Abstract
Despite a complex cascade of cellular events to reconstruct damaged extracellular matrix, ligament healing results in a mechanically inferior, scar-like tissue. During normal healing the number of macrophages significantly increases within the wound site. Then, granulation tissue expands into any residual, normal ligamentous tissue (creeping substitution), resulting in a larger region of healing, greater mechanical compromise, and an inefficient repair process. To study the effects of macrophages on the repair process, rats underwent bilateral, surgical rupture of their medial collateral ligaments. Treatment animals received liposome-encapsulated clodronate 2 days before rupture to ablate phagocytosing macrophages. Ligaments were then collected at day 5, 11, and 28 for immunohistochemistry and/or mechanical testing. Clodronate treatment reduced both the M1 and M2 macrophages at day 5 and altered early healing. However, the macrophages effectively returned to control levels after day 5 and reinitiated a wound healing response. Our results suggest that an early macrophage response, which is necessary for debridement of damaged tissue in the wound, is also important for cytokine release to mediate normal repair processes. Additionally, non-specific inhibition of macrophages (without regard to specific macrophage populations) can control excessive granulation tissue formation but is detrimental to early matrix formation and ligament strength.
Keywords: ligament healing, clodronate, macrophage, immunohistochemistry, rat
Introduction
Normal ligament healing after rupture undergoes inflammation, proliferation, and remodeling. Initially neutrophils, macrophages, and T-lymphocytes infiltrate the wound followed by fibroblasts, myofibroblasts, endothelial cells and additional macrophages. These cells form granulation tissue which expands to remodel residual extracellular matrix (ECM), i.e. “creeping substitution”, damaging intact portions of the ligament contributing to an inefficient healing response.2 As healing continues, type I procollagen decreases while type III collagen increases (manuscript in review). The newly formed type III collagen serves as a weak transient bridge for the injured ligament. As remodeling continues, the scar matures and the ratio of type I to type III collagen normalizes, improving the tensile strength of the compromised region, but the ligament never fully returns to its original values.13, 14 In an improved healing scenario granulation tissue would develop without excessive creeping substitution, collagen type I would regenerate rapidly and native tissue would regenerate to demonstrate pre-damage laxity and strength.
Macrophages are a heterogeneous group of cells having different phenotypes and functions. During creeping substitution, macrophages accumulate within the healing region to play a central role in wound healing via phagocytosis of cellular debris, apoptosis, inflammatory cell and myofibroblast recruitment, angiogenesis regulation, and scar tissue formation. Three distinct populations of activated macrophages are known to exist: classically-activated, alternatively activated, and regulatory macrophages. Classically activated (M1) macrophages participate in cellular debris clearance, host defense, and pro-inflammatory cytokine release. Alternatively activated (M2) macrophages less effectively kill pathogens, but instead secrete components of extracellular matrix (ECM), produce anti-inflammatory cytokines, and are involved primarily in the wound healing response. Regulatory (type II) macrophages have been recently distinguished from the M2 phenotype. These type II macrophages retain the ability to produce pro-inflammatory cytokines, but they simultaneously inhibit inflammation. They do not contribute to ECM production and are considered pertinent in immune regulation. Collectively, the macrophage populations form a carefully balanced regulatory network for the immune responses as the healing process progresses. Although the multifunctional role of macrophages is generally well reported, a more detailed understanding of their intricate role in ligament healing could generate new therapeutic approaches and clinical treatments.
The beneficial influence of macrophages during healing is controversial. Many suggest that the invading cells are detrimental to regeneration.3, 48, 10, 23 The presence of macrophages and their resultant cytokines have been linked to chronic inflammation and tissue fibrosis.5, 8, 12 Conversely, embryonic wounds heal scar-free, repairing without the presence of immune cells.1, 9 Our prior research correlating macrophages with creeping substitution and delayed/impaired ligament healing strongly suggested that macrophages significantly affect healing in the ligament.2 The objective of this study was to determine the functional and biological relevance of macrophages during ligament healing using a surgically disrupted medial collateral ligament (MCL) rat model. We hypothesized that eliminating the macrophage populations after injury would alter inherent ligament wound healing and subsequent remodeling. Clodronate, a well-known inhibitor of macrophages, was liposome-encapsulated and administered i.v. to rats. Non-encapsulated clodronate is nontoxic, does not cross cell membranes and has an extremely short half-life in the circulation. In vivo administration of clodronate-encapsulated liposomes results in the phagocytosis of liposomes by the macrophage/monocytes. Lysosomal enzymes of the macrophages/monocytes degrade the liposome and macrophages undergo apoptosis after exposure to clodronate, creating a macrophage-depleted tissue. After successful macrophage depletion, alterations in the ligament healing response were determined.
Materials and Methods
Animals
This study was approved by the University of Wisconsin Institutional Animal Use and Care Committee. Thirty-eight skeletally mature male Wistar rats (275–299 g) were used as an animal model for ligament healing. The medial collateral ligament (MCL) was chosen as a model for extra-capsular ligament healing. All rats were purchased with fitted external jugular catheters to facilitate i.v. treatment administration.
Surgical Procedure
Rats were separated into 2 treatment groups (n=19 rats/treatment group). Two days prior to surgery, animals were administered 1 ml/100 g body weight liposome-encapsulated clodronate (Clod-Lip) or liposome-encapsulated PBS which serves as the control (PBS-Lip). All rats were then subjected to bilateral transection (day 0) of the MCL. Rats were anesthetized via isofluorane. A surgically transected, rather than torn, MCL was used as an experimental model to create a uniform defect for healing. A 1 cm skin incision was made over the medial aspect at both the left and right stifles. The subcutaneous tissue was dissected to expose the sartorius muscle and underlying MCL. The mid-point of the MCL (determined using a scaled scalpel handle) was completely transected and the muscular, subcutaneous and subdermal tissue layers were each closed with 4-0 Dexon suture. All animals were allowed unrestricted cage movement immediately after surgery. At 5, 11, and 28 days post-injury, animals were sacrificed and the MCLs collected. MCLs were assayed via immunohistochemistry and mechanical testing. Three animals per treatment were used for immunohistochemistry and histology, and five animals per treatment were used for mechanical testing at each time point except for day 5. Mechanical testing was not performed on day 5 because the ligament is too structurally compromised for meaningful data with our methods of in vitro testing.
Macrophage Depletion
Circulating monocytes/macrophages were reduced in vivo using clodronate (dichloromethylene bisphosphonate). Clodronate was a gift of Roche Diagnostics GmbH (Mannheim, Germany) and was encapsulated into liposomes as previously described.24 Animals received one intravenous injection (1 ml/100 g body weight) of liposome-encapsulated clodronate or liposome-encapsulated PBS, 2 days prior to injury.
Tissue harvest
At the time of sacrifice the MCLs used for immunohistochemistry (IHC) were carefully dissected, measured, weighed, and immediately placed in optimal cutting temperature medium (OCT) for flash freezing. Longitudinal cryosections were then cut at a 5 µm thickness, mounted on Superfrost plus microscope slides and maintained at −70 C. Animals used for mechanical testing, were sacrificed and limbs were stored in toto at −70 C until used.
Histology
Ligament cryosections were H&E stained to observe general morphology of the healing ligaments. After staining, images were captured and the granulation tissue regions were measured using Image J (National Institutes of Health, NIH). Granulation tissue area was normalized to the total MCL area and expressed as the percent normalized granulation tissue.
Immunohistochemistry/Immunofluorescence (IHC/IF)
Immunostaining was performed on frozen sections using mouse monoclonal or rabbit polyclonal antibodies. Cryosections were fixed 10 minutes with acetone, exposed 5 minutes to 3% hydrogen peroxide to eliminate endogenous peroxidase activity, blocked 30 minutes with Background Buster (Innovex Biosciences, Richmond, CA) and incubated with rabbit or mouse primary antibody. Sections were then incubated with biotin, and streptavidin-conjugated to horseradish peroxidase using the Stat Q staining kit (Innovex Biosciences, Richmond, CA). The bound antibody complex was then visualized using diaminobenzidine (DAB). Stained sections were dehydrated, cleared, cover-slipped and viewed using light microscopy. M1 macrophages were also stained using double immunoflourescence (IF) to compare total day 5 cell numbers with M1 macrophages. For IF, sections were stained with mouse monoclonal antibody CD68, incubated with goat anti-mouse Alexa Fluor 555 secondary antibody (1:200, Invitrogen, Eugene, OR) and mounted in Prolong Gold anti-fade reagent containing DAPI (Invitrogen, Eugene, OR). Negative controls omitting the primary antibody were included with each experiment. Positive controls of gut or spleen were also included.
Mouse monoclonal antibodies to cell surface markers, CD68, CD163, and CD3 were utilized to identify the following leukocytes, respectively: classically activated macrophages (M1), alternatively activated macrophages (M2), and T-lymphocytes, (all from Abcam-Serotec, Raleigh, NC at a dilution of 1:100). Molecular markers for type II macrophages remain scarce and were not used in this study. To identify collagen production, type I procollagen (straight; SP1.D8; Developmental Hybridoma, Iowa City, Iowa) and type III collagen (1:8000, Sigma-Aldrich, St. Louis, MO) mouse antibodies were used. Endothelial cells were identified using the polyclonal rabbit antibody thrombomodulin (1:2500; American Diagnostica, Stamford, CT) and myofibroblasts were identified using α-smooth muscle actin (straight; Abcam-Serotec, Raleigh, NC).
Quantification
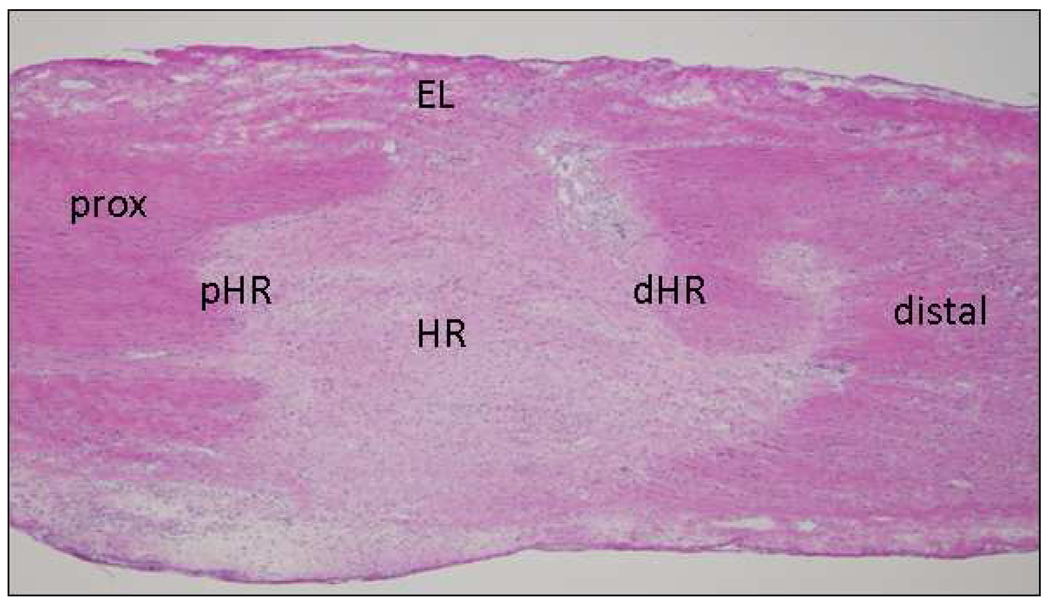
After IHC/IF staining, micrographs were collected using a camera assisted microscope (Nikon Eclipse microscope, model E6000 with an Olympus camera, model DP79). Six blocked random pictures were obtained from each stained cryosection. Images were captured at the healing region (HR), distal healing region (dHR) and proximal healing region (pHR) edges, epiligament (EL), and distal and proximal (prox) ends of the MCL (Fig. 1). Two to three sections were counted per animal. Each captured image for granulation tissue size, endothelial cells, myofibroblasts, type I procollagen, and type III collagen was then quantified with Image J (NIH). Images captured for T-lymphocytes, and M1 and M2 macrophages were quantified manually.
Figure 1.
Representative cross-section of the H&E-stained MCL, indicating the approximate locations images were captured for later cell enumeration. Two to three sections from each animal were captured accordingly (HR, healing region; pHR, proximal healing region; dHR, distal healing region; prox, proximal; EL, epiligament; Original magnification 400×).
Mechanical Testing
The functional mechanical properties of the ligament were tested to determine the influence of macrophage inhibition on mechanical performance. Pull-to-failure testing was performed as previously described by Provenzano et al. 18–20 After sacrifice the MCL was removed with both femoral and tibial insertion sites intact and the surrounding tissue was carefully excised with special care being taken to avoid damaging the insertion sites. During preparation the femur-MCL-tibia (FMT) complex was kept hydrated using phosphate-buffered saline. The width and thickness of the ligament was measured optically and the cross-sectional area for the ligament was estimated assuming an elliptical cross section. The FMT complex was mounted in a custom made testing bath and mechanical testing machine. Optical markers were applied to the ligament on the insertion sites and the displacement was recorded optically. A pre-load of 0.1 N was applied to the ligament and the MCL was preconditioned (cyclically loaded to 1% strain for 10 cycles). Dimension measurements for the ligament were recorded at the pre-load. The ligament was then pulled to failure at a rate of 10% strain per second.
Failure force was recorded as the highest load prior to failure of the ligament and failure stress was calculated by dividing the failure force by the initial cross-sectional area of the ligament. Failure strain was calculated by subtracting the initial ligament length from the ligament length at failure divided by the initial length of the ligament.
Statistical Analysis
Images for each antibody were obtained at the healing region (HR), proximal healing region (pHR), distal healing region (dHR), proximal (prox), distal (distal), and epiligament (EL) from 2–3 sections per animal with 3 animals per treatment/day (e.g. see Fig. 1). To determine any spatial treatment differences within the ligament, regions were subgrouped into the total, MCL, granulation tissue, ligament ends, or epiligament regions. The total region comprises the average of all images within the MCL (combined average of HR, pHR, dHR, prox, distal, and EL). The MCL region includes the average of all the groups excluding the epiligament. Granulation tissue includes the HR, pHR, and dHR regions. The ligament ends include the prox and distal ends (away from the granulation tissue). Finally the epiligament only takes into the account the epiligament measurements. All IHC assays were analyzed using One-Way Analysis of Variance (ANOVA) to compare the means of the total, MCL, granulation tissue, ligament ends, and epiligament regions between the PBS liposome and clodronate liposome treated groups. Specifically, an F-test was used for testing the overall difference among treatments. A pair wise-contrast F-test was used for testing the pair wise differences between groups. These analyses were conducted using the statistical software package R-2.9.1. Mechanical testing data were analyzed via t-tests, using Microsoft Office Excel (2007).
Results
Morphological measurements/granulation tissue size
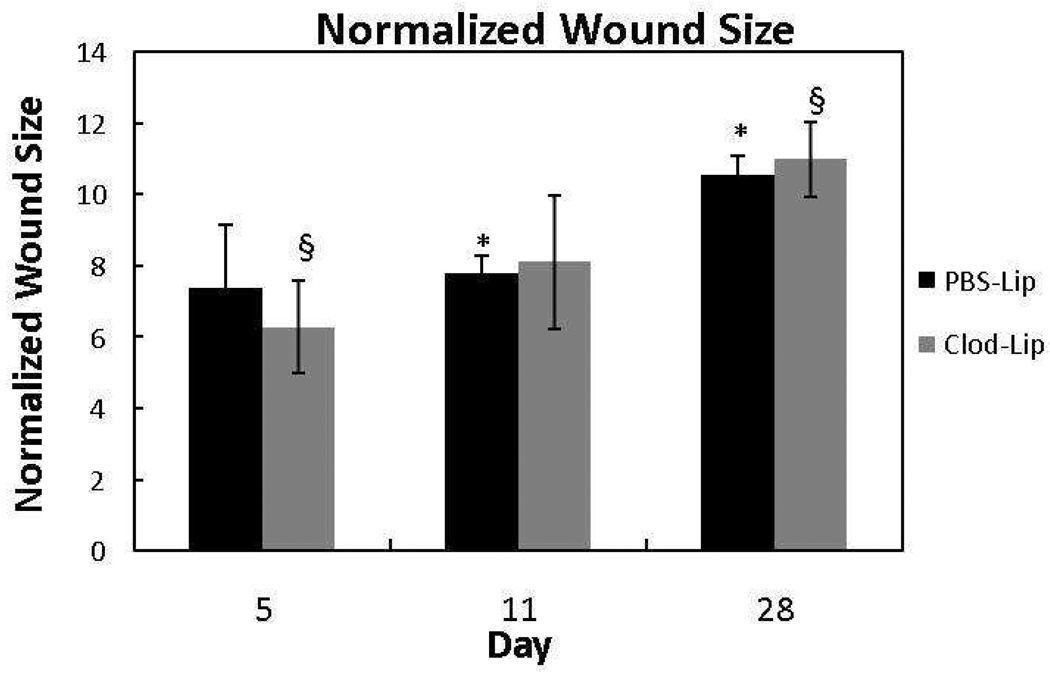
To determine if creeping substitution was altered by macrophage inhibition, the size of the granulation tissue region was measured by normalizing it with the total ligament area. At no time interval under investigation was the normalized granulation tissue size of the clodronate liposome-treated animals significantly different from the PBS liposome controls (Fig. 2; p > .05). The PBS-treated granulation tissue area significantly enlarged from day 11 to day 28 (p < .05) whereas, significant enlargement of the clodronate-treated granulation tissue was only observed when comparing day 5 and day 28 samples (p < .05). Additionally, day 5 ligaments exposed to clodronate liposomes weighed significantly less compared to the PBS liposome-treated animals (p < .05). No other time points showed significant differences.
Figure 2.
Graph of the normalized granulation tissue size at 5, 11, and 28 days post-injury after treatment with liposome-encapsulated clodronate (Clod-Lip) or liposome encapsulated PBS (PBS-Lip). Between treatments, granulation tissue area did not change in response to macrophage inhibition (p > .05). Within treatments, granulation tissue area experienced a significant change between the day 5 and day 28 samples after clodronate liposomes. In contrast, granulation tissue growth was most significant between day 11 and day 28 after PBS liposome treatment. * indicates significance between the day 11 and day 28 PBS-liposome groups. § indicates significant difference between the day 5 and day 28 Clodronate-liposome treated samples.
Macrophage Depletion
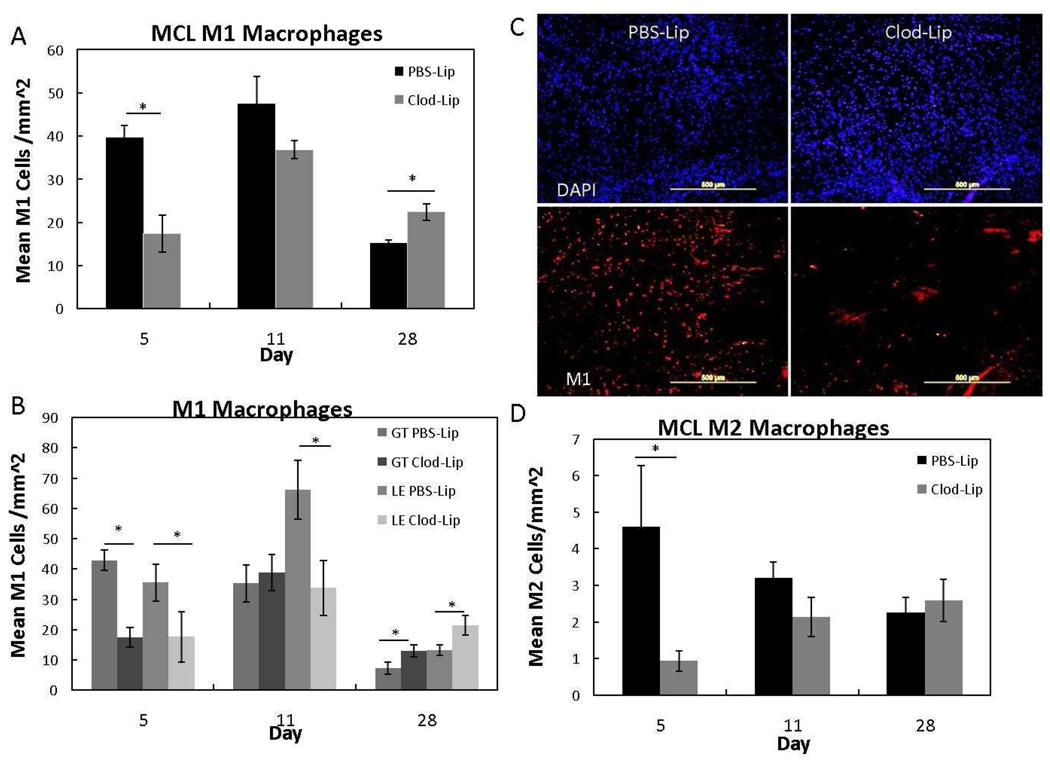
To evaluate the spatial and temporal effects of clodronate liposome treatment on classically and alternatively activated macrophage production, IHC was performed to identify M1 and M2 macrophages, respectively (Fig. 3A–D). Clodronate liposome treatment depleted M1 cells within the day 5 MCL (Fig. 3A); the effects were specifically observed within the granulation tissue and ligament ends (Fig. 3B). Double immunofluorescence of the M1 macrophages and overall cells, further indicates the depletion of macrophages by clodronate at day 5 (Fig. 3C). By day 11, M1 cells within the MCL were not significantly different except within the ligament ends (Fig. 3A–B). The inhibitory effects of Clod-Lip treatment were no longer observed at day 28 and significantly more M1 cells were present in the Clod-Lip group versus control (Fig. 3A; p < .05). The overall M2 cell numbers were likewise reduced by Clod-Lip treatment, but no specific differences were indicated within the granulation tissue, or ligament ends (Fig. 3D). No significant treatment changes were observed by the M1 or M2 cells within the epiligament at any day (p > .05). These results indicate that a single I.V. injection of Clod-Lip temporally and spatially reduces the number of M1 cells during early healing but does not significant affect macrophage numbers later.
Figure 3.
Graph of the M1 macrophages within the MCL (A), granulation tissue and ligament ends (B) at 5, 11, and 28 days post-injury. Double immunofluorescence image of total cells and M1 macrophages after PBS liposome- or clodronatete liposome-treatment (C). Graph of the MCL M2 macrophages at 5, 11, and 28 days post-injury after liposome-encapsulated clodronate treatment (D). A) Clodronate-liposomes (Clod-Lip) reduced MCL M1 macrophages 5 days postinjury compared to the PBS liposome controls (PBS-Lip) but increased cells at 28 days postinjury (p < .05). B) Within the MCL, clodronate liposome treatment reduced M1 macrophages within the granulation tissue (GT-Clod-Lip) and ligament ends (LE Clod-Lip) at day 5 but only depleted macrophages within the ligament ends on day 11. Both LE- and GT-clodronate liposome treated regions experienced an increased in M1 macrophages at day 28. C) Representative day 5 MCL images of DAPI (top row) and M1 macrophage (bottom row) double immunoflourescence comparing the number of M1 macrophages to total cells after PBSliposome (left column) or clodronate-liposome (right column) administration. D) Clodronate liposomes (Clod-Lip) reduced M2 macrophages at day 5, but no other time points were significant. * Indicates significance (p< .05) between Clod-Lip and PBS-Lip at day 5 (A, B, D) and day 28 (A). Values are expressed as mean cell numbers ± S.E.M.
T-lymphocyte Infiltration
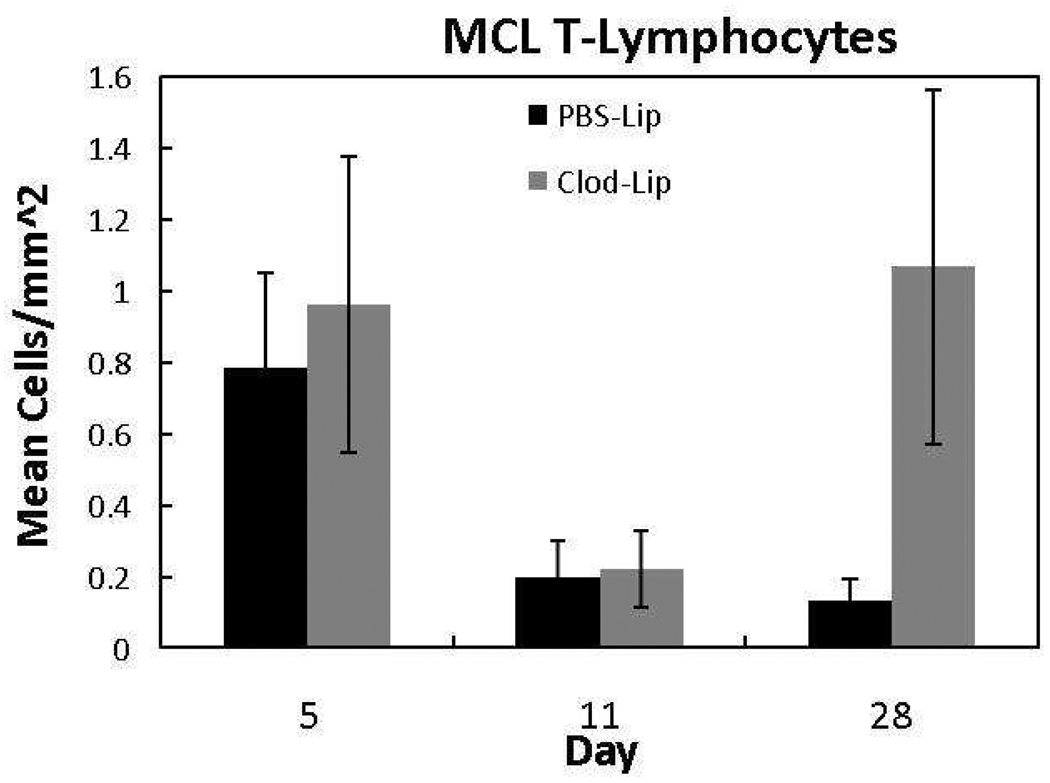
To determine if macrophage depletion influences T-lymphocyte numbers, tissue sections were subjected to CD3 IHC, identifying mature T-lymphocytes (Fig. 4). Very few T-lymphocytes were detected in the healing ligament, regardless of treatment or time of collection. T-lymphocytes were greatest in number at day 5 and decreased thereafter. Macrophage inhibition did not appear to have any influence (p >.05) on T-lymphocyte production.
Figure 4.
Response of T-lymphocytes to liposome-encapsulated clodronate (Clod-Lip) or PBSLip (PBS-Lip) treatment. Macrophage depletion did not significantly influence ligament T-lymphocytes across time (p > .05). Values are expressed as mean cell numbers ± S.E.M.
Endothelial Cell and Myofibroblast Production
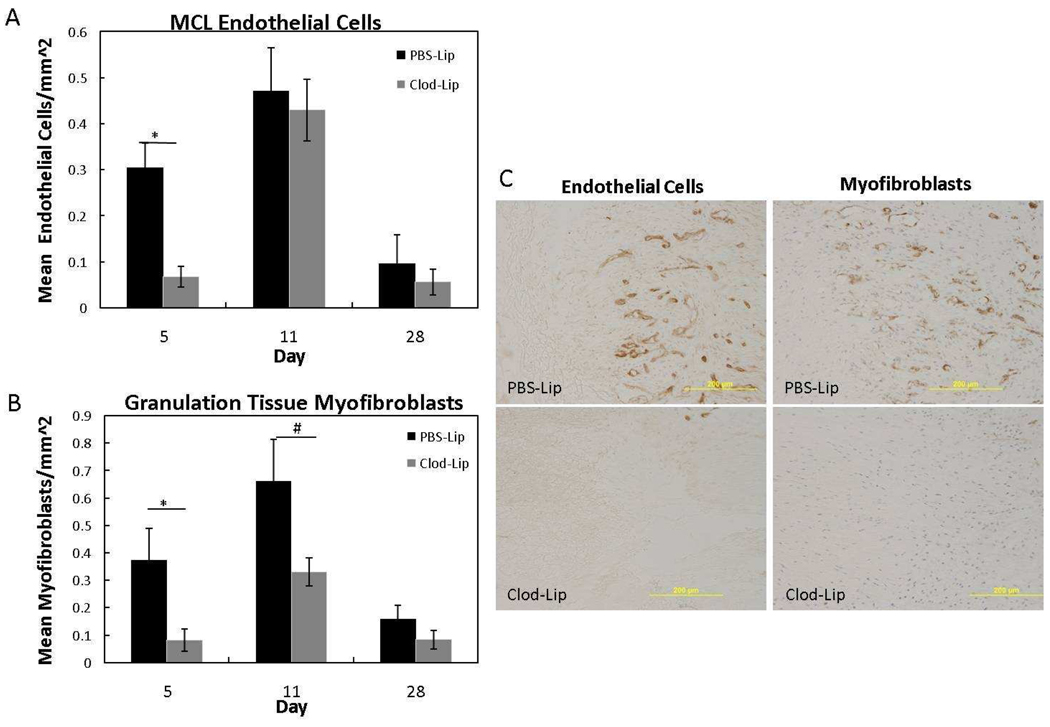
Granulation tissue formation involves an increase in vascularity, myofibroblasts and fibroblasts. To evaluate the effects of macrophage inhibition on granulation tissue factors, endothelial cell and myofibroblast localization was analyzed. Similar to normal granulation tissue formation 2, both cell types were increased at day 11. Macrophage inhibition significantly reduced endothelial cell and myofibroblast numbers in the day 5 healing ligament (Fig. 5A–C; p < .05). By day 11 and day 28 MCL endothelial cells were similar between the Clod-Lip and PBS-Lip groups. Myofibroblasts in the granulation tissue tended (p = .057) to remain low at day 11 after clodronate liposome treatment, suggesting a prolonged inhibitory effect on myofibroblasts but not endothelial cells. No treatment differences were observed at day 28.
Figure 5.
Endothelial cell (A) and myofibroblast (B) response to liposome-encapsulated clodronate (Clod-Lip) or PBS (PBS-Lip) treatment. A) Macrophage depletion significantly suppressed endothelial cells at day 5 (p <.05). B) Myofibroblasts significantly decreased at day 5 (p < .05) and tended to decrease at day 11 (p=.056) after clodronate liposome treatment. Treatment did not significantly influence endothelial cells or myofibroblasts at day 28 postinjury (p >.05). C) Representative images of endothelial cell (right column) and myofibroblast (left column) immunohistochemistry after PBS-liposome treatment (top row) or clodronate-liposome (bottom row) treatment (C). *Indicates significant difference (p < .05) between Clod-Lip and PBS-Lip at day 5. #Indicates a trend (p = .056) between Clod-Lip and PBS-Lip at day 11. Values are expressed as mean cell numbers ± S.E.M.
Collagen Production
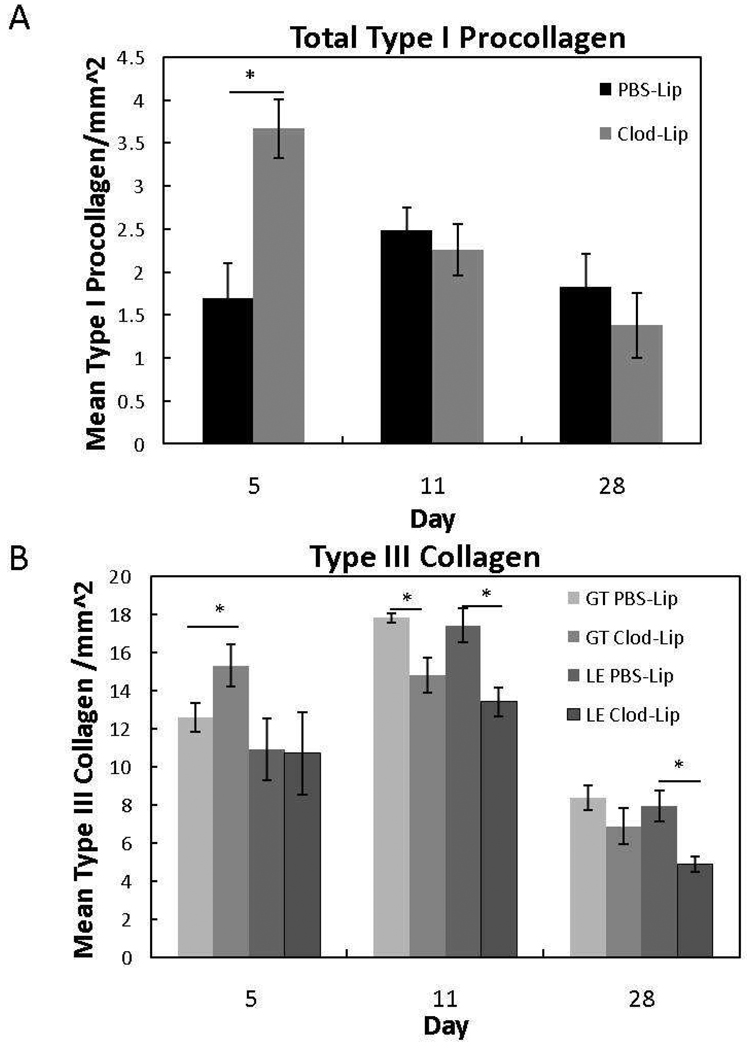
The native MCL is primarily composed of type I collagen whereas type III collagen is indicative of scar formation. To evaluate the effects of macrophage depletion on collagen production, IHC for type I procollagen and type III collagen was performed. Macrophage depletion stimulated total type I procollagen (p < .05) at day 5 but was ineffective thereafter (Fig. 6A; p < .05). Additionally, macrophage inhibition spatially and temporally influenced type III collagen localization (Fig. 6B). At day 5, the lower levels of macrophages resulted in an increase in type III collagen within the granulation tissue; no other regions were affected. By day 11, Clod-Lip reduced type III collagen within the granulation tissue and ligament ends. Type III collagen remained decreased within the ligament ends and epiligament (data not shown) but not the granulation tissue at day 28.
Figure 6.
Type I procollagen (A) and type III collagen (B) at 5, 11, and 28 days post-injury after liposome-encapsulated clodronate (Clod-Lip) or PBS (PBS-Lip) treatment. A) Macrophage depletion significantly increased total type I procollagen (A) at day 5 (p<.05). B) Within the granulation tissue (GT Clod-Lip), clodronate liposome treatment initially increased type III collagen at day 5. Levels then decreased within the granulation tissue (GT Clod-Lip) and/or ligament ends (LE Clod-Lip) at day 11 and 28 when compared to PBS-liposome treatment (GT PBS-Lip, LE PBS-Lip). *Indicates significant difference (p < .05) between Clod-Lip and PBSLip. Values are expressed as mean collagen/mm2 ± S.E.M.
Spatial Localization of IHC Factors
To target any cellular or ECM spatial differences after clodronate liposome treatment, the total ligament, MCL body, granulation tissue, ligament ends (outside of the granulation tissue) and the epiligament were analyzed. Treatment primarily affected IHC factors within the MCL body, including both the granulation tissue and ligament ends. Clodronate liposome treatment only influenced epiligament day 28 type III collagen and day 5 myofibroblasts. The spatial and temporal M1 macrophage and type III collagen results are of particular interest. Macrophage cell number reduction was initially observed within the MCL body (excluding the epiligament). By day 11, only the M1 macrophages within the ligament ends were significantly different. Additionally, type III collagen decreased within the MCL by day 11. Levels remained low within the ligament ends on day 28. These results suggest transient macrophage inhibition altered the typical creeping substitution pattern and downstream localization of type III collagen.
Mechanical Testing
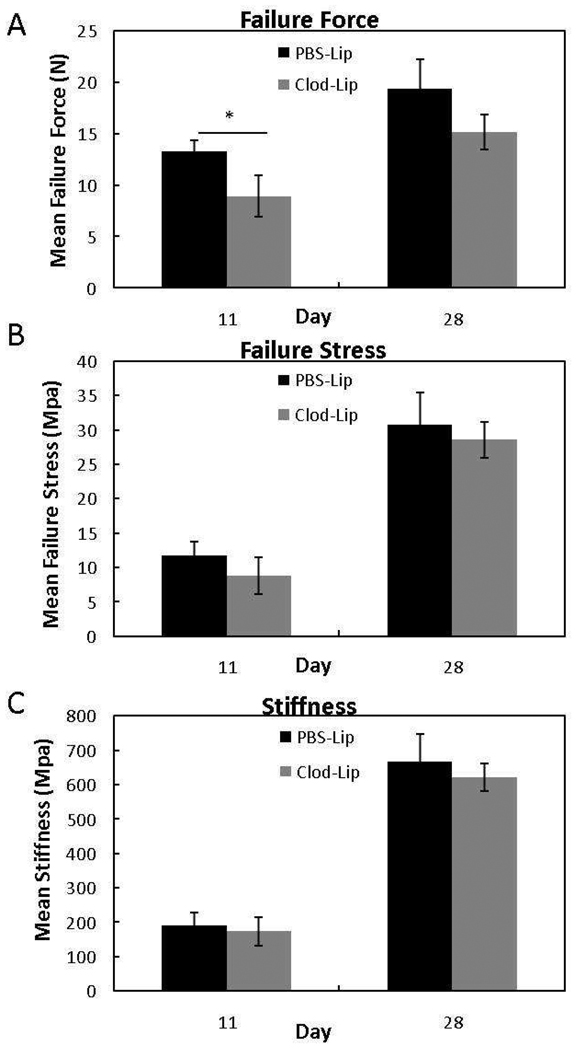
Analysis of the ligament mechanics after clodronate liposome treatment revealed changes in ligament healing. Failure force for a ligament is an indicator of ligament healing, since structural behavior is dependent on the collagen content and organization. The compositional and micro-structural changes induced by clodronate liposome treatment significantly decreased the ultimate failure force at day 11 (p< .05) but had no effect at day 28 (p > .05) when compared to the PBS liposome-treated ligaments (Fig. 7). This drop in force indicates the early depletion of macrophages compromises mechanical strength, but these effects are transient. No differences were observed in failure stress or stiffness between treatment groups (Fig. 7B, C).
Figure 7.
Response of failure force (A), failure stress (B), and stiffness (C) to liposome-encapsulated clodronate (Clod-Lip) or PBS (PBS-Lip) treatment. Macrophage inhibition reduced failure force at day 5 (p < .05). No significance in failure stress (B) or stiffness (C) was observed. *Indicates significant difference (p < .05) between Clod-Lip and PBS-Lip at day 5. Values are expressed as mean output ± S.E.M.
Discussion
The aim of this study was to determine the biological and functional effects of early macrophage depletion on ligament healing. Our previous research established a localized increase in the M1 macrophages during ligament creeping substitution.2 Therefore, we hypothesized that early depletion of macrophages would significantly alter ligament healing and scar formation. Liposome encapsulated clodronate is commonly used for macrophage depletion and angiogenesis inhibition.7, 22 Our results demonstrated that a single clodronate liposome injection did not fully ablate the M1 and M2 macrophages, but significantly reduced numbers in a spatial and temporal manner. The reduction in macrophages was sufficient to decrease myofibroblasts and endothelial cells and to increase type I procollagen at day 5. Although a single dose of clodronate liposome was not able to maintain macrophage inhibition to day 28, the early inhibition of macrophages resulted in a later decrease in type III collagen and ligament strength. These results support the role of macrophages in controlling angiogenesis, fibroblast differentiation, and collagen production during early ligament healing.
A desired healing scenario after injury would have little or no scar tissue, organized collagen fibers, restored concentrations of type I collagen, and limited creeping substitution. Such behavior, we hypothesize, would reduce type III collagen and regenerate the ligament with nearly normal composition and mechanical properties. In the current study, depletion of macrophages resulted in up-regulated type I procollagen (although concentrations are unknown) and reduced type III collagen, but these changes were either transient or not sufficient to improve ligament strength. Horn et al. showed that multiple clodronate liposome injections over time inhibits macrophages and controls axonal retraction (a process physically similar to ligament creeping substitution) between days 4 and 7 post-injury.10 We did not repeatedly inject our animals. Perhaps the remaining macrophages after one treatment and the later influx of M1 cells may have sufficiently maintained the creeping substitution phenomenon into the residual native tissue.
Clodronate liposomes are commonly used in vivo to reduce the number of macrophages.8, 10, 17, 23,25 Liposome-encapsulated clodronate, used in this study, cannot cross the vascular endothelium of capillaries and has no access to the currently inhabiting resident macrophages. However, monocytes in the circulation are depleted after intravenous administration of clodronate liposomes; any circulating monocytes/future resident cells migrating to the injury will be inhibited. Therefore, clodronate liposomes initially and non-specifically deplete all circulating phagocytosing macrophages and monocytes rather than specific phenotypes of macrophages. Macrophages confer remarkable plasticity and respond to the cytokine environment by modulating their phenotype and functionality. To date, three different macrophage populations with distinct biological functions have been identified: the M1, the M2, and the type II-activated macrophages (for review see 16). Activated M1 cells promote ECM degradation by producing nitric oxide, acquiring an enhanced capability to kill intracellular microorganisms, and stimulating a number of pro-inflammatory cytokines. The combination of IFN-γ and TNF-α stimulates the M1 macrophages. Macrophage phagocytosis of apoptotic cells results in a phenotypic transition from a M1 to a M2 macrophage phenotype.6 The M2 macrophages promote angiogenesis and matrix remodeling by releasing anti-inflammatory cytokines and inhibiting nitric oxide release. An experiment inhibiting gene expression of the M2 macrophages, but not the M1 macrophages, reported the removal of necrotic tissue from injured tissue, but severe defects in repair.21 These results substantiate the functional difference of the macrophage classes. The less known type II activated macrophages are stimulated by the combination of Fcγ receptors and the toll-like receptors. Activation of the type II macrophages induces an anti-inflammatory response and a Th2-like response that may be used to suppress autoimmune diseases. Based on these functional characterizations, modulation of the specific macrophage phenotypes may offer better control over healing rather than non-specific macrophage inhibition. Our study further demonstrates that clodronate liposomes do not distinguish the different macrophage phenotypes and overall depletion produced suboptimal mechanical properties. Thus, an optimal regenerative environment may involve a timed inhibition of the M1 macrophages and a concomitant stimulation of the M2 macrophages (rather than the non-specific depletion).
Bone-tendon healing and chronic fibrosis models improve healing after macrophage inhibition unlike the MCL model.5, 8, 8, 12 During normal healing, macrophage infiltration is important for wound debridement and subsequent cytokine production. In contrast, chronic fibrosis represents an exaggerated macrophage response in the absence of a wound to debride. Macrophage inhibition during chronic fibrosis permits down-regulation of the imposing inflammatory and fibrotic signals without the concern of ablating the cells necessary for wound debridement. Conversely, inhibition of macrophages before debris clearance in this study stimulated some repair factors but reduced ligament strength. Our results contrast with Hays et al 8 who demonstrated improved healing after macrophage depletion in a reconstructed ACL bone-tendon model. The disparity in results may be attributed to the different experimental models used (tendon to bone healing versus ligament to ligament healing). A number of studies have successfully used bisphosphonates in bone healing.11, 15 ACL reconstruction involves tendon-bone interface healing. Clodronate liposome administration to the reconstructed ACL model may have ablated the osteoclasts, thereby stimulating an altered repair process in bone versus soft tissue. Bone is also highly vascular compared to the ligament. Generally, tissues with more vascularity exhibit better healing than avascular tissues. Additionally, the inflammatory response is extended for a greater time period within the ACL reconstruction model than the MCL healing model (because the ACL graft is avascular to start and the synovial environment delays remodeling). Similar to the fibrotic model, general inhibition of the inflammatory response in a reconstructed ACL appears more beneficial than in an MCL.
In conclusion, early macrophage depletion limits granulation tissue formation and appears to alter downstream scar formation but not without compromising ligament strength. To improve the biological and mechanical function of the healing ligament, modulation of the specific macrophage phenotypes rather than nonspecific depletion, may be a more productive path to reduce scar formation and regenerate the native tissue.
Acknowledgement
The authors acknowledge that financial support was provided by the National Institutes of Health (NIH), Grant No. R01 AR049266 and R01 AR059916.
References
- 1.Burrington JD. Wound healing in the fetal lamb. J Pediatr Surg. 1971;6(5):523–528. doi: 10.1016/0022-3468(71)90373-3. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain CS, Crowley E, Vanderby R. The spatio-temporal dynamics of ligament healing. Wound Repair Regen. 2009;17(2):206–215. doi: 10.1111/j.1524-475X.2009.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi BM, Kwak HJ, Jun CD, et al. Control of scarring in adult wounds using antisense transforming growth factor-beta 1 oligodeoxynucleotides. Immunol Cell Biol. 1996;74(2):144–150. doi: 10.1038/icb.1996.19. [DOI] [PubMed] [Google Scholar]
- 4.Connors D, Gies D, Lin H, et al. Increase in wound breaking strength in rats in the presence of positively charged dextran beads correlates with an increase in endogenous transforming growth factor-beta1 and its receptor TGF-betaRI in close proximity to the wound. Wound Repair Regen. 2000;8(4):292–303. doi: 10.1046/j.1524-475x.2000.00292.x. [DOI] [PubMed] [Google Scholar]
- 5.Dayer JM. Aspects of resorption and formation of connective tissue during chronic inflammation in rheumatoid arthritis. Eur J Rheumatol Inflamm. 1982;5(4):457–468. [PubMed] [Google Scholar]
- 6.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104(1):27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 7.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, et al. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(8):3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 8.Hays PL, Kawamura S, Deng XH, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90(3):565–579. doi: 10.2106/JBJS.F.00531. [DOI] [PubMed] [Google Scholar]
- 9.Hopkinson-Woolley J, Hughes D, Gordon S, et al. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107(Pt 5):1159–1167. doi: 10.1242/jcs.107.5.1159. (Pt 5) [DOI] [PubMed] [Google Scholar]
- 10.Horn KP, Busch SA, Hawthorne AL, et al. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28(38):9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koivukangas A, Tuukkanen J, Kippo K, et al. Long-term administration of clodronate does not prevent fracture healing in rats. Clin Orthop Relat Res. 2003;408:268–278. doi: 10.1097/00003086-200303000-00036. (408) [DOI] [PubMed] [Google Scholar]
- 12.Laurent GJ. Biochemical pathways leading to collagen deposition in pulmonary fibrosis. Ciba Found Symp. 1985;114:222–233. doi: 10.1002/9780470720950.ch15. [DOI] [PubMed] [Google Scholar]
- 13.Levenson SM, Geever EF, Crowley LV, et al. The Healing of Rat Skin Wounds. Ann Surg. 1965;161:293–308. doi: 10.1097/00000658-196502000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech. 2004;37(6):865–877. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Madsen JE, Berg-Larsen T, Kirkeby OJ, et al. No adverse effects of clodronate on fracture healing in rats. Acta Orthop Scand. 1998;69(5):532–536. doi: 10.3109/17453679808997793. [DOI] [PubMed] [Google Scholar]
- 16.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 17.Popovich PG, Guan Z, Wei P, et al. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158(2):351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 18.Provenzano PP, Heisey D, Hayashi K, et al. Subfailure damage in ligament: a structural and cellular evaluation. J Appl Physiol. 2002;92(1):362–371. doi: 10.1152/jappl.2002.92.1.362. [DOI] [PubMed] [Google Scholar]
- 19.Provenzano PP, Martinez DA, Grindeland RE, et al. Hindlimb unloading alters ligament healing. J Appl Physiol. 2003;94(1):314–324. doi: 10.1152/japplphysiol.00340.2002. [DOI] [PubMed] [Google Scholar]
- 20.Provenzano PP, Rueden CT, Trier SM, et al. Nonlinear optical imaging and spectral-lifetime computational analysis of endogenous and exogenous fluorophores in breast cancer. J Biomed Opt. 2008;13(3) doi: 10.1117/1.2940365. 031220. [DOI] [PubMed] [Google Scholar]
- 21.Ruffell D, Mourkioti F, Gambardella A, et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A. 2009;106(41):17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai E, Anand A, Ambati BK, et al. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(8):3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 23.van Amerongen MJ, Harmsen MC, van Rooijen N, et al. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170(3):818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174(1–2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 25.Wolff RA, Tomas JJ, Hullett DA, et al. Macrophage depletion reduces monocyte chemotactic protein-1 and transforming growth factor-beta1 in healing rat vein grafts. J Vasc Surg. 2004;39(4):878–888. doi: 10.1016/j.jvs.2003.11.039. [DOI] [PubMed] [Google Scholar]