Abstract
Background
Antidepressant drugs (ADs) have been shown to activate BDNF (brain-derived neurotrophic factor) receptor TrkB in the rodent brain but the mechanism underlying this phenomenon remains unclear. ADs act as monoamine reuptake inhibitors and after prolonged treatments regulate brain bdnf mRNA levels indicating that monoamine-BDNF signaling regulate AD-induced TrkB activation in vivo. However, recent findings demonstrate that Trk receptors can be transactivated independently of their neurotrophin ligands.
Methodology
In this study we examined the role of BDNF, TrkB kinase activity and monoamine reuptake in the AD-induced TrkB activation in vivo and in vitro by employing several transgenic mouse models, cultured neurons and TrkB-expressing cell lines.
Principal Findings
Using a chemical-genetic TrkBF616A mutant and TrkB overexpressing mice, we demonstrate that ADs specifically activate both the maturely and immaturely glycosylated forms of TrkB receptors in the brain in a TrkB kinase dependent manner. However, the tricyclic AD imipramine readily induced the phosphorylation of TrkB receptors in conditional bdnf −/− knock-out mice (132.4±8.5% of control; P = 0.01), indicating that BDNF is not required for the TrkB activation. Moreover, using serotonin transporter (SERT) deficient mice and chemical lesions of monoaminergic neurons we show that neither a functional SERT nor monoamines are required for the TrkB phosphorylation response induced by the serotonin selective reuptake inhibitors fluoxetine or citalopram, or norepinephrine selective reuptake inhibitor reboxetine. However, neither ADs nor monoamine transmitters activated TrkB in cultured neurons or cell lines expressing TrkB receptors, arguing that ADs do not directly bind to TrkB.
Conclusions
The present findings suggest that ADs transactivate brain TrkB receptors independently of BDNF and monoamine reuptake blockade and emphasize the need of an intact tissue context for the ability of ADs to induce TrkB activity in brain.
Introduction
TrkB (tropomyosin-related kinase B) neurotrophin receptor transduces intracellular signaling events that are critical for neuronal differentiation, survival and plasticity throughout life [1]–[5]. Brain-derived neurotrophic factor (BDNF) is the main endogenous ligand for TrkB [3], but recent evidence demonstrates that TrkB can also be transactivated independently of BDNF or other neurotrophins through neuromodulator receptors [6], [7] and small molecules [8], [9].
Abnormal TrkB receptor signaling has been linked to a number of central nervous system (CNS) diseases such as mood and memory disorders and addiction [10]–[12]. Accumulating evidence suggests that antidepressant drugs (AD) that regulate the brain levels of monoamine neurotransmitters serotonin and norepinephrine, act at least partially by activating TrkB receptor signaling in brain [13], [14]. ADs have been shown to rapidly induce the phosphorylation and activation of TrkB receptors in the rodent cortex and hippocampus [13], [15]. When administered chronically, ADs also increase BDNF mRNA and protein levels and TrkB phosphorylation in brain [13], [15], [16]. Furthermore, animal studies suggest that many of the behavioral and functional actions of ADs are attenuated in mice with reduced BDNF signaling in brain [13], [17]. Levels of BDNF are reduced in the brain and serum of depressed patients and the levels are returned back to normal range upon a successful treatment with ADs [18], [19].
In this study we have examined several potential molecular mechanisms of AD-induced TrkB activation in vitro and in vivo. We show that both mature and immature forms of TrkB can be specifically tyrosine phosphorylated by ADs, but neither the endogenous ligand BDNF, nor the serotonin transporter (SERT), the principal target of many ADs, is required for this effect. However, the observation that ADs or serotonin (5-HT) or norepinephrine (NE) do not activate TrkB phosphorylation in vitro argues that ADs do not directly bind to TrkB receptors.
Results
Antidepressant drugs specifically activate TrkB receptors in mouse brain
Previous studies suggest that BDNF-TrkB signaling is critical for the behavioral effects of ADs [13], [17] and that ADs activate Trk receptors in vivo. We have previously shown that several independent antibodies raised against phosphorylated tyrosines Y705/706 or Y816 within the intracellular domain of TrkB all show increased phospho-TrkB levels after acute and chronic AD treatment [13], [15], while no increase is detected with antibodies against the shc binding site at pY515. Furthermore, immunoprecipitation with Trk specific antibodies and probing with pTyr-antibodies also reveals increased TrkB phosphorylation [13], [15], [20]. However, since phospho-Trk antibodies are not completely specific for TrkB, we investigated whether the protein phosphorylated by the acute AD treatment is indeed TrkB. We pretreated TrkBF616A knock-in mice [21] with NaPP1, a chemical that specifically inhibits TrkB kinase activity in these mutant mice, and then injected the mice acutely with imipramine. Whereas imipramine readily induced rapid activation of brain TrkB in vehicle-treated TrkBF616A knock-in mice, NaPP1 treatment abolished this effect ( Figure 1A–B ). Furthermore, when we treated transgenic mice over-expressing flag-tagged TrkB receptors in adult neurons (TrkB.TK+) [22], [23] with imipramine, we observed enhanced phosphorylation of the TrkB specific band when compared to wild-type mice (data not shown). Importantly, when TrkB receptors were immunoprecipitated with a Flag antibody from TrkB.TK+ mouse brain homogenates, phospho-TrkB signal was more intense in samples of imipramine treated animals ( Figure 1C ). Collectively, these data demonstrate that imipramine specifically induce phosphorylation of TrkB receptors in mouse brain.
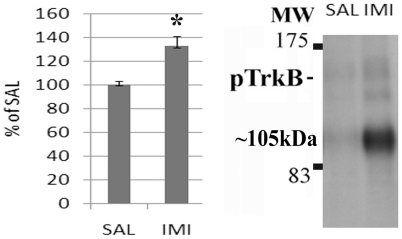
Figure 1. Imipramine specifically activates TrkB receptors in the mouse brain.
A) The ability of imipramine (30 mg/kg, i.p., 30 min; n = 6/group) to induce rapid TrkB phosphorylation in the hippocampus and medial prefrontal cortex of TrkBF616A mutant mice is abolished with 1NaPP1 pretreatment (25 µM for 1-week in drinking water+83 ng/g co-injection with imipramine). B) A representative blot showing TrkB kinase dependent action of imipramine-induced phosphorylation of TrkB (Y816) and the ∼105 kDa protein. C) A representative blot showing imipramine-induced TrkB phosphorylation in flag-precipitated pool of protein from the brains of mice over-expressing flag-tagged catalytic TrkB receptors. Data is presented as percentage of control/saline ± standard error of mean (SEM). *<0.05, **<0.01; two-way ANOVA with Newmann-Keuls post hoc test.
The immaturely glycosylated form of TrkB is phosphorylated by antidepressants
As we have previously shown [13], an additional low-molecular weight (LMW) phospho-Trk –immunoreactive protein (about 105 kDa) is robustly phosphorylated in the rodent brain after single or repeated AD treatment ( Figure 2A ). This phosphorylated protein is detected by the same antibodies that demonstrate the phosphorylation of TrkB after AD treatment (Figure S1A–B) and has been detected following TrkB immunoprecipitation and hybridization to pTyr antibodies [13], [20]. AD-induced phosphorylation of both the full-length TrkB and the 105 kDa protein is also readily detected in different brain regions including striatum, midbrain and cerebellum (data not shown), but, similar to full-length TrkB, its phosphorylation is diluted in whole brain homogenate (Figure S1C). However, this band cannot be reliably detected by antibodies against the non-phosphorylated intracellular domain of Trk receptors ( Figure 2A ).
Figure 2. Antidepressant drugs activate the immaturely glycosylated form of TrkB.
A) Acute imipramine treatment induces the phosphorylation (Y816) of full-length and low-molecular weight (LMW; ∼105 kDa) TrkB receptors in mouse brain. n = 6/group. B) Antidepressant-induced ∼105 kDa protein is sensitive to Endo-H digestion. A representative blot of triplicate data. C) Total TrkB, phosphorylated TrkB (Y816) and phosphorylated ∼105 kDa protein levels are increased in the brains of mice over-expressing catalytic TrkB receptors. n = 5/group. Data is presented as percentage of control ± standard error of mean (SEM). *<0.05, ***0.005; unpaired two-tailed t-test.
This lower molecular weight protein might represent an immaturely glycosylated form of catalytic TrkB [24], as TrkB transactivation has been shown to coincide with accumulation of intracellular immaturely glycosylated TrkB species [6], [7], [25]. We therefore further examined the glycosylation structure of this protein using endoglycosidase-H (Endo-H) that cleaves immature high-mannose rich N-glycans out of proteins. Endo-H digestion produced a slight reduction in the molecule weight of the mature full-length TrkB, suggesting that the mature TrkB still contains immature-type glycan residues ( Figure 2B ), as also observed before for TrkA [26]. Importantly, Endo-H treatment strongly reduced the molecular weight of 105 kDa protein ( Figure 2B ), suggesting that essentially all the glycan residues in this protein represent immature high-mannose rich N-glycans. These data are consistent with the interpretation that the 105 kDa protein represents an immaturely glycosylated and intracellularly located species of TrkB. This interpretation is further supported by the observations that the basal phosphorylation levels of this phosphoprotein are increased in the brains of TrkB over-expressing mice ( Figure 2C ) and that the activation of this band is lost after 1NaPP1 treatment in the TrkBF616A mice ( Figure 1B ).
Antidepressant-induced TrkB activation does not require BDNF
Previous studies have shown that acute AD treatment does not influence BDNF mRNA or protein levels [13], [16]. Since it has recently been suggested that pro and mature forms of BDNF might have different capacities to activate TrkB [27], we investigated the effects of acute antidepressant treatment on proBDNF cleavage in brain. However, we were not able to detect any proBDNF signal in wild-type mouse brain, even if the antibody readily detected the recombinant proBDNF control protein (Figure S2A). Nevertheless, acute fluoxetine treatment, with a dose and time point (30 mg/kg; 1 hour) which induced TrkB phosphorylation in mouse hippocampus [13], [15], failed to produce any significant changes in the levels of the mature BDNF (mBDNF) in mouse brain as detected with western blotting (Figure S2A). Furthermore, fluoxetine did not influence the activity of tissue plasminogen activator (tPA), the major regulator of pro-BDNF cleavage into mBDNF (Figure S2B). These data suggest that ADs do acutely not influence BDNF levels or processing in brain.
Although BDNF is the main ligand of TrkB, recent evidence suggests that TrkB can also be activated independently of BDNF in neurons [6], [8], [9]. We therefore used conditional BDNF mutant mice (BDNF2L/2LCk-cre) lacking BDNF in forebrain regions [28] to investigate whether BDNF is required for the AD-induced TrkB activation in brain in vivo. Imipramine readily induced tyrosine phosphorylation of both the mature and the immature glycosylated forms of TrkB in the hippocampus of conditional BDNF2L/2LCk-cre mice ( Figure 3 ). Similarly, imipramine produced an increase in brain TrkB phosphorylation in heterozygous bdnf +/− null mice and wild-type mice (data not shown). These data demonstrate that ADs activate TrkB receptors in the mouse brain in a manner independent of BDNF.
Figure 3. Role of BDNF in antidepressant-induced rapid TrkB activation in brain.
Imipramine (30 mg/kg, 30 min, i.p.) readily increases the phosphorylation of TrkB receptors (Y816) in forebrain specific BDNF−/− knock-out mice (BDNF2L/2LCk-cre) n = 4/group. Data is presented as percentage of control ± standard error of mean (SEM). *<0.05; unpaired two-tailed t-test.
Adenosine has been shown to transactivate TrkB receptors via adenosine-2A signaling in the absence of BDNF in vitro and in vivo [6], [29] and to enhance TrkB signaling [30]. Furthermore, some ADs have been shown to acutely increase the extracellular levels of adenosine by reducing adenosine reuptake [31]. We therefore tested whether prior pharmacological inhibition of adenosine A2A receptors with ZM241385 might block the acute effects of ADs on TrkB phosphorylation. We found that, imipramine increased the phosphorylation of TrkB receptors similarly in mice pretreated with saline or active dose [32], [33] of ZM241385 (Figure S3), suggesting that A2A receptors were not involved.
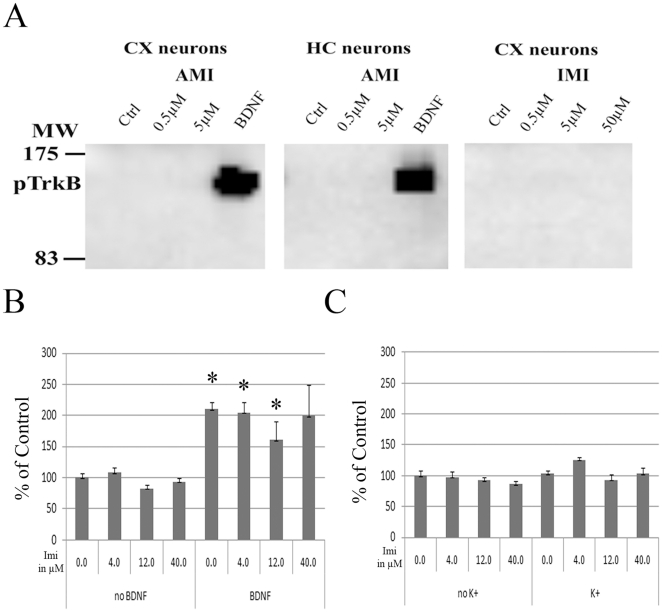
Amitriptyline, but not imipramine, was recently shown to directly bind and transactivate TrkB receptors in vitro [9]. We therefore tested whether amitriptyline, imipramine or other selected drugs could directly phosphorylate TrkB receptors in two different cell models: fibroblast expressing catalytic TrkB receptors and E18 rat primary hippocampal and cortical neuronal cultures. In both of these cells, BDNF produces a robust phosphorylation of TrkB. However, exposure to tested ADs, including amitriptyline, or other tested drugs did not regulate TrkB phosphorylation status in these cultures ( Figures 4A , S4). We further tested whether ADs might potentiate the pTrkB response induced by a small dose of BDNF or whether depolarization of neurons might render them sensitive to ADs in vitro. Imipramine did not facilitate BDNF-induced TrkB phosphorylation in vitro ( Figure 4B ), which is in line with the findings in BDNF deficient mice. Similarly, even when ADs were coupled with depolarization stimuli (50 mM K+), no significant changes in TrkB phosphorylation were seen ( Figure 4C ).
Figure 4. Antidepressant drugs amitriptyline and imipramine do not regulate TrkB phosphorylation in primary neurons.
A) Whereas BDNF (20 ng/ml; 15 min) robustly increases the phosphorylation of TrkB (Y816) in E18 rat cortical and hippocampal neurons (14 DIV), amitriptyline (left & middle; 0.5 µM, 5 µM; 15 min) and imipramine (0.5 µM, 5 µM; 50 µM; 15 min) produces no change on TrkB phosphorylation. Representative blot of triplicate data. B) Imipramine pre-treatment (4, 12, 40 µM; 15 min) did not facilitate BDNF-induced (5 ng/ml; 15 min) TrkB phosphorylation in E18 rat cortical neurons as measured with phospho-TrkB ELISA. n = 4/group. C) Imipramine pre-treatment (4, 12, 40 µM; 15 min) did not regulate TrkB phosphorylation in its own or in combination with depolarization stimuli (50 mM KCl; 15 min) as measured with phospho-TrkB ELISA. n = 4/group. Data is presented as percentage of control ± standard error of mean (SEM). *<0.05; one-way ANOVA with Newmann-Keuls post hoc test.
TrkB activation by antidepressant drugs is not mediated by the serotonin transporter or monoamine transmitters
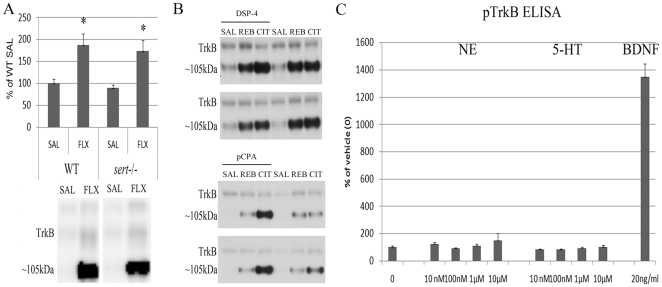
Essentially all clinically used antidepressant drugs acutely increase the extracellular levels of NE and/or 5-HT in brain and we therefore investigated the role of these monoamines in the AD-induced TrkB transactivation in vivo and in vitro. First we examined the effect of fluoxetine, a prototypic SERT selective reuptake inhibitor (SSRI), on TrkB receptor phosphorylation in SERT knockout mice (sert −/−). A 6–10 fold up-regulation of extracellular 5-HT levels in sert −/− mice [34] did not regulate basal TrkB phosphorylation levels in hippocampus when compared to the wild-type controls (89.43%±6.43% of wild-type, P = 0.19, Student t-test). Importantly, fluoxetine readily induced the phosphorylation of both the mature and immature forms of TrkB in the brains of sert −/− mice in a manner indistinguishable of the wild-type mice ( Figure 5A ), indicating that SERT is dispensable to the fluoxetine-induced TrkB autophosphorylation.
Figure 5. Monoamines and monoamine reuptake in TrkB activation in vitro and in vivo.
A) Serotonin selective reuptake inhibitor fluoxetine produced essentially similar changes on hippocampal TrkB phosphorylation in the brains of wild-type and serotonin transporter KO mice, sert −/−. n = 3–5/group. B) Whereas the ability of serotonergic antidepressant citalopram (20 mg/kg, i.p., 60 min) and norepinephrinergic antidepressant reboxetine (20 mg/kg, i.p., 30 min) to induce full-length TrkB receptor phosphorylation (Y816) in mice depleted of serotonin (with pCPA) or norepinephrine (with DSP-4) are reduced, ∼105 kDa protein is heavily phosphorylated by both drugs in the hippocampi of these mice. Representative blots. n = 6–7/group. C) Norepinephrine (NE; 10 nM-10 µM; 15 min) and serotonin (5-HT; 10 nM-10 µM; 15 min) produced no changes on TrkB phosphorylation in BDNF-responsive E18 rat cortical neurons (15DIV) as measured with phospho-TrkB ELISA. n = 4/group. Data is presented as percentage of control ± standard error of mean (SEM). *<0.05; two-way ANOVA with Newmann-Keuls post hoc test.
Because the selectivity of the ADs against different transporters is only relative, we performed chemical lesion experiments to reduce brain 5-HT (by pCPA treatment) and NE levels (by DSP4) and used selective 5-HT and NE transporter blockers citalopram and reboxetine, respectively. As noted before [15], citalopram and reboxetine produced a non-significant trend of increase in TrkB autophosphorylation levels in pCPA and DSP4 treated mice, respectively ( Figure 5B ). However, citalopram and reboxetine induced a strong and highly significant increase in the phosphorylation of the immaturely glycosylated form of TrkB in pCPA and DSP4 treated mice, respectively ( Figure 5B ). These observations suggest that even when brain 5-HT and NE levels are very low, ADs can activate at least the immaturely glycosylated form of TrkB.
Finally, we tested whether NE or 5-HT would directly regulate TrkB phosphorylation in cultured primary neurons. Under conditions where BDNF robustly induced TrkB phosphorylation, incubation with different concentrations of NE or 5-HT did not regulate TrkB phosphorylation levels in primary neuronal cultures ( Figure 5C ). Collectively, these data suggest that TrkB receptor is activated in mouse brain by ADs independent of monoamine reuptake inhibition.
Discussion
Emerging evidence suggests a key role of the BDNF-TrkB signaling in the regulation of many of the molecular and behavioral actions of ADs. ADs acutely and chronically increase TrkB signaling [13], [15]. Moreover, chronic, but not acute, AD treatment increases BDNF synthesis in the rodent brain [16], [35]. BDNF injection and TrkB activation produce AD-like responses in rodents [36]–[38], while mice deficient of BDNF or with inhibited TrkB signaling do not respond to ADs in the forced swim test [13], [17], the classical paradigm for AD effectiveness. These data suggest that, at least in rodents, activation of TrkB receptors induced by BDNF is essential for the antidepressant effect. However, we show here that rapid activation of TrkB in response to AD administration in vivo does not require BDNF release. This finding does not rule out the role of BDNF in regulating TrkB activation following chronic AD treatment. Since acute AD treatment increases phosphorylation of CREB, a critical upstream regulator of BDNF synthesis in a TrkB dependent manner [13], it is tempting to speculate that this ligand-independent TrkB activation is contributing the AD-induced BDNF synthesis in brain [39] which further leads to BDNF-dependent TrkB phosphorylation after prolonged AD administration.
Fluoxetine and SSRIs act primarily by blocking 5-HT reuptake in brain and BDNF, through TrkB, is a crucial regulator of serotonergic innervation [40], [41]. However, neither the SERT nor the monoamines 5-HT or NE appear to be required for the activation or TrkB by the ADs. We have previously shown that representatives of all the different chemical classes of ADs similarly increase TrkB phosphorylation in mouse brain, suggesting that the monoamine independent TrkB activation may be a common feature for all the ADs. Accumulating evidence has shown that ADs, including fluoxetine and tricyclic ADs, have several additional targets in cells such as neurotransmitter receptors [42], [43], ion channels [44], Sigma-1 receptors [45] and adenosine reuptake proteins [31] that could potentially be involved in regulating TrkB signaling. Since adenosine-A2A receptor signaling has been linked to TrkB signaling [30], we tested the role of this receptor in AD-induced TrkB response by pharmacologically blocking this receptor before imipramine challenge. However, no change was observed compared to control treatment indicating that adenosine is not a critical regulator of TrkB activation in response to AD treatments.
While all the different ADs readily induce TrkB autophosphorylation in rodent brain [13], [15], neither these compounds nor monoamines do, in our hands, induce TrkB phosphorylation in vitro in cultured cortical or hippocampal neurons, or in cell lines stably expressing TrkB receptors. A recent study reported that amitriptyline, but not imipramine, binds to TrkB receptors, induces their dimerization and autophosphorylation in cultured hippocampal neurons [9]. The reasons that underlie the discrepancy between that study and our results with amitriptyline are currently unclear, however, they may be related to the culture conditions used. The lack of activation of TrkB by ADs in cultured neurons is in line with our recent unpublished observations showing that the ability of ADs to activate TrkB in vivo is developmentally regulated: ADs do not activate TrkB in embryonic or early postnatal mice, but the ability of these compounds to induce pTrkB response appears only around postnatal day 15 (P15) (ADL, TR and EC, submitted). Taken together, these data suggests us that ADs do not directly bind to TrkB, but, instead, emphasize the importance of developmental processes and intact tissue context in the ability of small molecule weight drugs such as ADs to activate TrkB autophosphorylation in vivo.
Materials and Methods
Animal experiments - Wild-type (C57/BL6), TrkB.TK+ mutant [22], [23], trkB F616A knock-in [21], BDNF2L/2LCk-cre [28] and sert −/− knock-out mice [46], [47] were used in animal experiments. Mice were group-housed in standard laboratory conditions and food and water were freely available. All the experiments were carried out according to the guidelines of the Society for Neuroscience and were specifically approved by the University of Helsinki Committee on Animal Experiments (permit: HY 137-05) or the County Administrative Board of Southern Finland (Permit: ESLH-2007-09085/Ym-23). Unless otherwise stated all the tested chemicals used in these studies are purchased from Sigma-Aldrich.
In vivo drug treatments - Mice received a single i.p. injection of tested chemical or saline and after indicated time the mice were killed with CO2 and brain area of interest dissected on a cooled plastic dish. Next the samples were lyzed in NP++ buffer (137 mM NaCl, 20 mM Tris, 1% NP-40, 10% glycerol, 48 mM NaF, H2O, 2× Complete inhibitor mix (Roche) and 2 mM Na3VO4), incubated on ice (>30 min) and centrifugated (+4°C for 15 min, 16100 g). The supernatant was processed further as described below. The following chemicals were used: fluoxetine-HCl (Orion Pharma), citalopram-HBr (GlaxoSmithKline; GSK), imipramine-HCl, moclobemide (kind gift from F Hoffmann-La Roche Ltd), clomipramine-HCl, amitriptyline-HCl, reboxetine (GSK). Reboxetine and moclobemide were first stock-dissolved in DMSO, citalopram in ethanol, others directly in saline. In order to inhibit TrkB kinase activity prior drug administration in TrkBF616A knock-in mice, mice were pre-treated with 25 µM of 1NaPP1 (kindly provided by Prof. Jari Yli-Kauhaluoma, Univ. Helsinki, Finland) for 7 days (in drinking solution) and further co-injected i.p. (83 ng/g) with imipramine or vehicle. ZM241358 was injected i.p. to block adenosine A2A receptors 30 min prior imipramine treatment [33]. Brain NE and 5-HT levels were depleted using DSP-4 (brain NE levels <20% of control; P<0.01, t-test) and pCPA (brain 5-HT levels <15% of control; P<0.005, t-test) injections as described previously [15].
Tissue plasminogen activator (tPA) SDS-PAGE zymography - For tPA activity assay, freshly dissected brain samples were homogenized into buffer consisting of 0.1 M Tris-HCl (pH 8.0), 2.5% Triton-X-100, 10 µM leupeptin, 10 µg/ml aprotinin, 1 mM phenylmethanesulfonylfluoride (PMSF). Samples and controls (human recombinant tPA) were loaded under non-reducing conditions in SDS-PAGE containing±human plasminogen (Sigma-Aldrich) and pre-heated non-fat dry milk at low current (∼15–20 mA) over night (O/N) at cold bath. Next the gels were rinsed thoroughly with 2.5% Triton X-100 to remove SDS and allow proteins to renaturate. Next the gels were rinsed thoroughly with 10 mM CaCl2 50 mM Tris-HCl (pH 7.6) to remove Triton X-100 and the caseinolysis was allowed to occur by incubating the gels at +37°C for 16–24 h in the same solution. Caseinolytic areas were shown as translucent areas when the gels were stained with Coomassie Brilliant Blue.
In vitro experiments - For the primary neuronal cultures, hippocampi or cortex was dissected from E18 rat embryos and the tissue dissociated in a papain solution (in mg: 10 DL-Cystein-HCl, 10 bovine serum albumin (BSA), 250 glucose, ad 50 ml PBS; 10 min, 37°C). Next the cells were triturated and suspended in a medium containing 9.8 ml of Ca2+/Mg2+ free HBBS, 1 mM sodium pyruvate, 10 mM HEPES and 10 µl DNAse I. The cells were plated onto poly-L-lysine coated 12–24 well culture plates at a cell density of 0.5×106 ml−1 (hippocampal) or 1×106 ml−1 (cortical). Cells were maintained in neurobasal medium (+2% B27 supplement, 1% penicillin/streptomycin, 1% glutamine and 25 µM glutamic acid; 5% CO2, +37°C) for 14 to 15 days in vitro (DIV) before treatments. Parental MG87 and MG87-trkB fibroblasts [48] were cultured in 12–48 well culture plates in Dulbecco's Modified Eagle's Medium (DMEM) (+10% fetal calf serum, 1% PEST, 1% L-Glutamine, 400 µg/ml G418; 5% CO2, +37°C) and were stimulated under confluent conditions. The following chemicals were used for the experiments: BDNF (Peprotech), imipramine, amitriptyline, desipramine, chlorpromazine, phenelzine, clozapine, lithium (chloride salt). After the treatments, the medium was discarded and the cells lyzed in NP++ buffer and processed for western blot analysis or for phospho-Trk ELISA described below.
Phospho-Trk ELISA - An enzyme-linked immunosorbent assay (ELISA) method was developed to easily measure the level of phosphorylated Trk receptors from cultivated cells (Figure S4A). Whereas in Trk expressing cells the assay readily detects BDNF- or NGF-induced Trk phosphorylation, such induction is not detected in cells not expressing Trk receptors (data not shown). Moreover, BDNF-induced TrkB phosphorylation is lost if the cells are pretreated with Trk kinase inhibitor k252a (data not shown). Stimulation experiments were initiated by adding the drugs at different concentrations onto confluent cell line cultures or with primary neurons. After indicated incubation period at +37°C, the medium was removed and the cells were lyzed with cold NP++ buffer (50–100 µl). In a set of experiments, these steps were carried out by using Biomek FX workstation (Beckman Coulter) for the liquid handling and incubation. Following >30 min incubation on ice all material were transferred to pre-coated (sc-11-R, 1∶500, Santa Cruz Biotechnology; O/N at +4°C) and pre-blocked (2% BSA/PBS-T; 2 h at RT) white 96-well Optiplate™ (PerkinElmer) plates and 2–3% BSA/PBS-T (+2 mM Na3VO4) added ad 200 µl. The plates were incubated O/N at +4°C and thereafter the wells washed with PBS-T (4×300 µl) and anti-phosphotyrosine antibody added to the wells (4G10, Upstate; 1∶1000 in 5% NFDM/PBS-T or in house biotinylated PY20, AbD Serotec, 1∶1000 in 2% BSA/PBS-T; both O/N at +4°C). Following sequential washes and HRP-coupled tertiary antibody incubations (sheep anti-mouse-HRP, 1∶5000 in 5% NFDM/PBS-T or Streptavidin-HRP, 1∶10000 in 2% BSA/PBS-T; O/N at +4°C) 200 µl of ECL substrate (Pierce) was added to the wells and luminescence measured after 5 min with Varioskan Flash (Thermo Fisher Scientific) plate reader.
Western blotting and sugar digestions – Lectin precipitation was carried essentially as previously described [15] using triticum vulgaris (Amersham or EY Laboratories). A set of lectin precipitated samples were incubated with endoglycosidase-A (Endo-H) according to manufacturer's instructions (New England Biolabs). Proteins were separated in a SDS-PAGE under reducing conditions and blotted onto polyvinylidene difluoride (PVDF; Amersham) membrane. After blocking (3% BSA/TBST, 1 h, RT), the membranes were incubated with primary antibodies: anti-pY705/6 (own 1∶500–1000, [49]; Cell Signaling 1∶1000), anti-pY816 (1∶5000, a kind gift from Dr. Moses Chao, Skirball Institute, NY, USA), sc-11-R (1∶2000, Santa Cruz Biotechnology), anti-TrkB (1∶2000, BD Biosciences) or anti-BDNF (N-20/sc-546, Santa Cruz Biotechnology). Moreover, the membranes were washed with TBST and incubated with HRP-conjugated secondary antibody (1∶10000 in NFDM/TBST, 1 h, RT, Biorad). After subsequent washes, the secondary antibodies were visualized using ECL kits (Amersham Biosciences) followed by exposure to an X-ray film or Fuji LAS-3000 camera (Tamro Medlabs, Finland) for ECL detection.
Data quantitation and statistical analysis - Immunoblots were quantitated using NIH ImageJ 1.32. Statistical analyses were done using two-sample two-tailed Student t-test or when appropriate with one or two-way ANOVA followed with Newmann-Keuls post hoc test. Statistically significant P-value was set to 0.05. Data are presented as mean ± standard error of mean and as percentage of respective control.
Supporting Information
Diverse antidepressant drugs induce ∼105 kDa protein phosphorylation in the mouse brain. A) Representative blot showing the time-response (30 min, 60 min, 120 min) of fluoxetine-induced (20/30 mg/kg, i.p.) phosphorylation of TrkB and ∼105 kDa protein (Y816 in left; Y705/6 in right) in mouse hippocampus. B) Representative blots showing antidepressant-induced phosphorylation of ∼105 kDa in mouse hippocampus C) Representative blots showing imipramine-induced phosphorylation of ∼105 kDa in mouse striatum, midbrain and whole brain homogenate. Abbreviations: FLX = fluoxetine, SAL = saline; CIT = citalopram; AMI = amitriptyline; CLO = clomipramine; MOC = moclobemide; REB = reboxetine; MW = molecular weight; TrkB.extr = antibody directed against the extracellular portion of TrkB receptors.
(TIF)
Acute fluoxetine treatment did not regulate BDNF protein levels or tPA (tissue plasminogen activator) activity. A) Representative blot showing mature-BDNF specific band in western blot from brain homogenates and pro- and mature-BDNF specific bands from respective control lanes as detected with polyclonal BDNF antibody (N-20/sc-546; Santa Cruz). Acute fluoxetine did not regulate mature-BDNF levels in mouse hippocampus. n = 6/group. B) Representative zymography showing caseinolysis at the level of recombinant tPA. Acute fluoxetine did not regulate tPA activity levels in mouse hippocampus. n = 6/group. Data is presented as percentage of control ± standard error of mean (SEM).
(TIF)
Blockade of adenosine-A2A receptor signaling does not prevent antidepressant-induced TrkB activation. Acute imipramine treatment (30 mg/kg, i.p., 30 min; n = 6/group) induces essentially similar changes on TrkB phosphorylation in vehicle and adenosine-A2A receptor antagonist (ZM241358; 1 mg/kg, i.p., 30 min) pre-treated mice. A representative blot in left showing imipramine-induced phosphorylation of TrkB and ∼105 kDa protein in mouse brain. Data is presented as percentage of control/saline ± standard error of mean (SEM). *<0.05; two-way ANOVA with Newmann-Keuls post hoc test.
(TIF)
Phospho-TrkB enzyme-linked immunosorbent assay (ELISA). A) Dose-response of BDNF (2, 8, 20 ng/ml, 15 min) on TrkB phosphorylation in TrkB expressing fibroblasts cultivated in 48-well plates. n = 4/group. B) Whereas BDNF produces robust TrkB phosphorylation in TrkB expressing fibroblasts cultivated in 24-well plates, all the tested drugs at selected doses did not have any effect on TrkB phosphorylation (compounds incubated for 15 min). n = 3/group. Data is presented as percentage of control ± standard error of mean (SEM). Abbreviations: IMI = imipramine; PHE = phenelzine; FLX = fluoxetine; CLOZ = clozapine; Li = lithium chloride; CHLOR = chlorpromazine.
(TIF)
Acknowledgments
The authors would like to thank the laboratory members of Professor. Eero Castrén, especially Outi Nikkilä, Henri Autio, Ettore Tiraboschi and Juha Knuuttila for excellent technical and academic assistance. Professor Jari Yli-Kauhaluoma (University of Helsinki, Finland) is thanked for providing the NaPP1 inhibitor, and Professor Moses Chao (Skirball Institute, NY, USA) is thanked for providing phospho-TrkBY816 antibody.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by Sigrid Juselius Foundation (EC), Academy of Finland (EC), Finnish Cultural Foundation (TR), the Finnish Graduate School for Neuroscience (FGSN; LV) and the Deutsche Forschungsgemeinschaft (Le 629/4-2; KPL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 2.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 3.Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 5.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 6.Lee FS, Chao MV. Activation of trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YZ, McNamara JO. Mutual regulation of src family kinases and the neurotrophin receptor TrkB. J Biol Chem. 2010;285:8207–8217. doi: 10.1074/jbc.M109.091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang SW, Liu X, Chan CB, Weinshenker D, Hall RA, et al. Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chem Biol. 2009;16:644–656. doi: 10.1016/j.chembiol.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pezet S, Malcangio M. Brain-derived neurotrophic factor as a drug target for CNS disorders. Expert Opin Ther Targets. 2004;8:391–399. doi: 10.1517/14728222.8.5.391. [DOI] [PubMed] [Google Scholar]
- 11.Schindowski K, Belarbi K, Buee L. Neurotrophic factors in Alzheimer's disease: Role of axonal transport. Genes Brain Behav. 2008;7(Suppl 1):43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rantamäki T, Castrén E. Targeting TrkB neurotrophin receptor to treat depression. Expert Opin Ther Targets. 2008;12:705–15. doi: 10.1517/14728222.12.6.705. [DOI] [PubMed] [Google Scholar]
- 13.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;2:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rantamäki T, Hendolin P, Kankaanpää A, Mijatovic J, Piepponen P, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- 16.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–97. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 19.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyneken U, Sandoval M, Sandoval S, Jorquera F, Gonzalez I, et al. Clinically relevant doses of fluoxetine and reboxetine induce changes in the TrkB content of central excitatory synapses. Neuropsychopharmacology. 2006;31:2415–23. doi: 10.1038/sj.npp.1301052. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Koponen E, Voikar V, Riekki R, Saarelainen T, Rauramaa T, et al. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci. 2004;26:166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Koponen E, Lakso M, Castrén E. Overexpression of the full-length neurotrophin receptor trkB regulates the expression of plasticity-related genes in mouse brain. Brain Res Mol Brain Res. 2004;130:81–94. doi: 10.1016/j.molbrainres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Watson FL, Porcionatto MA, Bhattacharyya A, Stiles CD, Segal RA. TrkA glycosylation regulates receptor localization and activity. J Neurobiol. 1999;39:323–336. doi: 10.1002/(sici)1097-4695(199905)39:2<323::aid-neu15>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Lee FS, Rajagopal R, Chao MV. Distinctive features of trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002;13:11–17. doi: 10.1016/s1359-6101(01)00024-7. [DOI] [PubMed] [Google Scholar]
- 26.Miranda C, Di Virgilio M, Selleri S, Zanotti G, Pagliardini S, et al. Novel pathogenic mechanisms of congenital insensitivity to pain with anhidrosis genetic disorder unveiled by functional analysis of neurotrophic tyrosine receptor kinase type 1/nerve growth factor receptor mutations. J Biol Chem. 2002;277:6455–6462. doi: 10.1074/jbc.M110016200. [DOI] [PubMed] [Google Scholar]
- 27.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 28.Rios M, Fan G, Fekete C, Kelly J, Bates B, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 29.Wiese S, Jablonka S, Holtmann B, Orel N, Rajagopal R, et al. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc Natl Acad Sci U S A. 2007;104:17210–17215. doi: 10.1073/pnas.0705267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebastiao AM, Ribeiro JA. Triggering neurotrophic factor actions through adenosine A2A receptor activation: Implications for neuroprotection. Br J Pharmacol. 2009;158:15–22. doi: 10.1111/j.1476-5381.2009.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillis JW, Wu PH. The effect of various centrally active drugs on adenosine uptake by the central nervous system. Comp Biochem Physiol C. 1982;72:179–187. doi: 10.1016/0306-4492(82)90082-x. [DOI] [PubMed] [Google Scholar]
- 32.Pires VA, Pamplona FA, Pandolfo P, Fernandes D, Prediger RD, et al. Adenosine receptor antagonists improve short-term object-recognition ability of spontaneously hypertensive rats: A rodent model of attention-deficit hyperactivity disorder. Behav Pharmacol. 2009;20:134–145. doi: 10.1097/FBP.0b013e32832a80bf. [DOI] [PubMed] [Google Scholar]
- 33.Lobato KR, Binfare RW, Budni J, Rosa AO, Santos AR, et al. Involvement of the adenosine A1 and A2A receptors in the antidepressant-like effect of zinc in the forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:994–999. doi: 10.1016/j.pnpbp.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, et al. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- 35.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 37.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koponen E, Rantamäki T, Voikar V, Saarelainen T, Macdonald E, et al. Enhanced BDNF signaling is associated with an antidepressant-like behavioral response and changes in brain monoamines. Cell Mol Neurobiol. 2005;25:973–980. doi: 10.1007/s10571-005-8468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saarelainen T, Vaittinen S, Castrén E. TrkB-receptor activation contributes to the kainate-induced increase in BDNF mRNA synthesis. Cell Mol Neurobiol. 2001;21:429–435. doi: 10.1023/a:1012775808253. [DOI] [PubMed] [Google Scholar]
- 40.Martinowich K, Lu B. Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 41.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Lucchelli A, Santagostino-Barbone MG, Barbieri A, Candura SM, Tonini M. The interaction of antidepressant drugs with central and peripheral (enteric) 5-HT3 and 5-HT4 receptors. Br J Pharmacol. 1995;114:1017–1025. doi: 10.1111/j.1476-5381.1995.tb13307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raabe R, Gentile L. Antidepressant interactions with the NMDA NR1-1b subunit. J Biophys. 2008;2008:474205. doi: 10.1155/2008/474205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rammes G, Rupprecht R. Modulation of ligand-gated ion channels by antidepressants and antipsychotics. Mol Neurobiol. 2007;35:160–174. doi: 10.1007/s12035-007-0006-1. [DOI] [PubMed] [Google Scholar]
- 45.Narita N, Hashimoto K, Tomitaka S, Minabe Y. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur J Pharmacol. 1996;307:117–119. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- 46.Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 47.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: Insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 48.Vesa J, Kruttgen A, Shooter EM. p75 reduces TrkB tyrosine autophosphorylation in response to brain-derived neurotrophic factor and neurotrophin 4/5. J Biol Chem. 2000;275:24414–24420. doi: 10.1074/jbc.M001641200. [DOI] [PubMed] [Google Scholar]
- 49.Rantamäki T, Knuuttila JE, Hokkanen ME, Castrén E. The effects of acute and long-term lithium treatments on trkB neurotrophin receptor activation in the mouse hippocampus and anterior cingulate cortex. Neuropharmacology. 2006;50:421–427. doi: 10.1016/j.neuropharm.2005.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diverse antidepressant drugs induce ∼105 kDa protein phosphorylation in the mouse brain. A) Representative blot showing the time-response (30 min, 60 min, 120 min) of fluoxetine-induced (20/30 mg/kg, i.p.) phosphorylation of TrkB and ∼105 kDa protein (Y816 in left; Y705/6 in right) in mouse hippocampus. B) Representative blots showing antidepressant-induced phosphorylation of ∼105 kDa in mouse hippocampus C) Representative blots showing imipramine-induced phosphorylation of ∼105 kDa in mouse striatum, midbrain and whole brain homogenate. Abbreviations: FLX = fluoxetine, SAL = saline; CIT = citalopram; AMI = amitriptyline; CLO = clomipramine; MOC = moclobemide; REB = reboxetine; MW = molecular weight; TrkB.extr = antibody directed against the extracellular portion of TrkB receptors.
(TIF)
Acute fluoxetine treatment did not regulate BDNF protein levels or tPA (tissue plasminogen activator) activity. A) Representative blot showing mature-BDNF specific band in western blot from brain homogenates and pro- and mature-BDNF specific bands from respective control lanes as detected with polyclonal BDNF antibody (N-20/sc-546; Santa Cruz). Acute fluoxetine did not regulate mature-BDNF levels in mouse hippocampus. n = 6/group. B) Representative zymography showing caseinolysis at the level of recombinant tPA. Acute fluoxetine did not regulate tPA activity levels in mouse hippocampus. n = 6/group. Data is presented as percentage of control ± standard error of mean (SEM).
(TIF)
Blockade of adenosine-A2A receptor signaling does not prevent antidepressant-induced TrkB activation. Acute imipramine treatment (30 mg/kg, i.p., 30 min; n = 6/group) induces essentially similar changes on TrkB phosphorylation in vehicle and adenosine-A2A receptor antagonist (ZM241358; 1 mg/kg, i.p., 30 min) pre-treated mice. A representative blot in left showing imipramine-induced phosphorylation of TrkB and ∼105 kDa protein in mouse brain. Data is presented as percentage of control/saline ± standard error of mean (SEM). *<0.05; two-way ANOVA with Newmann-Keuls post hoc test.
(TIF)
Phospho-TrkB enzyme-linked immunosorbent assay (ELISA). A) Dose-response of BDNF (2, 8, 20 ng/ml, 15 min) on TrkB phosphorylation in TrkB expressing fibroblasts cultivated in 48-well plates. n = 4/group. B) Whereas BDNF produces robust TrkB phosphorylation in TrkB expressing fibroblasts cultivated in 24-well plates, all the tested drugs at selected doses did not have any effect on TrkB phosphorylation (compounds incubated for 15 min). n = 3/group. Data is presented as percentage of control ± standard error of mean (SEM). Abbreviations: IMI = imipramine; PHE = phenelzine; FLX = fluoxetine; CLOZ = clozapine; Li = lithium chloride; CHLOR = chlorpromazine.
(TIF)