Abstract
Objective
To estimate if echogenic bowel diagnosed on second-trimester ultrasound has an independent risk association with intrauterine growth restriction (IUGR) and intrauterine fetal demise.
Methods
This is a retrospective cohort study of all patients with singleton gestations who presented to our institution for second-trimester ultrasound between 1990 and 2008. Study groups were defined by the presence or absence of echogenic bowel. Primary outcomes were IUGR, defined as birth weight less than the 10th percentile, and intrauterine fetal demise at 20 weeks or more of gestation. Univariable and multivariable logistic regression analyses were used to estimate the risk of intrauterine fetal demise and IUGR in fetuses with echogenic bowel. Analyses were repeated after excluding cases of aneuploidy, cytomegalovirus (CMV) infection, other major congenital anomalies, and abnormal second-trimester serum screening results.
Results
Of 64,048 patients, the incidence of echogenic bowel was 0.4%. Of these, echogenic bowel was an isolated finding in 188 (72.3%) cases. There were 579 (0.9%) cases of intrauterine fetal demise and 8,173 (12.8%) cases of IUGR in the entire cohort. After excluding cases of aneuploidy and CMV infection, the incidence of IUFD was 7.3% in the echogenic bowel group compared to 0.9% in the non-echogenic bowel group, translating to an absolute risk increase (ARI) of 6.4%. The incidence of IUGR in the echogenic bowel group was 19.5% compared to 12.9% in the non-echogenic bowel group (ARI=6.6%). After controlling for potential confounders, echogenic bowel was signficantly associated with both IUFD (aOR 9.6, 95% CI 5.8–15.9) and IUGR (aOR 2.1, 95% CI 1.5–2.9) This risk association remained significant even when evaluating echogenic bowel as an isolated sonographic finding.
Conclusion
The presence of echogenic bowel on ultrasound is independently associated with an increased risk for both IUGR and IUFD. Serial growth assessment and antenatal testing may be warranted in these patients.
Introduction
The prevalence of echogenic bowel on routine second-trimester ultrasound ranges from 0.2 to 1.8%. (1) The differential diagnosis for this finding is broad and includes normal variant, primary gastrointestinal pathology, congenital viral infection, cystic fibrosis (CF), aneuploidy, and intra-amniotic bleeding. (2–7) Previous reports have also suggested an increased incidence of intrauterine growth restriction (IUGR) and intrauterine fetal demise (IUFD) in fetuses with echogenic bowel, with reported incidences ranging from 14.0–23.3% for IUGR and from 3.8–8.0% for IUFD. (7–11) Fetal redistribution of blood flow to vital organs with resultant bowel hypoperfusion and ischemia is the proposed explanation for the hyperechoic appearance of the fetal bowel in these situations. (8,10,12)
Despite these associations, prior studies have not been able to estimate a risk for each of these adverse outcomes with any precision. This is likely due to the overall rarity of the primary outcomes in relation to the small number of cases of echogenic bowel observed in these studies. Estimating these risks will be useful in counseling patients and may also provide insight into the appropriate antenatal management strategy for these patients. The aims of this study were to estimate the risk of IUGR and IUFD associated with the finding of echogenic bowel on second-trimester ultrasound using a robust ultrasound/genetics database and then to further refine those risk estimates by performing sub-analyses excluding fetuses with chromosomal abnormalities, congenital infections, and associated anomalies.
Materials and Methods
This is a retrospective cohort study of consecutive patients with singleton gestations referred to Washington University Medical Center in St. Louis for second-trimester ultrasound between 1990 and 2008. Study approval was obtained from the institutional review board at Washington University. Demographic information, maternal medical and obstetrical history, indication for ultrasound, ultrasound findings, and any genetic screening or diagnostic results are entered into a perinatal database at the time of the ultrasound for all patients seen at our institution. All pregnancy and neonatal outcome information is also routinely obtained and entered into this database by dedicated outcome coordinators. For the greater than 90% of patients who deliver within our healthcare system, outcome information is obtained from the electronic medical record. The small remainder of patients who do not deliver within our healthcare system are given a standardized questionnaire regarding pregnancy outcome and neonatal follow up. If a questionnaire is not returned within 4 weeks from the anticipated date of delivery, the patient is then contacted by telephone. In cases where a patient cannot be reached, the referring provider is contacted to obtain outcome information. Patients with multiple gestations and those with incomplete outcome information were excluded from the study.
The primary exposure in this study was the presence of fetal echogenic bowel, defined as bowel with a sonographic density equal to or greater than that of surrounding bone, at the time of second- trimester ultrasound. (Figure 1) The frequency of the ultrasound transducer ranged from 3.5–5.0 MHz, depending on maternal body habitus. In cases where the bowel was suspected to be echogenic, the ultrasound gain was turned down as low as possible. If the bowel continued to meet this criterion, then the diagnosis of echogenic bowel was made. All diagnoses of echogenic bowel were confirmed by a maternal-fetal medicine sub-specialist at the time of the ultrasound. Our primary outcomes of interest included IUGR and IUFD. IUGR was defined as birth weight less than the 10th percentile, using national standards derived by the Alexander growth curve. (13) IUFD was defined as fetal death at 20 weeks or more of gestation.
Figure 1.
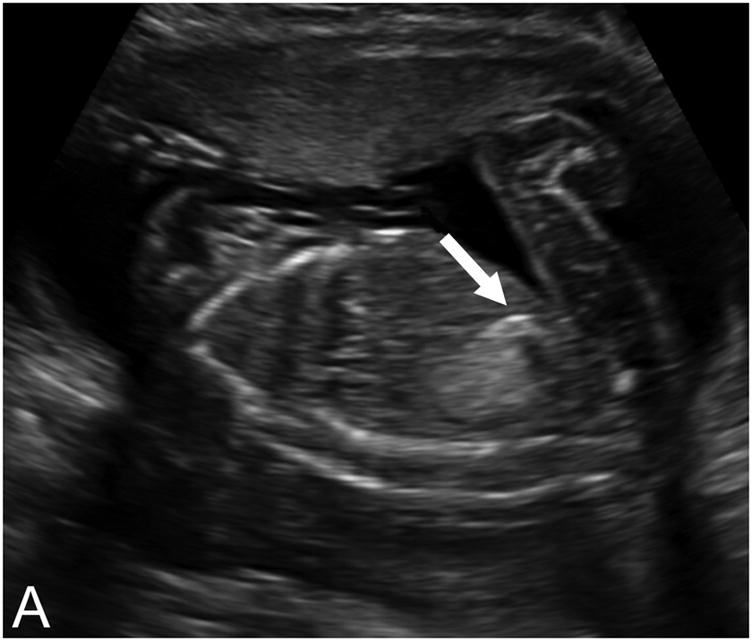

Echogenic bowel diagnosed on second-trimester ultrasound exam. A) Sagittal view; B) axial view.
Baseline characteristics as well as the incidence of the primary outcomes were compared between patients with and without fetal echogenic bowel. Student’s t tests were used to compare continuous variables, and chi-square and Fisher’s exact tests were used to compare categorical variables. The incidence of aneuploidy, cytomegalovirus (CMV) infection, CF, and associated anomalies in patients with fetal echogenic bowel was determined. Univariable analysis was used to estimate the relative risk (RR) of the primary outcomes. Multivariable logistic regression was then used to further define these risks, controlling for potential confounding factors. Factors identified as significant in the univariable analysis as well as those with biologic plausibility or historic significance were considered in the logistic regression models. Non-significant variables were removed from the models in a backwards, stepwise fashion to obtain adjusted odds ratios (aOR) and 95% confidence intervals (CI). Differences in the hierarchical models were tested using the likelihood ratio test or Wald test. Only variables that were statistically significant were included in the final model. To further examine the association between echogenic bowel and IUFD, stratified analyses based on the presence or absence of IUGR were also performed.
Given the known risk of IUGR and IUFD in patients with aneuploidy and/or CMV infection, a sub-analysis was performed after excluding these cases. Our institutional standard for the evaluation of echogenic bowel found on second-trimester ultrasound includes patient counseling toward invasive diagnostic testing for aneuploidy and congenital infection as well as parental CF carrier status determination. Patients who accept amniocentesis have their amniotic fluid sent for karyotype as well as CMV, toxoplasmosis, and parvovirus polymerase chain reaction (PCR). Patients who decline amniocentesis are offered maternal serologic testing for congenital infection, recognizing that this is not diagnostic. The finding of echogenic bowel is also relayed to the pediatricians for post-natal evaluation which includes CMV PCR from a neonatal specimen such as urine or saliva. Postnatal karyotype is also sent if newborn exam findings are suspicious for aneuploidy. Cases without a documented newborn exam were considered to have an unknown chromosomal status and were also excluded in this sub-analysis. CF cases were not excluded from the sub-analysis as there is currently no known evidence-based increased risk for IUGR or IUFD in these patients.
Finally, in order to evaluate the primary outcomes in relationship to cases of isolated echogenic bowel, an additional sub-analysis was performed after excluding all major structural anomalies. Given the association between abnormal serum analytes and adverse pregnancy outcomes, particularly elevated maternal serum alpha-fetoprotein (MSAFP) in cases of echogenic bowel, the analysis was also repeated after excluding all patients with abnormal second-trimester serum screening results. (8,10) As the approach to serum screening has evolved over the 18-year period of this study, an abnormal second-trimester serum screen was defined as 1) an abnormal triple screen (β-hCG, estriol, and MSAFP), 2) an abnormal quadruple screen (β-hCG, estriol, MSAFP, and inhibin), or 3) an isolated abnormal MSAFP result.
The approach to the primary and sub-analyses is outlined in Figure 2. P-values <0.05 were considered statistically significant. All statistical analyses were performed using STATA 10, Special Edition (College Station, TX).
Figure 2.
Flow chart showing the approach to the analysis. *Sequential subanalysis with serial exclusions listed.
Results
Of 72,368 patients in the cohort, complete pregnancy outcome data was available for 64,048 patients (88.5%). There were 260 cases of echogenic bowel, giving an incidence of 0.4% in our patient population. Of these 260 cases of echogenic bowel, there were 13 cases (5%) of trisomy 21, 1 case (0.4%) of trisomy 18, 2 cases (0.8%) of trisomy 13, 1 case (0.4%) of chromosomal mosaicism, 5 documented cases (1.9%) of CMV infection, 6 documented cases (2.3 %) of CF, and 1 case (0.4%) of both documented CF and chromosomal mosaicism. Of the remaining fetuses with echogenic bowel, 43 cases had associated anomalies, and 188 (72.3%) cases had echogenic bowel as an isolated finding. There were 579 cases (0.9%) of intrauterine fetal demise (IUFD) and 8,173 cases (12.8%) of intrauterine growth restriction (IUGR) in the study cohort. Maternal demographics and pregnancy characteristics for our population are shown in Table 1. Compared to the 260 patients with echogenic bowel, the 63,788 patients without echogenic bowel were of similar age, gravidity, parity, gestational age at the time of ultrasound, and also had similar rates of tobacco use, chronic hypertension, pre-eclampsia, pre-existing diabetes mellitus, and gestational diabetes. However, patients with a diagnosis of echogenic bowel were statistically more likely to be of Caucasian race. While patients with a diagnosis of echogenic bowel were more likely to have reported vaginal bleeding during the pregnancy, only 15% of patients with echogenic bowel who actually experienced an IUFD reported a positive history of vaginal bleeding. 47.3% of the echogenic bowel cases were diagnosed in the first 9 years of the study, and 52.7% were diagnosed during the subsequent 9 years.
Table 1.
Maternal demographics and pregnancy characteristics comparing patients with echogenic bowel to those without echogenic bowel diagnosed on second-trimester ultrasound
| Variable | Echogenic Bowel (n=260) | Non-Echogenic Bowel (n=63,788) | p-value |
|---|---|---|---|
| Mean Maternal Age (years) | 29.5 ± 6.2 | 30.2 ± 6.3 | 0.10 |
| Mean Gestational Age at Exam (weeks) | 18.4 ± 1.8 | 18.5 ± 1.7 | 0.62 |
| Mean Gravidity | 2.7 ± 1.7 | 2.7 ± 1.6 | 0.68 |
| Mean Parity | 1.1 ± 1.1 | 1.2 ± 1.1 | 0.88 |
| Caucasian Race | 73% | 63% | <0.001 |
| African American Race | 11% | 21% | <0.001 |
| Tobacco Exposure | 15% | 11% | 0.07 |
| Alcohol Exposure | 21% | 19% | 0.57 |
| Maternal Chronic Hypertension | 1% | 2% | 0.19 |
| Maternal Pre- gestational Diabetes | 0.4% | 2% | 0.09 |
| Pre-eclampsia | 7% | 7% | 0.75 |
| Gestational Diabetes | 3% | 5% | 0.08 |
| History of Vaginal Bleeding | 9% | 6% | 0.01 |
Patients with echogenic bowel were at an increased risk for IUFD compared to those without echogenic bowel (RR 8.6, 95% CI 5.6–13.2). This association remained statistically significant even after adjusting for African American race (aOR 10.3, 95% CI 6.5–16.5). Similarly, patients with echogenic bowel were also at an increased risk for IUGR compared to those without echogenic bowel (RR 1.6, 95% CI 1.3–2.1). After adjusting for African American race, tobacco use, pre-eclampsia and pre-existing diabetes mellitus, echogenic bowel remained associated with an increased risk for IUGR (aOR 2.1, 95% CI 1.5–3.0).
In order to further delineate this relationship, all patients with documented cases of CMV infection, aneuploidy, or unknown chromosomal status were excluded, leaving 50,171 patients for analysis. Results from this sub-analysis are shown in Table 2. The association between echogenic bowel and both IUFD (aOR 9.6, 95% CI 5.8–15.9) and IUGR (aOR 2.1, 95% CI 1.5–2.9) remained statistically significant after controlling for potential confounders. The median gestational age at the time of IUFD in the patients with echogenic bowel was 24.1 weeks (range 20.1–30.0 weeks). Given the well-established association between IUGR and IUFD, the relationship between echogenic bowel and IUFD was also evaluated after stratifying patients based on the presence or absence of IUGR. While the risk for IUFD was four-fold higher in the growth-restricted fetuses, there still remained a statistically significant risk of IUFD (RR 6.2, 95% CI 2.0–19.2) in those fetuses whose growth was appropriate for gestational age. Given the relatively small number of fetal demise cases in each stratum, an adjusted analysis could not reliably be performed.
Table 2.
Unadjusted and Adjusted Risk Estimates for IUGR and IUFD in Fetuses With Echogenic Bowel Compared to Those Without Echogenic Bowel After Excluding Cases of Aneuploidy and CMV Infection
| Incidence in EB group (n=231) | Incidence in Non-EB group (n=49,940) | Unadjusted RR (95% CI) | aOR (95% CI) | p-value | |
|---|---|---|---|---|---|
| IUGR | 19.5% | 12.9% | 1.6 (1.2–2.0) | 2.1* (1.5–2.9) | <0.001 |
| IUFD | 7.3% | 0.9% | 8.0 (5.0–12.8) | 9.6† (5.8–15.9) | <0.001 |
| IUFD+IUGR | 6.1% | 0.2% | 27.5 (16.0–47.2) | --- | --- |
| IUFD-IUGR | 1.3% | 0.2% | 6.2 (2.0–19.2) | --- | --- |
Adjusted for African American race, tobacco use, pre-eclampsia and pre-gestational diabetes
Adjusted for African American race
P-values are from the Wald test of the adjusted odds ratios.
IUGR=intrauterine growth restriction; IUFD=intrauterine fetal demise; EB=echogenic bowel; CMV=cytomegalovirus; RR=relative risk; aOR=adjusted odds ratio; CI=confidence interval
Finally, in order to estimate the risk of IUFD and IUGR in patients with isolated echogenic bowel, all patients with major anomalies (n=1,180) were excluded from the cohort. There were 188 cases of isolated echogenic bowel in the remaining patient population. Patients with isolated echogenic bowel were at increased risk for IUGR compared to those with no major fetal anomalies (19.7% versus 12.9%; aOR 2.1, 95% CI 1.4–3.0) after adjusting for African American race, tobacco use, pre-existing diabetes mellitus and pre-eclampsia. Patients with isolated echogenic bowel were also at increased risk for IUFD compared to those with no major fetal anomalies (5.3% versus 0.8%; aOR 7.4, 95% CI 3.9–14.1). After stratification, this risk association remained significant in both the growth-restricted and appropriate for gestational age groups. In the final sub-analysis, all patients with abnormal second-trimester serum screening results (n=5,148) were excluded. The positive risk association between isolated echogenic bowel and both IUGR and IUFD remained significant in patients with normal serum screening results. (Table 3)
Table 3.
Unadjusted and Adjusted Risk Estimates for IUGR and IUFD in Fetuses With Isolated Echogenic Bowel (excluding cases of aneuploidy, CMV infection and major anomalies)
| All cases of isolated echogenic bowel | |||||
|---|---|---|---|---|---|
| Incidence in EB group (n=188) | Incidence in Non-EB group (n=48,803) | Unadjusted RR (95% CI) | aOR (95% CI) | p-value | |
| IUGR | 19.7% | 12.9% | 1.6 (1.2–2.1) | 2.1* (1.4–3.0) | <0.001 |
| IUFD | 5.3% | 0.8% | 6.3 (3.4–11.5) | 7.4† (3.9–14.1) | <0.001 |
| IUFD+IUGR | 4.2% | 0.2% | 21.7 (10.7–44.0) | --- | --- |
| IUFD-IUGR | 1.3% | 0.2% | 5.8 (1.4–23.5) | --- | --- |
| Cases of isolated echogenic bowel with normal serum screening results | |||||
| Incidence in EB group (n=166) | Incidence in Non-EB group (n=43,677) | Unadjusted RR (95% CI) | aOR (95% CI) | p-value | |
| IUGR | 18.0% | 12.8% | 1.4 (1.1–2.0) | 1.9* (1.2–2.9) | 0.002 |
| IUFD | 4.2% | 0.8% | 5.3 (2.5–11.0) | 6.2† (2.9–13.4) | <0.001 |
| IUFD+IUGR | 3.0% | 0.2% | 17.3 (7.1–42.3) | --- | --- |
| IUFD-IUGR | 1.2% | 0.2% | 6.4 (1.6–25.9) | --- | --- |
Adjusted for African American race, tobacco use, pre-eclampsia and pre-gestational diabetes
Adjusted for African American race
P-values are from the Wald test of the adjusted odds ratios.
IUGR=intrauterine growth restriction; IUFD=intrauterine fetal demise; EB=echogenic bowel; RR=relative risk; aOR=adjusted odds ratio; CI=confidence interval
The median gestational age at the time of IUFD in patients with isolated echogenic bowel was 23.7 weeks (range 20.1–27.0 weeks) compared to 23.6 weeks (range 20.1–40.6 weeks) in patients without isolated echogenic bowel. (p=0.80)While the median gestational age at the time of IUFD was not significantly different between these groups, the range of gestational ages at which IUFD occurred was much wider in those without isolated echogenic bowel. Of the 149 patients with isolated echogenic bowel who did not have either IUGR or an IUFD, 93 (62.4%) had resolution of the echogenic bowel on follow-up ultrasound, 18 (12.1%) had no resolution, and 38 (25.5%) did not have a follow-up ultrasound at our institution.
Discussion
Our study demonstrates that the finding of echogenic bowel on second-trimester ultrasound, even when isolated, is associated with an increased risk for both IUGR and IUFD. Using a rigorous stepwise approach to the analysis, we observed a two-fold increase in IUGR in patients with both isolated echogenic bowel as well as in patients with associated anomalies and an even more substantial risk of IUFD in these patients.
Using a large retrospective cohort of patients from our institution’s 18-year experience, our study was able to evaluate adverse outcomes and quantify risks for IUGR and IUFD in pregnancies with fetal echogenic bowel diagnosed on a second-trimester ultrasound. In 1993, Nyberg et al. reported a 6.5-fold increased risk for adverse outcomes in 95 fetuses with echogenic bowel compared to 110 control fetuses. This relative risk decreased to 4.9, but remained statistically significant, when evaluating only isolated cases of echogenic bowel. (7) While this study did produce quantitative estimates, their definition of adverse outcome was a composite which included aneuploidy and CF in addition to IUGR and IUFD. Our study demonstrates that the association between echogenic bowel and IUGR and IUFD persists even when patients with other risk factors are excluded from the analysis. This suggests an association which is independent of aneuploidy, congenital infection, and associated anomalies.
In a case-control study of 156 fetuses diagnosed with echogenic bowel before 24 weeks’ gestation, IUGR, oligohydramnios, and an elevated MSAFP level were observed more frequently in cases of fetal demise compared to live-born infants. Despite these trends, only oligohydramnios and elevated MSAFP levels were found to be independently associated with IUFD in their logistic regression model. (10) In contrast, IUGR was present in 2 out of 4 (50%) cases of fetal demise in a prospective study by Ghose et al. (11) Findings from our stratified analysis demonstrated a significant risk association between echogenic bowel, both isolated and non-isolated, and IUFD regardless of the presence or absence of fetal growth restriction. These findings suggest that the presence of IUGR does not completely account for the increased IUFD risk in patients with echogenic bowel, although causal inferences should only be made with caution in observational studies such as ours. A similar phenomenon has also been observed in cases of gastroschisis in which perinatal death is postulated to occur through a pathway that is independent of fetal growth restriction. (14,15)
One of the most clinically important findings from our study is the early gestational age at which the fetal demises occurred in patients with echogenic bowel. The median gestational age of demise in the non-isolated group was 24.1 weeks versus 23.7 weeks in the isolated group. Of note, all fetal demises occurred before 30 weeks’ gestation in those with echogenic bowel in our population. Al-Koutaly et al. observed a similar median gestational age of fetal demise (22.0 weeks), although the upper limit of the gestational age range in that study was 39 weeks. (10) Our finding certainly has clinical implications for the antenatal monitoring of these fetuses. Given that most antenatal surveillance protocols do not begin until 28–32 weeks’ of gestation, this may preclude the use of antenatal surveillance as a means to identify and intervene on those fetuses at risk for imminent demise.
In patients with CF and/or aneuploidy, the characteristic ultrasound appearance of echogenic bowel is thought to be due to thickened, viscous meconium caused by decreased levels of microvillar enzymes in the gut and hypoperistalsis. (7,16,17) Ewer et al. proposed that echogenic bowel may also be a reflection of intrauterine gut ischemia, an effect that may be observed in growth-restricted fetuses who are exhibiting a “brain-sparing” circulatory response with resultant hypoperfusion of the splanchnic vessels. (12) More recently, Doppler studies of the splanchnic circulation in growth-restricted fetuses with echogenic bowel have demonstrated the opposite effect, a relative hyperperfusion of the fetal gut circulation represented by a low pulsatility index in both the celiac trunk and superior mesenteric artery.(18) While the precise mechanism is still unclear, it appears that abnormal vascular supply to the fetal gut contributes to the echogenic appearance of fetal bowel, thereby providing biologic plausibility to the association with IUGR observed in our study. In fetuses that experience IUFD in the absence of growth restriction, the mechanism is less clear. Prior studies have demonstrated that elevated MSAFP levels are associated with a worse prognosis in fetuses with echogenic bowel. (8,10) Elevated MSAFP levels can be seen in cases of intra-amniotic bleeding and may represent an alteration in the maternal-placental barrier. Al-Kouatly et al. speculated that echogenic bowel may appear before fetal demise in such cases, although only 1 of 9 patients (11.1%) with fetal demise in that study reported a history of vaginal bleeding during the pregnancy. (10) This is similar to the findings from our study in which only 15% of patients with echogenic bowel who had a subsequent IUFD reported a history of vaginal bleeding.
Strengths of our study include our robust ultrasound and genetics database from which obstetrical history, pregnancy outcome, and neonatal outcome data is extracted. Our large sample size allowed us to estimate risks for our primary outcomes, and our staged approach to the analysis further allowed us to determine whether these associations were independent of karyotype, congenital infection, other associated malformations, and abnormal second-trimester serum screening results. In addition, the incidence of aneuploidy, infection, and CF observed in our study is within similar ranges as those reported in prior studies, thereby validating our cohort.(5,10,11,19) Despite our large numbers, the analysis was still somewhat limited by the rarity of both the exposure and the outcome of IUFD, resulting in wide confidence intervals and preventing adjusted subgroup analysis.
Limitations of our study include its retrospective design in which data collection is limited to chart review and is contingent upon the correct coding of ultrasonographic findings and karyotype data. In addition, all patients in the cohort did not undergo invasive aneuploidy screening or testing for congenital infection or CF; however, our institution employs coordinators who are dedicated to obtaining neonatal outcomes; therefore, the possibility of missing an affected child diagnosed after birth is minimal. Furthermore, if there were any undiagnosed cases of CMV and aneuploidy in the non-echogenic bowel group, who did not undergo a standardized testing approach, our results would be biased towards the null hypothesis, producing an underestimation of association. If this misclassification bias does exist in our cohort, then our reported estimates of risk may actually be conservative. To further ensure the validity of our cohort, all patients with incomplete outcome information and unknown chromosomal status were excluded in the sub-analysis. Finally, echogenic bowel may be perceived as a somewhat subjective diagnosis. All ultrasounds in our unit are interpreted by a maternal-fetal medicine sub-specialist, and all ultrasound data is entered into our database prospectively at the time of the exam. Again, if misclassification of echogenic bowel diagnoses did occur in our cohort, this would also bias our results towards the null hypothesis. When we divided our 18-year study cohort in half, we noticed a comparable proportion of echogenic bowel cases being diagnosed in the first and second halves of our study, indicating that the diagnosis has been stable over time as ultrasound technology and practice have advanced.
In conclusion, our large retrospective study confirms the association between echogenic bowel diagnosed on second-trimester ultrasound and an increased risk for IUGR and IUFD. Our study also demonstrates that this risk association is independent of karyotypic abnormalities and congenital infections and even persists when echogenic bowel is observed as an isolated finding. Serial growth ultrasounds may be warranted in these patients, and although antenatal surveillance may be considered, its value is inconclusive given the early gestational age at which fetal demises occur in these patients.
Acknowledgments
Dr. Goetzinger is supported by a training grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5 T32 HD055172–02) and from a NIH/NCRR Washington University ICTS grant (UL1 RR024992). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented, in part, as a poster presentation at the 31st Annual Meeting of the Society for Maternal-Fetal Medicine on February 11, 2011 in San Francisco, CA.
References
- 1.Bronshtein M, Blazer S, Zimmer EZ. The Gastrointestinal Tract and Abdominal Wall. In: Callen PW, editor. Ultrasonography in Obstetrics and Gynecology. 5. Philadelphia: Saunders Elsevier; 2008. pp. 587–639. [Google Scholar]
- 2.Fakhary J, Reiser M, Shapiro LR, Scheuchter A, Pait LP, Glennon A. Increased echogenicity in the lower fetal abdomen: A common normal variant in the second trimester. J Ultrasound Med. 1986;5:489–92. doi: 10.7863/jum.1986.5.9.489. [DOI] [PubMed] [Google Scholar]
- 3.Caspi B, Elchalal U, Lancet M, Chemke J. Prenatal diagnosis of cystic fibrosis. Ultrasonographic appearance of meconium ileus in the fetus. Prenat Diagn. 1998;8:379–82. doi: 10.1002/pd.1970080508. [DOI] [PubMed] [Google Scholar]
- 4.Forouzan I. Fetal abdominal echogenic mass: An early sign of intrauterine cytomegalovirus infection. Obstet Gynecol. 1994;83:647–51. [PubMed] [Google Scholar]
- 5.Strocker AM, Snijders RJ, Carlson DE, Greene N, Gregory KD, Walla CA, Platt LD. Fetal echogenic bowel: parameters to be considered in the differential diagnosis. Ultrasound Obstet Gynecol. 2000;16:519–23. doi: 10.1046/j.1469-0705.2000.00241.x. [DOI] [PubMed] [Google Scholar]
- 6.Sepulveda W, Reid R, Nicolaidis P, Prendiville O, Chapman RS, Fisk NM. Second-trimester echogenic bowel and intraamniotic bleeding: association between fetal bowel echogenicity and amniotic fluid spectrophotometry at 410 nm. Am J Obstet Gynecol. 1996;174:839–42. doi: 10.1016/s0002-9378(96)70310-1. [DOI] [PubMed] [Google Scholar]
- 7.Nyberg DA, Dubinsky T, Resta RG, Mahony BS, Hickok DE, Luthy DA. Echogenic fetal bowel during the second trimester: clinical importance. Radiology. 1993;188:527–31. doi: 10.1148/radiology.188.2.8327709. [DOI] [PubMed] [Google Scholar]
- 8.Achiron R, Seidman D, Horowitz A, Mashiach S, Goldman B, Lipitz S. Hyperechoic fetal bowel and elevated serum alpha-fetoprotein: a poor fetal prognosis. Obstet Gynecol. 1996;88:368–71. doi: 10.1016/0029-7844(96)00162-7. [DOI] [PubMed] [Google Scholar]
- 9.MacGregor SN, Tamura R, Sabbagha R, Brenhofer JK, Kambich MP, Pergament E. Isolated hyperechoic fetal bowel: significance and implications for management. Am J Obstet Gynecol. 1995;173:1254–8. doi: 10.1016/0002-9378(95)91365-3. [DOI] [PubMed] [Google Scholar]
- 10.Al-Kouatly HB, Chasen ST, Karam AK, Ahner R, Chervenak FA. Factors associated with fetal demise in fetal echogenic bowel. Am J Obset Gynecol. 2001;185:1039–43. doi: 10.1067/mob.2001.117641. [DOI] [PubMed] [Google Scholar]
- 11.Ghose I, Mason GC, Martinez D, Harrison L, Evans JA, Ferriman EL, Stringer MD. Hyperechogenic fetal bowel: a prospective analysis of sixty consecutive cases. BJOG. 2000;107:426–9. doi: 10.1111/j.1471-0528.2000.tb13242.x. [DOI] [PubMed] [Google Scholar]
- 12.Ewer AK, McHugo J, Chapman S, Newwell SJ. Fetal echogenic gut: a marker of intrauterine gut ischemia? Arch Dis Child. 1993;69:510–3. doi: 10.1136/adc.69.5_spec_no.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 14.Towers CV, Carr MH. Antenatal fetal surveillance in pregnancies complicated by gastroschisis. Am J Obstet Gynecol. 2008;198:686e1–5. doi: 10.1016/j.ajog.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Adair CD, Rosnes J, Frye AH, Burrus DR, Nelson LH, Veille JC. The role of antepartum surveillance in the management of gastroschisis. Int J Gynaecol Obstet. 1996;52:141–4. doi: 10.1016/0020-7292(95)02551-0. [DOI] [PubMed] [Google Scholar]
- 16.Scioscia AL, Pretorius DH, Burdorick NE, Cahill TC, Axelrod FT, Leopold GR. Second-trimester echogenic bowel and chromosomal abnormalities. Am J Obstet Gynecol. 1992;167:889–94. doi: 10.1016/s0002-9378(12)80007-x. [DOI] [PubMed] [Google Scholar]
- 17.Brock DJH. A comparative study of microvillar enzyme activities in the prenatal diagnosis of cystic fibrosis. Prenat Diagn. 1985;5:129–34. doi: 10.1002/pd.1970050206. [DOI] [PubMed] [Google Scholar]
- 18.Achiron R, Mazkereth R, Orvieto R, Kuint J, Lipitz S, Rotstein Z. Echogenic bowel in intrauterine growth restriction fetuses: Does this jeopardize the gut? Obstet Gynecol. 2002;100:120–4. doi: 10.1016/s0029-7844(02)02038-0. [DOI] [PubMed] [Google Scholar]
- 19.Scotet V, Dugueperoux I, Audrezet MP, Audebert-Bellanger S, Muller M, Blayau M, Ferec C. Focus on cystic fibrosis and other disorders evidenced in fetuses with sonographic finding of echogenic bowel: 16-year report from Brittany, France. Am J Obstet Gynecol. 2010;203:592e1–6. doi: 10.1016/j.ajog.2010.08.033. [DOI] [PubMed] [Google Scholar]



