Abstract
Background
Approximately 15% of colorectal cancers develop because of defective function of the DNA mismatch repair (MMR) system. We determined the association of MMR status with colon cancer recurrence and examined the impact of 5-fluorouracil (FU)-based adjuvant therapy on recurrence variables.
Methods
We included stage II and III colon carcinoma patients (n = 2141) who were treated in randomized trials of 5-FU-based adjuvant therapy. Tumors were analyzed for microsatellite instability by polymerase chain reaction and/or for MMR protein expression by immunohistochemistry to determine deficient MMR (dMMR) or proficient MMR (pMMR) status. Associations of MMR status and/or 5-FU-based treatment with clinicopathologic and recurrence covariates were determined using χ2 or Fisher Exact or Wilcoxon rank-sum tests. Time to recurrence (TTR), disease-free survival (DFS), and overall survival (OS) were analyzed using univariate and multivariable Cox models, with the latter adjusted for covariates. Tumors showing dMMR were categorized by presumed germline vs sporadic origin and were assessed for their prognostic and predictive impact. All statistical tests were two-sided.
Results
In this study population, dMMR was detected in 344 of 2141 (16.1%) tumors. Compared with pMMR tumors, dMMR was associated with reduced 5-year recurrence rates (33% vs 22%; P < .001), delayed TTR (P < .001), and fewer distant recurrences (22% vs 12%; P < .001). In multivariable models, dMMR was independently associated with delayed TTR (hazard ratio = 0.72, 95% confidence interval = 0.56 to 0.91, P = .005) and improved DFS (P = .035) and OS (P = .031). In stage III cancers, 5-FU-based treatment vs surgery alone or no 5-FU was associated with reduced distant recurrence for dMMR tumors (11% vs 29%; P = .011) and reduced recurrence to all sites for pMMR tumors (P < .001). The dMMR tumors with suspected germline mutations were associated with improved DFS after 5-FU-based treatment compared with sporadic tumors where no benefit was observed (P = .006).
Conclusions
Patients with dMMR colon cancers have reduced rates of tumor recurrence, delayed TTR, and improved survival rates, compared with pMMR colon cancers. Distant recurrences were reduced by 5-FU-based adjuvant treatment in dMMR stage III tumors, and a subset analysis suggested that any treatment benefit was restricted to suspected germline vs sporadic tumors.
CONTEXT AND CAVEATS
Prior knowledge
A major percentage of colorectal cancers have proficient function of the DNA mismatch repair (pMMR) system but about 15% cancers have deficient MMR (dMMR) function. Whether there is an association between MMR status and cancer recurrence or survival in patients treated with 5-fluorouracil (FU)-based adjuvant therapy in colorectal cancer patients is not well understood.
Study design
MMR status (dMMR vs pMMR) was determined in stage II and III colonic adenocarcinoma patients who previously participated in randomized adjuvant therapy trials evaluating 5-FU-based therapy. Associations between MMR status and colon cancer recurrence (rate, site, and time to recurrence) were analyzed by cancer stage and adjuvant treatment. The prognostic and predictive impact of MMR status on 5-FU-based adjuvant therapy was also evaluated.
Contribution
Patients with dMMR colon cancers showed reduced rates of tumor recurrence, delayed time to recurrence, and improved stage- and treatment-adjusted disease-free and overall survival compared with pMMR colon cancers. Distant recurrences were reduced by 5-FU-based adjuvant treatment in dMMR stage III, but not in dMMR stage II patients. Analysis of a patient subset suggested that any treatment benefit was restricted to patients with suspected germline vs sporadic dMMR tumors.
Implications
Patients with dMMR colon cancers show reduced rates of recurrence and improved survival. Patients with stage III dMMR cancers may benefit from 5-FU-based adjuvant therapy compared with observation or no 5-FU treatment.
Limitations
Retrospective study design, analysis restricted to available tumor tissues from adjuvant studies, and data pooled from different randomized trials.
From the Editors
Colorectal cancer (CRC) is the fourth most prevalent cancer and is second only to lung cancer as a cause of cancer-related mortality in the United States (1). Although the majority of CRCs show chromosomal instability, approximately 15% of cancers develop via an alternative pathway characterized by defective function of the DNA mismatch repair (MMR) system. These CRCs are known as deficient MMR (dMMR) tumors, whereas most CRCs have proficient MMR (pMMR) (2). MMR deficiency is most commonly caused by epigenetic inactivation of the MLH1 gene in sporadic CRCs, and the remainder of dMMR tumors are associated with Lynch syndrome that is caused by germline mutations in MMR genes such as mutL homolog 1 (MLH1), mutS homolog 2 (MSH2), mutS homolog 6 (MSH6), and postmeiotic segregation increased 2 (PMS2) (3). At least 90% of dMMR tumors are associated with inactivation of MLH1 or MSH2 (4). Colon cancers with dMMR show a high-frequency microsatellite instability (MSI-H) that develops because of an inability to repair single nucleotide DNA mismatches, resulting in inactivating mutations in multiple genes (2). Cancers with pMMR show low-frequency MSI (MSI-L) or in most cases are microsatellite stable (MSS) (2).
Colon cancers with dMMR have distinct clinical and pathological features that commonly include proximal colon predominance, poor differentiation, diploid DNA content, and increased numbers of tumor-infiltrating lymphocytes (5,6). To date, data are lacking regarding the rate, pattern, and time to recurrence (TTR) in dMMR vs pMMR colon cancers. However, multiple studies have shown that dMMR colon cancers have a better stage-adjusted survival compared with pMMR cancers. These data are largely from retrospective studies (7–11) that included patients treated in phase III randomized trials of 5-fluorouracil (FU)-based adjuvant therapy, a population-based study (12), and a meta-analysis (13). Not all studies, however, have shown a prognostic impact of MMR status (14,15). Factors that can contribute to discrepant study findings include the relatively small numbers of patients with dMMR colon cancers and the bimodal age distribution among these patients. Specifically, patients with sporadic dMMR tumors are older than those with germline mutations in MMR genes who generally show early age at the onset of colon cancer (3). Patients with sporadic dMMR tumors may, therefore, have comorbidities that could adversely affect their survival. These issues also pertain to determining the predictive impact of MMR status for 5-FU-based adjuvant therapy in colon cancer patients, which is controversial. Retrospective studies indicate that patients with dMMR colon cancers do not appear to benefit from 5-FU-based adjuvant chemotherapy in contrast to patients with pMMR cancers (16–19). Although the majority of studies showed a lack of benefit of adjuvant 5-FU in dMMR colon cancers, an analysis of patients treated in adjuvant trials conducted by the National Surgery Adjuvant Breast and Bowel Project (NSABP) reported no interaction between MMR status and 5-FU response (15), and some studies reported a better outcome for dMMR colon cancer patients treated with 5-FU (20–22). An important issue that has not been addressed is whether the prognosis or treatment response differs among patients with sporadic dMMR tumors where MLH1 is inactivated by methylation of the gene promoter or those arising in Lynch syndrome because of germline MMR mutations.
We determined the association of MMR status with colon cancer recurrence variables (rate, TTR, and site of recurrence) to further explore the impact of dMMR on clinical outcomes. We also examined the impact of 5-FU-based adjuvant therapy on these recurrence variables. Such data may provide further insights into the clinical behavior of dMMR vs pMMR tumors that may be useful in patient management and the clinical decision-making process.
Subjects and Methods
Patient Characteristics
The study population consisted of 2141 patients with pathologically confirmed stage II (n = 778) and stage III (n = 1363) colonic adenocarcinomas. Only patients with available tissue specimens from adjuvant therapy trials were included in our analysis, and therefore, represent a subset of the overall study cohorts. Patients were previously enrolled in randomized adjuvant therapy trials evaluating 5-FU with levamisole or leucovorin vs surgery alone [Mayo Clinic and the North Central Cancer Treatment Group (NCCTG) studies 78-48-52 (23,24), 84-46-52/Intergroup 0035 (25,26), and 89-46-51 (27)]. Other adjuvant studies included in our analysis evaluated the administration of portal venous 5-FU vs surgery alone (NCCTG 79-46-04) (27), 5-FU with leucovorin and interferon-gamma (IFN-γ) with or without levamisole (NCCTG 87-46-51) (28), and 5-FU plus leucovorin with high vs standard dose levamisole (NCCTG 91-46-53) (29). Studies conducted by the Federation Francophone de la Cancerologie Digestive (FFCD) 8802, Gruppo Italiano Valutazione Interventi in Oncologia [GIVIO] (19), and the National Cancer Institute of Canada () study C03 were included (24). We also included adjuvant trials conducted by the NSABP that randomly assigned patients to immunotherapy (Bacille Calmette-Guérin [BCG]), surgery alone or the combination of 5-FU and vincristine and semustine (MOF) (C-01), portal venous 5-FU vs surgery alone (C-02), MOF vs 5-FU plus leucovorin (C-03), or 5-FU plus leucovorin vs 5-FU plus leucovorin plus levamisole (C-04) (15). None of the study patients received oxaliplatin or irinotecan as adjuvant therapy.
The primary tumor site was categorized as proximal colon if the tumor was located above the splenic flexure or distal colon if it was located at or below the splenic flexure. Tumor histological grade was categorized as grade 1 or 2 if well or moderately differentiated and grade 3 or 4 if poorly differentiated or undifferentiated respectively. Patient clinical and pathological data from the adjuvant studies are shown in Table 1. Data were collected from the individual study protocol databases and were then pooled. Colon cancer recurrence data were prospectively collected and recorded in the study databases. Median follow-up on living patients was 8 years. We categorized the site of first recurrence in the full dataset as local if the recurrence was at/near the anastomotic site, intra-abdominal, or at distant sites inclusive of lymph node metastases. For the predictive analysis, adjuvant treatment status was categorized as observation (surgery alone) or no 5-FU vs intravenous 5-FU-based chemotherapy (Table 1). The original clinical trials were approved by the local Institutional Review Boards of each participating site. The protocol for the current pooled analysis was approved by the Institutional Review Board of Mayo Clinic.
Table 1.
Clinical and pathological characteristics of patients and tumors by DNA mismatch repair (MMR) status
| Total No. of patients (n = 2141)* |
|||
| Proficient MMR (n = 1797) | Deficient MMR (n = 344) | ||
| Variable | No. (%) | No. (%) | P |
| Study group† | .317‡ | ||
| FFCD | 124 (80.5) | 30 (19.5) | |
| NCIC | 244 (85.0) | 43 (15.0) | |
| GIVIO | 153 (83.6) | 30 (16.4) | |
| NSABP | 438 (81.9) | 97 (18.1) | |
| NCCTG | 838 (85.3) | 144 (14.7) | |
| Sex | .019‡ | ||
| Women | 817 (81.9) | 180 (18.1) | |
| Men | 980 (85.7) | 164 (14.3) | |
| Colon cancer stage | <.001‡ | ||
| III | 1183 (86.8) | 180 (13.2) | |
| II | 614 (78.9) | 164 (21.1) | |
| Tumor histological grade§ | <.001‡ | ||
| Grade 1 or 2‖ | 1113 (88.8) | 141 (11.2) | |
| Grade 3 or 4‖ | 239 (69.9) | 103 (30.1) | |
| Primary tumor site§ | <.001‡ | ||
| Distal | 1059 (93.9) | 69 (6.1) | |
| Proximal | 712 (72.6) | 269 (27.4) | |
| Treatment status | .322‡ | ||
| Observation ¶ or no 5-FU | 629 (82.9) | 130 (17.1) | |
| 5-FU-based | 1168 (84.5) | 214 (15.5) | |
| Recurrence status | <.001‡ | ||
| No recurrence | 1222 (81.8) | 271 (18.2) | |
| Recurrence | 575 (88.7) | 73 (11.3) | |
| Site of recurrence§ | .171‡ | ||
| Intra-abdominal | 122 (84.7) | 22 (15.3) | |
| Local only | 41 (91.1) | 4 (8.9) | |
| Distant | 387 (90.2) | 42 (9.8) | |
| Age, y | .947# | ||
| Mean (SD) | 60.8 (10.38) | 60.1 (12.26) | |
| Median (range) | 62 (21.0–88.0) | 62.0 (22.0–86.2) | |
Patients with stage II (n = 778) and III (n = 1363) colon cancers who participated in clinical trials of 5-FU-based adjuvant therapy. FFCD = Federation Francophone de la Cancerologie Digestive; FU = fluorouracil; GIVIO = Gruppo Italiano Valutazione Interventi in Oncologia; NCCTG = North Central Cancer Treatment Group; NCIC = National Cancer Institute of Canada; NSABP = National Surgery Adjuvant Breast and Bowel Project; SD = standard deviation.
Randomized clinical trials evaluating 5-FU-based adjuvant chemotherapy.
P values were calculated using a two-sided χ2 test.
Missing observations.
Grade 1 or 2 = well or moderate differentiation; grade 3 or 4 = poor differentiation or undifferentiated.
Observation is defined as surgery alone.
P value was calculated using a two-sided Wilcoxon rank-sum test.
Determination of MMR Status
MMR status was determined by MSI testing and/or analysis of MMR protein expression by immunohistochemistry (IHC). Formalin-fixed paraffin-embedded tumor blocks from stage II (n = 778) and III (n = 1363) colonic adenocarcinomas were used. These archival tissues were obtained from participants in the adjuvant trials, as per the specific study protocols.
MSI Testing.
MSI was analyzed by polymerase chain reaction amplification of microsatellite loci in microdissected tumor-enriched paraffin tissue. Specimens from the NCCTG studies including Intergroup 0035 (Table 1) were screened using 4–11 microsatellite markers, as previously described (9,30). In NCCTG 91-46-53, MMR status was determined by analysis of instability at BAT26 coupled with MLH1, MSH2, and MSH6 protein expression (29). Nearly all specimens collected from the National Cancer Institute of Canada C03 trial (Table 1) were amplified with 5–10 mono- and dinucleotide microsatellite loci derived from the panel recommended by the NCI that includes BAT26, BAT25, D5S346, D2S123, and D17S250 markers (31). Within the NSABP studies, MSI was also determined by amplification of the five-marker Bethesda/NCI panel (31) and the transforming growth factor-beta receptor II (RII) locus (15). Specimens obtained from the trial conducted by the FFCD were screened only with mononucleotide microsatellite markers BAT25 and BAT26 (32). MSI testing in the GIVIO study was performed as outlined for National Cancer Institute of Canada-03, except that 53 of 183 tumors were analyzed using only BAT25 and BAT26, and instability was required at both loci for tumors to be regarded as unstable and thus consistent with MSI-H.
IHC Analysis of MMR Expression.
Formalin-fixed paraffin-embedded tumors (n = 900) were stained for MLH1 and MSH2 proteins using an immunoperoxidase method, as previously described (33). Staining was performed using the following primary antibodies: mouse anti-human MLH1 (clone G168-728, 1:250; BD Pharmingen, San Diego, CA) and mouse anti-human MSH2 (clone FE11, 1:50; Oncogene Research Products, Cambridge, MA) monoclonal antibodies. Analysis of MSH6 expression in tumors (n = 387) was performed using a mouse anti-human MSH6 monoclonal antibody (clone 44; Transduction Laboratories, Lexington, KY) in one study (NCCTG 91-46-53). For patients enrolled in the FFCD 8802 study, the same mouse anti-human MLH1 antibody that is described above was used, but a different antibody was used to detect MSH2 (clone G219-1129, 1:200; BD Pharmingen). Protein expression data was interpreted as follows—loss of an MMR protein was defined as the absence of nuclear staining of tumor cells in the presence of positive nuclear staining in normal colonic epithelium and lymphocytes. Each slide was assigned a unique number that enabled blinding of the researchers to patient identity and clinical characteristics.
Definition of dMMR/pMMR.
In tumors, dMMR was defined by the presence of MSI-H if greater than 30% of the microsatellite markers showed instability (31) and/or by loss of MLH1 or MSH2 or MSH6 protein expression, or as outlined above. The pMMR tumors were defined as MSI-L (ie, instability at <30% of loci screened), MSS, and/or by intact MMR protein expression. MSI-L has been shown to be biologically similar to tumors exhibiting MSS at all loci tested, and these two molecular phenotypes can be grouped together (13).
Testing for BRAF Mutation
Testing for BRAFV600E mutation in exon 15 was performed in a tumor subset by conformation-sensitive gel electrophoresis analysis or DNA sequencing or both, irrespective of the MMR status. This was done as previously described (34). DNA sequence analysis was performed on all samples that showed an abnormally migrating fragment by conformation-sensitive gel electrophoresis, all ambiguous conformation-sensitive gel electrophoresis results, and in dMMR tumors (34). Colon cancers tested for BRAF mutations and included in this report were from NCCTG adjuvant studies, including NCCTG 91-46-53 (n = 111 tumors).
Statistical Analyses
The χ2 and Wilcoxon rank-sum tests were used to test for an association between MMR status and other clinicopathologic variables, as shown in Table 1. The χ2 or Fisher exact tests were used to test for an association between adjuvant treatment status with the site of recurrence by dMMR and pMMR status. For this association, a formal test of homogeneity across the study groups was assessed using the Breslow–Day test. The cumulative incidence of recurrence was analyzed and shown graphically over time to evaluate patterns of recurrence. Disease-free survival (DFS) or TTR, censored at 5 years, was calculated as the number of years from random assignment to the first event of either disease recurrence (DFS, TTR) or death (DFS). Overall survival (OS), censored at 8 years, was calculated as the number of years from random assignment to the date of death or last contact in the adjuvant trials. The distributions of OS and DFS were estimated using Kaplan–Meier methodology. Univariate and multivariable Cox proportional hazards models were used to explore the association of MMR status, treatment, and site of recurrence with DFS and OS and were stratified by adjuvant study. The score and likelihood ratio test P values were used to test the statistical significance of each covariate in the univariate and multivariable Cox models, respectively. Wald test P values from a Cox model were used to assess the statistical significance of each individual site of recurrence category. Interaction effects were evaluated using the likelihood ratio test from multivariate Cox models. Graphical and statistical methods were used to examine whether proportional hazards assumptions were satisfied (35). Patient survival following the date of recurrence was assessed using a landmark analysis to determine if the site of recurrence was prognostic. All statistical tests were two-sided, and P values less than or equal to .05 were considered statistically significant. P values were not adjusted for multiple comparisons. Statistical analyses were performed using SAS software (SAS Institute, Cary, NC).
Results
Patient Characteristics
The study population consisted of 2141 patients with pathologically confirmed stage II (n = 778 tumors) and III (n = 1363 tumors) colonic adenocarcinomas who previously participated in randomized adjuvant therapy trials evaluating 5-FU-based therapy vs surgery alone or comparing 5-FU-based treatment regimens (Table 1). Details of the patient characteristics are shown in Table 1.
Frequency of MMR and Tumor Recurrence in Stage II and III Colon Cancer
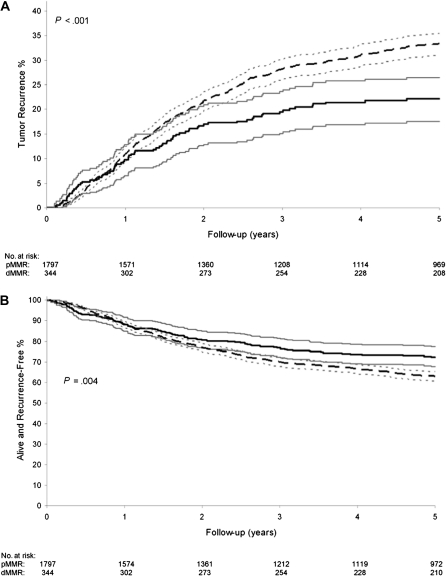
We detected dMMR in 344 (16.1%) of 2141 colon cancers, determined by MSI testing and/or IHC analysis for MMR proteins (Table 1). These tumors showed MSI-H (n = 210 tumors) and/or loss of either MLH1 (n = 112 tumors) or MSH2 (n = 22 tumors) protein expression. MSH6 expression was analyzed in 387 tumors in the NCCTG 91-46-53 study and of the 10 tumors that showed loss of MSH6, all showed concurrent loss of either MLH1 or MSH2 such that no additional dMMR cancers were identified. The frequency of dMMR was similar across the adjuvant study populations (P = .32) (Table 1). We stratified the clinical characteristics of the study population by MMR status. The dMMR vs pMMR colon cancers were more likely to be stage II vs III (P < .001), proximal vs distal (P < .001), from women vs men (P = .019), and poor or undifferentiated vs well or moderate differentiation (P < .001) (Table 1). Furthermore, fewer regional lymph node metastases were noted in patients with dMMR vs pMMR tumors (P < .001) (data not shown). Patients with dMMR vs pMMR tumors showed a statistically significant reduction in the rates of tumor recurrence at 5 years (22% vs 33%; P < .001) (Figure 1, A). The delay in recurrence for dMMR tumors became evident at 1 year of follow-up after primary tumor resection, and the divergence from pMMR tumors increased over time. Patients with stage II and III colon cancers with dMMR tumors also showed a statistically significant delay in TTR (hazard ratio [HR] = 0.65, 95% confidence interval [CI] = 0.51 to 0.83; P < .001) and DFS (HR = 0.73, 95% CI = 0.59 to 0.91; P = .004; Figure 1, B), compared with patients with pMMR tumors.
Figure 1.
Stage II and III colon cancer recurrence rates and survival by DNA mismatch repair (MMR) status. A) Tumor recurrence rates are shown for patients with deficient MMR (dMMR) (solid dark line) or proficient MMR (pMMR) tumors (dashed dark line) with the corresponding 95% confidence intervals (solid and dotted gray lines). Analysis was performed using Cox regression model, stratified by adjuvant study, and P value was calculated using a two-sided score test. B) Disease-free survival rates for patients with dMMR (solid dark line) or pMMR tumors (dashed dark line) with corresponding 95% confidence intervals (solid and dotted gray lines). Analysis was performed using Cox regression model, stratified by adjuvant study, and P value was calculated using a two-sided score test.
Association Between MMR Status and Colon Cancer Recurrence
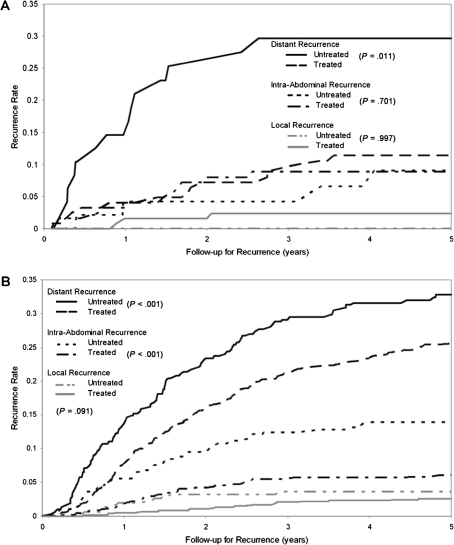
We examined the association between MMR status and the site of tumor recurrence in the study population. Primary tumors with dMMR had lower overall rates of tumor recurrence vs pMMR, with the largest reduction noted for recurrence at distant sites (12% vs 22%; P < .001) (Table 2). When analyzed by tumor stage, the association between MMR status and site of recurrence was limited to stage III vs II patients (P = .013). When stratifying by adjuvant treatment, patients with dMMR tumors receiving 5-FU-based therapy had higher rates of local and intra-abdominal recurrence but fewer distant metastases compared with patients with pMMR tumors (P = .006) (Table 2). However, MMR status was not associated with the site of recurrence in patients receiving observation (surgery alone) or no 5-FU treatment (P = .295). Analysis of the site of recurrence by stage showed that stage III patients with dMMR tumors treated with adjuvant 5-FU had a lower 5-year recurrence rate (22% vs 37%; P = .044) and recurred less frequently at distant sites (11% vs 29%; P = .011) vs patients receiving observation or no 5-FU (Table 2 and Figure 2, A). In addition, patients with dMMR tumors receiving 5-FU treatment were less likely to have a hepatic recurrence vs patients receiving observation or no 5-FU treatment (22% vs 56%; P = .005). An association between adjuvant 5-FU-based treatment and the site of recurrence was not observed in stage II patients with dMMR tumors (P = .570), and the association was only of marginal statistical significance for stage II pMMR tumors (P = .076) (data not shown). In stage III patients with pMMR tumors, 5-FU-based treatment reduced recurrence rates to all sites compared with patients receiving observation or no 5-FU treatment (P < .001) (Table 2 and Figure 2, B).
Table 2.
Association between DNA mismatch repair (MMR) and/or adjuvant treatment status on the site of tumor recurrence
| No. of patients (%) with recurrence |
|||||
| No. of patients (%) without recurrence | Site of recurrence (n = 618) |
||||
| MMR status | Local only | Intra-abdominal | Distant | P | |
| MMR status and site of tumor recurrence in all patients (N = 2111)* | |||||
| pMMR | 1222 (69) | 41 (2) | 122 (7) | 387 (22) | <.001† |
| dMMR | 271 (80) | 4 (1) | 22 (6) | 42 (12) | |
| Effect of 5-FU-based treatment by MMR status on site of recurrence in stage III (n = 1340)‡ | |||||
| pMMR | |||||
| Observation§ or no 5-FU | 160 (51) | 11 (4) | 42 (13) | 99 (32) | <.001‖ |
| 5-FU-based | 571 (67) | 21 (2) | 49 (6) | 210 (25) | |
| dMMR | |||||
| Observation§ or no 5-FU | 31 (63) | 0 (0) | 4 (8) | 14 (29) | .036¶ |
| 5-FU-based | 100 (78) | 3 (2) | 11 (9) | 14 (11) | |
Prevalence and site of tumor recurrence by MMR status in stage II and III colon cancer patients treated in 5-FU-based adjuvant therapy trials. dMMR = deficient MMR; FU = fluorouracil; pMMR = proficient MMR.
P value was calculated using the two-sided χ2 test for all sites or using only the distant recurrence category.
In patients with stage III colon cancers, the effect of 5-FU-based therapy on site of recurrence was determined.
Observation is defined as surgery alone.
P value was calculated using a two-sided χ2 test.
P value was calculated using the two-sided Fisher exact test. When only distant recurrence was analyzed, the P value was .011.
Figure 2.
Cumulative incidence of recurrence rates by tumor site and DNA mismatch repair (MMR) status in stage III colon cancer patients treated in adjuvant chemotherapy trials. A) Effect of 5-fluorouracil (FU)-based adjuvant therapy (treated) vs surgery alone or no 5-FU (untreated) by site of tumor recurrence in patients with deficient MMR colon cancers. B) Effect of 5-FU-based adjuvant therapy (treated) vs surgery alone or no 5-FU (untreated) by site of tumor recurrence in patients with proficient MMR colon cancers. P values were calculated using a two-sided χ2 test at 5 years.
We also determined whether the site of tumor recurrence was prognostic in our study population. A statistically significant reduction in OS after recurrence was observed in patients with intra-abdominal (P = .007) or distant (P = .001) sites of recurrence compared with those with local recurrence (Table 3). The best outcome was observed for local recurrence followed in order by lung, intra-abdominal, liver, and other distant sites. The worst outcome was observed for patients with recurrence to both liver and lung (P < .001) (Table 3).
Table 3.
Patient overall survival (OS) after colon cancer recurrence*
| OS |
||||
| Site of tumor recurrence | No. of patients | 5-y rate (95% CI) | HR (95% CI) | P |
| Overall <.001† | ||||
| Local (reference) | 45 | 22.7 (13.2 to 39.2) | — | |
| Intra-abdominal | 144 | 12.2 (7.7 to 19.3) | 1.72 (1.16 to 2.54) | .007‡ |
| Distant | 429 | 7.8 (5.4 to 11.1) | 1.79 (1.25 to 2.55) | .001‡ |
| Liver | 226 | 6.2 (3.7 to 10.6) | 1.91 (1.32 to 2.76) | <.001‡ |
| Lung | 94 | 7.4 (2.8 to 19.4) | 1.33 (0.89 to 1.99) | .168‡ |
| Liver and lung | 23 | 0.0 (0.0 to 0.0) | 3.55 (2.04 to 6.17) | <.001‡ |
| Other distant | 86 | 12.1 (6.7 to 21.9) | 1.98 (1.31 to 2.99) | .001‡ |
Impact of the site of tumor recurrence on OS after colon cancer recurrence was determined using a landmark analysis. A Cox model, stratified by study, was used to estimate the effect for each site of tumor recurrence. CI = confidence interval; HR = hazard ratio.
P value was calculated using a two-sided score test.
P values were calculated using a two-sided Wald χ2 test.
Next, we determined whether the effect of 5-FU-based treatment on site of tumor recurrence was consistent across the adjuvant study cohorts. No statistically significant differences were found for the effect of 5-FU-based treatment on the site of recurrence among the different study cohorts included in this analysis for dMMR (Phomogeneity = .45) and pMMR (Phomogeneity = .11) tumors. We also examined the distant recurrence rates in the largest adjuvant trials. In patients from the NCCTG studies with dMMR tumors, the distant recurrence rate was higher for those receiving observation or no 5-FU vs 5-FU-based treatment (17% vs 9%; P = .157). In patients from the NSABP studies with dMMR tumors, the distant recurrence rate was higher for observation or no 5-FU vs 5-FU-based treatment and was statistically significant (26% vs 10%; P = .036).
In an effort to further support our findings, we analyzed data for MMR status and tumor recurrence using only MMR protein expression because differences in MSI marker panels used in previous adjuvant studies have the potential for misclassification of some tumors. When the analysis was restricted to tumors with MLH1 and MSH2 protein expression data, the results were similar to the overall dataset (data not shown). Of 900 colon cancers with MLH1 and MSH2 expression data, 112 (12.4%) showed loss of MLH1 expression and 22 (2.4%) showed loss of MSH2 expression. In stage III colon cancers with dMMR because of loss of MLH1 or MSH2 proteins, higher rates of intra-abdominal recurrence (P = .011) but lower rates of distant recurrence (P = .031) were found compared with stage III pMMR tumors with intact MMR proteins. Furthermore, loss of MLH1 or MSH2 in patients treated with 5-FU-based therapy was associated with an increase in intra-abdominal recurrence and fewer distant recurrences compared with cancers with intact MMR proteins (P = .002) (data not shown). No statistically significant differences were seen in stage II patients. MMR protein status was not associated with the site of recurrence in patients receiving observation or no 5-FU (P = .707).
Assessment of MMR as Prognostic and Predictive Marker
MMR status provided prognostic information in colon cancer patients from adjuvant therapy trials. Patients with stage II and III colon cancers with dMMR tumors showed a statistically significant improvement in DFS (HR = 0.73, 95% CI = 0.59 to 0.91; P = .004) (Figure 1, B), OS (HR = 0.73, 95% CI = 0.59 to 0.90; P = .004), and TTR (HR = 0.65, 95% CI = 0.51 to 0.83; P < .001) (Table 4), compared with patients with pMMR tumors. When the data were analyzed by tumor stage in a univariate analysis (data not shown in the table), the association of dMMR and improved outcome was similar in stage II and III patients (Pinteractions ≥ .641) but was only statistically significant in stage III (TTR: HR = 0.70, 95% CI = 0.52 to 0.94, P = .016; DFS: HR = 0.76, 95% CI = 0.58 to 1.00, P = .047; OS: HR = 0.76, 95% CI = 0.59 to 0.99, P = .041) and not in stage II (TTR: HR = 0.73, 95% CI = 0.47 to 1.12, P = .148; DFS: HR = 0.83, 95% CI = 0.57 to 1.21, P = .339; OS: HR = 0.81, 95% CI = 0.55 to 1.18, P = .266) dMMR vs pMMR tumors. Other prognostic variables in a univariate analysis included age, sex, stage, and treatment status (Table 4). For treatment, a benefit of 5-FU-based therapy was seen for TTR (HR = 0.63, 95% CI = 0.52 to 0.77, P < .001), DFS (HR = 0.65, 95% CI = 0.54 to 0.77, P < .001), and OS (HR = 0.68, 95% CI = 0.57 to 0.82, P < .001) (Table 4). Concordant results were obtained using only MMR protein data where loss of either MLH1 or MSH2 was associated with a statistically significant improvement in DFS (HR = 0.67, 95% CI = 0.46 to 0.96; P = .027) and OS (HR = 0.69, 95% CI = 0.49 to 0.97, P = .031) rates compared with tumors with intact MMR proteins. Although the site of tumor recurrence was prognostic, analysis of patient survival after tumor recurrence showed no difference based upon MMR status (data not shown). In a multivariable analysis, MMR status was shown to be a statistically significant independent prognostic variable. Specifically, colon cancers with dMMR showed improved outcome compared with pMMR for TTR (HR = 0.72, 95% CI = 0.56 to 0.91, P = .005), DFS (HR = 0.80, 95% CI = 0.64 to 0.99, P = .035), and OS (HR = 0.79, 95% CI = 0.64 to 0.99, P = .031) (Table 5). Other statistically significant prognostic variables included age (P < .001), sex (P = .025), stage (P < .001), and treatment (P < .001) (Table 5).
Table 4.
Univariate analysis of the association of clinicopathologic features and DNA mismatch repair (MMR) status with patient outcome*
| TTR |
DFS |
OS |
||||||||
| Variable | Total No. | 5-y rate (95% CI) | HR (95% CI) | P† | 5-y rate (95% CI) | HR (95% CI) | P† | 5-y rate (95% CI) | HR (95% CI) | P† |
| Stage | ||||||||||
| II | 778 | 80.2 (77.3 to 83.1) | 0.40 (0.33 to 0.49) | <.001 | 75.9 (72.9 to 79.0) | 0.45 (0.38 to 0.54) | <.001 | 82.2 (79.5 to 85.0) | 0.47 (0.39 to 0.56) | <.001 |
| III | 1363 | 61.9 (59.3 to 64.6) | 1.00 (referent) | 58.1 (55.5 to 60.8) | 1.00 (referent) | 64.8 (62.3 to 67.4) | 1.00 (referent) | |||
| MMR status | ||||||||||
| pMMR | 1797 | 66.7 (64.5 to 69.0) | 1.00 (referent) | <.001 | 63.0 (60.8 to 65.3) | 1.00 (referent) | .004 | 69.9 (67.8 to 72.1) | 1.00 (referent) | .004 |
| dMMR | 344 | 77.9 (73.5 to 82.5) | 0.65 (0.51 to 0.83) | 72.4 (67.7 to 77.3) | 0.73 (0.59 to 0.91) | 77.1 (72.7 to 81.7) | 0.73 (0.59 to 0.90) | |||
| Sex | ||||||||||
| Women | 997 | 71.1 (68.3 to 74.1) | 1.00 (referent) | .0498 | 66.8 (63.9 to 69.9) | 1.00 (referent) | .111 | 72.1 (69.4 to 75.0) | 1.00 (referent) | .041 |
| Men | 1144 | 66.2 (63.5 to 69.1) | 1.17 (1.00 to 1.37) | 62.4 (59.6 to 65.4) | 1.13 (0.97 to 1.30) | 70.1 (67.4 to 72.8) | 1.16 (1.01 to 1.34) | |||
| Primary tumor site | ||||||||||
| Distal | 1128 | 67.0 (64.3 to 69.9) | 1.00 (referent) | .179 | 63.8 (61.0 to 66.7) | 1.00 (referent) | .436 | 71.9 (69.3 to 74.6) | 1.00 (referent) | .701 |
| Proximal | 981 | 70.3 (67.4 to 73.3) | 0.90 (0.77 to 1.05) | 65.7 (62.8 to 68.8) | 0.94 (0.81 to 1.09) | 70.5 (67.7 to 73.5) | 1.03 (0.89 to 1.19) | |||
| Adjuvant treatment | ||||||||||
| Observation‡ or no 5-FU | 759 | 63.2 (59.8 to 66.9) | 1.00 (referent) | <.001 | 59.2 (55.7 to 62.9) | 1.00 (referent) | <.001 | 67.2 (63.9 to 70.7) | 1.00 (referent) | <.001 |
| 5-FU-based | 1382 | 71.4 (69.0 to 73.8) | 0.63 (0.52 to 0.77) | 67.4 (64.9 to 69.9) | 0.65 (0.54 to 0.77) | 73.1 (70.8 to 75.5) | 0.68 (0.57 to 0.82) | |||
| Age, y | ||||||||||
| Continuous, increase of 1 y | 2141 | NA | 0.99 (0.99 to 1.00) | .026 | NA | 1.00 (0.99 to 1.01) | .734 | NA | 1.01 (1.00 to 1.02) | .002 |
A Cox model (stratified by study) was used to estimate the effect for each variable. 5-year survival rates indicate the percentage of stage II and III colon cancer patients who were alive at 5 years of follow-up. CI = confidence interval; DFS = disease-free survival; dMMR = deficient mismatch repair; FU = fluorouracil; HR = hazard ratio; NA = not applicable; OS = overall survival; pMMR = proficient mismatch repair; TTR = time to recurrence.
P values were calculated using a two-sided score test.
Observation is defined as surgery alone.
Table 5.
Multivariable analysis of time to recurrence (TTR), disease-free survival (DFS), and overall survival (OS) in all patients and by tumor stage*
| TTR |
DFS |
OS |
||||
| Variable | HR (95% CI) | P† | HR (95% CI) | P† | HR (95% CI) | P† |
| All patients (n = 2141)* | ||||||
| Tumor stage | ||||||
| Stage II vs III | 0.40 (0.32 to 0.48) | <.001 | 0.44 (0.36 to 0.52) | <.001 | 0.45 (0.37 to 0.54) | <.001 |
| MMR status | ||||||
| dMMR vs pMMR | 0.72 (0.56 to 0.91) | .005 | 0.80 (0.64 to 0.99) | .035 | 0.79 (0.64 to 0.99) | .031 |
| Sex | ||||||
| Men vs women | 1.18 (1.01 to 1.38) | .037 | 1.14 (0.98 to 1.32) | .081 | 1.18 (1.02 to 1.36) | .025 |
| Treatment | ||||||
| 5-FU vs observation‡ or no 5-FU | 0.58 (0.47 to 0.70) | <.001 | 0.60 (0.50 to 0.72) | <.001 | 0.64 (0.54 to 0.77) | <.001 |
| Age, y | ||||||
| Continuous, increase of 1 y | 0.99 (0.99 to 1.00) | .055 | 1.00 (1.00 to 1.01) | .547 | 1.01 (1.01 to 1.02) | <.001 |
| Stage II patients (n = 778) | ||||||
| MMR status | ||||||
| dMMR vs pMMR | 0.73 (0.47 to 1.13) | .143 | 0.86 (0.59 to 1.26) | .427 | 0.86 (0.59 to 1.25) | .417 |
| Sex | ||||||
| Men vs women | 0.88 (0.64 to1.22) | .457 | 0.95 (0.70 to 1.27) | .706 | 1.01 (0.75 to 1.35) | .966 |
| Treatment | ||||||
| 5-FU vs observation‡ or no 5-FU | 0.76 (0.54 to 1.07) | .119 | 0.77 (0.56 to 1.05) | .101 | 0.80 (0.58 to 1.09) | .156 |
| Age, y | ||||||
| Continuous, increase of 1 | 1.00 (0.99 to 1.02) | .647 | 1.02 (1.00 to 1.03) | .053 | 1.02 (1.01 to 1.04) | .003 |
| Stage III patients (n = 1363) | ||||||
| MMR status | ||||||
| dMMR vs pMMR | 0.72 (0.54 to 0.97) | .024 | 0.78 (0.60 to 1.02) | .063 | 0.78 (0.60 to 1.02) | .057 |
| Sex | ||||||
| Men vs women | 1.29 (1.08 to 1.54) | .006 | 1.20 (1.02 to 1.42) | .031 | 1.23 (1.04 to 1.45) | .015 |
| Treatment | ||||||
| 5-FU vs observation‡ or no 5-FU | 0.52 (0.41 to 0.66) | <.001 | 0.54 (0.43 to 0.68) | <.001 | 0.59 (0.47 to 0.74) | <.001 |
| Age, y | ||||||
| Continuous, increase of 1 y | 0.99 (0.98 to 1.00) | .026 | 1.00 (0.99 to 1.01) | .820 | 1.01 (1.00 to 1.02) | .007 |
Cox multivariable analysis, stratified by study, of the prognostic impact of clinicopathologic variables in stage II and/or III colon cancer patients treated in 5-FU-based adjuvant therapy trials. CI = confidence interval; dMMR = deficient mismatch repair; FU = fluorouracil; HR = hazard ratio; pMMR = proficient mismatch repair.
P values were calculated using a two-sided likelihood ratio test.
Observation is defined as surgery alone.
We also restricted the analysis to adjuvant studies (from NSABP and National Cancer Institute of Canada) that used at least five microsatellite markers that comprise the Bethesda panel (31) and an NCCTG study (91-46-53) that analyzed BAT26 and immunohistochemical expressions of MLH1, MSH2, and MSH6. Using this restricted dataset, we confirmed our major findings including the reduced recurrence rates and better DFS and OS in dMMR vs pMMR colon cancer patients, and the reduction in distant recurrence for 5-FU-based treatment in stage III patients (data not shown).
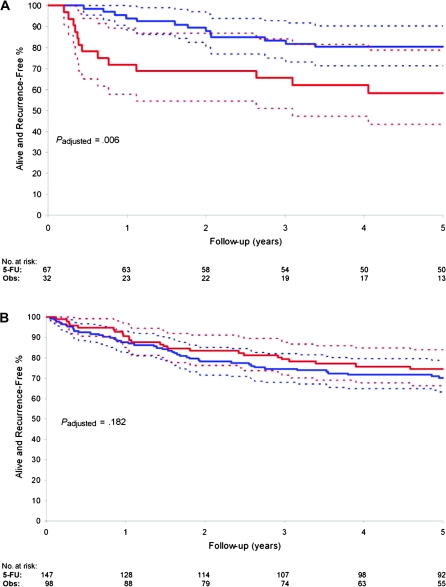
In a previous study, we performed a predictive analysis of MMR status and survival in patients treated in adjuvant 5-FU-based clinical trials with untreated control groups that represent a subset of our study population (36). Accordingly, we did not repeat the predictive analysis but sought to address whether a differential outcome from 5-FU-based chemotherapy exists for dMMR tumors of presumed sporadic vs germline (ie, Lynch syndrome) origin. We categorized patients as sporadic or germline using MSI and/or IHC data and age at adjuvant study randomization. We chose an age cutoff of 55 years based upon its inclusion in the modified Amsterdam criteria for the diagnosis of Lynch syndrome (37) and population-based data for the screening of CRCs in Lynch syndrome patients (38). Patients with suspected germline tumors showed loss of MSH2 expression or MSI-H and/or loss of MLH1 expression at age 55 years or less (n = 99 patients). None of the presumed germline tumors had a BRAFV600E mutation where such data were available. Patients with suspected sporadic tumors showed MSI-H and/or loss of MLH1 expression and were older than 55 years of age (n = 245 patients). The mean age of patients with suspected germline vs sporadic tumors was 44.9 vs 66.3 years (P < .001). We examined the prognostic impact of suspected germline vs sporadic dMMR tumors. Although patients with germline tumors showed improved survival compared with sporadic tumors, this association was no longer statistically significant after adjustment for patient age (data not shown). In an exploratory analysis, we examined the impact of 5-FU-based treatment on patient survival in these two groups. In patients with germline tumors, 5-FU-based treatment was associated with better TTR (HR = 0.25, 95% CI = 0.10 to 0.65, P = .003) and DFS (HR = 0.29, 95% CI = 0.11 to 0.72; P = .006) compared with observation or no 5-FU in a univariate analysis and after adjustment for stage, age, and sex (TTR: HR = 0.27, 95% CI = 0.12 to 0.63; P = .003; DFS: HR = 0.31, 95% CI = 0.14 to 0.70; P = .006) (Figure 3, A). In contrast, patients with suspected sporadic tumors receiving 5-FU-based treatment did not show a TTR or a DFS benefit compared with those receiving observation or no 5-FU (TTR: HR = 1.31, 95% CI = 0.64 to 2.70, P = .457; DFS: HR = 1.55, 95% CI = 0.85 to 2.83, P = .147) in a univariate analysis and after adjustment for stage, age, and sex (TTR: HR = 1.22, 95% CI = 0.59 to 2.51, P = .585; DFS: HR = 1.50, 95% CI = 0.82 to 2.74, P = .182) (Figure 3, B). In stage III patients with suspected germline tumors, a greater TTR and DFS benefit was observed for 5-FU-based treatment compared with those receiving observation or no 5-FU (DFS: HR = 0.26, 95% CI = 0.09 to 0.77, P = .009), whereas no treatment benefit was observed in patients with sporadic tumors (DFS: HR = 0.79, 95% CI = 0.35 to 1.80, P = .577).
Figure 3.
Effect of 5-fluorouracil (FU)-based adjuvant therapy on disease-free survival (DFS) in colon cancer patients with suspected germline mutations vs sporadic tumors. A) Effect of 5-FU-based therapy on DFS in colon cancer patients with suspected germline mutations. DFS rates with 95% confidence intervals (dotted lines) are shown in patients who received 5-FU-based treatment (blue) vs observation or no 5-FU (red). B) Effect of 5-FU-based therapy on DFS in patients with suspected sporadic colon cancer. DFS rate and 95% confidence intervals (dotted lines) are shown in patients who received 5-FU-based treatment (blue) vs observation or no 5-FU (red). P values were calculated using a two-sided likelihood ratio test after adjustment for stage, age, and sex. Obs = Observation.
To provide further support for our findings, we repeated the analysis of suspected germline vs sporadic dMMR tumors after restricting our study population to those studies (NSABP and National Cancer Institute of Canada), where MSI was determined using five or more microsatellite markers from the Bethesda panel (31) and a study that analyzed BAT26 and immunohistochemical expressions of MLH1, MSH2, and MSH6 proteins (NCCTG 91-46-53). When stratified by study, we found that patients (n = 66) with suspected germline mutations in dMMR tumors received a DFS benefit from 5-FU-based therapy (TTR: HR = 0.16, 95% CI = 0.05 to 0.47, P < .001; DFS: HR = 0.19, 95% CI = 0.07 to 0.55, P < .001), whereas patients (n = 120) with sporadic tumors did not (TTR: HR = 1.07, 95% CI = 0.40 to 2.86, P = .897; DFS: HR = 1.40, 95% CI = 0.64 to 3.06, P = .399) when compared with patients receiving observation or no 5-FU. Because our criteria do not provide definitive evidence of either germline or sporadic origin of the colon cancers, we further restricted the germline group to tumors with loss of MSH2 proteins (n = 22) that is consistent with germline mutations (3) and limited the sporadic group of tumors to those tested for BRAF and found to carry BRAFV600E mutations (n = 73) indicating a sporadic origin (34). Specifically, BRAF mutations are limited to sporadic dMMR colon cancers and can be used to differentiate sporadic from germline tumors with dMMR (34). Using this categorization and stratified by study, we found similar results (data not shown) for the predictive impact of dMMR for 5-FU-based adjuvant therapy.
We also examined the effect of 5-FU-based therapy on the site of tumor recurrence in suspected germline vs sporadic colon cancers. We found that 5-FU-based treatment vs observation or no 5-FU treatment was associated with a statistically significant reduction in distant recurrence rates in suspected germline (9% vs 33%; P = .003) but not in sporadic (10% vs 11%; P = .771) colon cancers. Restricting the analysis to stage III germline patients showed an even larger reduction in the distant recurrence rate for 5-FU-based treatment vs observation or no 5-FU treatment (14% vs 64%; P < .001). When germline dMMR cancers were removed from the analysis shown in Figure 2, A, an effect of 5-FU-based adjuvant therapy in stage III colon cancers was no longer evident.
Discussion
We determined the associations of MMR status with recurrence rates, sites of tumor recurrence, and survival of colon cancer patients treated in adjuvant chemotherapy trials. Colon cancer patients with dMMR tumors showed statistically significant reduced rates of recurrence, delayed TTR, and improved stage-adjusted rates of DFS and OS, compared with pMMR cancers. Furthermore, MMR status was a statistically significant and independent prognostic variable for TTR, DFS, and OS. Whereas dMMR tumors were diagnosed at an earlier stage (stage II vs III) and were less likely to recur, the prognosis after recurrence was similar to pMMR tumors. We found that the site of recurrence did not differ substantially based upon MMR status in colon cancers from patients receiving observation (surgery alone) or no 5-FU therapy. However, in stage III patients with dMMR tumors, those that received 5-FU-based adjuvant therapy were shown to have a statistically significant reduction in recurrence rates at distant sites compared with patients receiving observation or no 5-FU treatment.
Our findings suggest a treatment benefit in some stage III dMMR tumors, which is supported by recent data from the Pan European Trial Adjuvant Colon Cancer (PETACC-3) where a statistically significant improvement in relapse-free survival was observed for MSI-H compared with MSI-L/MSS patients treated with 5-FU and leucovorin (22). Patients with pMMR tumors receiving 5-FU-based therapy had a statistically significant reduction in recurrence rates to all sites in stage III cancers. When the analysis was restricted to patients who showed tumor recurrence despite adjuvant 5-FU-based therapy, patients with dMMR tumors showed increased intra-abdominal recurrence and reduced distant recurrence compared with pMMR tumors. Because this same pattern was not seen in patients receiving observation or no 5-FU treatment, this finding can possibly be attributed to an interaction of MMR deficiency and 5-FU-based chemotherapy. We found that the site of tumor recurrence was prognostic and that altering the pattern of tumor recurrence can affect patient survival. We sought to further support our results by restricting the analysis to colon cancers with MMR protein expression data, given the lack of uniformity of MSI marker panels used to analyze tumors in previous adjuvant studies, with the potential for misclassification of MSI status. We found entirely consistent results for the relationship of loss of MLH1 or MSH2 protein expression with reduced recurrence rates and better survival, as well as for the effect of 5-FU-based treatment upon the site of tumor recurrence compared with cancers with intact MMR proteins. The overall rate of dMMR was similar across studies, and a test for homogeneity found similar effects across the adjuvant study populations. We also restricted the analysis to studies that used five or more microsatellite markers and a study that used BAT26 and MLH1, MSH2, and MSH6 protein expression and found entirely consistent results with the full study population.
A predictive analysis of MMR status and survival from 5-FU-based adjuvant therapy was previously reported using adjuvant studies with untreated control arms from our overall study population (19). Therefore, we shifted our focus to determine whether differences in prognosis or response to 5-FU-based chemotherapy could be detected in suspected sporadic vs germline dMMR cancers. It remains uncertain whether the results of studies that included mainly dMMR sporadic colon cancers can be extrapolated to germline patients, as very limited and conflicting data have been reported for the latter group. (20,39). We categorized patients as sporadic or germline using MSI and/or IHC data and age at adjuvant study randomization. All tumors with loss of MSH2 were regarded as germline cases (4). For the others, we chose an age cutoff of 55 years based on its inclusion in the modified Amsterdam criteria for the diagnosis of Lynch syndrome and population-based data for the screening of CRCs for Lynch syndrome (38). Using our criteria and a non–population based cohort, the frequency of suspected germline cancers was 4.6% (99 of 2141 cancers), which is similar to the 3%–5% incidence reported in population-based studies where mutation analysis was performed on all MSI-H tumors (40). After adjustment for patient age, prognosis was similar for suspected sporadic and germline dMMR colon cancers. In our exploratory analysis, we found that 5-FU-based treatment (vs observation or no 5-FU) was associated with a statistically significant reduction in distant recurrence and an improvement in survival in suspected germline but not in sporadic cancers. The predictive impact of 5-FU-based therapy was even stronger in stage III germline cancers, whereas no benefit was observed for stage III sporadic tumors. Given the recognized limitations of our patient categorization, we also compared tumors with MSH2 loss consistent with germline mutation (4) with tumors previously shown to carry BRAFV600E mutations consistent with a sporadic origin (34) and found very similar results. BRAF mutation is strongly associated with sporadic dMMR colon cancers showing hypermethylation of multiple genes, known as the CpG island methylator phenotype (CIMP) (41), and are not detected in colon cancers due to germline mutations in MMR genes (42). These data suggest that differences in 5-FU response may be indirectly related to the mechanism of MMR deficiency. Potentially, BRAF mutations and/or CIMP in sporadic tumors may confer reduced sensitivity to adjuvant 5-FU that may account for our findings. Although data suggest that BRAF mutations are associated with worse outcome (43), their predictive impact for 5-FU is unknown. Conflicting data have been reported for CIMP and 5-FU response (44,45), a recent analysis of CIMP status in a population-based cohort of CRC patients found that CIMP-positive vs. negative tumors do not benefit from 5-FU-based adjuvant therapy (46). Accordingly, we cannot exclude a non-MSI/MMR mechanism as being responsible for our findings. Our results need to be confirmed and if so, further study is needed to address the mechanism underlying the observed effect.
This study has limitations that include the pooling of data from multiple trials including comparative treatment trials, the incomplete tissue availability from each study, and the retrospective study design. However, the effect of 5-FU-based treatment on the site of tumor recurrence was consistent across the adjuvant study cohorts, as determined by a test for homogeneity. We also found similar 5-year DFS rates when we compared our study cohort to the full study population from the adjuvant trials.
This study has several strengths that include clinical trials with meticulous data collection and extended follow-up, and our findings of concordant results for both tumor recurrence and patient survival. Our report is also the largest study of the relationship of MMR status with clinical outcome and the first to specifically address its impact upon rate and site of tumor recurrence. Our results have clinical implications for the use of adjuvant therapy and support our earlier recommendation (36,47,48) that stage II dMMR colon cancer patients should not receive adjuvant 5-FU-based therapy and that stage III dMMR patients should continue to be treated with 5-FU-based adjuvant chemotherapy per the current standard of care. Studies to determine the responsiveness of dMMR colon cancers to the standard FOLFOX (fluorouracil, leucovorin, oxaliplatin) adjuvant regimen are awaited. The pattern of tumor recurrence and better survival of dMMR colon cancers suggest that recurrent disease in these patients may warrant an aggressive surgical approach where feasible.
In conclusion, our data demonstrate that patients with dMMR colon cancers have a statistically significant reduction in their rates of tumor recurrence, a delayed TTR, and better survival rates, compared with pMMR colon cancers. Although no statistically significant differences were found for the site of recurrence stratified by MMR status in patients receiving observation or no 5-FU, patients with dMMR stage III tumors treated with 5-FU-based adjuvant therapy showed fewer distant metastases compared with similarly treated pMMR tumors. An exploratory analysis of cancers categorized as suspected germline vs sporadic suggests that any treatment benefit may be restricted to germline tumors. Studies are warranted to further address this potentially novel finding.
Funding
National Cancer Institute (CA104683-02 and 1 K05 CA142885-01 to FAS); Mayo Clinic Cancer Center (CA15083).
Footnotes
The authors wish to thank Dr Harry Yoon for his critical review of the article and Jody Clikeman for her very capable secretarial assistance. The authors were solely responsible for the study design, execution, management, analysis, data interpretation, preparation of the article, and the decision to submit the article for publication.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 3.Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3208–3215. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21(6):1174–1179. doi: 10.1200/JCO.2003.04.060. [DOI] [PubMed] [Google Scholar]
- 5.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 6.Jass JR, Do KA, Simms LA, et al. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42(5):673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinicrope FA, Rego RL, Halling KC, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 2006;131(3):729–737. doi: 10.1053/j.gastro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Gafa R, Maestri I, Matteuzzi M, et al. Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer. 2000;89(10):2025–2037. [PubMed] [Google Scholar]
- 9.Halling KC, French AJ, McDonnell SK, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999;91(15):1295–1303. doi: 10.1093/jnci/91.15.1295. [DOI] [PubMed] [Google Scholar]
- 10.Lanza G, Gafa R, Santini A, et al. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J Clin Oncol. 2006;24(15):2359–2367. doi: 10.1200/JCO.2005.03.2433. [DOI] [PubMed] [Google Scholar]
- 11.Kerr DGR, Quirke P, Watson D, et al. A. quantitative multigene RT-PCR assay for prediction of recurrence in stage II colon cancer: selection of the genes in four large studies and results of the independent, prospectively designed QUASAR validation study. J Clin Oncol. 2009;27(15s, suppl) Abstract 4000. [Google Scholar]
- 12.Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10(9):917–923. [PubMed] [Google Scholar]
- 13.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 14.Lamberti C, Lundin S, Bogdanow M, et al. Microsatellite instability did not predict individual survival of unselected patients with colorectal cancer. Int J Colorectal Dis. 2007;22(2):145–152. doi: 10.1007/s00384-006-0131-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim GP, Colangelo LH, Wieand HS, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007;25(7):767–772. doi: 10.1200/JCO.2006.05.8172. [DOI] [PubMed] [Google Scholar]
- 16.Benatti P, Gafa R, Barana D, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005;11(23):8332–8340. doi: 10.1158/1078-0432.CCR-05-1030. [DOI] [PubMed] [Google Scholar]
- 17.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jover R, Zapater P, Castells A, et al. The efficacy of adjuvant chemotherapy with 5-fluorouracil in colorectal cancer depends on the mismatch repair status. Eur J Cancer. 2009;45(3):365–373. doi: 10.1016/j.ejca.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Sargent DJ, Marsoni S, Thibodeau SN, et al. Confirmation of deficient mismatch repair (dMMR) as a predictive marker for lack of benefit from 5-FU based chemotherapy in stage II and III colon cancer (CC): a pooled molecular reanalysis of randomized chemotherapy trials. J Clin Oncol. 2008;26(20 suppl) Abstract 4008. [Google Scholar]
- 20.Hemminki A, Mecklin JP, Jarvinen H, et al. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119(4):921–928. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 21.Elsaleh H, Joseph D, Grieu F, et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355(9217):1745–1750. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 22.Tejpar SBF, Delorenzi M, Fiocca R, et al. Microsatellite instability (MSI) in stage II and III colon cancer treated with 5FU-LV or 5FU-LV and irinotecan (PETACC 3-EORTC 40993-SAKK 60/00 trial) J Clin Oncol. 2009;27(15s, suppl) Abstract 4001. [Google Scholar]
- 23.Laurie JA, Moertel CG, Fleming TR, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol. 1989;7(10):1447–1456. doi: 10.1200/JCO.1989.7.10.1447. [DOI] [PubMed] [Google Scholar]
- 24.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345(8955):939–944. [PubMed] [Google Scholar]
- 25.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322(6):352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 26.O’Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15(1):246–250. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 27.Beart RW, Jr., Moertel CG, Wieand HS, et al. Adjuvant therapy for resectable colorectal carcinoma with fluorouracil administered by portal vein infusion. A study of the Mayo Clinic and the North Central Cancer Treatment Group. Arch Surg. 1990;125(7):897–901. doi: 10.1001/archsurg.1990.01410190095015. [DOI] [PubMed] [Google Scholar]
- 28.Wiesenfeld M, O’Connell MJ, Wieand HS, et al. Controlled clinical trial of interferon-gamma as postoperative surgical adjuvant therapy for colon cancer. J Clin Oncol. 1995;13(9):2324–2329. doi: 10.1200/JCO.1995.13.9.2324. [DOI] [PubMed] [Google Scholar]
- 29.O’Connell MJ, Sargent DJ, Windschitl HE, et al. Randomized clinical trial of high-dose levamisole combined with 5-fluorouracil and leucovorin as surgical adjuvant therapy for high-risk colon cancer. Clin Colorectal Cancer. 2006;6(2):133–139. doi: 10.3816/ccc.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344(16):1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257. [PubMed] [Google Scholar]
- 32.Rosty C, Chazal M, Etienne MC, et al. Determination of microsatellite instability, p53 and K-RAS mutations in hepatic metastases from patients with colorectal cancer: relationship with response to 5-fluorouracil and survival. Int J Cancer. 2001;95(3):162–167. doi: 10.1002/1097-0215(20010520)95:3<162::aid-ijc1028>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 33.Thibodeau SN, French AJ, Roche PC, et al. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996;56(21):4836–4840. [PubMed] [Google Scholar]
- 34.French AJ, Sargent DJ, Burgart LJ, et al. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res. 2008;14(11):3408–3415. doi: 10.1158/1078-0432.CCR-07-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grambsch P, Therneau TM. Proportional. hazards test and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 36.Sargent DJ, Marsoni S, Monges G, et al. Defective. mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for herediatary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins MA, Dowty JG, Hopper JL, et al. Molecular screening of all colorectal tumors diagnosed before age 50 years followed by genetic testing efficiently identifies Lynch syndrome cases. Int J Cancer. 2009;124(5):x–i. doi: 10.1002/ijc.24173. [DOI] [PubMed] [Google Scholar]
- 39.de Vos tot Nederveen Cappel WH, Meulenbeld HJ, Kleibeuker JH, et al. Survival after adjuvant 5-FU treatment for stage III colon cancer in hereditary nonpolyposis colorectal cancer. Int J Cancer. 2004;109(3):468–471. doi: 10.1002/ijc.11712. [DOI] [PubMed] [Google Scholar]
- 40.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26(35):5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barault L, Charon-Barra C, Jooste V, et al. Hypermethylator phenotype in sporadic colon cancer: study on a population-based series of 582 cases. Cancer Res. 2008;68(20):8541–8546. doi: 10.1158/0008-5472.CAN-08-1171. [DOI] [PubMed] [Google Scholar]
- 42.Domingo E, Laiho P, Ollikainen M, et al. BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet. 2004;41(9):664–668. doi: 10.1136/jmg.2004.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58(1):90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen L, Catalano PJ, Benson AB, III, et al. Association between DNA methylation and shortened survival in patients with advanced colorectal cancer treated with 5-fluorouracil based chemotherapy. Clin Cancer Res. 2007;13(20):6093–6098. doi: 10.1158/1078-0432.CCR-07-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Rijnsoever M, Elsaleh H, Joseph D, et al. CpG island methylator phenotype is an independent predictor of survival benefit from 5-fluorouracil in stage III colorectal cancer. Clin Cancer Res. 2003;9(8):2898–2903. [PubMed] [Google Scholar]
- 46.Jover R, Nguyen T-P, Perez-Carbonell L. 5-Fluorouracil Adjuvant Chemotherapy Does Not Increase Survival in Patients with CpG Island Methylator Phenotype Colorectal Cancer. Gastroenterology. 2011;140:1174–1181. doi: 10.1053/j.gastro.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinicrope FA, Sargent DJ. Clinical implications of microsatellite instability in sporadic colon cancers. Curr Opin Oncol. 2009;21(4):369–373. doi: 10.1097/CCO.0b013e32832c94bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinicrope FA. DNA mismatch repair and adjuvant chemotherapy in sporadic colon cancer. Nat Rev Clin Oncol. 2010;7(3):174–177. doi: 10.1038/nrclinonc.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]