Abstract
Background
Transfer RNAs are synthesized as a primary transcript that is processed to produce a mature tRNA. As part of the maturation process, a subset of the nucleosides are modified. Modifications in the anticodon region often modulate the decoding ability of the tRNA. At position 34, the majority of yeast cytosolic tRNA species that have a uridine are modified to 5-carbamoylmethyluridine (ncm5U), 5-carbamoylmethyl-2′-O-methyluridine (ncm5Um), 5-methoxycarbonylmethyl-uridine (mcm5U) or 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U). The formation of mcm5 and ncm5 side chains involves a complex pathway, where the last step in formation of mcm5 is a methyl esterification of cm5 dependent on the Trm9 and Trm112 proteins.
Methodology and Principal Findings
Both Trm9 and Trm112 are required for the last step in formation of mcm5 side chains at wobble uridines. By co-expressing a histidine-tagged Trm9p together with a native Trm112p in E. coli, these two proteins purified as a complex. The presence of Trm112p dramatically improves the methyltransferase activity of Trm9p in vitro. Single tRNA species that normally contain mcm5U or mcm5s2U nucleosides were isolated from trm9Δ or trm112Δ mutants and the presence of modified nucleosides was analyzed by HPLC. In both mutants, mcm5U and mcm5s2U nucleosides are absent in tRNAs and the major intermediates accumulating were ncm5U and ncm5s2U, not the expected cm5U and cm5s2U.
Conclusions
Trm9p and Trm112p function together at the final step in formation of mcm5U in tRNA by using the intermediate cm5U as a substrate. In tRNA isolated from trm9Δ and trm112Δ strains, ncm5U and ncm5s2U nucleosides accumulate, questioning the order of nucleoside intermediate formation of the mcm5 side chain. We propose two alternative explanations for this observation. One is that the intermediate cm5U is generated from ncm5U by a yet unknown mechanism and the other is that cm5U is formed before ncm5U and mcm5U.
Introduction
Transfer RNAs are adapter molecules, which decode mRNA into protein and thereby play a central role in gene expression. The primary tRNA transcript is processed by different endo and exonucleases, and tRNA modifying enzymes to produce a mature tRNA [1], [2], [3]. In this maturation process, a subset of the four normal nucleosides adenosine (A), guanosine (G), cytidine (C) and uridine (U) are modified [2], [3]. The modifications are introduced post-transcriptionally, and the formation of a modified nucleoside may require one or several enzymatic steps [2], [3]. Of the 50 modified nucleosides so far identified in eukaryotic tRNAs, 25 are present in cytoplasmic tRNAs from S. cerevisiae [2], [4], [5]. In the anticodon region, especially in positions 34 (wobble position) and 37, nucleosides are frequently modified. Modified nucleosides in these positions are important for reading frame maintenance and efficient decoding during translation [2], [3]. In yeast, there are in total 42 cytosolic tRNA species, of which 11 have a uridine at position 34 modified to 5-carbamoylmethyluridine (ncm5U), 5-carbamoylmethyl-2′-O-methyluridine (ncm5Um), 5-methoxycarbonylmethyl-uridine (mcm5U) or 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U) [6]. The formation of these nucleosides requires addition of mcm or ncm side chains at the 5-position of the uracil moity and a subset of these tRNAs also have a thio (s2) group at the 2-position of U34 or a methylation at the 2′ position of the ribose.
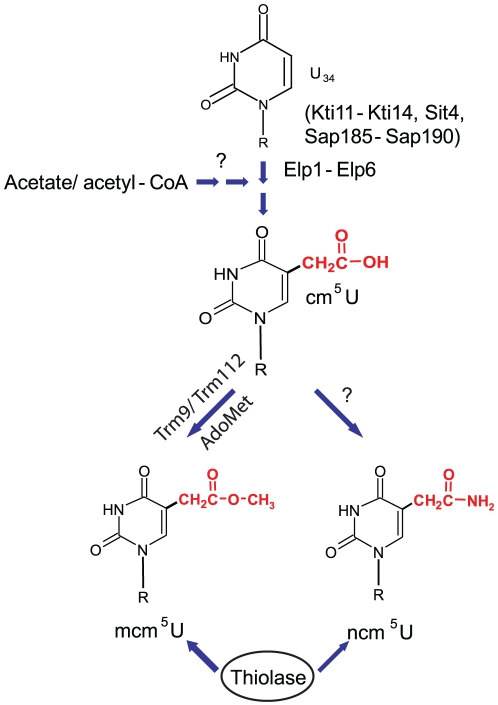
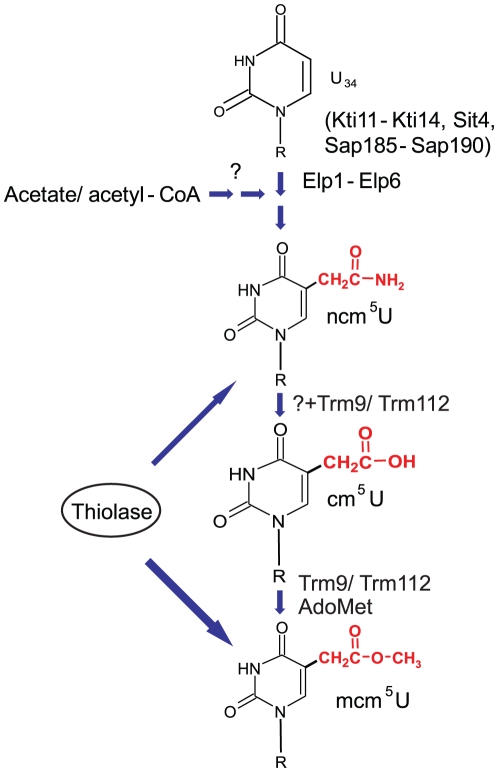
The common step in synthesis of ncm5 and mcm5 side chains at U34 in tRNAs requires at least 11 gene products (Figure 1). Deletion strains missing one of ELP1-ELP6, KTI11, KTI12, KTI14 or SIT4 genes, or both SAP185and SAP190 genes completely lack the mcm5U, mcm5s2U and ncm5U nucleosides, whereas a kti13 deletion mutant show dramatically reduced levels of these nucleosides [7], [8]. In strains with these genes mutated, no intermediates of mcm5U or ncm5U have been detected, whereas s2U is detected in tRNAs normally containing mcm5s2U [7], [8], [9], [10], [11], [12]. Thus, these gene products are required for an early step in synthesis of mcm5 and ncm5 groups (Figure 1). The earliest intermediate in the synthesis of mcm5U and ncm5U that has been detected is cm5U, and there is evidence that it originates from a metabolite related to acetyl-CoA [13] (Figure 1).
Figure 1. Model for formation of mcm5 side chain at wobble uridines.
The Elongator complex (Elp1-Elp6) and its potential regulators are required for the formation of cm5U. A methyl group is added to cm5U by Trm9p/Trm112p complex in tRNA species that in their mature form should have a mcm5 side chain. The cm5U in other tRNA species are converted to ncm5U by an unknown enzyme. For tRNAs that should contain a s2 group, presence of a mcm5 or ncm5 side chain is a prerequisite for efficient thiolation.
The ELP1-ELP6 gene products form the Elongator complex that consists of a core complex Elp1-Elp3 and a sub complex Elp4-Elp6 [14], [15], [16]. In the C-terminal part of Elp3p there is a potential acetyl-CoA binding domain [17], and the central region shares homology to the Radical SAM superfamily [18]. Members of this family contain an iron-sulphur (FeS) cluster and use S-adenosylmethionine (SAM) to catalyze a variety of radical reactions. The presence of a FeS cluster and ability to bind SAM has been verified for the M. jannaschii Elp3p homologue [18], whereas no binding of SAM to S. cerevisiae Elongator complex was observed [19]. At least Elp1 and Elp3 of Elongator core complex are in intimate contact with tRNA that is modified with a mcm side chain at U34 [7]. The KTI11-KTI14, SIT4 or SAP185 SAP190 gene products seem to regulate the activity of Elongator complex [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30].
The last step in formation of mcm5 side chain of U34 is a methyl esterification of cm5 [13], and requires Trm9p/Trm112p in yeast and ALKBH8/TRM112 in mammalians [31], [32], [33]. We confirm that Trm112p is also required for the last step of mcm5 side chain formation at position 34 in a subset of tRNAs. In vivo, Trm112p is essential for the methyl esterification to mcm5U34, and in vitro Trm112p improves the methyltransferase activity of Trm9p. The observation that the major intermediates accumulating in trm9 and trm112 mutants are ncm5U and ncm5s2U and not the expected cm5U and cm5s2U raises the question; what is the order of intermediates formed in biosynthesis of the mcm5 side chain of U34?
Materials and Methods
Yeast strains, media and genetic procedures
Strains used in this report, except those from the yeast deletion collection (Open Biosystems), are listed in Table S1A. Yeast media, genetic procedures and yeast transformation have been described previously [34]. To construct mtq2::KanMX6 and trm112::KanMX6 deletions, oligonucleotides (2104 and 2015, 1391 and 1392) in Table S1B containing 45nt sequence homology flanking the MTQ2 and TRM112 genes were used to amplify the KanMX6 cassette [35]. To delete TRM9, TRM11 and LYS9 in W303 strains, chromosomal DNA from the corresponding null mutants in the yeast deletion collection (Open Biosystems) were used as templates. The KanMX6 cassette together with 300–500 base pair flanking sequences to each gene were amplified with specific primers (1035 and 1036 for TRM9, 1950 and 1951 for TRM11, and 2059 and 2060 for LYS9) listed in Table S1B. The PCR products were introduced into diploid yeast strain UMY3104 and transformants were selected on YEPD plates containing 200 µg/ml Geneticin (G418). Transformants were sporulated and tetrad analysis verified a 2∶2 segregation of mating type and G418 resistance. Deletions were confirmed by PCR. The double mutants trm9Δ trm11Δ, trm9Δ lys9Δ, trm9Δ mtq2Δ, trm11Δ lys9Δ, trm11Δ mtq2Δ and lys9Δ mtq2Δ were generated by crossing the single mutants. The quadruple mutant was generated in a cross between trm9Δ lys9Δ and trm11Δ mtq2Δ.
Plasmid constructions
To generate the expression vector for the Trm9 protein, TRM9 gene was amplified by PCR using oligos 2015 and 2016 (Table S1B) and W303-1A genomic DNA as template. The PCR product was digested with BamH1 and HindIII, and subcloned to the corresponding sites of the expression vector pRSF-Duet1 (Novagen), generating an in frame fusion with the histidine tag. To construct the Trm9p-Trm112p co-expression vector, the TRM112 gene was amplified from W303-1A genomic DNA using oligos 2013 and 2014 (Table S1B) and cloned into the pRSF-Duet1-TRM9 vector using NdeI and XhoI.
Protein purification
The expression vectors were introduced into BL21(DE3)pLysS competent cells. Overnight cultures of transformed cells were grown in LB media containing 50 µg/ml Kanamycin at 37°C. Cultures were diluted to OD600 0.05 and grown to OD600 0.5 at 37°C. Cultures were placed on ice for 10 minutes. IPTG was added to a final concentration of 120 µg/ml and protein expression was induced at 15°C overnight. Harvested cell pellets were washed once by 0.9% NaCl and resuspended in breaking buffer (20 mM Tris pH 8.0, 10 mM imidazole, 150 mM NaCl, 0.2% NP-40, 2 mM β-mercaptoethanol) in the presence of proteinase inhibitor cocktail (Roche). Cells were broken by sonication and the cell extract was clarified by centrifugation at 16,000 g for 1 hour. The supernatant was mixed with TALON resin, equilibrated with breaking buffer and incubated at 4°C for 2 hours. The protein bound TALON resin was first washed with buffer 1 (20 mM Tris pH 8.0, 10 mM imidazole, 150 mM NaCl, 2 mM β-mercaptoethanol) and then with buffer 2 (20 mM Tris pH 8.0, 10 mM imidazole, 500 mM NaCl, 2 mM β-mercaptoethanol). Proteins were eluted with 330 mM imidazole and dialyzed overnight against storage buffer (25 mM Tris pH 8.0, 150 mM NaCl, 5 mM DTT, 10% glycerol) and kept at 4°C for future use.
Methyltransferase reaction
In the methyltransferase reaction, 50 µl of 2X reaction buffer (200 mM Tris 7.5, 0.2 mM EDTA, 20 mM MgCl2, 20 mM NH4Cl) was mixed with 20 µl [3H]AdoMet (0.55 mCi/ml, Perkin Elmer) and 20 µg tRNA, incubated at 37°C for 5 minutes. The methyltransferase reaction was initiated by adding 10 µg Trm9p or Trm9p-Trm112p. Aliquots of the reaction was withdrawn at different time points and mixed with 1 ml of 5% ice cold trichloroacetic acid (TCA). The tubes were incubated on ice for 10 minutes and samples were vacuum filtered through nitrocellulose filter (Millipore 0.45 µm). The [3H] incorporation was measured using a Wallac 1409 scintillation counter. To analyze [3H] incorporation in total tRNA by HPLC, 200 µg of tRNA was used. After 30 minutes of methyltransferase reaction, 2.5 volume of 99% ice cold ethanol was added into the reaction and samples were centrifuged for 30 minutes in eppendorf tubes at maximum speed. The pellet was resuspended in MQ water, digested with nuclease P1 and analyzed by HPLC [36]. The [3H] incorporation was monitored by a flow scintillation analyzer (Packard Bioscience).
Single tRNA isolation
Yeast cells were grown in 2L YEPD at 30°C to OD600 = 1.5. Total tRNA was prepared as described [36]. Single tRNA species were isolated from total tRNA by hybridizing to biotinylated complementary oligonucleotides [36] and separated from total tRNA by attachment to streptavidin coated Dynabeads M-280 (Invitrogen). The single tRNAs were digested to nucleosides with nuclease P1 followed by bacterial alkaline phosphatase (BAP) treatment [0.2 M (NH4)2SO4 pH 8.3], and analyzed by HPLC [37].
Results and Discussion
Trm112p is required for the methyl esterification of mcm5U and mcm5s2U
In a global analysis of protein complexes in yeast, Trm112p was found to interact
with three methyltransferases Trm9p, Trm11p and Mtq2p [38], [39], [40], [41]. In addition, Trm112p interacts
with the saccharopine dehydrogenase Lys9p, the essential DEAH-box ATP-dependent
RNA helicase Ecm16p and an essential component of the RSC chromatin remodeling
complex Sfh1p [38], [39], [40], [41]. The
N2-Monomethylguanosine-10
(m2G10) methyltransferase Trm11p, as well as the eRF1
methyltranferase Mtq2p, has to be in complex with Trm112p to be active [42],
[43]. Trm9p is required for the methyl esterification of
modified uridine nucleosides, resulting in the formation of
5-methylcarbonylmethyluridine (mcm5U34) and
5-methylcarbonylmethyl-2-thiouridine
(mcm5s2U34) present in a subset of tRNA species
in yeast, including 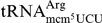 and
and
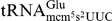 [31]. In the
methyl esterification reaction of these tRNAs, cm5U34 and
cm5s2U34 were suggested to be the
substrates [13], [31], [32,].
[31]. In the
methyl esterification reaction of these tRNAs, cm5U34 and
cm5s2U34 were suggested to be the
substrates [13], [31], [32,].
Both Trm9 and Trm112 are required for methyl esterification to mcm5U
and mcm5s2U [31], [32], [33]. To analyze the tRNA
modification status in these two mutants, total tRNA from
trm9Δ, trm112Δ and wild type strains
were isolated, digested to nucleosides and analyzed by HPLC. Similar to previous
reports [31],
[32],
[33],
total tRNA isolated from trm9 and trm112
deletion mutants lacked mcm5U and mcm5s2U
nucleosides (data not shown). In order to provide a more detailed analysis of
all possible nucleoside intermediates in trm9Δ and
trm112Δ mutants, single tRNA species,
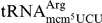 ,
, 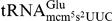 and
and
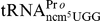 , were isolated from wild type,
trm9Δ and trm112Δ strains and the
purified tRNAs were digested to nucleosides and analyzed by HPLC (Figure 2, Table 1, data not shown). As
expected, the ncm5U nucleoside was present in
, were isolated from wild type,
trm9Δ and trm112Δ strains and the
purified tRNAs were digested to nucleosides and analyzed by HPLC (Figure 2, Table 1, data not shown). As
expected, the ncm5U nucleoside was present in
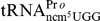 independent if the tRNA was isolated from
trm9Δ, trm112Δ or wild type
strains (data not shown). The mcm5U and mcm5s2U
nucleosides were present in
independent if the tRNA was isolated from
trm9Δ, trm112Δ or wild type
strains (data not shown). The mcm5U and mcm5s2U
nucleosides were present in 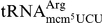 and
and
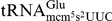 isolated from wild type but not from
trm9Δ and trm112Δ cells (Figure 2, Table 1). In
isolated from wild type but not from
trm9Δ and trm112Δ cells (Figure 2, Table 1). In
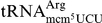 isolated from trm9Δ and
trm112Δ strains, we observed the appearance of
ncm5U and cm5U (Figure 2B, Table 1). Interestingly, the major
intermediate of the mcm5U nucleoside generated in the
trm9Δ and trm112Δ mutants is
ncm5U (Figure
2, Table 1).
The presence of ncm5U and cm5U has also been observed in
isolated from trm9Δ and
trm112Δ strains, we observed the appearance of
ncm5U and cm5U (Figure 2B, Table 1). Interestingly, the major
intermediate of the mcm5U nucleoside generated in the
trm9Δ and trm112Δ mutants is
ncm5U (Figure
2, Table 1).
The presence of ncm5U and cm5U has also been observed in
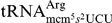 ,
, 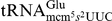 and
and
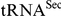 isolated from an
alkbh8
−/− mice [32], [44]. In
isolated from an
alkbh8
−/− mice [32], [44]. In
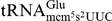 isolated from the trm9Δ and
trm112Δ strains, there was a complete lack of
mcm5s2U and a concomitant increase of cm5U,
ncm5U and ncm5s2U (Table 1). The presence of cm5U
supports the earlier observation that formation of a completed mcm5
side chain appears to be a prerequisite for efficient and complete thiolation of
position 2 in mcm5s2U containing tRNAs [10], [11], [12], [32]. An
unexpected observation was that the major species accumulating in
isolated from the trm9Δ and
trm112Δ strains, there was a complete lack of
mcm5s2U and a concomitant increase of cm5U,
ncm5U and ncm5s2U (Table 1). The presence of cm5U
supports the earlier observation that formation of a completed mcm5
side chain appears to be a prerequisite for efficient and complete thiolation of
position 2 in mcm5s2U containing tRNAs [10], [11], [12], [32]. An
unexpected observation was that the major species accumulating in
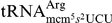 and
and 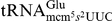 isolated from
trm9Δ and trm112Δ strains were
ncm5U and ncm5s2U, respectively (Table 1). We considered the
possibility that the ncm5 side chain was spontaneously generated from
cm5 by amidation during the bacterial alkaline phosphatase (BAP)
treatment in the digestion step of tRNA to nucleosides for HPLC analysis. To
test this hypothesis, synthetic cm5U nucleoside was treated in the
same way as in the digestion step of tRNA and analyzed by HPLC (Figure 3). We did not detect
any conversion of cm5U to ncm5U (Figure 3) indicating that formation of
ncm5U is enzymatically catalyzed and not an artifact of the
sample preparation procedure.
isolated from
trm9Δ and trm112Δ strains were
ncm5U and ncm5s2U, respectively (Table 1). We considered the
possibility that the ncm5 side chain was spontaneously generated from
cm5 by amidation during the bacterial alkaline phosphatase (BAP)
treatment in the digestion step of tRNA to nucleosides for HPLC analysis. To
test this hypothesis, synthetic cm5U nucleoside was treated in the
same way as in the digestion step of tRNA and analyzed by HPLC (Figure 3). We did not detect
any conversion of cm5U to ncm5U (Figure 3) indicating that formation of
ncm5U is enzymatically catalyzed and not an artifact of the
sample preparation procedure.
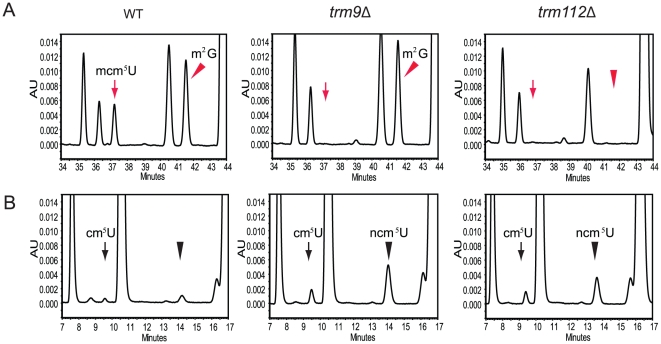
Figure 2.
trm9 and trm112 mutants are lacking
the mcm5 side-chain in 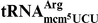 at wobble
uridines.
at wobble
uridines.
HPLC analysis of modified tRNA nucleosides from wild-type (UMY3169, left panels), trm9::KanMX4 (Open Biosystems, middle panels) and trm112::KanMX4 (UMY3330, right panels). Arrows in red and black indicate expected retention time of mcm5U and cm5U, respectively. Arrow heads in red and black indicate expected retention time of m2G and ncm5U, respectively. (A), Part of the chromatogram between retention times 34 and 44 min is shown. (B), Part of the chromatogram between retention times 7 and 17 min is shown. The small peak in wild-type at 14 min represents an unrelated compound with a spectrum different from ncm5U. The chromatograms were monitored at 254 nm.
Table 1. Relative amounts of various modified nucleosides in
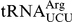 and
and
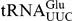 isolated
from wild type, trm9Δ and
trm112Δ strains.
isolated
from wild type, trm9Δ and
trm112Δ strains.
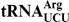
|
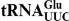
|
||||||||
| cm5U/Ψ | ncm5U/Ψ | mcm5U/Ψ | cm5U/Ψ | ncm5U/Ψ | mcm5U/Ψ | cm5s2U/Ψ | ncm5s2U/Ψ | mcm5s2U/Ψ | |
| WT | 0.016 | 0.044 | 0.183 | ND | ND | ND | 0.029 | ND | 0.220 |
| trm9Δ | 0.051 | 0.199 | ND | 0.065 | 0.028 | ND | 0.013 | 0.191 | ND |
| trm112Δ | 0.046 | 0.149 | ND | 0.044 | 0.044 | ND | 0.022 | 0.161 | ND |
Pseudouridine (Ψ) was used as the internal control. The numbers displayed are the ratios (modified nucleoside/Ψ). ND: not detected. The modified nucleosides cm5U, ncm5U, mcm5U and Ψ were monitored at 254 nm, and cm5s2U, ncm5s2U and mcm5s2U were monitored at 314 nm as thiolated nucleosides absorb well at this wavelength, while nonthiolated nucleosides do not.
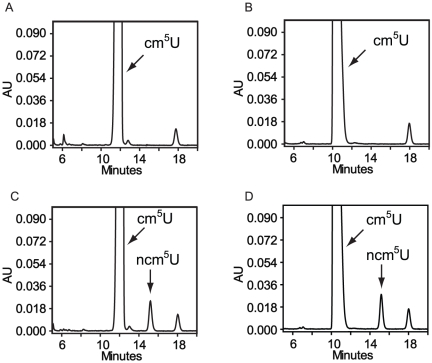
Figure 3. Nucleoside ncm5U is not generated by amidation of cm5U during conversion of tRNA into nucleosides.
Synthetic cm5U (A and B) or a mixture of synthetic cm5U and ncm5U (C and D) were treated with nuclease P1 for 16 hours, followed by a 2 hours incubation with either water (A and C) or bacterial alkaline phosphatase (BAP) (B and D). Parts of the chromatogram of HPLC analysis between 5 and 20 min are shown. The chromatograms were monitored at 254 nm.
In addition to Trm9p, Trm112p also interacts with Trm11p, Lys9p and Mtq2p encoded
by non-essential genes, and Ecm16p and Sph1p encoded by essential genes [38], [39], [40], [41]. Therefore, we
also analyzed single tRNA species 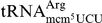 ,
,
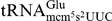 and
and 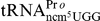 from
trm11Δ, lys9Δ and
mtq2Δ strains. Trm11p and Trm112p are essential for
formation of the m2G nucleoside [42]. Consistently,
from
trm11Δ, lys9Δ and
mtq2Δ strains. Trm11p and Trm112p are essential for
formation of the m2G nucleoside [42]. Consistently,
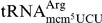 isolated from trm11Δ or
trm112Δ strains does not have the m2G
modified nucleoside, whereas the same tRNA from wild-type has m2G
(Figure 2 and S1). In
single tRNAs from lys9Δ and mtq2Δ
strains, there was no notable change in modified nucleosides as assessed by HPLC
analysis (Figure
S1, data not shown). A deletion of the TRM112 gene
causes a dramatic reduction in growth and a mtq2Δ strain
also shows a clear reduction in growth, whereas trm11Δ,
lys9Δ or trm9Δ strains show mild
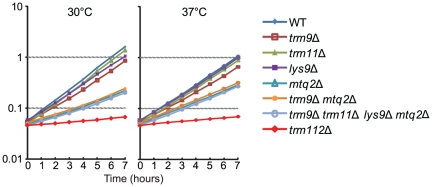
growth defects in YEPD medium at both 30°C and 37°C (Figure 4). We considered the
possibility that strains with multiple null alleles of genes encoding Trm112p
interacting proteins would show additive growth defects, possibly mimicking a
trm112Δ null allele. Since two Trm112p associated
proteins, Ecm16 and Sfh1, are encoded by essential genes, we were only able to
make strains with combinations of the trm11Δ,
lys9Δ, trm9Δ, and
mtq2Δ alleles. We first made the double mutants
trm11Δ lys9Δ,
trm11Δ trm9Δ,
trm11Δ mtq2Δ,
lys9Δ trm9Δ,
lys9Δ mtq2Δ and
trm9Δ mtq2Δ. No additive growth
reduction was observed in any of the constructs at both 30°C and 37°C
(Figure 4, data not
shown), in contrast to the previously observed growth defect of the
trm9Δ mtq2Δ mutant [33]. Further
we made a trm11Δ lys9Δ
trm9Δ, mtq2Δ quadruple mutant
strain that grew like a mtq2Δ strain at both 30°C and
37°C (Figure 4). These
data show that the poor growth of trm112Δ cells is not
entirely caused by defects in tRNA modification, eRF1 methylation and
dehydrogenase activity in the quadruple mutant. Possibly it is caused by reduced
function of Ecm16p or Sfh1p which might require the interaction with Trm112p to
be fully active.
isolated from trm11Δ or
trm112Δ strains does not have the m2G
modified nucleoside, whereas the same tRNA from wild-type has m2G
(Figure 2 and S1). In
single tRNAs from lys9Δ and mtq2Δ
strains, there was no notable change in modified nucleosides as assessed by HPLC
analysis (Figure
S1, data not shown). A deletion of the TRM112 gene
causes a dramatic reduction in growth and a mtq2Δ strain
also shows a clear reduction in growth, whereas trm11Δ,
lys9Δ or trm9Δ strains show mild
growth defects in YEPD medium at both 30°C and 37°C (Figure 4). We considered the
possibility that strains with multiple null alleles of genes encoding Trm112p
interacting proteins would show additive growth defects, possibly mimicking a
trm112Δ null allele. Since two Trm112p associated
proteins, Ecm16 and Sfh1, are encoded by essential genes, we were only able to
make strains with combinations of the trm11Δ,
lys9Δ, trm9Δ, and
mtq2Δ alleles. We first made the double mutants
trm11Δ lys9Δ,
trm11Δ trm9Δ,
trm11Δ mtq2Δ,
lys9Δ trm9Δ,
lys9Δ mtq2Δ and
trm9Δ mtq2Δ. No additive growth
reduction was observed in any of the constructs at both 30°C and 37°C
(Figure 4, data not
shown), in contrast to the previously observed growth defect of the
trm9Δ mtq2Δ mutant [33]. Further
we made a trm11Δ lys9Δ
trm9Δ, mtq2Δ quadruple mutant
strain that grew like a mtq2Δ strain at both 30°C and
37°C (Figure 4). These
data show that the poor growth of trm112Δ cells is not
entirely caused by defects in tRNA modification, eRF1 methylation and
dehydrogenase activity in the quadruple mutant. Possibly it is caused by reduced
function of Ecm16p or Sfh1p which might require the interaction with Trm112p to
be fully active.
Figure 4. Growth phenotypes.
Wild type (UMY2067), trm112Δ (UMY3679), trm9Δ (UMY3267), trm11Δ (UMY3677), lys9Δ (UMY3650), mtq2Δ (UMY3675), trm9Δ mtq2Δ (UMY3673) and trm9Δ trm11Δ lys9Δ mtq2Δ (UMY3680) strains were cultivated in YEPD at 30°C and 37°C.
Trm112p/Trm9p complex efficiently incorporates methyl groups into trm9 substrate tRNA in vitro
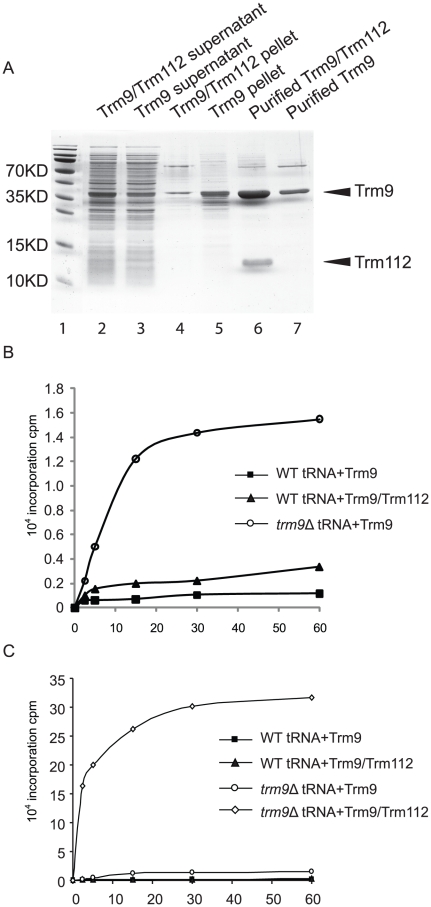
Trm9p has been shown to catalyze the methyl esterification to mcm5U and mcm5s2U in vitro [31]. We cloned the TRM9 gene into the expression vector pRSF duet to produce 6xHis-Trm9p recombinant protein in E. coli. We also made a pRSF duet vector construct, simultaneously expressing the 6xHis-Trm9p recombinant protein and a non-tagged Trm112p. When Trm9p was expressed alone, the majority of Trm9p recombinant protein was insoluble (Figure 5A), and the solubility of Trm9p dramatically improved when Trm112p was co-expressed with Trm9p. Purification of Trm9p by virtue of its 6xHis tag resulted in co-purification of Trm112p (Figure 5A), indicating that Trm9p forms a stable complex with Trm112p.
Figure 5. Trm9p/Trm112p complex efficiently catalyzes the methyl incorporation into trm9 substrate tRNA.
(A) SDS-PAGE analysis of histidine tagged Trm9p expressed alone or co-expressed with Trm112p and purified from E. coli. The gel was stained with Colloidal Blue (Invitrogen). Lane 1: Molecular weight standard (PageRuler prestained, Fermentas). Lane 2: Soluble fraction of extract from E. coli strains expressing Trm112p and histidine tagged Trm9p. Lane 3: Soluble fraction of extract from E. coli strains expressing histidine tagged Trm9p. Lane 4: Pellet from crude extract of E. coli strains expressing Trm112p and histidine tagged Trm9p. Lane 5: Pellet from crude extract of E. coli strains expressing histidine tagged Trm9p. Lane 6: Trm112p co-purified with histidine tagged Trm9 protein. Lane 7: Purified histidine tagged Trm9 protein. (B) [3H] methyl incorporation into tRNA as a function of time. Substrates were total tRNA preparations from strain UMY2067 (wild-type) and UMY3267 (trm9Δ). (▪) and (▴) are methyl incorporation reactions into wild-type tRNA by using Trm9p or Trm9p/Trm112p as enzyme. (○) is methyl incorporation reaction into trm9 tRNA by using Trm9p as enzyme. (C). The methyl incorporation into trm9 tRNA using Trm9p/Trm112p as enzyme (◊), in addition to the reactions in (B).
Purified Trm9p and Trm9/Trm112p complex was used to methylate total tRNA isolated from wild type and a trm9 deletion strains in vitro. Saponification of total tRNA with sodium hydroxide leads to the production of cm5U and cm5s2U from mcm5U and mcm5s2U, and this method has previously been used to generate substrates for Trm9p or ALKBH8 [31], [32]. However, saponification also efficiently degrades tRNA and we found that tRNA isolated from the trm9 deletion strain was a superior substrate in the methyl esterification assay (data not shown). To track methylation of tRNA substrates in vitro, S-adenosylmethionine containing a tritiated methyl donor group was used together with tRNA and purified enzyme. When total tRNA from wild type was used as a substrate, there was a small increase in incorporation of radioactive methyl groups with time using either Trm9p or the Trm9p/Trm112p complex (Figure 5B). In contrast, use of total tRNA from the trm9Δ strain and Trm9p leads to a clear but modest increase in the incorporation of radioactive methyl groups (Figure 5B). Moreover, the incorporation of radioactive methyl groups was 20-fold more efficient using Trm9p/Trm112p over Trm9p alone (Figure 5C). Thus, Trm112p is required for Trm9p to methylate its substrate tRNA more efficiently in vitro and is a prerequisite in vivo as no mcm5 nucleosides are formed in a trm112Δ mutant (Figure 2, Table 1). In the reaction using tRNA from the trm9Δ strain and Trm9p/Trm112p, there was a rapid incorporation of [3H] methyl groups in the first 5 minutes that entered to a plateau after 30 minutes (Figure 5C). The reduced incorporation was not due to enzyme inactivation with time as adding more enzyme at 30 minutes did not improve incorporation of radioactivity (data not shown).
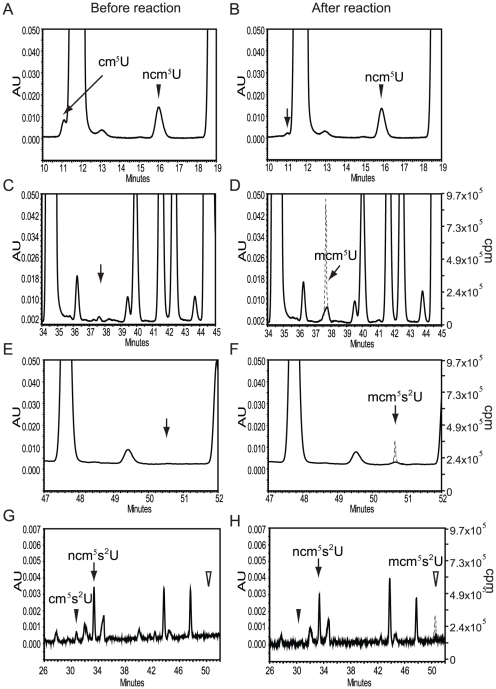
Based on HPLC analysis, there is an accumulation of cm5U, ncm5U, and ncm5s2U in total tRNA from a trm9Δ strain compared with a wild-type strain [33] (data not shown). When tRNA isolated from a trm9Δ strain was used as substrate in vitro, we observed a reduction of the cm5U nucleoside and appearance of mcm5U (Figure 6A-D, Table 2) consistent with cm5U being the substrate of Trm9 [31], [32], [33]. Furthermore, the relative amounts of ncm5U and ncm5s2U did not change after the methylation reaction, showing that these two nucleosides are not substrates of Trm9p/Trm112p under these conditions (Table 2) [33]. By using saponified tRNA, cm5s2U was suggested to be a substrate for Trm9p or ALKBH8/Trm112 [31], [32]. However, cm5s2U was not detected in total tRNA isolated from trm9 or trm112 mutants [33]. In our analysis of trm9 total tRNA, we observed a very small peak migrating in the position of cm5s2U, which was absent after the methylation reaction (Figure 6G-H, Table 2). When [3H]-CH3 was monitored by flow scintillation analyzer coupled to the HPLC, we found that the incorporated radioactivity migrated with retention times identical to those known for mcm5U and mcm5s2U nucleosides (Figure 6D, F and H). As the signal for the tentative cm5s2U is very weak, we cannot exclude the possibility that mcm5s2U originated from another species. These observations are consistent with those shown by Kalhor and Clarke [31], [32] and fully support the assertion that Trm9p is the methyltransferase catalyzing the formation of mcm5U from cm5U. Why and how ncm5U and ncm5s2U accumulates in tRNAs from strains lacking Trm9p or Trm112p, remains to be elucidated.
Figure 6. HPLC analysis of total trm9 tRNA after methyl incorporation by using Trm9p/Trm112p as enzyme.
(A–B) Part of the chromatogram between retention time 10 and 19 min is shown. The arrow in B indicates the expected retention time of cm5U. (C–D). Part of the chromatogram between retention time 34 and 45 min is shown. The arrow in C indicates the expected retention time of mcm5U. (E–F). Part of the chromatogram between retention time 47 and 52 min is shown. The arrow in E indicates the expected retention time of mcm5s2U. (G–H). Part of the chromatogram between retention time 26 and 52 min is shown. Open and closed arrowheads in G and H indicate the expected retention time of mcm5s2U and cm5U, respectively. Chromatograms in A–F were monitored at 254 nm and at 314 nm in G–H. The dashed line in D, F and H indicates the migration of isotope labeled nucleoside which overlaps with mcm5U and mcm5s2U, respectively. The Y axis to the left corresponds to absorbance units and the Y axis to the right shows the [3H] incorporation in cpm.
Table 2. Relative amounts of various modified nucleosides of total tRNA isolated from the trm9Δ strain before and after methylation reaction.
| cm5U/Ψ | ncm5U/Ψ | mcm5U/Ψ | cm5s2U/Ψ | ncm5s2U/Ψ | mcm5s2U/Ψ | |
| Before reaction | 0.01752 | 0.04634 | ND | 0.00081 | 0.00571 | ND |
| After reaction | 0.00227 | 0.04707 | 0.01720 | ND | 0.00521 | 0.00132 |
Pseudouridine (Ψ) was used as the internal control. The numbers displayed are the ratios (modified nucleoside/Ψ). ND: not detected. The modified nucleosides cm5U, ncm5U, mcm5U and Ψ were monitored at 254 nm, and cm5s2U, ncm5s2U and mcm5s2U were monitored at 314 nm as thiolated nucleosides absorb well at this wavelength, while nonthiolated nucleosides do not.
Alternative mechanisms in formation of the mcm5 side chain at wobble position
In trm9Δ or trm112Δ strains, the major species generated are ncm5U and ncm5s2U instead of the expected cm5U or cm5s2U. According to the model proposed in Figure 1, Elongator complex is required for and might directly catalyze the formation of cm5U. In the presence of Trm9 and Trm112p, cm5U is rapidly converted to mcm5U in tRNAs destined to contain a mcm5U nucleoside. Those tRNAs destined to contain ncm5U are not recognized by Trm9p/Trm112p and ncm5U is formed by an uncharacterized enzyme. In order to account for the presence of ncm5U and ncm5s2U in tRNAs that normally should contain mcm5U and mcm5s2U, one has to postulate that in the absence of Trm9p/Trm112p the uncharacterized enzyme responsible for amidation also recognizes these tRNA substrates (Figure 2). For tRNAs that should contain a s2 group, the presence of a mcm5 side chain has been suggested to be a prerequisite for efficient thiolation [10], [12]. We suggest that the presence of ncm5U, but not cm5U, in these tRNAs also promotes efficient thiolation, resulting in accumulation of ncm5s2U (Table 1).
The observation that the major U34 intermediates in
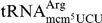 and
and 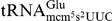 are
ncm5U and ncm5s2U in trm9
and trm112 mutants also supports an alternative model, i. e.
ncm5U is generated before cm5U (Figure 7). Such a model would require a
conversion of ncm5U to cm5U before the Trm9p/Trm112p
complex finally can form mcm5U. A similar mechanism has been
described in Eubacteria that have mnm5 instead of mcm5
side chains and the first intermediate in its synthesis is cmnm5U
[45].
The bi-functional MnmC demodifies cmnm5U to nm5U and
thereafter methylates nm5U to form mnm5U [45], [46], [47]. By
analogy, the Trm9p/Trm112p complex may be involved in two reactions; deamination
of ncm5U to cm5U, and then catalyzing formation of
mcm5U. The deaminase activity is not necessarily part of Trm9p or
Trm112p. In the absence of Trm9p or Trm112p, ncm5U accumulates in
tRNAs destined to contain mcm5s2U,
like
are
ncm5U and ncm5s2U in trm9
and trm112 mutants also supports an alternative model, i. e.
ncm5U is generated before cm5U (Figure 7). Such a model would require a
conversion of ncm5U to cm5U before the Trm9p/Trm112p
complex finally can form mcm5U. A similar mechanism has been
described in Eubacteria that have mnm5 instead of mcm5
side chains and the first intermediate in its synthesis is cmnm5U
[45].
The bi-functional MnmC demodifies cmnm5U to nm5U and
thereafter methylates nm5U to form mnm5U [45], [46], [47]. By
analogy, the Trm9p/Trm112p complex may be involved in two reactions; deamination
of ncm5U to cm5U, and then catalyzing formation of
mcm5U. The deaminase activity is not necessarily part of Trm9p or
Trm112p. In the absence of Trm9p or Trm112p, ncm5U accumulates in
tRNAs destined to contain mcm5s2U,
like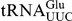 . As postulated in model 1, the presence of an
ncm5 side chain in these tRNAs promotes thiolation, generating
ncm5s2U. MnmC requires flavin adenine dinucleotide
(FAD) as co-factor in the de-modification reaction and SAM in the methylation
reaction. We performed an in vitro reaction with
[3H]AdoMet in the presence or absence of FAD. We
assumed if ncm5U is converted to cm5U in the presence of
FAD, more [3H]-methyl groups would be incorporated into
total tRNA isolated from trm9 deletion strain when FAD is
included in the reaction. Reactions conducted in the presence of FAD did not
increase the incorporation of [3H]-methyl into
trm9 deletion tRNA, nor did it decrease the overall amount
of ncm5U as analyzed by HPLC (data not shown). We also investigated
the potential use of other cofactors in the conversion of ncm5U to
cm5U such as NAD+ and NADP+
without success (data not shown). It remains to be elucidated which of these two
alternative pathways for formation of mcm5 side chains is used.
. As postulated in model 1, the presence of an
ncm5 side chain in these tRNAs promotes thiolation, generating
ncm5s2U. MnmC requires flavin adenine dinucleotide
(FAD) as co-factor in the de-modification reaction and SAM in the methylation
reaction. We performed an in vitro reaction with
[3H]AdoMet in the presence or absence of FAD. We
assumed if ncm5U is converted to cm5U in the presence of
FAD, more [3H]-methyl groups would be incorporated into
total tRNA isolated from trm9 deletion strain when FAD is
included in the reaction. Reactions conducted in the presence of FAD did not
increase the incorporation of [3H]-methyl into
trm9 deletion tRNA, nor did it decrease the overall amount
of ncm5U as analyzed by HPLC (data not shown). We also investigated
the potential use of other cofactors in the conversion of ncm5U to
cm5U such as NAD+ and NADP+
without success (data not shown). It remains to be elucidated which of these two
alternative pathways for formation of mcm5 side chains is used.
Figure 7. An alternative model for formation of mcm5 side chain at wobble uridines.
Elongator complex (Elp1-Elp6) and its potential regulators catalyzes the formation of ncm5U. The ncm5U is converted to cm5U by an unknown mechanism in tRNA species that in their mature form should have a mcm5 side chain. This unknown mechanism requires Trm9p/Trm112p. In the last step, a methyl group is added to cm5U by Trm9p/Trm112p complex in these tRNA species. For tRNAs that should contain a s2 group, presence of a mcm5 or ncm5 side chain is a prerequisite for efficient thiolation.
Supporting Information
HPLC analysis of modified nucleosides in
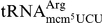 isolated
from wild-type,
trm11Δ,
lys9Δ and
mtq2Δ strains.
Arrows in red and black indicate expected retention time of mcm5U
and cm5U, respectively. Arrow heads in red and black indicate
expected retention time of m2G and ncm5U,
respectively. (A–D), Part of the chromatogram between retention times
34 and 44 min is shown. (B), Part of the chromatogram between retention
times 7 and 17 min is shown. The small peak in wild-type at 14 min
represents an unrelated compound with a spectrum different from
ncm5U. Absorbance at 254 nm (AU) was used to create the
chromatograms.
isolated
from wild-type,
trm11Δ,
lys9Δ and
mtq2Δ strains.
Arrows in red and black indicate expected retention time of mcm5U
and cm5U, respectively. Arrow heads in red and black indicate
expected retention time of m2G and ncm5U,
respectively. (A–D), Part of the chromatogram between retention times
34 and 44 min is shown. (B), Part of the chromatogram between retention
times 7 and 17 min is shown. The small peak in wild-type at 14 min
represents an unrelated compound with a spectrum different from
ncm5U. Absorbance at 254 nm (AU) was used to create the
chromatograms.
(EPS)
Acknowledgments
We are grateful to Gunilla Jäger for performing the HPLC analysis of tRNA. Members of the Byström lab are gratefully acknowledged for discussions. We thank Drs. Glenn Björk and Marcus Johansson for comments on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the Swedish Cancer Foundation (CAN 2007/890 to A.S.B.), the Swedish Science Research Council (2009-4761 to A.S.B.), Insamlingstiftelsen (223-27-10 to A.S.B.) and R01GM069949 from the National Institutes of Health, United States of America, to J.T.A. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hopper AK, Phizicky EM. tRNA transfers to the limelight. Genes Dev. 2003;17:162–180. doi: 10.1101/gad.1049103. [DOI] [PubMed] [Google Scholar]
- 2.Johansson MJO, Byström AS. Transfer RNA modifications and modifying enzymes in Saccharomyces cerevisiae. In: Grosjean H, editor. Fine-tuning of RNA functions by modification and editing. New York: Springer-Verlag; 2005. [Google Scholar]
- 3.Björk GR. tRNA: Structure, Biosynthesis, and Function. Washington, DC: ASM Press; 1995. Biosynthesis and Function of Modified Nucleosides. pp. 165–205. [Google Scholar]
- 4.Juhling F, Morl M, Hartmann RK, Sprinzl M, Stadler PF, et al. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozenski J, Crain PF, McCloskey JA. The RNA Modification Database: 1999 update. Nucleic Acids Res. 1999;27:196–197. doi: 10.1093/nar/27.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson MJO, Esberg A, Huang B, Björk GR, Byström AS. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol. 2008;28:3301–3312. doi: 10.1128/MCB.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang B, Johansson MJO, Byström AS. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11:424–436. doi: 10.1261/rna.7247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang B, Lu J, Byström AS. A genome-wide screen identifies genes required for formation of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine in Saccharomyces cerevisiae. RNA. 2008;14:2183–2194. doi: 10.1261/rna.1184108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Björk GR, Huang B, Persson OP, Byström AS. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA. 2007;13:1245–1255. doi: 10.1261/rna.558707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakai Y, Nakai M, Hayashi H. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J Biol Chem. 2008;283:27469–27476. doi: 10.1074/jbc.M804043200. [DOI] [PubMed] [Google Scholar]
- 11.Leidel S, Pedrioli PG, Bucher T, Brost R, Costanzo M, et al. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458:228–232. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- 12.Noma A, Sakaguchi Y, Suzuki T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 2009;37:1335–1352. doi: 10.1093/nar/gkn1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tumaitis TD, Lane BG. Differential labelling of the carboxymethyl and methyl substituents of 5-carboxymethyluridine methyl ester, a trace nucleoside constituent of yeast transfer RNA. Biochim Biophys Acta. 1970;224:391–403. doi: 10.1016/0005-2787(70)90572-1. [DOI] [PubMed] [Google Scholar]
- 14.Otero G, Fellows J, Li Y, de Bizemont T, Dirac AM, et al. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol Cell. 1999;3:109–118. doi: 10.1016/s1097-2765(00)80179-3. [DOI] [PubMed] [Google Scholar]
- 15.Krogan NJ, Greenblatt JF. Characterization of a six-subunit holo-elongator complex required for the regulated expression of a group of genes in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:8203–8212. doi: 10.1128/MCB.21.23.8203-8212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler GS, Petrakis TG, Ethelberg S, Tokunaga M, Erdjument-Bromage H, et al. RNA polymerase II elongator holoenzyme is composed of two discrete subcomplexes. J Biol Chem. 2001;276:32743–32749. doi: 10.1074/jbc.M105303200. [DOI] [PubMed] [Google Scholar]
- 17.Wittschieben BO, Otero G, de Bizemont T, Fellows J, Erdjument-Bromage H, et al. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol Cell. 1999;4:123–128. doi: 10.1016/s1097-2765(00)80194-x. [DOI] [PubMed] [Google Scholar]
- 18.Paraskevopoulou C, Fairhurst SA, Lowe DJ, Brick P, Onesti S. The Elongator subunit Elp3 contains a Fe4S4 cluster and binds S-adenosylmethionine. Mol Microbiol. 2006;59:795–806. doi: 10.1111/j.1365-2958.2005.04989.x. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood C, Selth LA, Dirac-Svejstrup AB, Svejstrup JQ. An iron-sulfur cluster domain in Elp3 important for the structural integrity of elongator. J Biol Chem. 2009;284:141–149. doi: 10.1074/jbc.M805312200. [DOI] [PubMed] [Google Scholar]
- 20.Jablonowski D, Butler AR, Fichtner L, Gardiner D, Schaffrath R, et al. Sit4p protein phosphatase is required for sensitivity of Saccharomyces cerevisiae to Kluyveromyces lactis zymocin. Genetics. 2001;159:1479–1489. doi: 10.1093/genetics/159.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jablonowski D, Fichtner L, Stark MJ, Schaffrath R. The Yeast Elongator Histone Acetylase Requires Sit4-dependent Dephosphorylation for Toxin-Target Capacity. Mol Biol Cell. 2004;15:1459–1469. doi: 10.1091/mbc.E03-10-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fichtner L, Frohloff F, Burkner K, Larsen M, Breunig KD, et al. Molecular analysis of KTI12/TOT4, a Saccharomyces cerevisiae gene required for Kluyveromyces lactis zymocin action. Mol Microbiol. 2002;43:783–791. doi: 10.1046/j.1365-2958.2002.02794.x. [DOI] [PubMed] [Google Scholar]
- 23.Fichtner L, Jablonowski D, Schierhorn A, Kitamoto HK, Stark MJ, et al. Elongator's toxin-target (TOT) function is nuclear localization sequence dependent and suppressed by post-translational modification. Mol Microbiol. 2003;49:1297–1307. doi: 10.1046/j.1365-2958.2003.03632.x. [DOI] [PubMed] [Google Scholar]
- 24.Frohloff F, Fichtner L, Jablonowski D, Breunig KD, Schaffrath R. Saccharomyces cerevisiae Elongator mutations confer resistance to the Kluyveromyces lactis zymocin. EMBO J. 2001;20:1993–2003. doi: 10.1093/emboj/20.8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frohloff F, Jablonowski D, Fichtner L, Schaffrath R. Subunit communications crucial for the functional integrity of the yeast RNA polymerase II elongator (gamma-toxin target (TOT)) complex. J Biol Chem. 2003;278:956–961. doi: 10.1074/jbc.M210060200. [DOI] [PubMed] [Google Scholar]
- 26.Bar C, Zabel R, Liu S, Stark MJ, Schaffrath R. A versatile partner of eukaryotic protein complexes that is involved in multiple biological processes: Kti11/Dph3. Mol Microbiol. 2008;69:1221–1233. doi: 10.1111/j.1365-2958.2008.06350.x. [DOI] [PubMed] [Google Scholar]
- 27.Jablonowski D, Taubert JE, Bar C, Stark MJ, Schaffrath R. Distinct subsets of Sit4 holophosphatases are required for inhibition of Saccharomyces cerevisiae growth by rapamycin and zymocin. Eukaryot Cell. 2009;8:1637–1647. doi: 10.1128/EC.00205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehlgarten C, Schaffrath R. Mutant casein kinase I (Hrr25p/Kti14p) abrogates the G1 cell cycle arrest induced by Kluyveromyces lactis zymocin in budding yeast. Mol Genet Genomics. 2003;269:188–196. doi: 10.1007/s00438-003-0807-5. [DOI] [PubMed] [Google Scholar]
- 29.Mehlgarten C, Jablonowski D, Breunig KD, Stark MJ, Schaffrath R. Elongator function depends on antagonistic regulation by casein kinase Hrr25 and protein phosphatase Sit4. Mol Microbiol. 2009;73:869–881. doi: 10.1111/j.1365-2958.2009.06811.x. [DOI] [PubMed] [Google Scholar]
- 30.Petrakis TG, Sogaard TM, Erdjument-Bromage H, Tempst P, Svejstrup JQ. Physical and functional interaction between Elongator and the chromatin-associated Kti12 protein. J Biol Chem. 2005;280:19454–19460. doi: 10.1074/jbc.M413373200. [DOI] [PubMed] [Google Scholar]
- 31.Kalhor HR, Clarke S. Novel methyltransferase for modified uridine residues at the wobble position of tRNA. Mol Cell Biol. 2003;23:9283–9292. doi: 10.1128/MCB.23.24.9283-9292.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Songe-Møller L, van den Born E, Leihne V, Vagbo CB, Kristoffersen T, et al. Mammalian ALKBH8 possesses tRNA methyltransferase activity required for the biogenesis of multiple wobble uridine modifications implicated in translational decoding. Mol Cell Biol. 2010;30:1814–1827. doi: 10.1128/MCB.01602-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazauric MH, Dirick L, Purushothaman SK, Björk GR, Lapeyre B. Trm112p is a 15-kDa zinc finger protein essential for the activity of two tRNA and one protein methyltransferases in yeast. J Biol Chem. 2010;285:18505–18515. doi: 10.1074/jbc.M110.113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dan Burke DD, Stearns T. Methods in yeast genetics. Cold Spring Harbor Laboratory Press. 2000.
- 35.Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Björk GR, Jacobsson K, Nilsson K, Johansson MJ, Byström AS, et al. A primordial tRNA modification required for the evolution of life? EMBO J. 2001;20:231–239. doi: 10.1093/emboj/20.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gehrke CW, Kuo KC, McCune RA, Gerhardt KO, Agris PF. Quantitative enzymatic hydrolysis of tRNAs: reversed-phase high-performance liquid chromatography of tRNA nucleosides. J Chromatogr. 1982;230:297–308. [PubMed] [Google Scholar]
- 38.Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–147. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- 39.Gavin AC, Aloy P, Grandi P, Krause R, Boesche M, et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440:631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- 40.Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- 41.Yu H, Braun P, Yildirim MA, Lemmens I, Venkatesan K, et al. High-quality binary protein interaction map of the yeast interactome network. Science. 2008;322:104–110. doi: 10.1126/science.1158684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purushothaman SK, Bujnicki JM, Grosjean H, Lapeyre B. Trm11p and Trm112p are both required for the formation of 2-methylguanosine at position 10 in yeast tRNA. Mol Cell Biol. 2005;25:4359–4370. doi: 10.1128/MCB.25.11.4359-4370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heurgue-Hamard V, Graille M, Scrima N, Ulryck N, Champ S, et al. The zinc finger protein Ynr046w is plurifunctional and a component of the eRF1 methyltransferase in yeast. J Biol Chem. 2006;281:36140–36148. doi: 10.1074/jbc.M608571200. [DOI] [PubMed] [Google Scholar]
- 44.van den Born E, Vagbo CB, Songe-Møller L, Leihne V, Lien GF, et al. ALKBH8-mediated formation of a novel diastereomeric pair of wobble nucleosides in mammalian tRNA. Nat Commun. 2011;2:172. doi: 10.1038/ncomms1173. [DOI] [PubMed] [Google Scholar]
- 45.Moukadiri I, Prado S, Piera J, Velazquez-Campoy A, Björk GR, et al. Evolutionarily conserved proteins MnmE and GidA catalyze the formation of two methyluridine derivatives at tRNA wobble positions. Nucleic Acids Res. 2009;37:7177–7193. doi: 10.1093/nar/gkp762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagervall TG, Edmonds CG, McCloskey JA, Björk GR. Transfer RNA(5-methylaminomethyl-2-thiouridine)-methyltransferase from Escherichia coli K-12 has two enzymatic activities. J Biol Chem. 1987;262:8488–8495. [PubMed] [Google Scholar]
- 47.Bujnicki JM, Oudjama Y, Roovers M, Owczarek S, Caillet J, et al. Identification of a bifunctional enzyme MnmC involved in the biosynthesis of a hypermodified uridine in the wobble position of tRNA. RNA. 2004;10:1236–1242. doi: 10.1261/rna.7470904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiorentini P, Huang KN, Tishkoff DX, Kolodner RD, Symington LS. Exonuclease I of Saccharomyces cerevisiae functions in mitotic recombination in vivo and in vitro. Mol Cell Biol. 1997;17:2764–2773. doi: 10.1128/mcb.17.5.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J, Huang B, Esberg A, Johansson MJO, Byström AS. The Kluyveromyces lactis g-toxin targets tRNA anticodons RNA. 2005;11:1648–1654. doi: 10.1261/rna.2172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HPLC analysis of modified nucleosides in
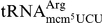 isolated
from wild-type,
trm11Δ,
lys9Δ and
mtq2Δ strains.
Arrows in red and black indicate expected retention time of mcm5U
and cm5U, respectively. Arrow heads in red and black indicate
expected retention time of m2G and ncm5U,
respectively. (A–D), Part of the chromatogram between retention times
34 and 44 min is shown. (B), Part of the chromatogram between retention
times 7 and 17 min is shown. The small peak in wild-type at 14 min
represents an unrelated compound with a spectrum different from
ncm5U. Absorbance at 254 nm (AU) was used to create the
chromatograms.
isolated
from wild-type,
trm11Δ,
lys9Δ and
mtq2Δ strains.
Arrows in red and black indicate expected retention time of mcm5U
and cm5U, respectively. Arrow heads in red and black indicate
expected retention time of m2G and ncm5U,
respectively. (A–D), Part of the chromatogram between retention times
34 and 44 min is shown. (B), Part of the chromatogram between retention
times 7 and 17 min is shown. The small peak in wild-type at 14 min
represents an unrelated compound with a spectrum different from
ncm5U. Absorbance at 254 nm (AU) was used to create the
chromatograms.
(EPS)