Abstract
Background
Salmonella enterica serotype Typhi is a human-restricted intracellular pathogen and the cause of typhoid fever. Cellular immune responses are required to control and clear Salmonella infection. Despite this, there are limited data on cellular immune responses in humans infected with wild type S. Typhi.
Methodology/Principal Findings
For this work, we used an automated approach to purify a subset of S. Typhi proteins identified in previous antibody-based immuno-affinity screens and antigens known to be expressed in vivo, including StaF-putative fimbrial protein-STY0202, StbB-fimbrial chaperone-STY0372, CsgF-involved in curli production-STY1177, CsgD- putative regulatory protein-STY1179, OppA-periplasmic oligopeptide binding protein precursor-STY1304, PagC-outer membrane invasion protein-STY1878, and conserved hypothetical protein-STY2195; we also generated and analyzed a crude membrane preparation of S. Typhi (MP). In comparison to samples collected from uninfected Bangladeshi and North American participants, we detected significant interferon-γ responses in PBMCs stimulated with MP, StaF, StbB, CsgF, CsgD, OppA, STY2195, and PagC in patients bacteremic with S. Typhi in Bangladesh. The majority of interferon-γ expressing T cells were CD4 cells, although CD8 responses also occurred. We also assessed cellular proliferation responses in bacteremic patients, and confirmed increased responses in infected individuals to MP, StaF, STY2195, and PagC in convalescent compared to acute phase samples and compared to controls. StaF is a fimbrial protein homologous to E. coli YadK, and contains a Pfam motif thought to be involved in cellular adhesion. PagC is expressed in vivo under the control of the virulence-associated PhoP-regulon required for intra-macrophage survival of Salmonella. STY2195 is a conserved hypothetical protein of unknown function.
Conclusion/Significance
This is the first analysis of cellular immune responses to purified S. Typhi antigens in patients with typhoid fever. These results indicate that patients generate significant CD4 and CD8 interferon-γ responses to specific S. Typhi antigens during typhoid fever, and that these responses are elevated at the time of clinical presentation. These observations suggest that an interferon-γ based detection system could be used to diagnose individuals with typhoid fever during the acute stage of illness.
Author Summary
Salmonella enterica serotype Typhi infection is a significant global public health problem and the cause of typhoid fever. Salmonella are intracellular pathogens, and cellular immune responses are required to control and clear Salmonella infections. Despite this, there are limited data on cellular immune responses during wild type S. Typhi infection in humans. Here we report the assessment of cellular immune responses in humans with S. Typhi bacteremia through a screening approach that permitted us to evaluate interferon-γ and proliferation responses to a number of S. Typhi antigens. We detected significant interferon-γ CD4 and CD8 responses, as well as proliferative responses, to a number of recombinantly purified S. Typhi proteins as well as membrane preparation in infected patients. Antigen-specific interferon-γ responses were present at the time of clinical presentation in patients and absent in healthy controls. These observations could assist in the development of interferon-γ-based diagnostic assays for typhoid fever.
Introduction
Salmonella enterica serotype Typhi is a human-restricted intracellular pathogen and the cause of typhoid fever. It is estimated that over 20 million cases of S. Typhi infection occur each year, resulting in approximately 200,000 deaths per year globally [1]. Current typhoid vaccines provide 50–75% protection for 2–5 years [2]. Mediators of protective immunity against typhoid are incompletely understood. S. Typhi is an invasive enteropathogen that, following ingestion, transits through intestinal epithelial cells, is taken up by professional phagocytic cells, survives within macrophages, and systemically circulates [3], [4], [5], [6]. Antibody responses to lipopolysaccharide (LPS), flagellin, Vi capsular polysaccharide, and crude whole cell preparations have been documented, and antibody responses are the basis of the Widal serologic diagnostic assay for typhoid fever [7], [8], [9], [10], [11]. However, with the exception of antibody responses against the S. Typhi capsule (Vi antigen) [12], antibody responses may play a limited role in mediating protective immunity during typhoid fever.
S. Typhi is an intracellular pathogen, and cellular immune responses are required to control and clear S. Typhi infections [10], [13], [14], [15]. Unfortunately, there are limited data on antigen-specific cellular responses during human wild type S. Typhi infection. What is known is largely derived from analyses of cellular responses in mice infected with S. Typhimurium [16], [17], [18]; however, S. Typhimurium does not cause a typhoidal illness in humans, and S. Typhi and S. Typhimurium differ significantly at the genomic level [17], [19], [20]. Direct analysis of cellular responses during S. Typhi infection in humans either pre-dates modern immunologic techniques [21] or involves characterizing immune responses in recipients of live attenuated oral typhoid vaccines [22], [23]. These analyses have shown that CD4+ and CD8+ T cells are critical to the development of protective immunity to Salmonella, and control of Salmonella infection involves prominent expression of interferon-γ by both CD4 and CD8 cells [24], [25], [26]. To date, however, there is less information on the cellular responses in humans during wild type infection, especially to purified S. Typhi antigens.
To address this, we used a modification of an automated approach to purify a subset of S. Typhi proteins for use in immunologic assays [27]. We selected antigens for evaluation based on our previous application of a high throughput immuno-affinity screen, In Vivo Induced Antigen Technology (IVIAT), to S. Typhi [28]. Here we describe purification of a subset of these proteins, and evaluation of interferon-γ and cellular proliferation responses to these antigens in humans with S. Typhi bacteremia in Bangladesh. We also assessed responses to a crude membrane preparation of S. Typhi.
Materials and Methods
Generation of expression clones for antigen production
We selected 58 S. Typhi proteins contained within operons identified during our previous application of IVIAT to S. Typhi [28]. IVIAT identifies proteins expressed in vivo during human infection and that generate an antibody response [28]. We obtained pDONR221 Gateway Based entry clones of the S. Typhi CT18 genes corresponding to selected proteins from the NIAID-sponsored Pathogen Functional Genomic Resource Center, J. Craig Venter Institute (JCVI, formerly The Institute for Genomic Research). We used LR clonase II enzyme reactions (Invitrogen, Carlsbad, CA) as per the manufacturer's instructions to move inserts into pDEST17 (Invitrogen, Carlsbad, CA) to generate a fusion containing an amino terminal 6× histidine (HIS) tag. We transformed DH5alpha-T1R competent cells with LR reactions and selected for ampicillin resistance. We confirmed insert presence by restriction digestion and PCR analysis and transformed purified plasmids into E. coli protein expression strain BL21 star (DE3) pLysS (Invitrogen).
Protein expression
We grew transformants harboring recombinant plasmids at 37°C as 1.5 ml cultures in 96-well blocks (Marsh Biomedical Products) to an OD600 of 0.6–0.8. We induced cultures with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) on a 96-well plate shaker (Multitron) (×900 rpm). After 3 hours at 37°C, we harvested cells at 4°C and stored preparations at −80°C for further use. We also induced BL21 star (DE3) pLysS containing pDEST17 but lacking an S. Typhi insert. This construct produced a truncated HIS-tagged protein MSYYHHHHHHLESTSLYKKAERERKMI that we recovered and used as a control protein in immunological assays.
Automated 96-well protein purification
We performed protein purifications in 96-well plates using a BiomekFx (Beckman Coulter) robotic liquid handler as previously described [27]. For this 6xHIS denaturing affinity purification, we thawed cell pellets at room temperature for 15 min, lysed them in the presence of protease inhibitors in 115 µl lysis buffer I (100 mM NaH2PO4, 10 mM Tris, pH 8.0), robotically resuspended product in a 96-well block and agitated at 900 rpm for 10 min (5 min in the clockwise direction and 5 min in the counterclockwise direction). We then added 10 µl of DNase mix (10 mg/ml DNase; Sigma Aldrich in 900 mM MgCl2, 100 mM MnCl2) to the lysate and agitated the preparation at 900 rpm for 10 min. Next, we added 115 µl of lysis buffer II (100 mM NaH2PO4, 10 mM Tris, 6 M guanidine hydrochloride, 10 mM, 2-mercaptoethanol, pH 8.0) to create denaturing conditions. We then allowed these cell lysates to bind to 30 µl of MagneHIS beads (Promega) with shaking at 900 rpm for 20 min (10 min clockwise, 10 min counterclockwise), and separated beads using a magnabot (24-pin magnet; Promega). The robotic liquid handler then washed the MagneHIS beads with bound protein three times with wash buffer (100 mM NaH2PO4, 10 mM Tris, 8 M urea). We prevented bead adherence to the walls during washing by shaking the samples at 900 rpm for 2.5 min clockwise and then 2.5 min counterclockwise. We then washed the beads with bound protein using 100 µl of distilled water, and added 50 µl distilled water to make the final suspensions for analysis. We repeated this extraction cycle six times.
Automated 96-well protein analysis
We analyzed proteins in a 96-well format using a capillary-based instrument, the LabChip90 (Caliper Sciences). We automated a system that resuspended 3 µl of protein sample in 7 µl analysis buffer (Caliper Sciences), heated these to 96°C for 5 min., cooled them to room temperature, and briefly centrifuged to collect the sample. We added distilled water (35 µl) to each sample prior to analysis. We primed the analysis chip (Caliper Sciences) according to the manufacturer’s instructions. The automated protein analysis generated three different forms of output: a chromatogram that showed migration time; a virtual gel that mimicked a Coomassie stained gel; and a results table that included the estimated size, quality, and quantity of each peak. The LabChip90 analyzed 96 proteins at a time with analysis time of 40 seconds per sample. We parsed the output results and imported them into the Harvard Institute of Proteomics protein database. We assessed for presence of contaminating E. coli LPS using a HEK-Blue LPS Detection kit (InvivoGen, San Diego, CA).
Production and mass spectrometric analysis of S. Typhi crude membrane preparation
We prepared S. Typhi membrane preparation as previously described [29], [30]. Briefly, we cultured S. Typhi Ty21a on sheep blood agar plates and harvested in Tris buffer (10 mM Tris, pH 8.0, 5 mM MgCl2). We sonicated the mixture, and centrifuged at 1400× g for 10 minutes and transferred the supernatant to fresh tubes, centrifuging at 14900× g for 30 minutes. We suspended the pellet in 10 ml Tris buffer, and determined the protein content by the BioRad Protein Assay per the manufacturer's instructions.
We performed mass spectrometric analysis of the S. Typhi membrane preparation as previously described using a LTQ-Orbitrap XL (Thermo Fisher Scientific) instrument [19], [31]. We identified peptides using SEQUEST (Thermo Fisher Scientific) through Bioworks Browser, version 3.3.1 SR1. MS/MS data were obtained using 10 ppm mass accuracy on precursor m/z and a 0.5 Da window on fragment ions. Fully enzymatic tryptic searches with up to three missed cleavage sites were allowed. Oxidized methionines were searched as a variable modification and alkylated cysteines were searched as a fixed modification. Salmonella databases for CT18 were downloaded from EMBL-EBI and supplemented with common contaminants. We employed a reverse database strategy [32] using concatenating reversed protein sequences for each database entry in SEQUEST. We filtered peptides for each charge state to a false discovery rate (FDR) of 1%, and then grouped peptides into proteins using Occam’s razor logic. A full listing of proteins identified in mass spectrometric analysis of Salmonella Typhi membrane preparation is available in the supplemental material (Table S1).
Collection of specimens from study subjects
Individuals (1–59 years of age) with fever of 3–7 days duration (≥39°C) having clinical symptoms and signs suggestive of typhoid fever and lacking an alternate diagnosis who presented to the Kamalapur field site of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) Dhaka hospital were eligible for enrollment. We collected venous blood (for children <5 years of age, 3 ml of blood; for older individuals, 5 ml of blood) for culture (n = 69). We used the BacT/Alert automated system and identified S. Typhi organisms using standard biochemical methods and by reaction with Salmonella-specific antisera [30], [33]. Following informed consent from patients or guardians in the case of children, we collected an additional 5 ml of blood from bacteremic individuals within 72 hours of the patient presenting for medical care, and a follow-up sample 21–28 days later (n = 16; ages 2–22 years). All patients with 3 days or longer of fever were treated initially with amoxicillin or cefixime at the discretion of the attending physician until scheduled follow-up 48–72 hours later, or sooner as clinically indicated. Individuals with documented S. Typhi bacteremia were continued on amoxicillin if they showed signs of improvement and their blood isolates showed sensitivity to first line treatment; or were switched to parenteral ceftriaxone or oral ciprofloxacin, if their isolates were not sensitive and/or they failed to improve by 72 hours; therapy was continued for up to 14 days, or up to 7 days beyond defervescence, whichever occurred first. All patients recovered. We also collected 5 ml of blood from North American volunteers (n = 3) without a history of international travel who had never received typhoid vaccination and who did not have previous known Salmonella infection, and we collected 5 ml of blood from healthy Bangladeshi volunteers (n = 4) who did not have illness, fever or diarrhea in the preceding three months [34]. Studies were approved by the Institutional Review Boards of the ICDDR,B and Massachusetts General Hospital.
PBMC isolation
We diluted heparinized blood in phosphate buffered saline (PBS; 10 mM, pH 7.2) and isolated peripheral blood mononuclear cell (PBMC) by gradient centrifugation on Ficoll-Isopaque (Pharmacia, Uppsala, Sweden). We re-suspended isolated PBMCs to a concentration of 1×106 cells/ml in RPMI complete medium RPMI-1640 (Gibco, Gaithersburg, Md) with 10% heat-inactivated fetal bovine serum (Hyclone-Thermo Scientific, Waltham, MA, USA), 100 units/ml penicillin, 100 µg/ml streptomycin, 100 mM pyruvate, and 200 mM L-glutamine (Gibco) [35].
Interferon gamma ELISPOT assay
We used PBMCs to measure human interferon-γ expression using an ELISPOT format with MabTech antibodies, according to the manufacturers’ instructions (Mabtech Inc, Cincinati, OH, USA). In brief, we coated 96-well nitrocellulose plates (Multiscreen HTS, Millipore) with 100 µl of 15 µg/ml human monoclonal anti-interferon-γ antibody (1-D1K) overnight at 4°C. Following washing the plates and subsequent blocking with 10% FBS for 2 h at room temperature, we added PBMCs from individual patients or controls at a concentration of 2×105 per well for each experimental condition. We added individual S. Typhi antigens or control protein to wells at a concentration of 140 ng/well of total preparation for each purified antigen (in 200 µl culture, final concentration 0.7 µg/ml). In separate wells, we also added S. Typhi membrane preparation at a final concentration of 10 µg/ml in 200 µl culture, phytohaemagglutinin (PHA; Murex Diagnostics Ltd, Temple Hill, UK) at a final concentration of 2.5 µg/ml in 200 µl culture, and keyhole limpet hemocyanin (KLH). We included additional control wells with media but lacking antigen. Following incubation of plates at 37°C in 5% CO2 for 20 hours, we washed plates, added biotinylated monoclonal anti-interferon-γ antibody (7-B6-1-biotin; 1∶500 dilution), incubated plates at room temperature for an additional 2 hours, washed them, added streptavidin-HRP (1∶500 dilution), and re-incubated for 1 hour at room temperature. We developed plates with aminoethylcarbazol plus H2O2, and counted interferon-γ secreting cells using a stereomicroscope. We subtracted results for wells containing media only and expressed results as the number of spots/106 PBMC in each experimental condition [36].
Intracellular cytokine staining
To characterize the interferon-gamma T cell response further, we resuspended PBMCs at a concentration of 1×106 cells/mL in RPMI medium (Gibco, Carlsbad, CA) and supplemented with 10% fetal calf serum (FCS, Gibco). We cultured PBMCs in U-bottom tissue culture plates (Nunc, Denmark) in the presence of Salmonella membrane preparation (MP; 10 µg/ml), StaF (7 µg/ml), PagC (7 µg/ml), KLH (2.5 µg/ml as a negative control) or PMA (5.0 ng/ml as a positive control; Phorbol 12-myristate 13-acetate) with ionomycin (1.0 µg/ml). Samples containing only unstimulated cells were included to assess in vivo stimulation. We used 1.0 µg/ml of anti-CD28 (clone 28.2; BD Pharmingen) and anti-CD49d (clone 9F10; BD Pharmingen) for co-stimulation. We incubated PBMCs and antigens for 2 hours at 37° C in 5% CO2. After 2 hours, we added 10 µg/mL of brefeldin A (BFA, Sigma) and continued incubating the plates for an additional 4 hours [37]. Following stimulation, we washed cells with PBS and 2% FCS. We then stained cells for 30 min at 4°C with the following surface monoclonal antibodies: anti-CD3-APC, anti-CD4–perCP, and anti-CD8-FITC (Becton Dickinson, San Jose, USA). Following surface staining, we washed the cells and incubated the preparations with FACS Lysing Solution (BD Bioscience) for 10 minutes, and then re-washed and permeabilized the preparations with FACS permeabilizing solution (BD Bioscience) for 10 min at room temperature. We washed the permeabilized cells and stained them for 30 min at 4°C with fluorochrome-conjugated anti-IFN-γ-PE (BD Bioscience). Following staining, we re-washed the cells, and fixed them in formaldehyde before performing flow cytometry using a FACS Calibur (BD, San Jose, CA) [37]. We identified the lymphocyte population on forward versus side scatter plot, then gated CD3+CD4+ and CD3+CD8+ subpopulations, and identified CD4+IFN-γ+ and CD8+IFN-γ+ subpopulations. We subtracted unstimulated responses, and expressed results as interferon-γ+ T cells per 10×6 PBMC.
T-cell proliferation assay
To evaluate proliferative responses to antigens, we cultured PBMCs (105 cell per well) in DMEM/F12 medium (Gibco, GlutaMAX) supplemented with 1% gentamicin and 5% human AB+ serum in triplicate wells in round-bottomed 96-well plates. We added S. Typhi antigens and controls to wells at the same concentrations used in the interferon-γ ELISPOT assay and with a final culture volume of 200 µl. We incubated plates at 37°C in 5% CO2 for 5 days. After 48 h incubation, we replaced 100 µl of the medium per well with fresh medium. After 5 days of incubation, we added 3H-thymidine (1 µCi) to each well under sterile conditions, incubated plates for an additional 8 hours, harvested cells in Bray’s scintillation fluid (Ultimagold, PerkinElmer, Boston, MA) using a cell harvester (Skatron instruments, Norway), and assessed [3H] thymidine incorporation using a liquid scintillation β-counter (Beckman LS6500 multipurpose scintillation counter, USA) as previously described [22], [38]. We expressed results as counts per minute (cpm), and calculated stimulation indices for each antigen according to the formula: net cpm with antigen /net cpm without antigen (media alone) for each individual on each day (day 5 and day 20) [39].
Statistical analysis
We used Prism4 (version 4.03, GraphPad Software, Inc.) for data management, analysis and graphical presentation. We used unpaired T tests to compare differences between groups, and paired T tests to evaluate differences between study days within groups.
Results
Automated production of S. Typhi proteins
We estimated that we required at least 20 µg of a specific protein for use in our planned immunological assays. Our six production runs resulted in the production of 20 µg or more for 25 of our selected 58 proteins; nine of these samples had purity by LC90 Caliper analysis of >90%, and 17 had purity greater than >80%. Purity was defined as the quantity of protein matching the molecular size of the desired product. The LPS contamination of all preparations was found to be less than the level of detection of our assay kit (<300 fg/µl). Of these 17 proteins with sufficient quantity and purity, we selected 7 proteins for our initial analysis (Table 1) representing a range of cellular location and function, including a number involved in fimbrial attachment or adhesion such as StaF (putative fimbrial protein encoded by STY0202), StbB (fimbrial chaperone encoded by STY0372), CsgF (involved in curli production encoded by STY1177), and CsgD (a putative regulatory protein encoded by STY1179), as well as OppA (a periplasmic oligopeptide binding protein precursor involved in peptide transport encoded by STY1304), a conserved hypothetical protein encoded by STY2195, and PagC, an outer membrane protein encoded by STY1878 whose expression is regulated by the PhoP regulon involved in intra-macrophage survival [19], [28].
Table 1. S. Typhi protein preparations used in this study.
| STY number | Annotated name | Protein |
| STY0202 | Putative fimbrial protein | StaF |
| STY0372 | Fimbrial chaperone protein | StbB |
| STY1177 | Assembly/transport component in curli production | CsgF |
| STY1179 | Putative regulatory protein | CsgD |
| STY1304 | Periplasmic oligopeptide-binding protein precursor | OppA |
| STY1878 | Outer membrane invasion protein | PagC |
| STY2195 | Conserved hypothetical protein | |
| Membrane preparation | Crude membrane preparation containing at least 934 S. Typhi proteins (see Tables 2 and 3) | MP |
Mass spectrometric analysis of the S. Typhi membrane preparation
Our mass spectrometric analysis of S. Typhi membrane preparation identified 934 S. Typhi proteins (636 with three or more spectral counts), including many involved in energy metabolism, protein synthesis and fate, cell envelope or peptidoglycan synthesis or maintenance, cellular processes, proteins involved in transport, proteins involved in regulatory functions, and proteins involved in virulence and pathogenesis (Table 2 and 3 and Table S1). We also identified two of our 7 selected proteins (OppA and PagC) in the S. Typhi membrane preparation.
Table 2. Functional categories of proteins detected in S. Typhi membrane preparation.
| Classification | Number of proteins |
| Energy metabolism | 134 |
| Unknown function or unclassified | 105 |
| Cell envelope | 90 |
| Hypothetical proteins | 83 |
| Protein synthesis | 77 |
| Transport and binding proteins | 74 |
| Protein fate | 65 |
| Pathogenesis/virulence/cellular processes | 59 |
| Central intermediary metabolism | 42 |
| Regulatory functions | 39 |
| DNA metabolism | 38 |
| Amino acid biosynthesis | 30 |
| Biosynthesis of cofactors, prosthetic groups, and carriers | 28 |
| Purines, pyrimidines, nucleosides, and nucleotides | 25 |
| Transcription | 19 |
| Fatty acid and phospholipid metabolism | 18 |
| Mobile and extrachromosomal element functions | 4 |
| Viral functions | 4 |
| Total | 934 |
Table 3. Proteins represented in the S. Typhi membrane preparations and selected S. Typhi antigens.
| Accession | Entry name | Protein name | Peptide Hits | Rank* |
| Most abundant proteins | ||||
| P0A1H6 | EFTU_SALTI | Elongation factor Tu (EF-Tu) | 380 | 1 |
| Q8Z7S0 | OMPA_SALTI | Outer membrane protein A (OmpA) | 342 | 2 |
| Q8Z8C8 | Q8Z8C8_SALTI | Succinate dehydrogenase flavoprotein subunit | 298 | 3 |
| Q8Z1T9 | LAMB_SALTI | Maltoporin (maltose-inducible porin) | 250 | 4 |
| Q8XGX4 | ATPB_SALTI | ATP synthase subunit beta | 201 | 5 |
| Q8XG95 | ATPA_SALTI | ATP synthase subunit alpha | 185 | 6 |
| P0A264 | OMPC_SALTI | Outer membrane protein C (porin ompC) | 170 | 7 |
| Q8Z9F0 | Q8Z9F0_SALTI | Pyruvate dehydrogenase E1 component | 135 | 8 |
| Q8Z4L6 | PUR4_SALTI | Phosphoribosylformylglycinamidine synthase | 132 | 9 |
| P0AA29 | THIO_SALTI | Thioredoxin-1 (Trx-1) | 125 | 10 |
| Q8Z9E6 | Q8Z9E6_SALTI | Aconitate hydratase 2 | 117 | 11 |
| Q8Z937 | FADE_SALTI | Acyl-coenzyme A dehydrogenase | 116 | 12 |
| P0A1D4 | CH60_SALTI | 60 kDa chaperonin pProtein Cpn60) (GroEL protein) | 105 | 13 |
| Q8Z9A3 | YAET_SALTI | Outer membrane protein assembly factor yaeT | 102 | 14 |
| Q8XH17 | Q8XH17_SALTI | Outer membrane protein x | 98 | 15 |
| Q8Z858 | Q8Z858_SALTI | D-alanyl-D-alanine carboxypeptidase (penicillin-binding protein 6) | 91 | 16 |
| Q8Z6J0 | Q8Z6J0_SALTI | Phosphoenolpyruvate synthase | 88 | 17 |
| Q8XFH6 | Q8XFH6_SALTI | Peptidoglycan-associated lipoprotein | 86 | 18 |
| Q8Z8C6 | Q8Z8C6_SALTI | 2-oxoglutarate dehydrogenase E1 component | 78 | 19 |
| Q8Z7D2 | Q8Z7D2_SALTI | Aconitate hydratase 1 (Aconitate hydratase 1 (citrate hydro-lyase 1)) | 75 | 20 |
| Selected virulence proteins | ||||
| Q8Z7H3 | PHOQ_SALTI | Virulence sensor histidine kinase (PhoQ) | 70 | 29 |
| Q8Z8L8 | Q8Z8L8_SALTI | Ferrienterobactin receptor (FepA) | 38 | 103 |
| Q8Z7H2 | PHOP_SALTI | Virulence transcriptional regulatory protein (PhoP) | 37 | 104 |
| Q8Z1P4 | Q8Z1P4_SALTI | Two-component response regulator (PmrA) | 4 | 487 |
| P61091 | SLYA_SALTI | Transcriptional regulator (SlyA) | 4 | 543 |
| Q8Z6B2 | Q8Z6B2_SALTI | Outer membrane invasion protein (PagC) | 4 | 544 |
| Q8Z3Y7 | Q8Z3Y7_SALTI | Putative uncharacterized protein associated with virulence (STY3182) | 2 | 749 |
| Q8Z727 | HLYE_SALTI | Hemolysin E (Cytotoxin ClyA) | 1 | 859 |
| S . Typhi proteins individually purified and also detected in S . Typhi membrane preparation | ||||
| Q8Z7F0 | Q8Z7F0_SALTI | Periplasmic oligopeptide-binding protein (OppA) | 15 | 225 |
| Q8Z6B2 | Q8Z6B2_SALTI | Outer membrane invasion protein (PagC) | 4 | 544 |
*Rank order of abundance.
Interferon-γ ELISPOT responses and T cell characterization
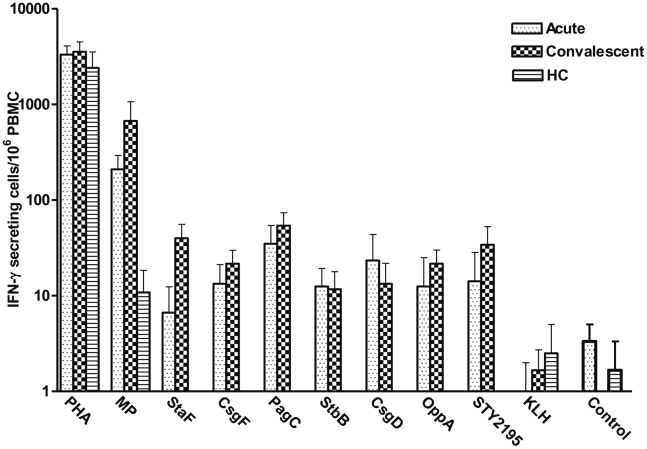
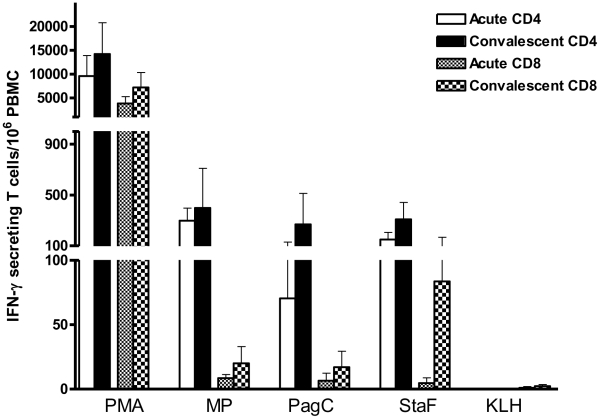
We found that patients with S. Typhi bacteremia had elevated interferon-γ ELISPOT responses at both acute and convalescent stages of infection compared to healthy controls for all seven of the purified S. Typhi proteins, as well as against S. Typhi crude membrane preparation (P<0.05) (Figure 1). In contrast, responses to PHA did not differ significantly between patients and healthy controls, and minimal responses were detected against control protein and KLH in both patients and healthy controls. To assess whether interferon-γ responses were CD4 or CD8-derived, we used intracellular cytokine staining following stimulation with a subset of proteins, and found that the majority of interferon-γ expressing cells were CD4-positive, although a CD8 positive response was also detected (Figure 2).
Figure 1. Interferon-γ ELISPOT responses to S. Typhi antigens including StaF (STY0202), CsgF (STY1177), PagC (STY1878), StbB (STY0372), CsgD (STY1179), OppA (STY1304), conserved hypothetical protein encoded by STY2195, S. Typhi membrane preparation (MP), control protein, and phytohaemagglutinin (PHA) and keyhole limpet hemocyanin (KLH) during acute and convalescent stage illness in S. Typhi bacteremic patients and in healthy controls (HC).
Mean and standard error of the mean represented.
Figure 2. Characterization of interferon-γ CD4 and CD8 responses to S. Typhi antigens including StaF (STY0202), PagC (STY1878), S. Typhi membrane preparation (MP), and PMA and keyhole limpet hemocyanin (KLH) during acute and convalescent stage illness in S. Typhi bacteremic patients.
Mean and standard error of the mean represented.
Proliferation responses
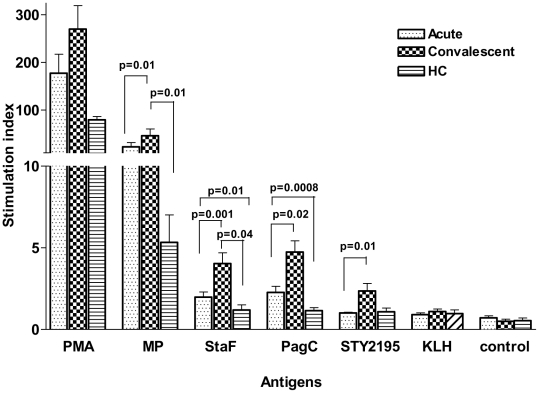
To further evaluate responses, we selected the three proteins associated with the highest interferon-γ expression levels in convalescent phase samples, as well as membrane preparation, for inclusion in cellular proliferation assays. In comparison to healthy Bangladeshi controls residing within the same S. Typhi endemic area, individuals with documented S. Typhi bacteremia had significantly elevated proliferation indices at the acute stage of illness to StaF and PagC (P<0.01−0.0008), but not to STY2195, or crude membrane preparation, and these acute stage responses further significantly increased within bacteremic individuals by the convalescent period compared to the acute stage responses (P≤0.02−0.001) (Figure 3). We also detected a significantly increased proliferation response to STY2195 and S. Typhi membrane preparation in bacteremic patients at convalescence compared to acute phase samples and compared to control patients (p≤0.01).
Figure 3. Cellular proliferation responses to S. Typhi antigens including StaF (STY0202), PagC (STY1878), conserved hypothetical protein encoded by STY2195, S. Typhi membrane preparation (MP), control protein, and phytohaemagglutinin (PHA) and keyhole limpet hemocyanin (KLH) during acute and convalescent stage illness in S. Typhi bacteremic patients and in healthy controls (HC).
Stimulation index: net cpm with antigen /net cpm without antigen (media alone) for each individual on each day (acute and convalescent). Mean and standard error of the mean represented.
Discussion
Cellular immune responses, including CD4 and CD8-mediated interferon-γ responses, play a critical role in clearing and controlling systemic Salmonella infections [23], [40]. Despite this, there has been limited evaluation of cellular responses in humans to wild-type S. Typhi. No animal model fully replicates host-pathogen interactions and immunologic events that occur during this human-restricted infection. Evaluation in humans has largely focused on characterizing responses in recipients of attenuated vaccine strains of S. Typhi [23], [38], [41], [42], [43], [44]. We report here a screening approach that permitted us to evaluate interferon-γ and proliferation responses to a number of bacterial antigens in S. Typhi-infected humans in Bangladesh. We selected proteins that we had previously identified in immuno-affinity screening assays for humoral responses [28], and we recovered these selected proteins using an automated system and high throughput genomic and proteomic technologies. Although we were able to generate adequate samples for only approximately a third of our selected proteins for evaluation in humans, we feel that high throughput approaches such as the one we describe will assist in accelerating analysis of pathogens that express thousands of antigens. For instance, S. Typhi contains approximately 4,400 open reading frames, and although protein microarrays can be used to screen for humoral responses across the immunoproteome, no comparable system has yet been developed to assess cellular immune responses in a high throughput manner, despite the critical role that cellular immune responses play against intracellular pathogens.
We recognize that high throughput purification techniques may be compromised by issues of contamination, including with LPS when expression occurs in E. coli vectors. However, LPS contamination of all preparations was found to be less than the level of detection of our assay kit (<300 fg/µl), we did not detect cellular immune responses to control protein expressed and purified from E. coli in the same manner as our S. Typhi proteins, and we detected cellular immune responses in patients but not healthy controls to purified S. Typhi proteins. All of these observations suggest that the responses we observed were antigen-specific and not due to contaminating LPS.
In the S. Typhimurium mouse model, CD4 and CD8 cells are critical to the development of protective immunity, and control of Salmonella infection involves prominent expression of interferon-γ by both CD4 and CD8 cells [24], [25], [26]. Overall, only a relatively few defined class I and class II epitopes have been identified in the S. Typhimurium mouse model, including epitopes in FliC and SipC for CD4 cells, and OmpC and GroEL for CD8 cells [24], [40], [45], [46], [47], [48]. A number of Salmonella antigens are also able to induce partially protective immunity when included in subunit-based vaccines in mice, including flagellin, MIG-14 and SseB (Salmonella antigens expressed in vivo), suggesting that immune responses against a number of Salmonella antigens could contribute to protective immunity [49], [50], [51].
In comparison to the murine data, evaluation of cellular responses to S. Typhi in humans have largely involved individuals who have received attenuated S. Typhi vaccine strains such as Ty21a and CVD908 [23], [38], [41], [42], [43], [44]. In concordance with the mouse data, these studies have shown induction of interferon-γ-expressing CD4 and CD8 responses following vaccination [23], [24], [38], [42], [52]. Interestingly, CD8 responses may involve both classical (HLA-A, B and C in humans) and non-classical (HLA–E, F, and G) mediated T cell recognition [43], [52]. Using an ex vivo model, Sztein and colleagues have also recently found that direct infection of antigen-presenting dendritic cells with S. Typhi leads to expression of high levels of TNF-α, IL-6 and IL-8, and low levels of interferon-γ and IL-12 p70, but that dendritic cells can also ingest other infected human cells leading to high level expression of interferon-γ and IL-12 p70, with subsequent induction of a population of CD3+CD8+CD45RA-CD62L- effector/memory T cells in co-cultured lymphocytes [53]. Based on these observations, we used recombinant antigens to assess and characterize interferon-γ and proliferation responses in infected humans in Bangladesh. To establish the feasibility of our approach, we focused our initial efforts on a subset of proteins that we had previously identified as generating humoral immunity and being expressed in vivo during human infection [28]. These included a number involved in fimbrial attachment or adhesion such as StaF, StbB, CsgF, and CsgD, as well as OppA, a conserved hypothetical protein encoded by STY2195, and PagC, an outer membrane protein encoded by STY1878. We previously found that humans infected with S. Typhi develop a serum antibody response to PagC and that this response increases at convalescence [28]. Here we furthered this observation and report detection of a parallel cellular response against PagC during human infection, including both interferon-γ and proliferative responses, and show that responses in convalescence were higher than during acute stage illness. Although the role of PagC during human infection is not fully understood, its expression is controlled by the PhoP-regulon involved in intra-macrophage survival [19], [54].
We also detected significant increases in cellular responses during convalescence against StaF, a fimbrial protein homologous to E. coli YadK that contains a Pfam motif believed to be involved in cellular adhesion [17], STY2195, a conserved hypothetical protein of unknown function, and a crude membrane preparation containing over 900 S. Typhi proteins, including GroEL, OmpC, OppA and PagC.
We found that S. Typhi proteins elicit both CD4+ and CD8+ interferon-γ expressing responses, with CD4 responses being more numerous than CD8 responses. Of interest, we were able to detect antigen-specific interferon-γ responses in patients, including at the time that patients presented for clinical care, but similar responses were not seen on controls. These observations suggest that an antigen-specific interferon-γ-based detection system might be used to diagnose individuals with typhoid fever during the acute stage of illness, similar to the approach used to diagnose infection with Mycobacterium tuberculosis [55], [56], [57]. Currently, all available diagnostic tests for typhoid fever lack either sensitivity and/or specificity, especially in areas of the world endemic for typhoid. For example, microbiological culturing of blood has approximately 30–70% sensitivity, depending on the volume of blood obtained and whether previous antibiotics have been administered, and the Widal assay has at best 85% specificity when analyzing both acute and convalescent phase responses in endemic zones where typhoid exacts its highest burden [58], [59], [60].
In summary, we have used a screening format to preliminarily characterize S. Typhi antigen-specific interferon-γ responses in patients with typhoid fever. This is the first characterization of such responses in humans, and further immunologic analysis will be required to assess the role, if any, that these responses play in controlling or clearing S. Typhi infection. Our study has a number limitations, including analysis of a relatively small number of purified S. Typhi antigens, characterization of a limited number of immunologic parameters, and the absence of the inclusion of febrile control patients confirmed not to be acutely infected with S. Typhi; however, our detection of antigen-specific interferon-γ responses could assist in the development of interferon-γ-based diagnostic assays for typhoid fever, and our overall approach could be used to identify antigens capable of inducing cellular immune responses during infection with other intracellular pathogens.
Supporting Information
Mass spectrometric analysis of S . Typhi membrane preparation.
(XLS)
Acknowledgments
S. Typhi gene inserts were obtained from the NIAID-sponsored Pathogen Functional Genomic Resource Center, J. Craig Venter Institute (JCVI, formerly The Institute for Genomic Research).
Footnotes
The authors have declared that no competing interests exist.
This work was supported by the International Centre for Diarrhoeal Disease Research, Bangladesh (www.icddrb.org) and grants from the National Institutes of Health, including the National Institute of Allergy & Infectious Diseases (AI072599 [ETR], AI077883 [ETR], AI058935 [RCC, SBC]); Swedish Sida (FQ); the PneumoADIP Project of the Johns Hopkins Bloomberg School of Public Health (WAB); a ICDDR,B-US Centers for Disease Control and Prevention Cooperative Agreement (WAB); ICDDR,B core funds (FQ, WAB); a Training Grant in Vaccine Development from the Fogarty International Center (TW05572 [AS, MSB, FQ]); an American Recovery and Reinvestment Act (ARRA) FIC Post-doctoral Fellowship in Global Infectious Diseases (TW05572 [RCC, DTL]); Career Development Awards (K01) from the Fogarty International Center (TW007409 [JBH], TW07144 [RCL]) and the Harvard Initiative for Global Health Post-doctoral Fellowship in Global Infectious Diseases (DTL); and a Physician Scientist Early Career Award from the Howard Hughes Medical Institute (RCL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, et al. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17(Suppl 2):S22–27. doi: 10.1016/s0264-410x(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 3.Alpuche-Aranda CM, Berthiaume EP, Mock B, Swanson JA, Miller SI. Spacious phagosome formation within mouse macrophages correlates with Salmonella serotype pathogenicity and host susceptibility. Infect Immun. 1995;63:4456–4462. doi: 10.1128/iai.63.11.4456-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser MJ, Newman LS. A review of human salmonellosis: I. Infective dose. Rev Infect Dis. 1982;4:1096–1106. doi: 10.1093/clinids/4.6.1096. [DOI] [PubMed] [Google Scholar]
- 5.Buchmeier NA, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwan WR, Huang XZ, Hu L, Kopecko DJ. Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect Immun. 2000;68:1005–1013. doi: 10.1128/iai.68.3.1005-1013.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang T, Puthucheary SD. Significance and value of the Widal test in the diagnosis of typhoid fever in an endemic area. J Clin Pathol. 1983;36:471–475. doi: 10.1136/jcp.36.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herath HM. Early diagnosis of typhoid fever by the detection of salivary IgA. J Clin Pathol. 2003;56:694–698. doi: 10.1136/jcp.56.9.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.House D, Wain J, Ho VA, Diep TS, Chinh NT, et al. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol. 2001;39:1002–1007. doi: 10.1128/JCM.39.3.1002-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez C, Calderon GM, Ximenez C, Melendro EI. Human cell-mediated immune responses to antigenic fractions of Salmonella typhi. Immunology. 1996;89:262–267. doi: 10.1046/j.1365-2567.1996.d01-723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jesudason MV, Sridharan G, Arulselvan R, Babu PG, John TJ. Diagnosis of typhoid fever by the detection of anti-LPS & anti-flagellin antibodies by ELISA. Indian J Med Res. 1998;107:204–207. [PubMed] [Google Scholar]
- 12.Sur D, Ochiai RL, Bhattacharya SK, Ganguly NK, Ali M, et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med. 2009;361:335–344. doi: 10.1056/NEJMoa0807521. [DOI] [PubMed] [Google Scholar]
- 13.Sarma VN, Malaviya AN, Kumar R, Ghai OP, Bakhtary MM. Development of immune response during typhoid fever in man. Clin Exp Immunol. 1977;28:35–39. [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopalan P, Kumar R, Malaviya AN. A study of humoral and cell-mediated immune response following typhoid vaccination in human volunteers. Clin Exp Immunol. 1982;47:275–282. [PMC free article] [PubMed] [Google Scholar]
- 15.Tiwari H, Kamat RS. Cross-reactions in cell-mediated immunity to Salmonella causing enteric fever. J Med Microbiol. 1986;21:233–237. doi: 10.1099/00222615-21-3-233. [DOI] [PubMed] [Google Scholar]
- 16.Ohl ME, Miller SI. Salmonella: a model for bacterial pathogenesis. Annu Rev Med. 2001;52:259–274. doi: 10.1146/annurev.med.52.1.259. [DOI] [PubMed] [Google Scholar]
- 17.Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 2001;413:848–852. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- 18.McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 19.Charles RC, Harris JB, Chase MR, Lebrun LM, Sheikh A, et al. Comparative proteomic analysis of the PhoP regulon in Salmonella enterica serovar Typhi versus Typhimurium. PLoS One. 2009;4:e6994. doi: 10.1371/journal.pone.0006994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos RL, Baumler AJ. Cell tropism of Salmonella enterica. Int J Med Microbiol. 2004;294:225–233. doi: 10.1016/j.ijmm.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Levine MM, DuPont HL, Hornick RB, Snyder MJ, Woodward W, et al. Attenuated, streptomycin-dependent Salmonella typhi oral vaccine: potential deleterious effects of lyophilization. J Infect Dis. 1976;133:424–429. doi: 10.1093/infdis/133.4.424. [DOI] [PubMed] [Google Scholar]
- 22.Kilhamn J, Lundin SB, Brevinge H, Svennerholm AM, Jertborn M. T- and B-cell immune responses of patients who had undergone colectomies to oral administration of Salmonella enterica serovar Typhi Ty21a vaccine. Clin Diagn Lab Immunol. 2003;10:426–430. doi: 10.1128/CDLI.10.3.426-430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salerno-Goncalves R, Wyant TL, Pasetti MF, Fernandez-Vina M, Tacket CO, et al. Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain CVD 908-htrA. J Immunol. 2003;170:2734–2741. doi: 10.4049/jimmunol.170.5.2734. [DOI] [PubMed] [Google Scholar]
- 24.Ravindran R, McSorley SJ. Tracking the dynamics of T-cell activation in response to Salmonella infection. Immunology. 2005;114:450–458. doi: 10.1111/j.1365-2567.2005.02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med. 2002;2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- 26.Mittrucker HW, Kohler A, Kaufmann SH. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect Immun. 2002;70:199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy TV, Wu W, Qiu QQ, Shi Z, LaBaer J, et al. Bacterial cell-free system for high-throughput protein expression and a comparative analysis of Escherichia coli cell-free and whole cell expression systems. Protein Expr Purif. 2004;36:217–225. doi: 10.1016/j.pep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Harris JB, Baresch-Bernal A, Rollins SM, Alam A, LaRocque RC, et al. Identification of in vivo-induced bacterial protein antigens during human infection with Salmonella enterica serovar Typhi. Infect Immun. 2006;74:5161–5168. doi: 10.1128/IAI.00488-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhuiyan TR, Qadri F, Saha A, Svennerholm AM. Infection by Helicobacter pylori in Bangladeshi children from birth to two years: relation to blood group, nutritional status, and seasonality. Pediatr Infect Dis J. 2009;28:79–85. doi: 10.1097/INF.0b013e31818a5d9d. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh A, Bhuiyan MS, Khanam F, Chowdhury F, Saha A, et al. Salmonella enterica serovar Typhi-specific immunoglobulin A antibody responses in plasma and antibody in lymphocyte supernatant specimens in Bangladeshi patients with suspected typhoid fever. Clin Vaccine Immunol. 2009;16:1587–1594. doi: 10.1128/CVI.00311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaRocque RC, Krastins B, Harris JB, Lebrun LM, Parker KC, et al. Proteomic analysis of Vibrio cholerae in human stool. Infect Immun. 2008;76:4145–4151. doi: 10.1128/IAI.00585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 33.Talawadekar NN, Vadher PJ, Antani DU, Kale VV, Kamat SA. Chloramphenicol resistant Salmonella species isolated between 1978 and 1987. J Postgrad Med. 1989;35:79–82. [PubMed] [Google Scholar]
- 34.Bhuiyan TR, Lundin SB, Khan AI, Lundgren A, Harris JB, et al. Cholera caused by Vibrio cholerae O1 induces T-cell responses in the circulation. Infect Immun. 2009;77:1888–1893. doi: 10.1128/IAI.01101-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qadri F, Ahmed T, Ahmed F, Bradley Sack R, Sack DA, et al. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi children 18-36 months of age. Vaccine. 2003;21:2394–2403. doi: 10.1016/s0264-410x(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 36.Sacre K, Carcelain G, Cassoux N, Fillet AM, Costagliola D, et al. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J Exp Med. 2005;201:1999–2010. doi: 10.1084/jem.20042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gauduin MC. Intracellular cytokine staining for the characterization and quantitation of antigen-specific T lymphocyte responses. Methods. 2006;38:263–273. doi: 10.1016/j.ymeth.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Lundin BS, Johansson C, Svennerholm AM. Oral immunization with a Salmonella enterica serovar Typhi vaccine induces specific circulating mucosa-homing CD4(+) and CD8(+) T cells in humans. Infect Immun. 2002;70:5622–5627. doi: 10.1128/IAI.70.10.5622-5627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. Cell-mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine. 2007;25:1416–1425. doi: 10.1016/j.vaccine.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo WF, Ong H, Metcalf ES, Soloski MJ. T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J Immunol. 1999;162:5398–5406. [PubMed] [Google Scholar]
- 41.Sztein MB, Tanner MK, Polotsky Y, Orenstein JM, Levine MM. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J Immunol. 1995;155:3987–3993. [PubMed] [Google Scholar]
- 42.Salerno-Goncalves R, Pasetti MF, Sztein MB. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2002;169:2196–2203. doi: 10.4049/jimmunol.169.4.2196. [DOI] [PubMed] [Google Scholar]
- 43.Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2004;173:5852–5862. doi: 10.4049/jimmunol.173.9.5852. [DOI] [PubMed] [Google Scholar]
- 44.Lundgren A, Kaim J, Jertborn M. Parallel analysis of mucosally derived B- and T-cell responses to an oral typhoid vaccine using simplified methods. Vaccine. 2009;27:4529–4536. doi: 10.1016/j.vaccine.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Lo WF, Woods AS, DeCloux A, Cotter RJ, Metcalf ES, et al. Molecular mimicry mediated by MHC class Ib molecules after infection with gram-negative pathogens. Nat Med. 2000;6:215–218. doi: 10.1038/72329. [DOI] [PubMed] [Google Scholar]
- 46.Diaz-Quinonez A, Martin-Orozco N, Isibasi A, Ortiz-Navarrete V. Two Salmonella OmpC K(b)-restricted epitopes for CD8+-T-cell recognition. Infect Immun. 2004;72:3059–3062. doi: 10.1128/IAI.72.5.3059-3062.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cookson BT, Bevan MJ. Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognized by T cells from orally immunized mice. J Immunol. 1997;158:4310–4319. [PubMed] [Google Scholar]
- 48.Musson JA, Hayward RD, Delvig AA, Hormaeche CE, Koronakis V, et al. Processing of viable Salmonella typhimurium for presentation of a CD4 T cell epitope from the Salmonella invasion protein C (SipC). Eur J Immunol. 2002;32:2664–2671. doi: 10.1002/1521-4141(200209)32:9<2664::AID-IMMU2664>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 49.Rollenhagen C, Sorensen M, Rizos K, Hurvitz R, Bumann D. Antigen selection based on expression levels during infection facilitates vaccine development for an intracellular pathogen. Proc Natl Acad Sci U S A. 2004;101:8739–8744. doi: 10.1073/pnas.0401283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect Immun. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasan A, Foley J, McSorley SJ. Massive number of antigen-specific CD4 T cells during vaccination with live attenuated Salmonella causes interclonal competition. J Immunol. 2004;172:6884–6893. doi: 10.4049/jimmunol.172.11.6884. [DOI] [PubMed] [Google Scholar]
- 52.Sztein MB. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica Serovar Typhi strains used as live oral vaccines in humans. Clin Infect Dis. 2007;45(Suppl 1):S15–19. doi: 10.1086/518140. [DOI] [PubMed] [Google Scholar]
- 53.Salerno-Goncalves R, Sztein MB. Priming of Salmonella enterica serovar typhi-specific CD8(+) T cells by suicide dendritic cell cross-presentation in humans. PLoS One. 2009;4:e5879. doi: 10.1371/journal.pone.0005879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller SI, Kukral AM, Mekalanos JJ. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2005;172:631–635. doi: 10.1164/rccm.200502-196OC. [DOI] [PubMed] [Google Scholar]
- 56.Goletti D, Vincenti D, Carrara S, Butera O, Bizzoni F, et al. Selected RD1 peptides for active tuberculosis diagnosis: comparison of a gamma interferon whole-blood enzyme-linked immunosorbent assay and an enzyme-linked immunospot assay. Clin Diagn Lab Immunol. 2005;12:1311–1316. doi: 10.1128/CDLI.12.11.1311-1316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hauer B, Loddenkemper R, Detjen A, Forssbohm M, Haas W, et al. [Interferon-gamma assays -- description and assessment of a new tool in the diagnosis of tuberculosis]. Pneumologie. 2006;60:29–44. doi: 10.1055/s-2005-919075. [DOI] [PubMed] [Google Scholar]
- 58.Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, et al. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol. 1998;36:1683–1687. doi: 10.1128/jcm.36.6.1683-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wain J, Hosoglu S. The laboratory diagnosis of enteric fever. J Infect Dev Ctries. 2008;2:421–425. doi: 10.3855/jidc.155. [DOI] [PubMed] [Google Scholar]
- 60.Baker S, Favorov M, Dougan G. Searching for the elusive typhoid diagnostic. BMC Infect Dis. 2010;10:45. doi: 10.1186/1471-2334-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mass spectrometric analysis of S . Typhi membrane preparation.
(XLS)