Abstract
Background
Acute myeloid leukemia (AML) is a genetically heterogeneous cancer that frequently exhibits aberrant kinase signaling. We investigated a treatment strategy combining sorafenib, a multikinase inhibitor with limited single-agent activity in AML, and cytarabine, a key component of AML chemotherapy.
Methods
Using 10 human AML cell lines, we determined the effects of sorafenib (10 μM) on antileukemic activity by measuring cell viability, proliferation, ERK1/2 signaling, and apoptosis. We also investigated the effects of sorafenib treatment on the accumulation of cytarabine and phosphorylated metabolites in vitro. A human equivalent dose of sorafenib in nontumor-bearing NOD-SCID-IL2Rγnull mice was determined by pharmacokinetic studies using high performance liquid chromatography with tandem mass spectrometric detection, and steady-state concentrations were estimated by the fit of a one-compartment pharmacokinetic model to concentration–time data. The antitumor activity of sorafenib alone (60 mg/kg) twice daily, cytarabine alone (6.25 mg/kg administered intraperitoneally), or sorafenib once or twice daily plus cytarabine was evaluated in NOD-SCID-IL2Rγnull mice bearing AML xenografts.
Results
Sorafenib at 10 μM inhibited cell viability, proliferation and ERK1/2 signaling, and induced apoptosis in all cell lines studied. Sorafenib also increased the cellular accumulation of cytarabine and metabolites resulting in additive to synergistic antileukemic activity. A dose of 60 mg/kg in mice produced a human equivalent sorafenib steady-state plasma exposure of 10 μM. The more dose-intensive twice-daily sorafenib plus cytarabine (n = 15) statistically significantly prolonged median survival in an AML xenograft model compared with sorafenib once daily plus cytarabine (n = 12), cytarabine alone (n = 26), or controls (n = 27) (sorafenib twice daily plus cytarabine, median survival = 46 days; sorafenib once daily plus cytarabine, median survival = 40 days; cytarabine alone, median survival = 36 days; control, median survival = 19 days; P < .001 for combination twice daily vs all other treatments listed).
Conclusions
Sorafenib in combination with cytarabine resulted in strong anti-AML activity in vitro and in vivo. These results warrant clinical evaluation of sorafenib with cytarabine-based regimens in molecularly heterogeneous AML.
Context and Caveats
Prior knowledge
Previous studies have indicated that aberrant kinase signaling in acute myeloid leukemia (AML) patients is associated with poor prognosis. Sorafenib is a multikinase inhibitor that has demonstrated limited efficacy in the clinic as a monotherapy for genetically heterogeneous AML and enhanced efficacy when combined with chemotherapeutic drugs.
Study design
The antileukemic activity of sorafenib alone and in combination with cytarabine, a widely used chemotherapeutic agent for the treatment of AML, was studied in heterogeneous AML cell lines, primary childhood AML blast samples, and in a mouse model. The effect of sorafenib on cell viability, proliferation, kinase signaling, and apoptosis, in vitro; and apoptosis were measured in vitro. The effects of sorafenib plus cytarabine on viability of AML cells and primary blasts in vitro, and on AML xenograft growth in vivo, were determined. Different doses and schedules were investigated.
Contribution
The addition of sorafenib administered at 10 μM statistically significantly enhanced the antileukemic activity of cytarabine in an additive to synergistic manner when delivered simultaneously in vitro and in vivo. The combination treatment increased the intracellular accumulation of cytarabine and its metabolites in vitro, indicating a potential mechanism behind the antileukemic activity.
Implications
Sorafenib combined with cytarabine may have potential clinical use as a treatment for AML.
Limitations
The exact mechanisms behind the enhanced antileukemic activity of the combination of sorafenib and cytarabine are unclear, and further studies investigating the role of sorafenib-mediated inhibition of kinase signaling pathways are warranted. Also, the most effective dose and schedule of the combination of sorafenib and cytarabine in molecularly heterogeneous AML (ie, leukemias with different FLT3 status) should be determined.
From the Editors
Acute myeloid leukemia (AML) is a genetically heterogeneous form of leukemia. Leukemic cells in many AML patients are characterized by aberrant signaling in downstream pathways such as RAF/MEK/ERK and PI3K/AKT (1–3), which can lead to uncontrolled cell proliferation and increased cell survival. Phosphorylated AKT and ERK are reportedly expressed in greater than 70% of primary AML samples (4–6), with high expression conferring a poor prognosis (7–9). The molecular basis for increased signaling in these pathways is variable. It may involve gain-of-function mutations in receptor tyrosine kinases (RTKs), such as FLT3 and c-KIT, increased expression or auto- and paracrine stimulation of RTKs, or may occur in the absence of detectable alterations in RTKs (1–3). Regardless of the molecular mechanism, inhibition of the deregulated signaling pathways is an attractive treatment strategy for AML, particularly considering the number of patient samples that show activated signaling overall.
At nanomolar concentrations, sorafenib is an inhibitor of C-RAF, B-RAF, c-KIT, FLT3, platelet-derived growth factor receptor-α and -β, and vascular endothelial growth factor receptor-1, -2, and -3. At a clinically achievable plasma concentration of 10 μM, sorafenib inhibits multiple additional RTKs and intracellular kinases (10). Sorafenib is approved for the treatment of advanced renal cell carcinoma and hepatocellular carcinoma and is being evaluated for the treatment of AML (11). In AML cells, sorafenib has been shown to inhibit phosphorylation of ERK1/2, inhibit cell growth, and induce apoptosis in vitro (12–14). The latter effect might be explained by increased expression of Bim and activation of proapoptotic proteins Bad, Bax, and Bak and decreased expression of Mcl-1 (11,13). In adults with relapsed or refractory AML, sorafenib as a single agent produced modest reductions in peripheral blood or bone marrow blasts, with low overall response rates (0%–3.8%) (15–17). It was previously shown that inhibitors of mitogen-activated protein kinase signaling can lower the apoptotic threshold of leukemic cells and sensitize them to the proapoptotic effects of chemotherapeutic agents (18). We therefore hypothesized that sorafenib might improve the antileukemic activity of standard chemotherapeutic drugs. In this study, we evaluated the activity of sorafenib in combination with cytarabine, one of the most widely used agents for the treatment of AML, using AML cell lines, primary childhood AML blast samples, and in an AML xenograft model. Pharmacokinetic studies were performed to determine a dose and schedule that produced human equivalent exposure for in vivo evaluation.
Materials and Methods
Cell Lines and Reagents
Sorafenib was purchased from Toronto Research Chemicals (Ontario, Canada). Cytarabine was purchased from Sigma-Aldrich (St Louis, MO). 3H cytarabine (with a specific activity of 14.9 Ci/mmol at 1.0 mCi/mL) was purchased from Moravek Biochemicals (Brea, CA). Cell culture reagents, including RPMI-1640, Dulbecco’s modified Eagle medium, and fetal bovine serum, were purchased from Invitrogen (Carlsbad, CA). The human AML cell lines HL-60, Kasumi-1, NB4, ML-2, MV4-11, KG-1, THP-1, U937, and M-07e were purchased from the American Tissue Culture Collection (Manassas, VA) and were cultured in RPMI-1640 containing 10% or 20% fetal bovine serum at 37°C in a humidified atmosphere with 5% CO2. M-07e cells were supplemented with interleukin-3 (10 ng/mL) (Sigma-Aldrich). OCI-AML3 cells were obtained from Dr Brian Sorrentino (St Jude Children’s Research Hospital, Memphis, TN) (19) and maintained inDulbecco’s modified Eagle medium containing 10% fetal bovine serum. Cytogenetic profiles, unique leukemogenic translocation/fusion genes, and mutations in tyrosine kinases were confirmed and described previously (14) and last verified in 2008, except for OCI-AML3 cells that were verified in 2009.
Primary AML Blast Samples
Leukemic blasts from bone marrow or peripheral blood were obtained from 25 children with newly diagnosed AML before treatment on the protocol AML02 (20), following informed consent according to institutional guidelines. Details of the study are provided in the Supplementary Materials (available online). Enrichment of blasts to more than 90% was carried out using a method based on magnetic-activated cell sorting, as previously described (14). Briefly, cells were suspended in magnetic-activated cell sorting buffer and were incubated with antibodies in the dark for 10 minutes at 4°C to deplete contamination from T cells, B cells, erythroids, neutrophils, and monocytes. After washing with 10 mL magnetic-activated cell sorting buffer once to remove unbound antibodies, 20 μL of anti-phycoerythrin microbeads (Miltenyi Biotec, Auburn, CA) per 1 × 107 cells were added for antibody selection. The phycoerythrin-labeled cell suspension was applied to a LS column (Miltenyi Biotec) and washed twice with 3 mL ice-cold magnetic-activated cell sorting buffer (Miltenyi Biotec).
Analysis of Cell Viability, Proliferation, and Apoptosis
Cell viability was determined in AML cell lines and primary blast samples using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Cell Proliferation Kit I (Roche, Indianapolis, IN), as previously described (14). Ten cell lines were treated with sorafenib or cytarabine at increasing concentrations (0.0001–40 μM) for 72 hours (three independent experiments were performed with eight replicates at each concentration). The concentration inhibiting cell viability by 50% compared with dimethyl sulfoxide-treated control cells (IC50) was determined using the software program GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA). Seven cell lines that grew well in culture and collectively had a drug sensitivity that spanned the entire range of single-agent sensitivity to sorafenib and cytarabine (eg, most sensitive, intermediately sensitive, and least sensitive cells) were chosen for drug combination studies. Cell lines were treated simultaneously with the combination of sorafenib and cytarabine for 72 hours at a fixed concentration ratio of sorafenib to cytarabine of 2:1 (OCI-AML3 and NB4 cells), 10:1 (HL-60), 100:1 (U937), 1:2 (THP-1), 1:5 (Kasumi-1), or 1:250 (MV4-11), with the concentrations selected on the basis of the results from experiments with individual drug treatments. We also evaluated the effect of sequence of drug administration in MV4-11 cells because this was the only cell line in which we previously observed a strong sorafenib-induced cell cycle block (14). We evaluated the sequences cytarabine for 24 hours before sorafenib for 48 hours (pre-cytarabine) and sorafenib for 24 hours before cytarabine for 48 hours (pre-sorafenib), as previously described for the tyrosine kinase inhibitor lestaurtinib (21). For the combination studies, two or three independent experiments were performed with eight replicates each. Two approaches were used to assess if combination drug effects were synergistic, additive, or antagonistic: 1) the universal response surface approach (22) and 2) the median effect approach (23). The response surface method was implemented in Matlab (version 7.11; MathWorks, Natick, MA). A synergism–antagonism parameter, α, and its associated 95% confidence intervals (CIs) were determined. A positive α indicated synergism, a negative α indicated antagonism, and a zero α indicated an additive effect. The median effect approach according to the method of Chou and Talalay (23) was implemented in the computer software CalcuSyn (version 2.1; Biosoft, Cambridge, UK). A combination index value and its associated 95% confidence interval was determined at the 50%, 75%, and 90% effective dose (ED) corresponding to ED50, ED75, and ED90. A combination index value of less than 0.9 indicated synergy, a combination index value of 0.90–1.1 indicated additive effects, and a combination index value of more than 1.1 indicated antagonism.
For primary AML blasts, duplicate samples were treated with sorafenib (10 μM) or cytarabine (10 μM) for 96 hours. A sorafenib concentration of 10 μM was used because this is an average steady-state plasma concentration achieved at the adult approved dose of 400 mg administered twice daily (24). A cytarabine concentration of 10 μM was chosen because this is the average steady-state plasma concentration achieved in children receiving cytarabine as a continuous infusion (25). When enough cells were available, the combination of sorafenib (10 μM) plus cytarabine (10 μM) was evaluated.
Cell proliferation was determined in HL-60, Kasumi-1, KG-1, M-07e, MV4-11, ML-2, NB4, OCI-AML3, THP-1, and U937 AML cell lines using a 5-ethynyl-2’-deoxyuridine (EdU) cell proliferation kit (Click-iT EdU Alexa Fluor 488 Flow Cytometry Assay Kit; Invitrogen), which measures newly synthesized DNA. Cells were seeded in six-well plates for 24 hours followed by a 72-hour incubation with sorafenib (10 μM). Cells were washed, incubated with EdU (10 μM) for 1 hour, and resuspended at 1 × 107 cells per milliliter in 1× phosphate-buffered saline (8 g sodium chloride, 0.2 g potassium chloride, 1.44 g sodium hydrogen phosphate, and 0.24 g potassium dihydrogen phosphate per 1 L of water), containing 1% bovine serum albumin. The cell suspension (100 μL) was incubated with 100 μL of fixative for 15 minutes, washed with 1% bovine serum albumin in phosphate-buffered saline, and incubated with a Triton X-100-based permeabilization reagent provided in the assay kit for 30 minutes. Cells were centrifuged at 500g for 5 minutes at room temperature, and the cell pellet was then resuspended with 0.5 mL of reaction cocktail for 30 minutes before detection of Alex Fluor 488 dye positive–staining cells by flow cytometry (Flow Cytometry and Cell Sorting Shared Resource, St Jude Children’s Research Hospital). Detection is based on a click reaction, which is a copper-catalyzed covalent reaction between an azide and an alkyne. In this application, the EdU contains the alkyne, whereas the Alexa Fluor 488 dye contains azide. Data were analyzed as the mean value of signal (% of cells staining positive with Alexa Fluor 488 dye) to background. Two independent experiments were performed in duplicate for each cell line.
Apoptosis was measured in HL-60, Kasumi-1, KG-1, M-07e, MV4-11, ML-2, NB4, OCI-AML3, THP-1, and U937 AML cell lines using an Annexin V-FITC/7-AAD kit (Beckman Coulter, Miami, FL), as previously described (14). Briefly, cells were seeded in six-well plates for 24 hours, followed by treatment with sorafenib (10 μM) for 72 hours. The percentage of cells with Annexin V–positive staining was determined by flow cytometry. Two independent experiments were performed in duplicate for each cell line.
Analysis of Kinase Phosphorylation
Sorafenib-mediated kinase inhibition in HL-60, Kasumi-1, KG-1, M-07e, MV4-11, ML-2, NB4, OCI-AML3, THP-1, and U937 AML cell lines was determined using a quantitative bead-based multiplex phosphoprotein assay (BioPlex Suspension Array System; Bio-Rad, Hercules, CA). Custom multiplex beads were obtained to measure levels of total and phosphorlyated ERK1/2 (T202/Y204 and T185/Y187), Akt (S473), and p70S6 kinase (T421/S424) (Bio-Rad). Cells at a density of 1.0 × 106 cells per milliliter per well were added to a six-well plate and grown overnight in a culture medium containing 2% fetal bovine serum. Cells were then treated with sorafenib (10 μM) for 1 hour. Protein lysates were prepared using a BioPlex cell lysis kit (Bio-Rad) and then stored at −80°C until analysis. Protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL). Protein (2.5 μg) was diluted in 50 μL of lysis buffer (Bio-Rad) and added to custom-made polystyrene beads coupled to highly specific polyclonal antibodies (exclusively developed and validated for Bio-Rad by Cell Signaling Technology, Danvers, MA) against human total and phosphorylated ERK, AKT, and p70S6 kinase. Samples were then incubated overnight at room temperature with constant shaking. After three washes with buffer (Bio-Rad), samples were incubated for 30 minutes with biotin-labeled detection antibodies specific for the secondary epitopes on the phosphorylated and total proteins listed above, followed by incubation with a fluorescence-labeled streptavidin reporter for 10 minutes. The signal intensity of fluorescence (arbitrary units [AU]) was measured using a BioPlex Array Reader (Bio-Rad). Data were presented as fluorescence units without correction for background. Two independent experiments were performed in duplicate.
Pharmacokinetics of Sorafenib in NOD-SCID-IL2Rγnull (NSG) Mice
To determine the sorafenib dose in mice that produces the human equivalent exposure, we conducted pharmacokinetic studies in 8- to 12-week-old female NSG mice (n = 90) (Taconic, Hudson, NY). This mouse strain was chosen as it was the same strain our AML xenograft studies were performed in (see below). Mice were housed in the Animal Resources Center, and all pharmacokinetic experiments were approved by the Institutional Animal Care and Use Committee of St Jude Children’s Research Hospital.
Sorafenib was administered by oral gavage once or twice daily at doses ranging from 10 to 60 mg/kg. Sorafenib was formulated in a 50% cremophor EL (Sigma-Aldrich) or 50% ethanol solution, which was diluted 1:4 with deionized water right before administration. Pharmacokinetic studies were performed after a single dose of 10, 40, and 60 mg/kg; after the first and second dose of 10 mg/kg administered twice daily; and after the first and fifth doses of 40 and 60 mg/kg administered twice daily. Blood was obtained from individual mice at two time-points from an orbital bleed and cardiac puncture. Groups of mice were sampled at five to six different sampling times over 8–24 hours with three mice per group, for a total of 15–18 mice per dose. Blood was centrifuged at 3000g for 5 minutes, and the plasma was removed and stored at −80°C until analysis. Sorafenib plasma concentrations were measured using a validated method based on high performance liquid chromatography (HPLC) with tandem mass spectrometric detection, as previously described (26). A one-compartment pharmacokinetic model with a bolus dose was fit to the plasma concentration–time data using population modeling techniques, which account for both the intersubject and intrasubject variability (27), as implemented in the software program NONMEM version V (University of California, San Francisco, CA). The area under the concentration–time curve from 0 to 24 hours (AUC[0–24h]) and the time for which plasma concentrations remained above 10 μM during a 24-hour dosing period were calculated from the model-estimated curve for each dose and schedule. The mean steady-state plasma concentration was calculated as AUC[0–24h] divided by 24 hours. Blood was also sampled 2 hours after the morning dose on day 12 or 19 in leukemia tumor–bearing NSG mice (n = 8) (described below) treated with sorafenib (60 mg/kg) twice daily plus cytarabine (6.25 mg/kg) once daily.
Leukemia Xenograft Model
U937 cells that were transfected with a murine stem cell virus-green fluorescent protein vector containing the firefly luciferase and green fluorescent protein genes (pMSCV-luc-IRES-GFP) were obtained from Dr D. Campana (28). The construct was made in the Vector Core Laboratory at St Jude Children’s Research Hospital. Green fluorescent protein–positive U937 cells were sorted by flow cytometry, expanded in culture, and 10 000 cells were injected intravenously into 8- to 12-week-old female NSG mice (n = 112). Studies were first conducted to determine a tolerable schedule for the combination of sorafenib plus cytarabine for 28 days. Sorafenib (60 mg/kg) administered twice daily was tolerable in eight of eight (100%) nontumor-bearing NSG mice for 28 days, with an average weight loss of 7%. The tolerability of sorafenib (60 mg/kg) twice daily in combination with cytarabine (6.25 mg/kg) once daily was then evaluated in 12 mice that were injected with U937 cells (10 000 cells). Cytarabine for intraperitoneal injection was formulated in phosphate-buffered saline. After 21 days of continuous daily treatment with the combination of sorafenib plus cytarabine, three of 12 mice lost between 15% and 20% of their body weight. In subsequent studies, tumor-bearing mice were treated using a schedule of 5 days treatment with 2 days off for up to 28 days for all treatment groups.
To evaluate antileukemic activity, groups of 12–15 tumor-bearing mice were randomly assigned to receive sorafenib (60 mg/kg twice daily), cytarabine (6.25 mg/kg once daily), or sorafenib (60 mg/kg) once or twice daily plus cytarabine (6.25 mg/kg) starting on day 3 after injection of U937 cells. A group of four to eight untreated mice were included as controls with each experiment. A control group treated with the sorafenib vehicle was not included because previous studies have shown that the cremophor EL solution is not absorbed intact after oral administration and hence was not expected to affect the outcome of the experiments (29,30). The experiment comparing cytarabine alone, sorafenib twice daily plus cytarabine, and the controls was repeated to obtain weekly complete blood counts to assess toxicity among the treatment groups. Organ size and tumor infiltration were also measured by immunohistochemistry in colonized tissue on days 24 and 26 of therapy. Images of hematoxylin and eosin staining of spleen longitudinal sections were taken using an Olympus BX41 light microscope (×10 magnification) equipped with a digital camera and Spot Imaging Software version 4.6 (Diagnostic Instruments, Sterling Heights, MI).
Noninvasive imaging was performed twice weekly in all experiments to monitor tumor engraftment. The luciferase substrate D-luciferin firefly potassium salt (Caliper, Hopkinton, MA) at a dose of 150 mg/kg was administered by intraperitoneal injection. The mice were then anesthetized by 1.5%–2.5% isoflurane inhalation, and bioluminescence was done 5 minutes later by use of a Xenogen in vivo imaging system (Hopkinton, MA) in the Animal Imaging Facility at St Jude Children’s Research Hospital. Total body bioluminescence was quantified for the body area that included each mouse in its entirety. Figures for biophotonic imaging data were generated with R software (Windows version 2.10.1) (31). Mice were monitored daily and were killed by CO2 asphyxiation when they showed signs of terminal illness, including hind leg paralysis, inability to eat or drink, and/or moribund. All animal experiments were approved by the Institutional Animal Care and Use Committee of St Jude Children’s Research Hospital.
Early Uptake of Cytarabine in AML Cells
Early cellular uptake of cytarabine in MV4-11 and U937 cells in the absence and presence of sorafenib was measured by the oil-stop transport method, as previously described (32–34). Briefly, a time course of cytarabine uptake was determined at 5, 15, 30, 45, 60, 90, 120, and 300 seconds. Reaction mixtures consisted of the addition of 150 μL of oil (consisting of 84.9 parts Dow Corning 550 silicone oil [Dow Corning Corporation, Midland, MI] and 15.1 parts Fisher 0-119 light paraffin oil [EMD Chemicals, Gibbstown, NJ]; specific gravity = 1.03 g/mL) and 100 μL of transport medium (NaHCO3-free RPMI-1640 with 20 mM HEPES [pH 7.4] containing 3H-cytarabine [1.25 μM] with or without sorafenib [5 μM]). Drug uptake was initiated by the addition of 100 μL of transport medium containing logarithmically growing 3 × 106 cells. At the specified incubation time, the reaction was ended by addition of 200 μL of transport medium containing nitrobenzylthioinosine (10 μM) (Sigma-Aldrich), followed immediately by centrifugation at 500g at room temperature for 30 seconds. The supernatant was removed, and the cell pellet was washed with 1.5 mL of water. Cell pellets were solubilized with 0.5 mL of 1N NaOH and incubated at room temperature overnight. An aliquot (25 μL) of the cell lysate was used to estimate protein concentration using a BCA protein assay kit. Radioactivity of a 400-μL aliquot of the cell lysate was measured by a LS 6500 liquid scintillation counter (Beckman Coulter, Brea, CA). Cellular uptake was expressed as picomoles per milligram of protein. Two independent experiments were performed in triplicate.
Intracellular Accumulation of Cytarabine and Metabolites in AML Cells
Logarithmically growing cells (10 × 106 cells) were washed and seeded in six-well plates with serum-free medium containing 3H-cytarabine (1.25 μM) and were incubated for 2 hours at 37°C with 5% CO2. Cells were also pretreated with sorafenib (5 μM) for 15 minutes followed by addition of 3H-cytarabine (1.25 μM) for 2 hours. Cells were washed twice with ice-cold phosphate-buffered saline and gradually shaken at 4°C for 10 minutes in 300 μL of buffer containing 70% methanol and 30% 15 mM Tris (pH 7.4). After centrifugation, 25 μL of cell extract was removed for protein concentration measurement using a BCA protein assay kit. Cytarabine and phosphorylated metabolites were measured in cell extracts by HPLC using a modification of previously described methods (35,36). Analyte reference material for cytarabine (Ara-C), Ara-C-monophosphate disodium salt, Ara-C-diphosphate trisodium salt, Ara-C triphosphate disodium salt, and cytidine 5′-disphophocholine sodium salt (internal standard) were obtained from Sigma-Aldrich. Standards were prepared at a concentration of 1 mg/mL in water, and a working solution containing 100 μg/mL of all analytes was prepared in Tris-buffered methanol (dilution, 70:30 vol/vol; pH 7.4). Working solution (20 μL) was added to 200 μL of cell extract before analysis. The HPLC system consisted of a Shimadzu SCL-10Avp system controller, SPD-10Avp UV-Visible detector, two LC-10ATvp HPLC pumps, and a Rheodyne manual injection valve model 7125 (Rheodyne LLC, Rohnert Park, CA). The system was controlled using Shimadzu Class VP HPLC software (Shimadzu, Columbia, MD), version 7.3. Analytes were separated by use of a binary gradient system on a Whatman Partisil SAX anion exchange column (Whatman, Clifton, New Jersey) (10 μm particle size, 250 × 4.6 mm internal diameter) with a precolumn packed with Whatman anion exchange silica. The mobile phase consisted of buffer A (0.5 mM NH2H2PO4 [pH 2.85]) and buffer B (500 mM NH2H2PO4 [pH 3.4]). The flow rate varied from 0.5 mL/min from 0 to 10 minutes, to 1.0 mL/min from at least 10 minutes throughout the remainder of the run. Elution times were monitored at 254 nm and the temperature was set at 40°C. Seventy-five fractions were collected over 50 minutes using a LKB model 2211 fraction collector (LKB Bromma, Uppsala, Sweden). Radioactivity was measured by an LS 6500 multipurpose liquid scintillation counter (Beckman Coulter, Brea, CA) after mixing the sample with 5 mL of Scinitisafe 30% scintillation fluid (PerkinElmer, Waltham, MA). Cellular accumulation was expressed as picomoles per milligram of protein. Two independent experiments were performed in triplicate.
Gene and Protein Expression of ABCC10 and ABCC11
Total RNA was extracted from HL-60, Kasumi-1, KG-1, M-07e, MV4-11, ML-2, NB4, OCI-AML3, THP-1, and U937 AML cell lines using TRIzol (Invitrogen). RNA was obtained from biological replicates for each cell line. The Affymetrix U133 plus 2.0 GeneChip array (Affymetrix, Santa Clara, CA) was used to determine gene expression according to the manufacturer’s protocol. The gene expression value (hybridization intensity) and detection call (present, absent, or marginal) were calculated using the Affymetrix MAS5 Statistical Algorithm Software Developer’s Kit (37). The average intensity of each array was normalized by global scaling to a target hybridization intensity of 100 AU. The expression signals and intensity calls for ABCC10 and ABCC1 were retrieved from the processed array data.
ABCC10 protein expression was determined in HL-60, Kasumi-1, KG-1, M-07e, MV4-11, ML-2, NB4, OCI-AML3, THP-1, and U937 AML cell lines by western blotting, with minor modifications to a previously described protocol (14). Briefly, whole-cell lysates were prepared using an M-PER mammalian protein extraction reagent (Thermo Scientific, Rockford, IL). The protein concentration was determined by a BCA protein assay kit, and 30 μg of protein was loaded on 4%–12% NuPAGE Bis-Tris gels (Invitrogen) and transferred onto a PVDF membrane (Invitrogen). Each lane was probed with a goat polyclonal antibody to human ABCC10 (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:1000. Assessment of human glyceraldehyde-3-phosphate dehydrogenase protein using a mouse primary monoclonal antibody (Santa Cruz Biotechnology) at a dilution of 1:2000 served as a loading control.
Statistical Analysis
Overall survival was defined as the time from injection of U937 cells to the time of death. The Kaplan–Meier method (38) was used to compute overall survival estimates, and the log-rank test was used to compare overall survival estimates across treatment groups. In these studies, 90 of 92 mice died of leukemia. One mouse treated with cytarabine alone died on day 26 due to an oral gavage problem, and one mouse treated with sorafenib twice daily plus cytarabine died on day 19 due to toxicity.
Biophotonic imaging data were normalized by applying the log transformation and then subtracting the mean of day 10 values among untreated controls within each chronologically defined period. For each time point, a one-way analysis of variance model was used to compare the mean of normalized biophotonic imaging values across treatment groups and to calculate 95% confidence intervals for the mean of each treatment group. The mean fluorescence estimates and 95% confidence intervals were then back-transformed by exponentiation. All statistical analyses were performed using SAS software (Windows version 9.2; SAS Institute, Cary, NC) and R software (31). All statistical tests were two-sided, and P values less than .05 were considered statistically significant.
Results
Viability, Proliferation, Apoptosis, and Signaling in AML Cell Lines After Sorafenib Treatment
We exposed 10 AML cell lines (nine cell lines with wild-type FLT3) representing a range of chromosomal aberrations and alterations in kinases (14) to sorafenib at a clinically relevant steady-state concentration (10 μM) for 72 hours and evaluated cell viability using the MTT cell proliferation assay. Sorafenib decreased cell viability to no more than 50% compared with untreated controls in all cell lines and to less than 10% compared with controls in six cell lines (Table 1 and Supplementary Figure 1, available online). Treatment with sorafenib (10 μM) for 72 hours also inhibited cell proliferation by more than 85% compared with untreated controls in all cell lines, except U937 cells (Table 1). Apoptosis was also seen in all cell lines, with at least 80% Annexin-positive cells observed for five cell lines (Table 1). Sorafenib-mediated decreases in cell viability and proliferation were paralleled by decreases in phospho-ERK1/2 or phospho-p70S6 kinase protein levels by 50% or greater compared with untreated controls in seven and six cell lines, respectively (Table 1), with no change in total protein levels (data not shown). Sorafenib also decreased phospho-AKT by 90% in the U937cell line, which had high endogenous protein levels of phospho-AKT.
Table 1.
Biological activity of sorafenib in acute myeloid leukemia cell lines*
| Cell line | Mean IC50† (95% CI), μM | Mean proliferation‡, % |
Apoptosis§ (95% CI), % |
Mean levels of phospho-ERK‖ (95% CI), AU |
Mean levels of phospho-p70S6K‖ (95% CI), AU |
Mean levels of phospho-AKT‖ (95% CI), AU |
|||||
| Concentration of sorafenib, μM | |||||||||||
| 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | ||
| HL-60 | 3.3 (2.2 to 4.4) | 61 | 6.2 | 12 (0 to 25) | 89 (77 to 101) | 982 (463 to 1502) | 159 (102 to 217) | 5698 (4263 to 7132) | 3024 (1149 to 4899) | 121 (62 to 179) | 95 (40 to 150) |
| Kasumi-1 | 0.04 (0.01 to 0.07) | 30 | 2.5 | 31 (9 to 53) | 87 (72 to 101) | 162 (117 to 208) | 133 (105 to 162) | 1441 (1226 to 1655) | 286 (245 to 328) | 99 (65 to 134) | 66 (46 to 86) |
| KG-1 | 2.6 (0.33 to 5.0) | 48 | 5.3 | 28 (13 to 43) | 45 (23 to 67) | 243 (179 to 308) | 139 (115 to 164) | 5370 (3191 to 7550) | 1954 (1013 to 2898) | 84 (66 to 102) | 84 (73 to 95) |
| ML-2 | 3.7 (2.0 to 5.4) | 131 | 4.5 | 9.4 (0 to 19) | 94 (92 to 97) | 116 (0 to 239) | 55 (20 to 90) | 61 (42 to 80) | 64 (36 to 91) | 53 (34 to 72) | 38 (27 to 50) |
| U937 | 6.9 (5.6 to 8.2) | 63 | 51 | 6.0 (1 to 11) | 56 (33 to 79) | 1192 (691 to 1693) | 155 (121 to 189) | 1183 (866 to 1500) | 752 (535 to 969) | 1451 (710 to 2192) | 148 (120 to 176) |
| MV4-11 | 0.002 (0.0002 to 0.004) | 28 | 0.5 | 15 (0 to 38) | 90 (69 to 111) | 1194 (650 to 1739) | 103 (82 to 124) | 5123 (2641 to 7604) | 779 (362 to 1196) | 230 (216 to 244) | 147 (88 to 206) |
| Mo7e | 9.1 (8.4 to 9.8) | 47 | 1.6 | 22 (17 to 27) | 42 (37 to 47) | 154 (121 to 187) | 143 (113 to 172) | 3179 (2535 to 3824) | 1252 (632 to 1872) | 114 (28 to 200) | 135 (41 to 229) |
| THP-1 | 6.2 (2.9 to 9.6) | 50 | 2.1 | 12 (0 to 34) | 36 (14 to 58) | 415 (271 to 559) | 86 (53 to 120) | 937 (0 to 2343) | 162 (13 to 310) | 180 (67 to 293) | 106 (48 to 164) |
| NB4 | 7.3 (3.0 to 11.5) | 74 | 10 | 27 (23 to 31) | 55 (37 to 74) | 1081 (978 to 1185) | 260 (187 to 332) | 6753 (4484 to 9023) | 3465 (2159 to 4770) | 179 (102 to 257) | 104 (58 to 151) |
| OCI-AML3 | 3.9 (3.3 to 4.4) | 140 | 4.2 | 13 (8 to 19) | 82 (33 to 81) | 1706 (910 to 2502) | 227 (48 to 405) | 281 (185 to 378) | 84 (61 to 107) | 199 (72 to 327) | 95 (61 to 129) |
The data are the mean with 95% confidence interval (CI) calculated using the t distribution at a confidence level of P = .05. When only two replicate measurements were obtained, the 95% confidence interval was not reported. AU = arbitrary units of fluorescence.
IC50 = the concentration (μM) inhibiting cell viability by 50% compared with dimethyl sulfoxide-treated control cells. Data represent the average of three independent experiments.
Proliferation was measured by use of a 5-ethynyl-2′-deoxyuridine cell proliferation kit and flow cytometer. The mean number of Alexa Fluor 488 positive–staining cells from treated vs untreated cells are shown. Values were calculated by comparing the treated cells to untreated cells. Data are from two or three independent experiments with one replicate each.
Apoptosis was measured by use of an Annexin V-FITC/7-AAD kit and flow cytometer. The mean number of Annexin V–positive staining cells from treated vs untreated control cells are shown. Data are from three independent experiments with one replicate each.
Mean levels of phosphoproteins were measured by a quantitative bead-based multiplex phosphoprotein assay. Data are from two independent experiments with two replicates each.
Sorafenib and Cytarabine Combination Treatment of AML Cell Lines and Primary Blast Samples
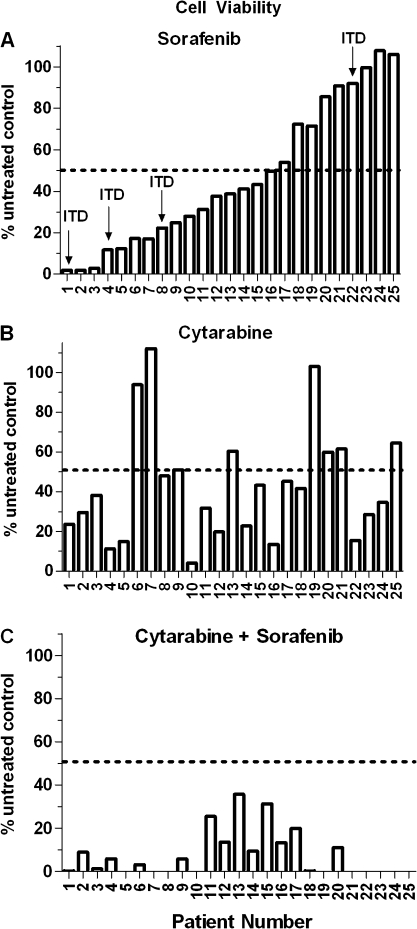
Twenty-five freshly isolated primary childhood AML samples (21 samples with wild-type FLT3) were treated with sorafenib (10 μM) for 96 hours and cell viability was assessed with an MTT assay. Cell viability ranged from 0% to 100% of untreated controls (median = 39%) (Figure 1, A). Sorafenib-mediated decreases in cell viability were not limited to samples with FLT3-ITD (FLT3 internal tandem duplications) mutations.
Figure 1.
Effect of sorafenib alone, cytarabine alone, and sorafenib plus cytarabine on cell viability in primary childhood acute myeloid leukemia blast samples. Freshly isolated primary leukemic blast cells were treated with A) sorafenib (10 μM), B) cytarabine (10 μM), or C) sorafenib plus cytarabine for 96 hours, and cell viability was determined using a MTT assay. Data in (A) are displayed in descending order of activity. Data are from one experiment with duplicate measurements; the average of the two measurements is shown. ITD = FLT3 internal tandem duplications.
Blast samples treated with cytarabine (10 μM) for 96 hours showed a wide range of drug sensitivity but had minimal cross-sensitivity to sorafenib (Figure 1, B). Likewise, minimal cross-sensitivity between the two drugs was observed in AML cell lines (Supplementary Figure 1, available online). The activity of sorafenib and cytarabine following simultaneous exposure for 72 hours was determined in seven AML cell lines. Combination index values at 50%, 75%, and 95% effective concentrations (ED50, ED75, and ED95) were calculated by the median effect approach, and values for the synergism–antagonism parameter, α, were determined using the universal response surface approach and are shown in Table 2. By assessing drug effects using the two approaches, the combination of sorafenib plus cytarabine was synergistic in four cell lines (OCI-AML3, MV4-11, NB4, and THP-1) and additive in three cell lines (HL-60, Kasumi-1, and U937). We also evaluated the effects of simultaneous exposure to sorafenib (10 μM) plus cytarabine (10 μM) in all primary samples that had a sufficient number of cells available. For 13 of 15 samples, the percentage of viable cells after drug treatment relative to untreated control cells was lower for the combination of sorafenib plus cytarabine than for either agent alone. Of the remaining two samples, there were no viable cells after treatment with sorafenib alone or sorafenib plus cytarabine for one sample, and the combination resulted in 8.8% cell viability for the other sample (sorafenib alone resulted in 1.3% cell viability and cytarabine alone resulted in 29.6% cell viability in this sample). Of the 15 samples, overall mean viability for sorafenib alone, cytarabine alone, and the combination sorafenib plus cytarabine was 34.2% (95% CI = 20.1% to 48.2%), 39.0% (95% CI = 27.1% to 51.0%), and 12.6% (95% CI = 6.5% to 18.7%), respectively.
Table 2.
Effect of sorafenib (0.00125–10 μM) plus cytarabine (0.0125–20 μM) for 72 hours on the viability of acute myeloid leukemia cell lines*
| Cell line | Sequence of drug administration | Ratio of concentration of sorafenib to cytarabine | Combination index† (95% CI) |
α‡ (95% CI) | ||
| ED50 | ED75 | ED90 | ||||
| OCI-AML3 | Simultaneous | 2:1 | 0.55 (0.01 to 1.2) | 0.48 (0.06 to 0.89) | 0.42 (0.16 to 0.67) | 2.1 (1.3 to 3.0) |
| MV4-11 | Simultaneous | 1:250 | 0.67 (0.12 to 1.2) | 0.58 (0.23 to 0.92) | 0.51 (0.29 to 0.73) | 1.7 (1.4 to 1.9) |
| MV4-11 | Pre-cytarabine | 1:250 | 0.78 (0.65 to 0.90) | 0.46 (0.38 to 0.54) | 0.28 (0.21 to 0.35) | 2.8 (2.0 to 3.6) |
| MV4-11 | Pre-sorafenib | 1:250 | 0.73 (0.44 to 1.0) | 0.62 (0.46 to 0.77) | 0.52 (0.38 to 0.67) | 1.0 (0.66 to 1.4) |
| NB4 | Simultaneous | 2:1 | 0.60 (0.35 to 0.86) | 0.57 (0.33 to 0.81) | 0.57 (0.33 to 0.81) | 0.85 (0.57 to 1.1) |
| HL-60 | Simultaneous | 10:1 | 1.00 (0.59 to 1.5) | 0.84 (0.44 to 1.2) | 0.68 (0.26 to 1.1) | −0.19(−0.23 to −0.14) |
| THP-1 | Simultaneous | 1:2 | 0.98 (0.90 to 1.1) | 0.89 (0.80 to 0.98) | 0.81 (0.68 to 0.93) | 0.40 (0.26 to 0.54) |
| Kasumi-1 | Simultaneous | 1:5 | 1.00 (0.84 to 1.2) | 0.90 (0.73 to 1.1) | 0.86 (0.65 to 1.1) | 0.082 (0.032 to 0.13) |
| U937 | Simultaneous | 100:1 | 0.85 (0.67 to 1.0) | 0.96 (0.79 to 1.1) | 1.0 (0.25 to 1.7) | −0.26 (−0.35 to −0.17) |
Data from two to three independent experiments each performed with eight replicates at each drug concentration. Ninety-five percent confidence intervals (CIs) were calculated by multiplying 1.96 by the SE of the mean estimate. α = synergism-antagonism parameter; ED50 = concentration producing 50% effect; ED75 = concentration producing 75% effect; ED90 = concentration producing 90% effect; pre-cytarabine = cytarabine before sorafenib, pre-sorafenib = sorafenib before cytarabine.
The median effect approach was used to assess synergism, additive, or antagonism of drug effect. Data from independent experiments were combined to estimate combination index. Combination index values <0.9 indicate synergy, 0.90–1.1 indicate additive effect, and >1.1 indicate antagonism.
The universal response surface approach was used to assess synergism, additive effect, or antagonism of drug effect. Data from independent experiments were combined to estimate α. A positive α indicates synergism, a negative α indicates antagonism, and a zero α indicates an additive effect.
Two additional sequences of drug administration were assessed in MV4-11 cells: cytarabine before sorafenib (pre-cytarabine) and sorafenib before cytarabine (pre-sorafenib) (Table 2). A synergistic interaction (defined as combination index value <1.0; α > 1.0) was observed with both sequences, although stronger synergy was observed when cytarabine was administered before sorafenib (combination index values of 0.78, 0.46, and 0.28 at the ED50, ED75, and ED90, respectively; α = 2.8) compared with after sorafenib (combination index values of 0.73, 0.62, and 0.52 at the ED50, ED75, and ED90, respectively; α = 1.0). This is on the basis of the observation that although at the ED50 and ED75 there is no difference in drug effects between the three different sequences of administration (similar combination index values and overlapping confidence intervals), stronger drug effects were observed at the ED90 for the pre-cytarabine sequence (lower combination index value and nonoverlapping confidence intervals). Using the universal response surface approach that accounts for all of the data over the entire concentration range, pre-cytarabine was more synergistic than other sequences, with a higher α (pre-cytarabine α = 2.8, simultaneous α = 1.7, and pre-sorafenib α = 1.0) and nonoverlapping confidence interval (Table 2). Overall, these data would suggest that the pre-cytarabine sequence is superior in mediating effects on cell viability relative to the pre-sorafenib and simultaneous sequences evaluated.
Pharmacokinetics of Sorafenib in NSG Mice
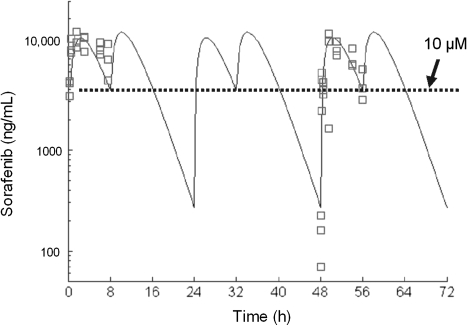
Pharmacokinetic studies were conducted in NSG mice to determine the sorafenib dose and schedule that could produce human equivalent plasma steady-state exposure. In humans, sorafenib has a long plasma half-life (24–48 hours), and when given twice daily, results in minimal fluctuations in plasma concentrations from peak to trough at the steady state (24). Therefore, we aimed to identify a sorafenib dose and schedule that achieved an average steady-state plasma concentration of 10 μM but also maximized the time that sorafenib concentrations remained above 10 μM. Sorafenib exposures achieved at different doses administered once or twice daily are shown in Table 3. Sorafenib (60 mg/kg) administered once daily achieved an average steady-state plasma concentration of 9.9 μM, but the time that plasma concentrations remained above 10 μM was longer with twice-daily vs once-daily administration (17 vs 8 hours). Therefore, sorafenib (60 mg/kg) administered twice daily was selected for in vivo evaluation. Figure 2 shows a model-estimated concentration–time curve following administration of sorafenib (60 mg/kg) twice daily at time 0 and 8 hours for 3 days when the steady state was reached. The maximum plasma concentration was 12 700 ng/mL (27 μM), which occurred 2 hours after oral administration. In tumor-bearing mice treated with sorafenib (60 mg/kg) twice daily plus cytarabine (6.25 mg/kg) once daily for 2 to 3 weeks, the mean sorafenib concentration measured 2 hours after drug administration was 9327 ng/mL (20 μM, 95% CI = 2478 to 16 177 ng/mL).
Table 3.
Sorafenib plasma exposure in NOD-SCID-IL2Rγnull mice at different doses and schedules of administration
| Dose | Schedule | Average steady-state plasma concentration*, μM | Time, h >10 μM*,† |
| 10 mg/kg‡ | Single dose | 1.4 | 0 |
| 10 mg/kg‡ | Twice daily at 0 and 12 h for 1 d | 2.7 | 0 |
| 30 mg/kg§ | Twice daily at 0 and 8 h for 3 d | 8.0 | 7 |
| 40 mg/kg‡ | Single dose | 5.4 | 5 |
| 40 mg/kg§ | Twice daily at 0 and 8 h for 3 d | 10.7 | 10 |
| 60 mg/kg‡ | Single dose | 9.9 | 8 |
| 60 mg/kg‡ | Twice daily at 0 and 8 h for 3 d | 14.5 | 17 |
Average steady-state plasma concentration and time >10 μM were determined from model-estimated concentrations.
Time (hours) more than 10 μM denotes the time that plasma concentrations remained above 10 μM.
Data from independent pharmacokinetic experiments.
Data from pharmacokinetic model simulations.
Figure 2.
Sorafenib plasma concentrations in NOD-SCID-IL2Rγnull mice (n = 15) treated with sorafenib (60 mg/kg) twice daily for 3 days. The symbols are the observed concentrations of sorafenib (nanograms per milliliter) at each time point (hours) as measured by high performance liquid chromatography with tandem mass spectrometric detection. The line is the fit of a first-order absorption, one-compartment model to the data.
Antitumor Activity of Sorafenib Combined With Cytarabine in a Xenograft Model of AML
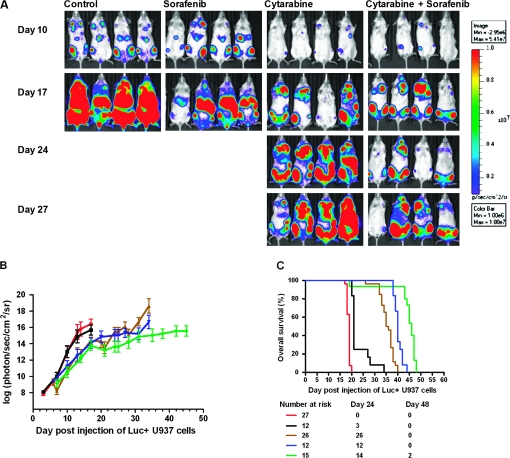
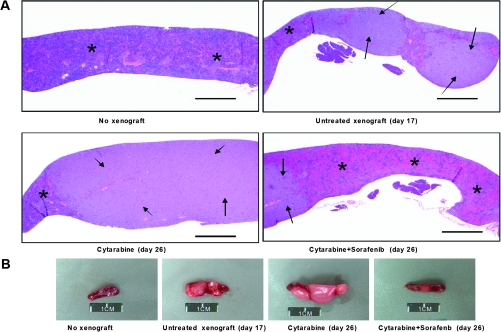
The antileukemic activity of cytarabine alone (once daily), sorafenib alone (twice daily), or the combination of sorafenib (once or twice daily) plus cytarabine compared with untreated controls was evaluated in NSG mice engrafted with the U937 cells. Differences in tumor engraftment and progression among the different treatment groups were monitored by bioluminescence imaging (Figure 3, A) and quantitative biophotonic imaging analysis (Figure 3, B), and the incidence of death due to leukemia progression was calculated (Figure 3, C). Death due to leukemia progression among untreated mice and mice treated with sorafenib alone was similar (19 vs 21 days, respectively). Tumor progression, quantified by assessment of the increase in mean log-transformed bioluminescence signal, was statistically significantly delayed by cytarabine-treated mice compared with untreated mice on day 17 (cytarabine alone vs untreated, mean = 14.4 photon/s/cm2/sr [95% CI = 13.9 to 14.8 photon/s/cm2/sr] vs mean = 16.4 photon/s/cm2/sr [95% CI = 15.8 to 17.0 photon/s/cm2/sr], P < .001). By day 25, statistically significant differences in tumor progression were observed between mice treated with sorafenib twice daily plus cytarabine compared with mice treated with cytarabine (sorafenib plus cytarabine vs cytarabine, mean = 13.6 photon/s/cm2/sr [95% CI = 12.9 to 14.3 photon/s/cm2/sr] vs mean = 15.5 photon/s/cm2/sr [95% CI = 15.0 to 16.0 photon/s/cm2/sr], P < .001). By pair-wise comparisons, the dose-intensive twice-daily sorafenib administration combined with cytarabine statistically significantly prolonged median survival compared with sorafenib once daily plus cytarabine (P < .001), cytarabine alone (P < .001), and the control (P < .001) (sorafenib twice daily plus cytarabine, median survival = 46 days; sorafenib once daily plus cytarabine, median survival = 40 days; cytarabine alone, median survival = 36 days; and control, median survival = 19 days). Figure 4, A shows representative images of hematoxylin and eosin staining of spleen longitudinal sections that demonstrate colonization of the spleen by leukemic cells. No notable changes in liver size or histology were observed among the mice on day 26 (not shown). However, spleens of cytarabine alone–treated mice on day 26 were (mean = 0.2 g, 95% CI = 0.1 g to 0.3 g) heavier than those of mice treated with sorafenib twice daily plus cytarabine (mean = 0.05 g, 95% CI = 0.01g to 0.06 g) (Figure 4, B).
Figure 3.
Antileukemic activity of sorafenib alone, cytarabine alone, and sorafenib plus cytarabine in a U937 xenograft model. NOD-SCID-IL2Rγnullmice were injected with luciferase-labeled (Luc+) U937 cells and treated 3 days later with sorafenib alone (60 mg/kg) twice daily by oral gavage, cytarabine alone (6.25 mg/kg) once daily by intraperitoneal injection, or sorafenib once or twice daily plus cytarabine for 5 days each week for 28 days. A) Serial bioluminescence images of representative mice receiving sorafenib alone twice daily (n = 12), cytarabine alone (n = 26), or sorafenib twice daily plus cytarabine (n = 15). B) Tumor progression monitored by quantitative biophotonic imaging analysis of control and treatment and groups. Red line, controls (n = 27); black line, sorafenib alone twice daily (n = 12); brown line, cytarabine alone (n = 26); blue line, sorafenib once daily plus cytarabine (n = 12); green line, sorafenib twice daily plus cytarabine (n = 15). The mean values and their 95% confidence intervals (error bars) are given. C) A plot of overall survival probability, estimated with the Kaplan–Meier method. Red line, controls (n = 27); black line, sorafenib alone (twice daily) (n = 12); brown line, cytarabine (n = 26); blue line, sorafenib once daily plus cytarabine (n = 12); green line, sorafenib twice daily plus cytarabine (n = 15).
Figure 4.
The effect of cytarabine alone compared with sorafenib plus cytarabine on leukemic cell colonization in the spleen and on spleen size. NOD-SCID-IL2Rγnull mice were injected with luciferase-labeled U937 cells and treated 3 days later with cytarabine (6.25 mg/kg) once daily by intraperitoneal injection, or sorafenib (60 mg/kg) twice daily plus cytarabine (6.25 mg/kg) for 5 days each week for 26 days. A) Representative images of hematoxylin and eosin staining of spleen longitudinal sections from nontumor mice (n = 5), untreated mice (day 17) (n = 3), mice treated with cytarabine alone (day 26) (n = 3), or sorafenib twice daily plus cytarabine (day 26) (n = 3). Images were taken by an Olympus BX41 light microscope (×2 magnification) equipped with a digital camera and Spot Imaging Software. Arrows indicate tumor infiltrate and asterisks indicate normal tissue. Scale bar = 1 mm. B) Pictures of whole spleens from mice in the indicated treatment groups. Data are representative of three mice in each treatment group. Scale bar = 1 cm.
Effect of Sorafenib on the Uptake and Accumulation of Cytarabine in AML Cell Lines
In vitro studies have shown that cytarabine-sensitive cells accumulate higher intracellular concentrations of Ara-C triphosphate (Ara-C-TP) than do resistant cells (39), and associations have been found between the accumulation of Ara-C-TP in blasts and clinical response in AML patients (40). Because sorafenib inhibits ATP-binding cassette (ABC) transporters involved in cellular drug efflux (41), and cytarabine has been shown to be a substrate for the efflux transporters ABCC10 (MRP7) and ABCC11 (MRP8) (42,43), we hypothesized that sorafenib may inhibit transporter-mediated efflux of cytarabine from AML cells, increase the accumulation of cytarabine phosphorylated metabolites, and thus improve cytotoxicity against AML blasts. Sorafenib (5 μM) did not change the early uptake (between 2 and 300 seconds) of cytarabine (1.25 μM) in MV4-11 and U937 cells (Supplementary Figure 2, available online). However, when the accumulation of cytarabine (1.25 μM) at 2 hours (representing steady-state conditions; data not shown) was evaluated in AML cell lines, sorafenib (5 μM) increased Ara-C-TP concentrations in four of the five cell lines tested (Table 4). The greatest increase in Ara-C-TP concentrations was observed in cells with lower basal accumulation of Ara-C-TP (<12 000 disintegrations per minute [DPM]/mg protein in OCI-AML3, MV4-11, and THP-1 cells, respectively) compared with those with higher basal accumulation Ara-C-TP (more than 133 000 DPM/mg protein in HL-60 and U937 cells).
Table 4.
Effect of sorafenib on the accumulation of cytarabine and phosphorylated metabolites in human acute myeloid leukemia cell lines*
| Cell line | Mean intracellular levels (95% CI), DPM/mg of protein |
|||||||
| Cytarabine |
Ara-C-monophosphate |
Ara-C-diphosphate |
Ara-C-triphosphate |
|||||
| 0 μM sorafenib | 5 μM sorafenib | 0 μM sorafenib | 5 μM sorafenib | 0 μM sorafenib | 5 μM sorafenib | 0 μM sorafenib | 5 μM sorafenib | |
| MV4-11 | 3585 (599 to 6570) | 8732 (4389 to 13 076) | 254 (210 to 299) | 588 (326 to 850) | 395 (248 to 543) | 1357 (499 to 2215) | 5059 (3729 to 6388) | 16 451 (12 788 to 20 113) |
| OCI-AML3 | 5611 (4114 to 7107) | 17 416 (15 051 to 19 781) | 466 (192 to 739) | 4312 (3396 to 5227) | 5445 (4006 to 6884) | 25 458 (19 863 to 31 053) | 11 190 (2149 to 20 231) | 61 581 (51 685 to 71 477) |
| THP-1 | 4372 (2650 to 6093) | 13 935 (7567 to 20 302) | 265 (82 to 447) | 1811 (0 to 3862) | 2433 (1376 to 3489) | 5269 (2652 to 7887) | 11 057 (6165 to 15 949) | 32 407 (18 027 to 46 788) |
| HL-60 | 11 335 (10 045 to 12 625) | 21 672 (13 849 to 29 494) | 9674 (0 to 19 931) | 26 783 (8582 to 44 985) | 24 615 (10 447 to 38 783) | 86 912 (42 915 to 130 909) | 133 247 (112 106 to 154 388) | 210 139 (173 147 to 247 131) |
| U937 | 674 (0 to 1690) | 903 (0 to 1825) | 5157 (1381 to 8933) | 10 677 (6840 to 14 513) | 21 921 (11 167 to 32 676) | 33 708 (26 984 to 40 431) | 163 975 (107 570 to 220 379) | 140 242 (104 545 to 175 940) |
Values represent two independent experiments with two replicates each. CI = confidence interval; DPM = disintegrations per minute.
To determine if expression of ABCC10 and ABCC11 were plausible efflux transporters involved in sorafenib-induced cellular retention of cytarabine, we assessed for gene and protein expression in 10 AML cell lines. We determined that ABCC10, but not ABCC11, was present in the 10 AML cell lines by use of a gene expression array (not shown). ABCC10 protein expression was found in four of the 10 AML cell lines by western blotting (Supplementary Figure 3, available online).
Discussion
Sorafenib demonstrated in vitro activity in a panel of molecularly heterogeneous AML cell lines and primary childhood AML samples at a clinically achievable concentration of 10 μM. When given simultaneously with cytarabine, synergistic to additive inhibition of cell viability was observed. In an AML xenograft model, administration of sorafenib (10 μM) plus cytarabine prolonged survival compared with cytarabine alone.
Although sorafenib appears to be more active in leukemias with the FLT3-ITD abnormality, antileukemic activity has been reported in several patients with AML and wild-type FLT3 (15,17). Our study shows the in vitro antileukemic activity of sorafenib in 10 genetically heterogeneous AML cell lines as well as in 25 primary AML blast samples in which activity was observed in both FLT3-ITD and wild-type FLT3 AML cells. Sorafenib alone also exhibited a different spectrum of resistance compared with cytarabine alone, indicating that sorafenib may be active in some cells with de novo resistance to cytarabine. Sorafenib alone effectively inhibited ERK signaling in AML cell lines, consistent with previous studies (12–14). Also, sorafenib alone inhibited AKT phosphorylation in one cell line, which may represent an additional mechanism of sorafenib-induced apoptosis in which AKT-mediated negative regulation of proapoptotic BAD is suppressed (11). Treatment with sorafenib alone also inhibited phosphorylation of p70S6K, a downstream effector of both ERK and AKT signaling that is involved in transcription and translation of genes that regulate cell growth, proliferation, and survival. The precise mechanism of action of sorafenib in AML is unknown but likely involves inhibition of RTKs and downstream signaling pathways, the latter, which is supported by the results of our study.
Because sorafenib previously demonstrated modest single-agent activity in relapsed or refractory AML (15,17), we evaluated its activity in combination with cytarabine, one of the most widely used chemotherapeutic agents for AML. In our study, sorafenib and cytarabine had a synergistic to additive activity in seven AML cell lines, and inhibition of cell viability was greater after treatment with sorafenib plus cytarabine in 13 of 15 childhood primary blast samples compared with either agent alone. In MV4-11 AML cells and BaF3 cells with a FLT3-ITD mutation, administration of the kinase inhibitor lestaurtinib simultaneously with or immediately following treatment with cytarabine produced cytotoxicity in a synergistic fashion, in contrast to the antagonist effect observed when lestaurtinib was administered before cytarabine (21). In MV4-11 cells, we observed a synergistic interaction when sorafenib was administered before cytarabine, although stronger synergy was observed when cytarabine was administered before sorafenib. Our data suggest that the sequence of sorafenib and cytarabine administration may not be a critical factor to consider in FLT3-ITD AML. Our data showed that in wild-type FLT3 U937 AML tumor-bearing NSG mice, sorafenib did not prolong survival compared with untreated controls, which is generally consistent with the limited single-agent activity observed in phase I trials (15–17). However, we observed that sorafenib administered simultaneously with cytarabine statistically significantly prolonged median survival compared with cytarabine alone in this AML xenograft model, findings that are consistent with the results of a recent phase II study in 51 adults with previously untreated AML receiving sorafenib simultaneously in combination with cytarabine and idarubicin during the first week of induction therapy. Overall, 38 (75%) of 51 patients achieved a complete response (44), and the response rate was higher in patients with FLT3-ITD AML (14 of 15, 92%), but 24 (66%) of 36 of patients with wild-type FLT3 achieved a complete response, demonstrating the potential utility of sorafenib in combination with chemotherapy in some patients with FLT3 wild-type disease.
The twice-daily administration schedule of sorafenib plus cytarabine was more active than sorafenib given once daily plus cytarabine. Pharmacokinetic studies in NSG mice demonstrated that the time sorafenib plasma concentrations remained above 10 μM was longer with twice-daily vs once-daily administration (17 vs 8 hours), and exposure with the former schedule was more similar to human steady-state exposure with minimal fluctuations from peak to trough with an approximate average concentration of 10 μM. These pharmacokinetic studies provide a preliminary benchmark for effective sorafenib concentrations in patients receiving sorafenib in combination with cytarabine, particularly in wild-type FLT3.
Our study identified a potential mechanism underlying the interaction between sorafenib and cytarabine. Previous studies have demonstrated that protein kinase inhibitors can inhibit nucleoside transport into cells (45,46), indicating that caution should be taken when combining a kinase inhibitor with a nucleoside analogue. However, our study shows that sorafenib did not decrease the initial uptake of cytarabine in AML cell lines but increased the cellular accumulation of cytarabine triphosphate, the active moiety of cytarabine, by up to three- to fivefold in cells in which synergistic drug effects were observed with combination treatment compared with cells treated with cytarabine alone. Because cytarabine-sensitive cells accumulate higher intracellular concentrations of cytarabine triphosphate than resistant cells (39), it is plausible that enhanced accumulation of cytarabine triphosphate by sorafenib contributes to the synergistic to additive activity observed with the drug combination. The precise mechanism of this intracellular pharmacokinetic interaction is unknown but may involve enhanced formation of cytarabine to its phosphorylated metabolites or reduced cellular efflux. Members of the ABCC efflux transporter family have been shown to transport a range of base, nucleoside, and nucleotide analogs (47,48), and cytarabine has recently been shown to be a substrate for ABCC10 (MRP7) and ABCC11 (MRP8) (42,43). We determined that whereas ABCC11 was not expressed, ABCC10 protein was expressed in four of 10 AML cell lines studied. In addition to ABCC10, other ABCC family members may efflux cytarabine from AML cells, and studies are currently under way to investigate this mechanism. In U937 cells, the combination of sorafenib plus cytarabine resulted in additive drug effects in vitro, and survival was prolonged in a U937 xenograft model. Because sorafenib treatment did not increase the cellular retention of cytarabine triphosphate in U937 cells with high basal accumulation of the active metabolite, it is likely that other mechanisms are contributing to the antileukemic activity of the combination therapy. Recently, it was shown that the mitogen-activated protein kinase pathway was activated in NB4 and HL-60 AML cells treated with cytarabine (49). A selective MEK inhibitor, AZD624 (AstraZeneca Pharmaceuticals), effectively blocked cytarabine-induced MEK/ERK activation and enhanced growth arrest and apoptosis in both the NB4 and HL-60 AML cell lines. Future investigations are needed to characterize the role of sorafenib in the inhibition of activated signaling pathways in drug-resistant leukemic cells.
Our study is not without several limitations. We evaluated different sequences of sorafenib and cytarabine combination treatment in one AML cell line with an FLT3-ITD mutation. The superiority of one sequence over the others in cells with wild-type FLT3 is unknown and may differ from that of AML cells with the FLT3-ITD mutation. Also, we suggest a target effective sorafenib plasma concentration of 10 μM in AML patients based on in vitro data in 10 molecularly heterogeneous AML cell lines (one with FLT3-ITD) and 25 primary blast samples (four with FLT3-ITD), and in vivo data in one AML xenograft with wild-type FLT3. Based on these data, it cannot be determined if the requirements for optimal sorafenib plasma exposure and/or sequence of drug administration with cytarabine (eg, simultaneous vs sequential administration) are different for FLT3-ITD and wild-type FLT3 AML. Current preclinical investigations are aimed to define these parameters in mouse models of AML.
In conclusion, sorafenib has antileukemic activity in molecularly heterogeneous AML in vitro and in vivo. Sorafenib enhances the antitumor activity of cytarabine in vitro and in vivo when administered simultaneously in combination. Our study provides the foundation for the future clinical evaluation of sorafenib in combination with cytarabine-based regimens in frontline and relapsed/refractory childhood AML.
Funding
National Institutes of Health (R01 CA138744 to S.D.B.); United States Public Health Service Cancer Center Support Grants P30 CA021765 (Pharmacokinetics Core) (S.D.B.); American Lebanese Syrian Associated Charities.
Supplementary Material
Footnotes
The study sponsors were not involved in the design, collection, analysis and interpretation of data, writing of the article, or the decision to submit the article for publication.
References
- 1.Scholl C, Gilliland DG, Frohling S. Deregulation of signaling pathways in acute myeloid leukemia. Semin Oncol. 2008;35(4):336–345. doi: 10.1053/j.seminoncol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Park S, Chapuis N, Tamburini J, et al. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica. 2010;95(5):819–828. doi: 10.3324/haematol.2009.013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101(12):4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 4.Min YH, Eom JI, Cheong JW, et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia. 2003;17(5):995–997. doi: 10.1038/sj.leu.2402874. [DOI] [PubMed] [Google Scholar]
- 5.Milella M, Kornblau SM, Estrov Z, et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest. 2001;108(6):851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandage VL, Gale RE, Linch DC, et al. PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-kappaB, Mapkinase and p53 pathways. Leukemia. 2005;19(4):586–594. doi: 10.1038/sj.leu.2403653. [DOI] [PubMed] [Google Scholar]
- 7.Kornblau SM, Womble M, Qiu YH, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108(7):2358–2365. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornblau SM, Tibes R, Qiu YH, et al. Functional proteomic profiling of AML predicts response and survival. Blood. 2009;113(1):154–164. doi: 10.1182/blood-2007-10-119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallay N, Dos Santos C, Cuzin L, et al. The level of AKT phosphorylation on threonine 308 but not on serine 473 is associated with high-risk cytogenetics and predicts poor overall survival in acute myeloid leukaemia. Leukemia. 2009;23(6):1029–1038. doi: 10.1038/leu.2008.395. [DOI] [PubMed] [Google Scholar]
- 10.Fabian MA, Biggs WH, III, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23(3):329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 11.Mori S, Cortes J, Kantarjian H, et al. Potential role of sorafenib in the treatment of acute myeloid leukemia. Leuk Lymphoma. 2008;49(12):2246–2255. doi: 10.1080/10428190802510349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auclair D, Miller D, Yatsula V, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21(3):439–445. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Konopleva M, Ruvolo VR, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008;22(4):808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 14.Hu S, Niu H, Minkin P, et al. Comparison of antitumor effects of multitargeted tyrosine kinase inhibitors in acute myelogenous leukemia. Mol Cancer Ther. 2008;7(5):1110–1120. doi: 10.1158/1535-7163.MCT-07-2218. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100(3):184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 16.Pratz KW, Cho E, Levis MJ, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24(8):1437–1444. doi: 10.1038/leu.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crump M, Hedley D, Kamel-Reid S, et al. A randomized phase I clinical and biologic study of two schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: a NCIC (National Cancer Institute of Canada) Clinical Trials Group Study. Leuk Lymphoma. 2010;51(2):252–260. doi: 10.3109/10428190903585286. [DOI] [PubMed] [Google Scholar]
- 18.Dent P, Grant S. Pharmacologic interruption of the mitogen-activated extracellular-regulated kinase/mitogen-activated protein kinase signal transduction pathway: potential role in promoting cytotoxic drug action. Clin Cancer Res. 2001;7(4):775–783. [PubMed] [Google Scholar]
- 19.Abbott BL, Colapietro AM, Barnes Y, et al. Low levels of ABCG2 expression in adult AML blast samples. Blood. 2002;100(13):4594–4601. doi: 10.1182/blood-2002-01-0271. [DOI] [PubMed] [Google Scholar]
- 20.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543–52. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levis M, Pham R, Smith BD, et al. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104(4):1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 22.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47(2):331–385. [PubMed] [Google Scholar]
- 23.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12(4):426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 25.Ozkaynak MF, Avramis VI, Carcich S, et al. Pharmacology of cytarabine given as a continuous infusion followed by mitoxantrone with and without amsacrine/etoposide as reinduction chemotherapy for relapsed or refractory pediatric acute myeloid leukemia. Med Pediatr Oncol. 1998;31(6):475–482. doi: 10.1002/(sici)1096-911x(199812)31:6<475::aid-mpo3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhao M, Rudek MA, He P, et al. A rapid and sensitive method for determination of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846(1-2):1–7. doi: 10.1016/j.jchromb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Hing JP, Woolfrey SG, Greenslade D, et al. Is mixed effects modeling or naive pooled data analysis preferred for the interpretation of single sample per subject toxicokinetic data? J Pharmacokinet Pharmacodyn. 2001;28(2):193–210. doi: 10.1023/a:1011507100493. [DOI] [PubMed] [Google Scholar]
- 28.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer research. 2009;69(9):4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meerum Terwogt JM, Beijnen JH, ten Bokkel Huinink WW, et al. Co-administration of cyclosporin enables oral therapy with paclitaxel. Lancet. 1998;352(9124):285. doi: 10.1016/s0140-6736(98)24030-x. [DOI] [PubMed] [Google Scholar]
- 30.Malingre MM, Terwogt JM, Beijnen JH, et al. Phase I and pharmacokinetic study of oral paclitaxel. J Clin Oncol. 2000;18(12):2468–2475. doi: 10.1200/JCO.2000.18.12.2468. [DOI] [PubMed] [Google Scholar]
- 31.The R Project for Statistical Computing. http://www.r-project.org/ Accessed April 1, 2011. [Google Scholar]
- 32.Harley ER, Paterson AR, Cass CE. Initial rate kinetics of the transport of adenosine and 4-amino-7-(beta-D-ribofuranosyl)pyrrolo[2,3-d]pyrimidine (tubercidin) in cultured cells. Cancer Res. 1982;42(4):1289–1295. [PubMed] [Google Scholar]
- 33.Graham KA, Leithoff J, Coe IR, et al. Differential transport of cytosine-containing nucleosides by recombinant human concentrative nucleoside transporter protein hCNT1. Nucleosides Nucleotides Nucleic Acids. 2000;19(1-2):415–434. doi: 10.1080/15257770008033018. [DOI] [PubMed] [Google Scholar]
- 34.Boleti H, Coe IR, Baldwin SA, et al. Molecular identification of the equilibrative NBMPR-sensitive (es) nucleoside transporter and demonstration of an equilibrative NBMPR-insensitive (ei) transport activity in human erythroleukemia (K562) cells. Neuropharmacology. 1997;36(9):1167–1179. doi: 10.1016/s0028-3908(97)00136-6. [DOI] [PubMed] [Google Scholar]
- 35.Wang LM, Woodward CN, White JC, et al. Determination of 3H-labelled cytosine arabinoside and eight metabolites in cell extracts by high-performance liquid chromatography and scintillation spectrometry. J Chromatogr. 1989;491(2):331–340. doi: 10.1016/s0378-4347(00)82851-0. [DOI] [PubMed] [Google Scholar]
- 36.Parker WB, Shaddix SC, Chang CH, et al. Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5′-triphosphate. Cancer Res. 1991;51(9):2386–2394. [PubMed] [Google Scholar]
- 37. http://www.affymetrix.com/partners_programs/programs/developer/stat_sdk/index.affx method. Accessed June 1, 2010. [Google Scholar]
- 38.Kaplan EL, Meier P. Nonparamteric estimation from incomplete observations. J Amer Stat Assoc. 1958;53:457–481. [Google Scholar]
- 39.Kufe D, Spriggs D, Egan EM, et al. Relationships among Ara-CTP pools, formation of (Ara-C)DNA, and cytotoxicity of human leukemic cells. Blood. 1984;64(1):54–58. [PubMed] [Google Scholar]
- 40.Estey E, Plunkett W, Gandhi V, et al. Fludarabine and arabinosylcytosine therapy of refractory and relapsed acute myelogenous leukemia. Leuk Lymphoma. 1993;9(4-5):343–350. doi: 10.3109/10428199309148532. [DOI] [PubMed] [Google Scholar]
- 41.Hu S, Chen Z, Franke R, et al. Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transporters. Clin Cancer Res. 2009;15(19):6062–6069. doi: 10.1158/1078-0432.CCR-09-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopper-Borge E, Xu X, Shen T, et al. Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 2009;69(1):178–184. doi: 10.1158/0008-5472.CAN-08-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Y, Kotova E, Chen ZS, et al. MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3’-dideoxycytidine and 9′-(2’-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003;278(32):29509–29514. doi: 10.1074/jbc.M304059200. [DOI] [PubMed] [Google Scholar]
- 44.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28(11):1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang M, Wang Y, Mitchell BS, et al. Regulation of equilibrative nucleoside uptake by protein kinase inhibitors. Nucleosides Nucleotides Nucleic Acids. 2004;23(8-9):1445–1450. doi: 10.1081/NCN-200027667. [DOI] [PubMed] [Google Scholar]
- 46.Huang M, Wang Y, Cogut SB, et al. Inhibition of nucleoside transport by protein kinase inhibitors. J Pharmacol Exp Ther. 2003;304(2):753–760. doi: 10.1124/jpet.102.044214. [DOI] [PubMed] [Google Scholar]
- 47.Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch - Eur J Physiol. 2007;453(5):661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 48.Kruh GD, Guo Y, Hopper-Borge E, et al. ABCC10, ABCC11, and ABCC12. Pflugers Arch. 2007;453(5):675–684. doi: 10.1007/s00424-006-0114-1. [DOI] [PubMed] [Google Scholar]
- 49.Nishioka C, Ikezoe T, Yang J, et al. Inhibition of MEK signaling enhances the ability of cytarabine to induce growth arrest and apoptosis of acute myelogenous leukemia cells. Apoptosis. 2009;14(9):1108–1120. doi: 10.1007/s10495-009-0372-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.