Abstract
HIV partner services can effectively reach populations with high HIV prevalence. However, located and notified sex and needle-sharing partners of persons infected with HIV often fail to test. Field testing may increase the proportion of notified partners who test for HIV. In 2008, New York City's health department incorporated field testing into partner services. After the introduction of field testing, the proportion of notified partners who tested for HIV rose from 52% to 76% (P<.001). HIV prevalence fell slightly among notified partners who accepted testing (12% to 9%, P=.82), but we identified more than double the number of new positives (11 vs 25). All positive and 97% of negative results were received by the person tested.
KEY FINDINGS
Field testing can be a simple addition to a preexisting partner services program
The OraSure conventional oral fluid HIV test is well suited for field testing
The proportion of notified partners who tested increased from 52% to 76% after field testing was introduced
Implementing field testing may not increase the proportion of newly diagnosed partners identified per test, but did increase the overall number of newly diagnosed partners
Start-up costs were manageable and training of staff was simple
PARTNER SERVICES CAN effectively reach a population with high HIV prevalence.1 However, located and notified partners of persons infected with HIV often fail to test.1 Field testing may help reduce the barriers to testing for notified partners. Although the Centers for Disease Control and Prevention's Recommendations for Partner Services Programs discusses field testing for HIV infection,2 we only identified 1 published report of a program that used field testing as part of a partner services program.3,4 That study used rapid-testing technology in both field and clinic settings and found that 78% of notified partners tested for HIV. However, the study did not report on changes in the proportion of partners who tested compared with pre-existing procedures, nor did it discuss operational aspects of field testing. In 2006, the New York City Department of Health and Mental Hygiene (DOHMH) created the Field Services Unit (FSU) to assist HIV-diagnosing providers at non-DOHMH facilities with partner services.5 The FSU notified persons designated as sex and needle-sharing partners of a recently confirmed HIV-positive person of their exposure to HIV and referred or escorted them to a clinic for HIV testing. In its first full year of operation (September 1, 2006–August 31, 2007), only 52% (95 of 181) of FSU-notified partners not known to be HIV-infected at the time of notification tested for HIV. FSU staff reported during case review meetings that partners refused escort to clinics for testing mostly because of perceived inconvenience or fear of being seen by people they knew at a clinic. FSU staff also noted during supervisory review that, in their experience, repeat visits to partners who initially refused testing were time-consuming and did not lead to greater testing acceptance. To increase the proportion of partners notified in the field who tested, the FSU staff supplemented clinic referrals with the introduction of field testing in February 2008. We evaluated the change in proportion of partners tested for HIV before and after the introduction of field testing. This study is important because it evaluates a practical alternative to referring partners notified in the field of their exposure to HIV to a clinic for testing and outlines the methods and cost implications of adopting conventional oral fluid HIV-testing in a field setting.
FIELD TESTING
The FSU considered using rapid testing in the field (e.g., residence, car), but found the conventional OraSure HIV-1 (OraSure Technologies, Inc., Bethlehem, PA) tests to be operationally simpler and better adapted to the field environment. OraSure requires 1 oral specimen collection to obtain a confirmatory result.6,7 Specimens can be collected in various settings without requirement for a level clean surface, and staff training is simple.6,7 Tests are portable and stable at room temperature for up to 30 days after specimen collection.
A written protocol outlined procedures; staff were trained by FSU management and OraSure representatives. FSU staff carried test kits to every field-based HIV partner notification in a thermal lunch bag. Before notification, FSU staff confirmed a partner's identity and ensured confidentiality of location or invited the partner to an official car for the notification. HIV testing was offered after notification. Written consent and specimen collection took approximately 15 minutes. Specimens were packaged and dropped off at a courier box for delivery to a commercial laboratory where enzyme immunoassays were used for screening and Western blots confirmed positive results. Quality assurance was performed by the laboratory according to their routine, clinical testing protocols. Results were available in 2 to 5 days. FSU staff verified contact information and made arrangements with tested individuals for the location and timing of delivery of test results. FSU staff delivered positive results in person; negative results were delivered either in person or by telephone, depending on partners’ preference. FSU staff assisted–partners who tested HIV-positive with entry into HIV medical care.
The impact of the field testing intervention was evaluated by comparing testing data collected from preintervention (September 1, 2006–August 31, 2007) and intervention (September 1, 2008–August 31, 2009) periods. These periods allow for programmatic phase-in of field testing and maintain comparable durations. We excluded partners tested before the index patient interview or those referred to another jurisdiction for notification. All HIV-positive results were reported to DOHMH. DOHMH documented negative tests if (1) the FSU staff performed the test, (2) a medical provider confirmed test results, or (3) partners showed the FSU staff written test results or signed a New York State release of medical information permitting staff to access medical records. Self-reported results were not considered valid.
EVALUATION
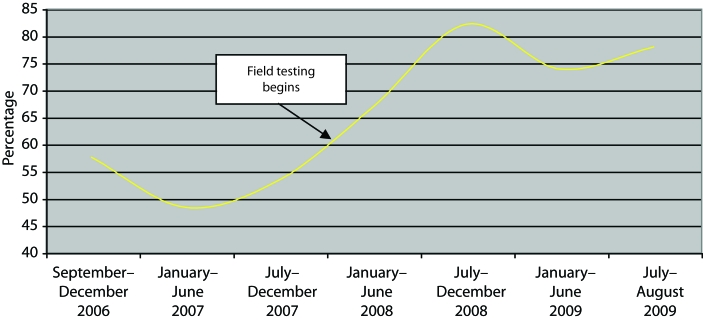
We notified more partners in the intervention phase (351) compared with the preintervention period (181), with a significant increase in the proportion of young (aged 13–29 years) partners notified (P=.004) but no change in the proportion of partners notified by sex or race/ethnicity. Introduction of field testing had a significant effect on testing. In the preintervention phase, 52% (95 of 181) of partners not known to be HIV-infected at notification were tested (Table 1). After implementation of field testing, this proportion rose to 76% (268 of 351; P<.001). Testing proportions increased significantly for women and men, male sexual partners of male patients, all age groups, Blacks, and Hispanics. Although testing proportions varied slightly in the preintervention period, the increase in the proportion of notified partners who tested was substantial and sustained in the intervention period (Figure 1). During the intervention period, partners chose a field test over a clinic test 84% of the time. All 12 partners who field-tested HIV-positive and 97% (206 of 212) who field-tested negative received their results. Those who did not receive results refused them or could not be located. Overall seroprevalence of tested partners decreased slightly from 12% (11 new diagnoses per 95 clinic tests only) to 9% (25 new diagnoses per 268 field and clinic tests; P=.82) after the introduction of field testing. Despite this drop in seroprevalence, the number of newly diagnosed persons more than doubled (11 vs 25) as a result of increased testing.
TABLE 1.
Proportion of Notified Partners of HIV-Infected Patients Who Tested for HIV Before (September 1, 2006–August 31, 2007) and After (September 1, 2008–August 31, 2009) the Introduction of HIV Field Testing: New York, NY
| Tested Before Introduction, No. (%)a | Tested After Introduction, No. (%) | Pb | |
| Total tested of those notified | 95 (52) | 268 (76) | <.01 |
| Sex | |||
| Female | 40 (59) | 99 (80) | <.01 |
| Male | 55 (49) | 169 (74) | <.01 |
| Age, y | |||
| 13–29 | 26 (49) | 107 (78) | <.01 |
| ≥30 | 68 (57) | 160 (77) | <.01 |
| Unknown | 1 (11) | 1 (20) | |
| Race/ethnicity | |||
| Black | 53 (56) | 158 (76) | <.01 |
| Hispanic | 37 (54) | 91 (79) | <.01 |
| Other | 5 (36) | 17 (65) | .07 |
| Unknown | 0 (0) | 2 (67) | .4 |
| Men who have sex with menc | 13 (38) | 47 (61) | .03 |
Proportion of notified partners (number notified not shown) with negative or unknown status at time of notification.
χ2 and Fisher's exact tests were used to calculate significant changes between preintervention and intervention. periods.
Denominator is notified male sex partners.
FIGURE 1.
Proportion of notified partners of HIV-infected patients who tested for HIV before (September 1, 2006–August 31, 2007) and after (September 1, 2008–August 31, 2009) the introduction of HIV field testing: New York, NY.
TESTING COSTS
We estimated testing costs of referring a notified partner to a clinic for field testing (based on testing in New York City DOHMH sexually transmitted disease [STD] clinics) to testing them in the field, using the actual number of tests performed in each period (Table 2). Direct material costs were capitalized over 3 years. Because the STD clinics performed ongoing testing, start-up costs were not included in the preintervention period. Time allocated for each activity was based on interviews with staff and observation in the field. HIV-negative tests, which represented the vast majority of tests, averaged $119 per clinic test versus $123 per field test, whereas HIV-positive tests averaged $126 per clinic test versus $167 per field test. The salary of the field testing coordinator had the largest impact on the cost differential between clinic and field testing. Because the majority of costs were personnel-related and labor costs are high in New York City, other jurisdictions may find implementing field testing less costly.
TABLE 2.
Costs of Referring Notified Partners to a Health Department Sexually Transmitted Disease (STD) Clinic for Testing Compared with Testing Partners in the Field: New York, NY
| STD Clinic Testinga |
Field Testingb |
|||
| Description of Cost | Cost per Unit, US$ or Minutes | Cost per Test, 2009 US$ | Cost per Unit, US$ or Minutes | Cost per Test, 2009 US$ |
| Start-up costs | ||||
| Fax machine, incubator, cooler bags, timers, thermometersc | $0 | $0 | $1435 | $6 |
| Recurring costs | ||||
| Test kits | $12d | $39e | ||
| Costs for confirmatory results | $46 | $5 | $70f | $4 |
| Personnel costs | ||||
| Field testing coordinator | g | $28 | h | $46 |
| Escort partner to clinic for test | 120 | $60 | 0 | $0 |
| Triage and registration | 10 | $4 | 0 | $0 |
| DIS to collect specimen | 15 | $8 | 15 | $8 |
| DIS to fill in paperwork and drop off specimen | 0 | $0 | 30 | $15 |
| DIS to return a positive result | 20 | $10 | 45 | $23 |
| Supervising DIS to accompany DIS for positive result | 0 | 0 | 45 | $26 |
| DIS to return a negative result | 5 | $3 | 10 | $5 |
| Total costs | ||||
| Cost per positive test | $126 | $167 | ||
| Cost per negative test | $119 | $123 | ||
Note. Salaries were estimated for staff 2 years on board: testing coordinatorshairsp;=hairsp;$52 462, clerk for triage and registration=$31 852, phlebotomist=$38 113, interviewing staff=$45 585, supervising staff=$53 000. Times were estimated based on interviews with staff and observation in the field.
Costs for testing in a clinic were estimated using the number of tests conducted in the preintervention period of September 1, 2006–August 30, 2007 (95 tests, 11 positive results) for notified partners referred to a health department STD clinic.
Costs for testing in the field were estimated using the number of field tests conducted during the intervention period of September 1, 2008–August 30, 2009 (226 field tests, 12 positive results). 226 of 268 (84%) of the tests performed during the intervention were conducted in the field.
Direct materials costs for field testing were capitalized over 3 years. STD clinics had ongoing testing, so start-up costs were not included.
OraQuick rapid test.
OraSure conventional test.
A surcharge of $70 was assessed for all positive tests above a 4% HIV prevalence. Below the 4% threshold, the cost of a confirmatory test was included in the OraSure test cost of $39.
5% full-time equivalent.
20% full-time equivalent.
LIMITATIONS
In both periods, only verified results were documented. During the preintervention period, we relied on provider report, partner-provided written documentation, or release of medical records to obtain negative results. All positive results are reported to the health department as required by law. In the intervention period, as the testing provider, the FSU staff had access to all field test results. This may have led us to underestimate clinic-based negative tests and, therefore, overestimate the difference in testing proportions observed between intervention periods. Another limitation is that we did not have a control group, and thus observed improvements between periods could be a result of improvements in staff performance after training and experience gained in the field. Though these limitations may overestimate the difference in partners tested between periods, we believe that field testing did lead to more notified partners tested because of the substantial increase in the number of tests performed. Another limitation is that in June of 2008, a few months after the FSU staff introduced field testing, the New York City health department launched a testing campaign in the Bronx, one of the city's 5 boroughs. Though advertising associated with this campaign may have potentially increased partners’ willingness to test for HIV in that borough, we saw no difference in the proportion of notified partners willing to test by borough.
CHALLENGES
The largest challenge the FSU faced was convincing staff that field testing would not add significantly to workload nor create risk for personnel. Staff were particularly concerned about returning HIV-positive results in an uncontrolled environment where they would be alone with a partner. Staff fears were allayed by the several-day lag between specimen collection and return of results (given that a conventional test was used instead of a rapid one) and a policy that included supervisory or medical review to prepare and accompany staff delivering a positive test result. Outlining field testing procedures in a written protocol assisted us with staff training and facilitated a consistent approach in the field.
NEXT STEPS
Based on our findings that field testing was operationally manageable and costs were feasible, we will continue to offer OraSure field testing to partners not known to be HIV infected at the time of their notification. Testing partners at notification improved testing outcomes, including among populations disproportionately affected by HIV, and we were able to deliver almost all results, including all positive results. Other partner services programs wishing to improve the proportion of exposed partners tested for HIV should consider field testing.
Acknowledgments
This work was funded by the Centers for Disease Control and Prevention (CDC; grant 07768). Data were presented as an abstract at the 2010 CDC National Sexually Transmitted Disease Prevention Conference.
We acknowledge the Field Services Unit (FSU) staff who skillfully implemented field testing, especially Elena Flores, who pioneered the program for the FSU and Mildred McNeal, our field testing coordinator. We also thank Charu Jain Sabharwal, Samuel Jenness, and Colin Shepard for reviewing drafts.
Human Participant Protection
No institutional review board approval was necessary because this was a programmatic evaluation of adding field testing into partner services, an ongoing Department of Health and Mental Hygiene program. Partners provided written informed consent prior to HIV testing as per current New York State law requirements.
References
- 1.Hogben M, McNally T, McPheeters M, Hutchinson AB. The effectiveness of HIV partner counseling and referral services in increasing identification of HIV-positive individuals: a systematic review. Am J Prev Med. 2007;33(2 Suppl):S89–S100 [DOI] [PubMed] [Google Scholar]
- 2.Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83; quiz CE1-4 [PubMed] [Google Scholar]
- 3.Begley EB, Oster AM, Song B, et al. Incorporating rapid HIV testing into partner counseling and referral services. Public Health Rep. 2008;123(Suppl 3):126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrestha RK, Begley EB, Hutchinson AB, et al. Costs and effectiveness of partner counseling and referral services with rapid testing for HIV in Colorado and Louisiana, United States. Sex Transm Dis. 2009;36(10):637–641 [DOI] [PubMed] [Google Scholar]
- 5.Udeagu CC, Bocour A, Gale I, Begier EM. Provider and client acceptance of a health department enhanced approach to improve HIV partner notification in New York City. Sex Transm Dis. 2010;37(4):266–271 [DOI] [PubMed] [Google Scholar]
- 6.Orasure. IV-1 Oral Specimen Collection Device Product Information. Available at: http://www.orasure.com/products-infectious/products-infectious-oralfluid.asp. Accessed March 7, 2011.
- 7.Vargo S, Agronick G, O'Donnell L, Stueve A. Using peer recruitment and OraSure to increase HIV testing. Am J Public Health. 2004;94(1):29–31 [DOI] [PMC free article] [PubMed] [Google Scholar]