comparative study. Stats Med. 1995;14:2685–2699. doi: 10.1002/sim.4780142408. [DOI] [PubMed] [Google Scholar]
- 6.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trial. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 7.Rothman KJ. Modern epidemiology. Boston: MA Little and Brown; 1986. [Google Scholar]
- 8.Robins J, Breslow N, Greenland S. Estimators of the Mantel-Haenszel variance consistent in both sparse data and large strata models. Biometrics. 1986;42:311–323. [PubMed] [Google Scholar]
- 9.Fleiss J, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44:127–139. doi: 10.1016/0895-4356(91)90261-7. [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.Carlin JB. Meta analysis for 2 β 2 tables: a Bayesian approach. Stats Med. 1992;11:141–158. doi: 10.1002/sim.4780110202. [DOI] [PubMed] [Google Scholar]
- 12.Hardy RJ, Thompson SG. A likelihood approach to meta analysis with random effects. Stats Med. 1996;15:619–629. doi: 10.1002/(SICI)1097-0258(19960330)15:6<619::AID-SIM188>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Spiegelhalter D, Thomas A, Best N, Gilks W. Cambridge: Medical Research Council Biostatistics Unit; 1995. BUGS: Bayesian inference using Gibbs sampling, Version 0.50. [Google Scholar]
- 14.Ioannidis JPA, Cappelleri JC, Lau J, Skolnik PR, Melville B, Chalmers TC, et al. Early or deferred zidovudine therapy in HIV-infected patients without an AIDS defining illness. Ann Intern Med. 1995;122:856–866. doi: 10.7326/0003-4819-122-11-199506010-00009. [DOI] [PubMed] [Google Scholar]
- 15.Freemantle N, Mason JM, Eccles M. Deriving treatment recommendations from evidence within randomised trials: the role and limitation of meta analysis. Intern J Technol Assess Health Care (in press). [PubMed]
- 16.Second International Study of Infarct Survival Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;ii:349–360. [PubMed] [Google Scholar]
- 17.The Thrombolysis in Myocardial Infarction Study Group. Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction: results of the thrombolysis in myocardial infarction (TIMI) phase II trial. N Engl J Med. 1989;320:618–627. doi: 10.1056/NEJM198903093201002. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelmsson C, Vedin JA, Wilhelmsen L, Tibblin G. Reduction of sudden deaths after myocardial infarction by treatment with alprenolol: preliminary results. Lancet. 1974;ii:1157–1160. doi: 10.1016/s0140-6736(74)90807-1. [DOI] [PubMed] [Google Scholar]
- 19.Feely J, de Vane PJ, Maclean D. β-Blockers and sympathomimetics. BMJ. 1983;286:1043–1047. doi: 10.1136/bmj.286.6370.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDevitt DG. The assessment of β-adrenoceptor-blocking drugs in man. Br J Clin Pharmacol. 1977;4:413–425. doi: 10.1111/j.1365-2125.1977.tb00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen A. Intravenous β-blockade in acute myocardial infarction: should be used in combination with thrombolysis. BMJ. 1998;317:226–227. doi: 10.1136/bmj.317.7153.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy. I. Prevention of death, myocardial infarction and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 23.β-Blocker Pooling Project Research Group. The β-blocker pooling project (BBPP): subgroup findings from randomized trials in post infarction patients. Eur Heart J. 1988;9:8–16. [PubMed] [Google Scholar]
- 24.Gottlieb SS, McCarter RJ, Vogel RA. Effect of β-blockade on mortality among high risk and low risk patients after myocardial infarction. N Engl J Med. 1998;339:489–497. doi: 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]
- 25.First International Study of Infarct Survival Collaborative Group. Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. Lancet. 1986;ii:57–67. [PubMed] [Google Scholar]
- 26.Doughty RN, Rodgers A, Sharpe N, MacMahon S. Effects of β-blocker therapy on mortality in patients with heart failure: a systematic overview of randomized controlled trials. Eur Heart J. 1997;18:560–565. doi: 10.1093/oxfordjournals.eurheartj.a015297. [DOI] [PubMed] [Google Scholar]
- 27.Heidenreich PA, Lee TT, Massie BM. Effect of β-blockade on mortality in patients with heart failure: a meta analysis of randomized clinical trials. J Am Coll Cardiol. 1997;30:27–34. doi: 10.1016/s0735-1097(97)00104-6. [DOI] [PubMed] [Google Scholar]
- 28.Cleland JGF, Freemantle N, McGowan J, Clark A. The evidence for β blockers in heart failure. BMJ. 1999;318:824–825. doi: 10.1136/bmj.318.7187.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latest trials on statins show large benefits? Lancet. 1997;350:1525. [Google Scholar]
- 30.The Multicenter Ditiazem Postinfarction Trial (MDPIT) Research Group. The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med. 1988;319:385–392. doi: 10.1056/NEJM198808183190701. [DOI] [PubMed] [Google Scholar]
- 31.Smith P, Arnesen H, Holme I. The effect of warfarin on mortality and reinfarction after myocardial infarction. N Engl J Med. 1990;323:147–152. doi: 10.1056/NEJM199007193230302. [DOI] [PubMed] [Google Scholar]
- 32.Anticoagulants in the Secondary Prevention of Events in Coronary Thrombosis (ASPECT) Research Group. Effect of long term oral anticoagulant treatment on mortality and cardiovascular morbidity after myocardial infarction. Lancet. 1994;343:499–503. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.