Abstract
The synthesis of glutathione, a major cellular antioxidant with a critical role in T cell proliferation, is limited by cysteine. In this study, we evaluated the contributions of the xC- cystine transporter and the transsulfuration pathway to cysteine provision for glutathione synthesis and antioxidant defense in naïve versus activated T cells and in the immortalized T lymphocyte cell line, Jurkat. We show that the xC- transporter, although absent in naïve T cells, is induced after activation, releasing T cells from their cysteine dependence on antigen-presenting cells. We also demonstrate the existence of an intact transsulfuration pathway in naïve and activated T cells and in Jurkat cells. The flux through the transsulfuration pathway increases in primary but not in transformed T cells in response to oxidative challenge by peroxide. Inhibition of the transsulfuration pathway in both primary and transformed T cells decreases cell viability under oxidative-stress conditions. Antioxid. Redox Signal. 15, 39–47.
Introduction
T cells play important roles in innate and adaptive immune responses, and their dysfunctions, due to excessive sensitivity to self-antigens or to deficiency, are associated with pathologies. Redox modulation has emerged as a key strategy in regulation of T cell functions (18, 28). Glutathione (GSH), a major cellular antioxidant and a cysteine reservoir, is an important component of redox signaling pathways and plays an essential role in T cell function. An increase in intracellular GSH levels is needed for the proliferative response of T cells to mitogens and antigens (24). Perturbation of intracellular GSH levels and the GSH/GSSG redox status dramatically affects DNA synthesis, T cell proliferation, and the cytotoxic T cell response (8, 17).
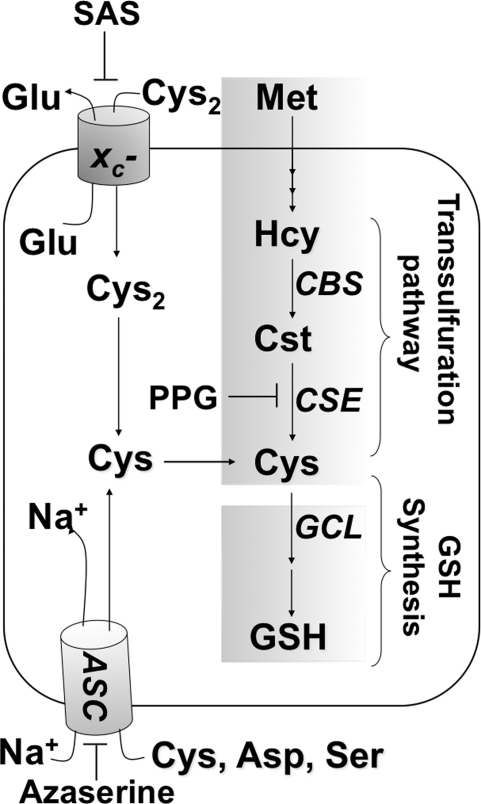
Low cysteine levels allow activation of NF-κB–dependent transcription in the early G1 phase of the cell cycle, whereas in the late G1 and S phases, IL-2–dependent cell proliferation is correlated with higher cysteine/GSH levels (3). GSH depletion restricts cell-cycle progression from the G1 to the S phase (13). Cysteine, the limiting amino acid for GSH synthesis, can be obtained from metabolism through the transsulfuration pathway, or via transport. The ASC system imports cysteine, whereas the xC- antiporter uses the transmembrane glutamate gradient to drive import of cystine into the cell, where it is subsequently reduced to cysteine (Fig. 1). The xC- cystine transporter is composed of two subunits, the transmembrane xCT light chain, which houses the transporter activity, and the regulatory extracellular 4F2 heavy chain (22). In the extracellular compartment, cysteine exists predominantly in its oxidized form, and the concentration of plasma cysteine (10 to 25 μM) is significantly lower than of cystine (100 to 200 μM) (28).
FIG. 1.
Glutathione homeostasis in T cells by the xC- transporter and the transsulfuraton pathway. PPG, SAS, and azaserine are inhibitors of γ-cystathionase, the xC- transporter, and the neutral amino acid transporter ASC, respectively. CBS, CSE, and GCL denote cystathionine β-synthase, γ-cystathionase, and γ-glutamylcysteine ligase, respectively.
Naïve T cells reportedly lack the xC- transporter and are thus metabolically dependent on antigen-presenting cells (APCs) for meeting their cysteine needs during activation and proliferation (1, 16, 29). Endowed with the xC- transporter, APCs take up cystine, and using a convoluted metabolic route involving conversion to GSH followed by its secretion and cleavage, furnish extracellular cysteine for uptake by T cells (29). GSH levels in APCs influence T cell response patterns, with low GSH favoring a Th1- over a Th2-associated cytokine-secretion pattern (19). The contribution, if any, of the transsulfuration pathway, which converts methionine to cysteine, to redox metabolism in naïve T cells is not known. Methionine is an essential amino acid that is converted via the methionine cycle to homocysteine. Two enzymes in the transsulfuration pathway, cystathionine β-synthase (CBS) and γ-cystathionase, convert homocysteine to cysteine and play a quantitatively significant role in supplying cysteine needed for GSH synthesis in several cell types (5, 15).
In the present study, we evaluated changes in the transsulfuration pathway and the xC- system during transformation of naïve T cells to the activated state. We demonstrate, by using metabolic labeling and pharmacologic inhibition studies, the presence of an intact transsulfuration pathway in both naïve and activated T cells and in Jurkat cells. We found that the expression of xCT is induced on T cell activation, weaning T cells off their metabolic dependence on APCs. Under oxidative-stress conditions, the transsulfuration pathway is upregulated in naïve but not transformed T cells, whereas its inhibition enhances cellular susceptibility to death in both naïve and transformed cells.
Materials and Methods
Mice and cell lines
Male BALB/c mice (7 to 10 weeks) were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained in pathogen-free animal facilities at the University of Michigan. The University's Committee on Use and Care of Animals approved the protocol for animal handling used in this study. Jurkat cells were obtained from ATCC (Manassas, VA).
Isolation and preparation of murine primary cells
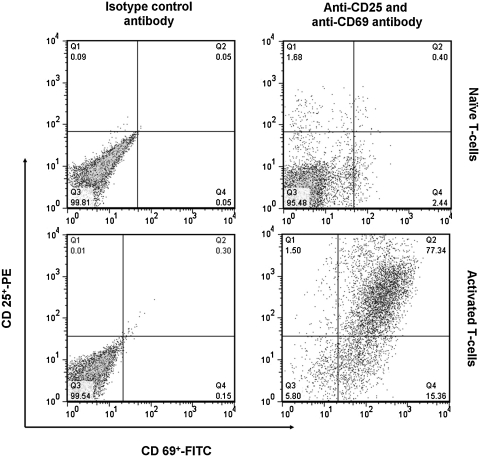
CD3+ T lymphocytes were prepared from lymph nodes and spleen that were harvested and mashed. T cells were enriched by negative selection on T cell columns (R&D Systems, Minneapolis, MN) as per the vendor's protocol (6). For in vitro activation, as isolated naïve T cells were suspended in RPMI medium containing 2.5% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 1 × penicillin/streptomycin (Invitrogen), 50 μM 2-mercaptoethanol (T cell media), and incubated with either anti-CD3 + anti-CD28 antibodies (eBiosciences) or with anti-CD3 antibody + dendritic cells or irradiated splenocytes (as APC, 1:4 ratio) for 48 to 72 h. After incubation, activated T cells were separated from APCs by gentle pipetting, and T cells were collected with low-speed centrifugation at 200 g for 10 min at 4°C. Activation of T cells was assessed by monitoring the activation markers CD25 and CD69 with flow cytometry (Fig. 2). Numbers inside panels indicate the percentage of naïve and activated CD3+ T cells positive for CD25-FITC and CD69-PE.
FIG. 2.
Upregulation of activation-associated markers on T cell surfaces. Naïve and dendritic cell-activated T cells were stained with anti-CD25-PE and anti-CD69-FITC or the corresponding isotype controls and analyzed with flow cytometry. Numbers inside the boxes indicate percentage of CD3+ naïve and activated T cells positive for CD25-FITC and CD69-PE.
Dendritic cells were obtained from femurs and tibias removed from adult male mice and from the bone marrow were flushed out with Ca2+- and Mg2+-free phosphate-buffered saline (PBS). Red blood cells were lysed with ACK buffer (Lonza, Walkersville, MD). Bone marrow cells were plated at 2–5 × 106 cells/ml in a 250-ml flask (total, 15 ml) in DMEM supplemented with 100 μg/ml penicillin/streptomycin, 2 mM l-glutamine, 50 μM β-mercaptoethanol, 1 mM pyruvate, 1:100 nonessential amino acids, and 10% heat-inactivated FBS (dendritic cell medium). Recombinant murine granulocyte macrophage colony-stimulating factor and recombinant murine interleukin 4 (R&D Systems), at 20 ng/ml each, were added on day 0. On days 2 and 4, the culture medium was replaced with dendritic cell medium supplemented with cytokines, and floating cells were discarded. On day 7, cells were collected with Accutase (eBioscience) to harvest adherent cells and were centrifuged; the pellet was resuspended in fresh dendritic cell medium and used in T cell co-cultures as APCs.
Cell-culture conditions
Jurkat cells were cultured in DMEM medium containing 10% FBS, 2 mM l-glutamine, and 1 × penicillin/streptomycin (Jurkat medium). To activate Jurkat cells, phorbol 12-myristate 13-acetate (PMA) was added to the culture medium at a final concentration of 100 ng/ml for 48 h. PMA was washed out, and cells were incubated with fresh culture medium at the start of the experimental treatment. Naïve or preactivated T cells were cultured in T cell medium, and at the indicated time points, an aliquot of the medium was collected for extracellular cysteine, cystine, and glutamate measurement, rapidly frozen, and stored at −80°C until further use. To measure the intracellular thiol concentration and to estimate flux through the transsulfuration pathway, naïve or activated T cells were incubated with 2 μCi/ml l-[35S]-methionine (specific activity of 1,175 Ci/mmol; Perkin Elmer) in the presence or absence of 2.5 mM propargylglycine (PPG) for 6 or 12 h. The final concentration of l-[35S]-methionine was 2 nM, and the final specific activity, 20 μCi/μmol. To measure the effect of oxidative stress on the transsulfuration flux, the same experiments were repeated except in the presence of 20 μM and 100 μM t-BuOOH for naïve and activated T cells, respectively. At the indicated time points, cells were washed with ice-cold PBS, harvested, and frozen at −80°C until further use.
Metabolite analysis
Samples for analysis of extracellular cysteine, cystine, and glutamate were prepared by mixing conditioned media with an equal volume of metaphosphoric acid solution, and the precipitated proteins were sedimented by centrifugation at 13,000 g for 10 min at 4°C. Protein-free extracts were alkylated with monoiodoacetic acid, derivatized with 2,4-dinitrofluorobenzene solution, and analyzed with HPLC, as previously described (6, 15). The concentration of metabolites in the control medium was subtracted from the final values. Intracellular GSH and [35S]-methionine incorporation into GSH was quantified as described previously (6, 15). The intracellular values were normalized to the protein concentration in each sample.
Western blot analysis
T cells (naïve or activated T cells) and Jurkat cells were harvested and lysed on ice, as described previously (5). Antibodies against methionine synthase, CBS, xCT (Novus), γ-glutamylcysteine ligase (γ-GCL, Lab Vision) and actin (Sigma) were used to monitor expression of the respective protein antigens and detected by using the Dura chemiluminescent horseradish peroxidase system (Pierce), as per the vendor's protocol.
[14C]Cystine-uptake assay
Naïve or activated T cells (2 × 106) were incubated with 0.1 μCi/ml, [14C]-cystine (250 mCi/mmol, Perkin Elmer,) in the presence or absence of 500 μM sulfasalazine (SAS) for the indicated times. The final concentration of [14C]-cystine was 400 nM, and the final specific activity was 0.5 μCi/mmol. Cells were collected, washed 3 times with ice-cold phosphate-buffered saline, and then lysed with 400 μl of 500 mM NaOH. Scintillation cocktail was added, and total intracellular [14C] radioactivity was recorded. Data were normalized to cell number.
Cell-viability assay
Cells were incubated with the indicated concentrations of tertiary butyl hydroperoxide (t-BuOOH) in the presence or absence of 2.5 mM PPG for 12 to 14 h. Cell viability was determined by incubating the cells with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide dye (0.5 mg/ml), followed by washing, solubilizing in dimethylsulfoxide, and reading the optical density at 553 nm.
Statistical analyses
Comparison between groups was done by using the Student t test. A value of p < 0.05 was considered to be statistically significant.
Results
The xC- transporter is induced during APC-independent activation of T cells
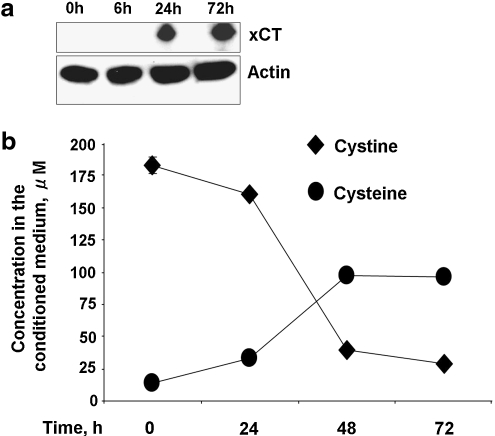
Although T cells depend on APCs for their cysteine needs (1, 29), it is known that T cells can be activated ex vivo in an APC-independent fashion (i.e., by antibodies to the T cell receptor, CD3, and to the co-stimulatory molecule, CD28). This raises the obvious question as to how cysteine needs are met under these activation conditions. To assess the involvement of the xC- transporter in cystine uptake, we used Western blot analysis to monitor expression of the xCT subunit in naïve and anti-CD3/anti-CD28 activated T cells. As reported previously (25), xCT expression was not detectable in naïve T cells but was highly induced in activated T cells (Fig. 3a).
FIG. 3.
T cells induce the cystine transporter during activation. (a) xCT expression increases upon activation of T cells by anti-CD3/anti-CD28 antibodies. Cell lysate from either naïve T cells or T cells activated by anti-CD3/anti-CD28 antibodies for 6, 24, and 72 h were subjected to immunoblotting and probed with anti-xCT antibody. Actin is shown as an equal loading control. (b) Naïve CD3+ T cells were incubated with anti-CD3 and anti-CD28 antibodies, and the concentrations of cystine and cysteine were measured at the indicated time points. Data are shown as mean ± SD and are representative of two (a) and three (b) independent experiments performed in triplicates (b).
To evaluate whether induction of xCT is correlated with a functional difference in cystine transport in activated but not naïve T cells, we measured the kinetics of cystine consumption. During incubation with anti-CD3 and anti-CD28 antibodies, T cells progressively consume increasing amounts of cystine and accumulate cysteine in the extracellular compartment (Fig. 3b), consistent with the induction of the xC- transporter. Cysteine accumulation plateaued after 48 h, at which time, the extracellular cystine concentration was very low.
The xc- transporter is induced during APC-dependent activation of T cells
Next, we examined whether xC- expression is also induced in T cells activated in the presence of dendritic cells and anti-CD3. We found that xCT was strongly induced after 48 h of incubation (Fig. 4a). We focused the remainder of the study on APC-activated T cells.
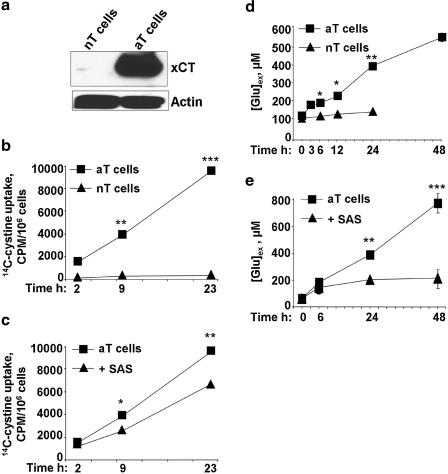
FIG. 4.
Activated but not naïve T cells mediate cystine/glutamate exchange via xC-. (a) xCT expression increases upon activation of T cells by dendritic cells. Cell lysate from either naïve T cells or T cells collected from a dendritic cell and T cell co-culture were subjected to immunoblotting and probed with anti-xCT antibody. Actin is shown as an equal loading control. Naïve (b, d) or activated (b, c, d, e) T cells were incubated with 0.1 μCi/ml [14C]cystine in the presence or absence of 500 μM SAS (c, e) for the indicated times. An aliquot of the medium at indicated times was removed for glutamate analysis (d, e), and cells were collected for intracellular radioactivity measurement (b, c). For this, cells were collected in a tube, washed 3 times with cold phosphate-buffered saline, and then lysed with 400 μl NaOH (500 mM). Scintillation cocktail was added, and total intracellular [14C] radioactivity was recorded and normalized to the cell number. Data are shown as mean ± SD and are representative of two (a, b, e) and three (c, d) independent experiments performed in duplicates. *p ≤ 0.05; **p ≤ 0.01; and ***p ≤ 0.001.
Cystine/glutamate exchange is mediated by the xc- transporter
We compared the kinetics of [14C]-cystine uptake in naïve versus activated T cells. Under these conditions, activated T cells showed a time-dependent increase in intracellular radiolabel accumulation (Fig. 4b), which was sensitive to inhibition by SAS, an inhibitor of xC- (Fig. 4c). In contrast, negligible uptake of radioactivity was observed with naive T cells. Because xC- is an antiporter that exchanges cystine stoichiometrically with glutamate, we assessed extracellular glutamate levels in the conditioned media from activated T cells cultured after their separation from APCs. Under these conditions, activated T cells showed a time-dependent increase in extracellular glutamate levels, which was sensitive to SAS treatment (Fig. 4d and e). Naïve T cells do not export glutamate, and the basal concentration in the culture medium (∼100 μM) is unchanged over a 24-h incubation time (Fig. 4d).
The endogenous transsulfuration pathway is a source of cysteine for GSH in naïve T cells
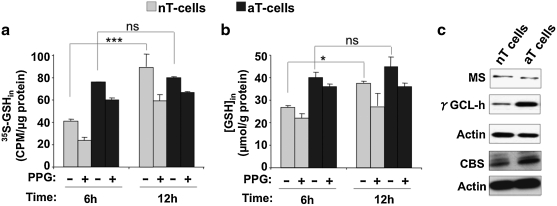
Because naïve T cells do not express xC-, we wondered whether the endogenous transsulfuration pathway is a source of cysteine needed for GSH and other biosynthetic purposes in these cells. To test this, we measured the transfer of radioactivity from [35S]-methionine to GSH in the presence and absence of PPG, a suicide inhibitor of γ-cystathionase, the second enzyme in the transsulfuration pathway (Fig. 1). The transsulfuration pathway is clearly intact in both naïve and activated T cells, as evidenced by the incorporation of radiolabel from [35S]-methionine into GSH and its inhibition by PPG (Fig. 5a). Radiolabel incorporation in naïve cells was inhibited to ∼50% and ∼33% of the untreated values at 6 h and 12 h, respectively, by PPG. In activated T cells, accumulation of radioactivity in GSH was less sensitive to PPG inhibition.
FIG. 5.
The transsulfuration pathway is intact in both naïve and activated T cells. Naïve (gray) and activated (black bar) T cells were incubated with 2 μCi/ml l-[35S]-methionine in the presence or absence of 2.5 mM PPG for 6 and 12 h. At the indicated time, cells were harvested, and radioactivity incorporation in GSH (a) and the intracellular GSH concentration (b) were measured and normalized to protein. Data are shown as mean ± SD and are representative of three independent experiments performed on different batches of cells. Statistical analysis with the Student t-test revealed significant changes in intracellular GSH synthesis and radioactive labeling over time in both naïve and activated T cells and its inhibition by PPG. *p ≤ 0.05; ***p ≤ 0.001; and ns, not significant. Panel c shows the comparison of expression level of CBS, methionine synthase, and γGCL (heavy subunit) between naïve and activated T cells with Western blot analysis.
The 100% increase in [35S]-labeling of GSH in naïve T cells between 6 and 12 h was accompanied by an ∼40% increase in GSH concentration during the same time period (Fig. 5b). The GSH concentration was higher at 6 h in activated compared with naïve T cells and increased only marginally (∼12%) at 12 h. We examined the expression of some key regulatory enzymes in sulfur metabolism: methionine synthase, CBS, and γ-GCL in naive and activated T cells. Whereas methionine synthase expression was unchanged, activation was accompanied by increased expression of CBS and γ-GCL (Fig. 5c).
T cells activate the transsulfuration pathway on peroxide challenge
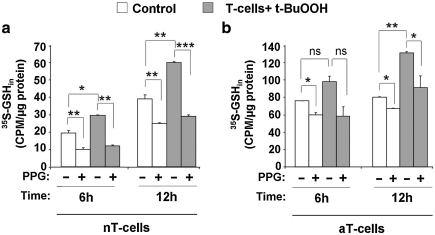
Mammalian cells are known to upregulate flux through the transsulfuration pathway with oxidative-stress conditions and, in the short term, increase GSH concentration by ∼40% (12, 27). We assessed whether the transsulfuration pathway responds similarly in naïve and activated T cells to peroxide challenge. For this, naïve or APC-activated T cells were incubated with [35S]-methionine and challenged with the indicated concentration of t-BuOOH in the presence or absence of PPG for 6 h and 12 h (Fig. 6). t-BuOOH treatment induced a 50% increase in radiolabel incorporation in naïve T cells both at 6 and 12 h after stimulation, which was completely inhibited by PPG (Fig. 6a). Activated T cells showed a modest increase (∼25%) in radiolabel incorporation into GSH at 6 h and a more sizeable increase (∼65%) after 12 h of t-BuOOH exposure (Fig. 6b). These results demonstrate that T cells activate the transsulfuration pathway in response to peroxide-induced oxidative stress.
FIG. 6.
Peroxide stress activates the transsulfuration pathway in both naïve and activated T cells. Naïve T cells (a) ± 20 μM t-BuOOH (gray bar) were incubated with 2 μCi/ml l-[35S]-methionine ± 2.5 mM PPG. Activated T cells (b) ± 100 μM t-BuOOH (gray bar) were incubated with 2 μCi/ml l-[35S]-methionine ± 2.5 mM PPG. At the indicated time, cells were harvested, and radioactivity incorporation into GSH was measured and normalized to protein concentration. Data are shown as mean ± SD and are representative of three independent experiments performed on different batches of cells. Statistical analysis with the Student t-test revealed significant changes in intracellular radiolabeling with t-BuOOH treatment and inhibition by PPG. *p ≤ 0.05; **p ≤ 0.01; and ***p ≤ 0.001.
Transformed T cells do not activate the transsulfuration pathway with peroxide challenge
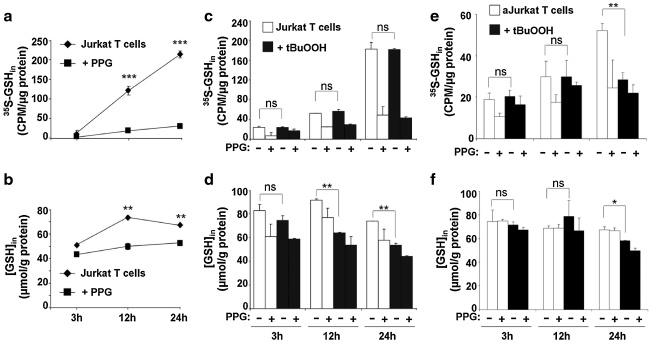
Jurkat cells are commonly used as a model for T cells in laboratory experiments. Because the metabolism of transformed cells is often distinct from that of the primary cells from which they are derived, we investigated whether Jurkat cells have an intact transsulfuration pathway that is responsive to oxidative-stress conditions. A time-dependent increase in radiolabel incorporation from [35S]-methionine into GSH that was sensitive to PPG inhibition confirmed the presence of an intact transsulfuration pathway in these cells (Fig. 7a). The intracellular GSH concentration initially increased (50%) between 3 and 12 h of incubation, after which it stabilized (Fig. 7b). The sensitivity of the intracellular GSH pool to PPG, which diminished to ∼50% and ∼30% of control values at 12 and 24 h, respectively, indicated that between 50 and 70% of the cysteine used for GSH synthesis is derived via other routes. The presence of the xCT subunit of the cystine transporter, as detected with Western blot analysis (not shown), suggests that Jurkat cells can derive cysteine from imported cystine. Unlike primary T cells, the transsulfuration pathway in Jurkat cells is unresponsive to peroxide stress, as evidenced by the lack of increased radiolabel incorporation (Fig. 7c) and decreased intracellular GSH on t-BuOOH exposure (Fig. 7d).
FIG. 7.
Jurkat cells have an intact transsulfuration pathway that is unresponsive to peroxide stress. Resting Jurkat cells (a–d) or PMA-activated Jurkat cells (e–f ) were either untreated (a, b) or treated with 10 μM t-BuOOH (c–f ) and incubated with 2 μCi/ml l-[35S]-methionine ± 2.5 mM PPG, as described under Methods. At the indicated time, cells were harvested, and radioactivity incorporation into GSH (a, c, e) and the intracellular GSH concentration (b, d, f ) was measured and normalized to protein concentration. Data are shown as mean ± SD and are representative of three independent experiments performed in duplicates. Statistical analysis with the Student t-test revealed a significant time-dependent increase in intracellular GSH synthesis and radiolabeling in Jurkat cells and inhibition by PPG. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ns, not significant.
PMA-activated Jurkat cells also showed a time-dependent increase in radiolabel incorporation from [35S]-methionine into GSH (Fig. 7e), although the extent of incorporation was considerably lower than that in unactivated cells (Fig. 7c). As with resting Jurkat cells, the transsulfuration pathway in activated Jurkat cells was unresponsive to oxidative stress, at least for the first 12 h after peroxide treatment. Thereafter, an ∼45% decrease in radiolabel incorporation into GSH was seen between 12 and 24 h (Fig. 7e), although the intracellular GSH pool size decreased only marginally over the same time period (Fig. 7f). This suggests that cysteine derived from some other source, possibly transport, might be responsible for maintaining intracellular GSH concentrations under these conditions.
Transsulfuration pathway blockade increases T cell susceptibility to oxidative stress–induced cell death
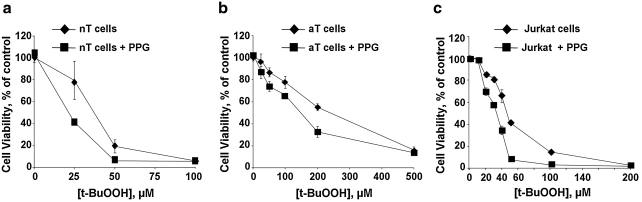
Because the transsulfuration pathway is important in many cell types for cysteine provision, especially under oxidative-stress conditions, we compared the viability of T cells exposed to varying concentrations of t-BuOOH in the presence and absence of PPG (Fig. 8). Both naive T cells and Jurkat cells are significantly more susceptible to peroxide-induced cell death (LD50 = 35 μM and 42 μM, respectively) than are activated T cells (LD50 = 240 μM). Inhibition of the transsulfuration pathway increased the sensitivity of all three cell types (LD50 = 21 μM, 32 μM, and 150 μM for naïve, Jurkat, and activated T cells, respectively).
FIG. 8.
Blockade of the transsulfuration pathway enhances T cell susceptibility to oxidative stress–induced cell death. Naïve (a) or activated (b) T cells or Jurkat cells (c) were incubated with the indicated concentrations of t-BuOOH ± 2.5 mM PPG for 12–14 h. Cell viability was determined as described under Methods. Data are shown as mean ± SD and are representative of at least two independent experiments performed in duplicates.
Discussion
The requirement for a reducing extracellular microenvironment for T cell activation and proliferation has been suspected from their dependence on an exogenous reductant, such as β-mercaptoethanol, added to the culture medium (20). The basis of this dependence has been ascribed to the inability of naïve T cells to efficiently transport cystine, the oxidized form of the amino acid that is relatively abundant in circulation. More recent studies demonstrated that APCs, especially dendritic cells, provide the extracellular reducing milieu to facilitate an immune response (1, 29). In the present study, we demonstrated that during APC-independent activation of T cells by anti-CD3 and anti-CD28 antibodies, cystine uptake and extracellular cysteine accumulation, hallmarks of APC metabolism during activation, are observed. This begs the obvious question as to what is the primary sulfur metabolic status of T cells (i.e., what pathways exist for provision of cysteine needed for GSH and other biosynthetic needs in naïve cells, and what routes for cysteine acquisition are added on activation). To address these questions, we characterized the transsulfuration pathway in naïve and activated T cells, its contribution to GSH homeostasis and antioxidant capacity under oxidative-stress conditions, and the induction of the xC- transporter system that leads to APC independence. We also compared the sulfur metabolic status of Jurkat cells, a transformed cell line that is widely used as an experimental model system for T cells.
Activation of T cells is accompanied by upregulation of pathways of nutrient uptake, ATP production, macromolecule synthesis, and programming of T cells for proliferation (10). We show that xCT, the catalytic subunit of the xC- cystine transporter, is highly expressed in T cells activated in an APC-dependent or -independent manner, but is not detectable in naïve T cells. Earlier studies have shown that the mRNA levels for xCT are very low in naïve T cells, whereas both xCT mRNA and protein are expressed in dendritic cells (2, 16, 29). Mice with homozygous disruption of the xC- transporter (xCT-/-) exhibit redox imbalance in the plasma, with high cystine and low GSH in comparison to xCT+/+ mice. Embryonic fibroblasts from xCT-/- mice fail to survive in culture unless supplemented with cysteine derivatives or antioxidants (21). Enhanced xCT activity in astrocytes increases GSH synthesis and protects neurons from oxidative stress (23). Expression of xCT is regulated by Nrf-2 (nuclear factor erythroid 2–related factor-2), which binds to the antioxidant response element (11) in response to oxidative stress. Although the mechanism of transcriptional induction of xCT during T cell activation awaits elucidation, it is possible that an initial prooxidant response during T cell activation leads to the transcriptional activation of xCT.
Clearly, in the absence of xC-, T cells must obtain cysteine needed for protein synthesis and for maintaining GSH at the levels seen in naïve cells from elsewhere. One such avenue is the transporter for cysteine. In T cells, cysteine uptake is mediated mainly by the sodium-dependent ASC system (for alanine, serine, and cysteine) (9). The cystine-transport activity is extremely low in resting T cells compared with the cysteine-transport activity. When T cells are activated, both cystine and cysteine transporter activities are upregulated, with the cystine-uptake rate still being lower than the cysteine-uptake rate (9).
Another route for provision of cysteine is the transsulfuration pathway, the presence of which in T cells is controversial. Human lymphoid cell lines from normal subjects but not from a cystathionuric patient with γ-cystathionase deficiency, were able to convert [35S]-homocysteine to [35S]-cysteine, consistent with the presence of the transsulfuration pathway (26). However, other studies using the PCR and flow-cytometry techniques reported that T cells lack γ-cystathionase and concluded that these cells lack the transsulfuration pathway (4, 25). With a sensitive metabolic radiolabeling method, we confirmed the presence of an intact transsulfuration pathway in naïve and activated T cells, as well as in Jurkat cells (Figs. 5 and 6). The transsulfuration pathway is estimated to contribute ∼50% of the cysteine in the GSH pool in hepatoma cells lines and in macrophages (5, 15). Our studies demonstrate the quantitative significance of the transsulfuration pathway to GSH homeostasis in T cells in which inhibition by PPG caused an ∼35%, ∼25%, and ∼50% decrease in GSH concentration within 12 h in naïve, activated, and Jurkat T cells, respectively. Furthermore, increased flux through the transsulfuration pathway is observed under oxidative-stress conditions in primary T cells and represents an autocorrective response for rebuilding antioxidant capacity (i.e., regaining the GSH pool size compromised by oxidizing conditions). However, even in the absence of increased flux through this pathway, its importance to the cellular capacity for countering oxidative stress is exemplified by the decrease in the LD50 for peroxide in all three cell types treated with the transsulfuration inhibitor, PPG (Fig. 8).
The transsulfuration pathway and the xC- transporter play important roles in several settings of intercellular communication. For example, the transsulfuration pathway is induced during monocyte differentiation and plays an important role in intracellular killing of mycobacteria (5). Inhibition of the transsulfuration pathway during mycobacterial infection allows bacteria to proliferate in the hostile environment of the host. Redox signaling between astrocytes and T cells endows astrocytes with a neuroprotective phenotype (6), and blockade of the xC- transporter is detrimental for this phenotype. Immunosuppression of autoreactive T cells by regulatory T cells involves interference with extracellular cysteine accumulation by dendritic cells (29).
Because naïve T cells have the capacity for cysteine synthesis via the endogenous transsulfuration route, why then are they dependent on APCs for provision of cysteine during activation? We posit that the key role for APC-derived extracellular cysteine might be to create a reducing microenvironment needed to facilitate inter- and intracellular signaling. The cysteine/cystine redox couple is an important redox buffer and is considered to be an important indicator of the extracellular redox poise (7). Human cells in the culture maintain an extracellular redox potential of about −80 mV, which is associated with growth arrest/differentiation. This value is similar to the plasma cysteine/cystine redox potential in young healthy adults (14). More-negative redox potential values favor cellular proliferation, whereas more-positive values favor cell death. Hence, cysteine accumulation by APCs is instrumental in fashioning a reductive shift in the extracellular microenvironment that is conducive for subsequent T cell proliferation. In addition to remodeling of the extracellular redox potential, it is possible that flux through the transsulfuration pathway is insufficient for meeting the cysteine needs of T cells that receive activation signals, which eventually leads to an increase in cell size in addition to triggering proliferation. The relative importance of APC-derived cysteine for extracellular redox remodeling versus supporting intracellular biosynthetic needs awaits further investigation.
Abbreviations Used
- APC
antigen-presenting cell
- CBS
cystathionine β-synthase
- FBS
fetal bovine serum
- GCL
γ-glutamylcysteine ligase
- GSH
glutathione
- PBS
phosphate-buffered saline
- PMA
phorbol 12-myristate 13-acetate
- PPG
propargylglycine
- SAS
sulfasalazine
- t-BuOOH
tert-butyl hydroperoxide
Footnotes
The first two authors contributed equally to this work.
Acknowledgment
This work was supported in part by a grant from the National Institutes of Health (HL58984 and DK64959).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Angelini G. Gardella S. Ardy M. Ciriolo MR. Filomeni G. Di Trapani G. Clarke F. Sitia R. Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A. 2002;99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellani P. Angelini G. Delfino L. Matucci A. Rubartelli A. The thiol redox state of lymphoid organs is modified by immunization: role of different immune cell populations. Eur J Immunol. 2008;38:2419–2425. doi: 10.1002/eji.200838439. [DOI] [PubMed] [Google Scholar]
- 3.Droge W. Eck HP. Gmunder H. Mihm S. Modulation of lymphocyte functions and immune responses by cysteine and cysteine derivatives. Am J Med. 1991;91:140S–144S. doi: 10.1016/0002-9343(91)90297-b. [DOI] [PubMed] [Google Scholar]
- 4.Eagle H. Washington C. Friedman SM. The synthesis of homocystine, cystathionine, and cystine by cultured diploid and heteroploid human cells. Proc Natl Acad Sci U S A. 1966;56:156–163. doi: 10.1073/pnas.56.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg S. Vitvitsky V. Gendelman HE. Banerjee R. Monocyte differentiation, activation, and mycobacterial killing are linked to transsulfuration-dependent redox metabolism. J Biol Chem. 2006;281:38712–38720. doi: 10.1074/jbc.M606235200. [DOI] [PubMed] [Google Scholar]
- 6.Garg SK. Banerjee R. Kipnis J. Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J Immunol. 2008;180:3866–3873. doi: 10.4049/jimmunol.180.6.3866. [DOI] [PubMed] [Google Scholar]
- 7.Go YM. Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimble RF. The effects of sulfur amino acid intake on immune function in humans. J Nutr. 2006;136:1660S–1665S. doi: 10.1093/jn/136.6.1660S. [DOI] [PubMed] [Google Scholar]
- 9.Ishii T. Sugita Y. Bannai S. Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. J Cell Physiol. 1987;133:330–336. doi: 10.1002/jcp.1041330217. [DOI] [PubMed] [Google Scholar]
- 10.Jones RG. Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM. Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 12.Martinov MV. Vitvitsky VM. Banerjee R. Ataullakhanov FI. The logic of the hepatic methionine metabolic cycle. Biochim Biophys Acta. 2009;1804:89–96. doi: 10.1016/j.bbapap.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messina JP. Lawrence DA. Cell cycle progression of glutathione-depleted human peripheral blood mononuclear cells is inhibited at S phase. J Immunol. 1989;143:1974–1981. [PubMed] [Google Scholar]
- 14.Moriarty-Craige SE. Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 15.Mosharov E. Cranford MR. Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:1300–5711. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- 16.Pacheco R. Oliva H. Martinez-Navio JM. Climent N. Ciruela F. Gatell JM. Gallart T. Mallol J. Lluis C. Franco R. Glutamate released by dendritic cells as a novel modulator of T cell activation. J Immunol. 2006;177:6695–6704. doi: 10.4049/jimmunol.177.10.6695. [DOI] [PubMed] [Google Scholar]
- 17.Pallardo FV. Markovic J. Garcia JL. Vina J. Role of nuclear glutathione as a key regulator of cell proliferation. Mol Aspects Med. 2009;30:77–85. doi: 10.1016/j.mam.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Pani G. Colavitti R. Borrello S. Galeotti T. Redox regulation of lymphocyte signaling. IUBMB Life. 2000;49:381–389. doi: 10.1080/152165400410227. [DOI] [PubMed] [Google Scholar]
- 19.Peterson JD. Herzenberg LA. Vasquez K. Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pruett SB. Obiri N. Kiel JL. Involvement and relative importance of at least two distinct mechanisms in the effects of 2-mercaptoethanol on murine lymphocytes in culture. J Cell Physiol. 1989;141:40–45. doi: 10.1002/jcp.1041410107. [DOI] [PubMed] [Google Scholar]
- 21.Sato H. Shiiya A. Kimata M. Maebara K. Tamba M. Sakakura Y. Makino N. Sugiyama F. Yagami K. Moriguchi T. Takahashi S. Bannai S. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280:37423–37429. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- 22.Sato H. Tamba M. Ishii T. Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 23.Shih AY. Erb H. Sun X. Toda S. Kalivas PW. Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci. 2006;26:10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth MJ. Glutathione modulates activation-dependent proliferation of human peripheral blood lymphocyte populations without regulating their activated function. J Immunol. 1991;146:1921–1927. [PubMed] [Google Scholar]
- 25.Srivastava MK. Sinha P. Clements VK. Rodriguez P. Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sturman JA. Beratis NG. Guarini L. Gaull GE. Transsulfuration by human long term lymphoid lines: normal and cystathionase-deficient cells. J Biol Chem. 1980;255:4763–4765. [PubMed] [Google Scholar]
- 27.Vitvitsky V. Thomas M. Ghorpade A. Gendelman HE. Banerjee R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem. 2006;281:35785–35793. doi: 10.1074/jbc.M602799200. [DOI] [PubMed] [Google Scholar]
- 28.Yan Z. Banerjee R. Redox remodeling as an immunoregulatory strategy. Biochemistry. 2010;49:1059–1066. doi: 10.1021/bi902022n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Z. Garg SK. Kipnis J. Banerjee R. Extracellular redox modulation by regulatory T cells. Nat Chem Biol. 2009;5:721–723. doi: 10.1038/nchembio.212. [DOI] [PMC free article] [PubMed] [Google Scholar]