Abstract
Objectives. We examined relationships between herpes simplex virus type 2 (HSV-2), a biomarker for sexual risk, and HCV, a biomarker for injecting risk, with HIV among injecting drug users (IDUs) who began injecting after large-scale expansion of syringe exchange programs in New York City.
Methods. We recruited 337 heroin and cocaine users who began injecting in 1995 or later from persons entering drug detoxification. We administered a structured interview covering drug use and HIV risk behavior and collected serum samples for HIV, HCV, and HSV-2 testing.
Results. HIV prevalence was 8%, HSV-2 39%, and HCV 55%. We found a significant association between HSV-2 and HIV (odds ratio [OR] = 7.9; 95% confidence interval [CI] = 2.9, 21.4) and no association between HCV and HIV (OR = 1.14; 95% CI = 0.5, 2.6). Black IDUs had the highest prevalence of HSV-2 (76%) and HIV (24%) but the lowest prevalence of HCV (34%).
Conclusions. Most HIV infections among these IDUs occurred through sexual transmission. The relative importance of injecting versus sexual transmission of HIV may be critical for understanding racial/ethnic disparities in HIV infection.
Persons who inject drugs, or injecting drug users (IDUs), are at risk for HIV infection through both multiperson use (sharing) of needles and syringes and unprotected sex. Sharing needles and syringes is a considerably more efficient mode of HIV transmission than is heterosexual intercourse,1,2 so in most epidemiological situations, injecting-related transmission is much more important than is sexual transmission. This relative efficiency of transmission is reflected in the current Centers for Disease Control and Prevention transmission classification system, in which persons with both injecting drug risk and heterosexual risk behavior are placed in the injecting drug use transmission category only.3
However, several factors may change the relative importance of injecting versus sexual transmission of HIV among IDUs. First, programs to prevent injecting-related transmission can be quite effective. In areas where combined HIV prevention programs (including syringe exchange, drug abuse treatment, community outreach, and voluntary HIV counseling and testing)4 have been implemented, injecting-related transmission has been substantially reduced and sexual transmission can be more important among IDUs. This effect appears to have occurred in Amsterdam5,6 and Chicago.7
Second, use of certain drugs may be associated with unsafe sexual behaviors and thus increase the importance of sexual transmission of HIV in populations of injecting and noninjecting drug users. Crack cocaine8,9 and, more recently, methamphetamine10,11 are probably the 2 most important examples of this phenomenon.
Third, some sexually transmitted diseases, such as syphilis and herpes simplex virus type 2 (HSV-2), may increase HIV transmission among both injecting and noninjecting drug users. There is considerable biological and epidemiological evidence that HSV-2 infection facilitates both acquiring and transmitting HIV. Two meta-analyses and a recent qualitative review have concluded that prevalent HSV-2 infection is associated with a two- to threefold increased likelihood of acquiring HIV.12–14 Although most research on HSV-2 and HIV has been conducted in Africa, several studies indicate positive associations between HSV-2 and HIV among injecting and noninjecting drug users in the United States.15 Because HSV-2 is transmitted sexually but not through sharing drug injection equipment, it can be used as a biomarker for sexual risk among IDUs.16
Assessing the relative importance of injecting versus sexual transmission of HIV among IDUs may also have great importance for understanding racial/ethnic differences in HIV infection among injecting and noninjecting drug users.
We examined relationships between HSV-2 and HIV among IDUs who began injecting after arge-scale implementation of syringe exchange programs from 1992 to 1998 in New York City. The expanded programs included not only a much greater volume of syringes exchanged but also increases in services such as voluntary HIV counseling and testing and referrals to drug abuse treatment. Thus, the large-scale expansion of the syringe exchange programs can be seen as the beginning of combined HIV prevention programs for IDUs17–19 in New York City. The expansion of the syringe exchange programs was followed by a reduction in HIV incidence from approximately 4/100 person-years to 1/100 person-years among IDUs in New York City.20
We chose the term “persons who inject drugs,” which emphasizes that these individuals should be considered persons first and that they are much more than the behavior of injecting drug use. However, we address official classification of HIV transmission routes, so we use the current standard terms “injecting drug use” and “injecting drug users” and the abbreviation “IDU.” We want to emphasize that HIV prevention for persons who inject drugs should fully consider their human rights.
METHODS
We derived our data from drug users entering the Beth Israel Medical Center drug detoxification program in New York City. The methods for this risk factors study have been described in detail,16,21 so we will summarize the most relevant aspects here. The Beth Israel detoxification program serves New York City as a whole, and approximately half of its patients live in Manhattan, one quarter in Brooklyn, one fifth in the Bronx, and the remainder (5%) elsewhere. Patients enter the program voluntarily.
Trained interviewers, who were all phlebotomists, rotated through the general admission wards of the program in a preset order. When starting work in a specific ward, the interviewer examined all intake records of that ward to construct a list of patients admitted within the prior 3 days. The interviewer then asked all the patients on this list to participate in the study. Of the patients our interviewers approached, more than 95% were willing to participate. After the interviewer asked all the patients on the list to participate and interviewed those who agreed, the interviewer moved to the next ward in the preset order. Because there was no relationship between assigning patients to wards and the order in which the staff rotated through the wards, these procedures should have produced an unbiased sample of persons entering the detoxification program.
The interviewer administered a structured questionnaire covering demographics, drug use, sexual risk behavior, and use of HIV prevention services. The questionnaire was used in previous research with drug users in New York and internationally, and there is considerable evidence of construct validity for this questionnaire.20,22,23 We included participants in the analyses if they had injected illicit drugs in the 6 months before the interview and had began injecting in 1995 or later. Most HIV risk behavior questions referred to the 6 months before the interview. We assessed the sexual behavior of men who have sex with men (MSM) by asking men about sex with other men in the 5 years before the interview. The questionnaire included the date of birth and age when illicit drugs were first injected to determine the year of first injection.
After the interview, an HIV counselor met with the participant for pretest counseling and specimen collection. We conducted HIV testing at the New York City Department of Health Laboratory using a commercial enzyme-linked immunosorbent assay (ELISA) test with Western blot confirmation (BioRad Genetic Systems HIV-1–2+0 ELISA and HIV-1 Western blot; BioRad Laboratories, Hercules, CA). BioReference Laboratories performed HSV-2 testing using the Focus HerpeSelect 1 and 2 ELISA. We used an optical density value of 1.1 or higher to classify a participant as HSV-2-seropostive. We also examined the distribution of the optical density scores. Of the participants, 10% had optical density values between 1.1 and 3.5 and were potentially false positives. If we had removed these participants from the analyses, it would have reduced the HSV-2 prevalence by approximately 4% but would not have changed any of the relationships between HSV-2 and HIV.
We tested samples for HCV antibody with the Ortho HCV enzyme immunoassay 4.0 (Ortho-Clinical Diagnostics, Raritan, NJ; Abbott Laboratories, Abbott Park, IL). We considered samples ≥ 8.0 positive. We confirmed samples with values of 1.0 to 8.0 with recombinant immunoblot assays. We considered samples with values < 1.0 negative. We did not perform confirmatory testing, as our previous studies have shown high sensitivity and specificity of this test in populations of IDUs.24,25
We have collected serial cross-sectional data in the project since 1990. We permitted individuals to participate in the study in different years. For the analyses reported here, however, we used only the first interview from persons who participated more than once between July 2005 and August 2009.
The analyses presented here focus on the relationships between HSV-2 and HIV and between HCV and HIV among IDUs who began injecting in New York City in 1995 or later. We chose the year 1995 because it is the midpoint for the large-scale expansion of syringe exchange programs in New York City.20 A previous analysis of a much smaller sample of IDUs who began injecting in 1995 or later also suggests that this was the period when the dominant mode of HIV transmission among IDUs in New York City shifted from injecting to sexual transmission.16 For convenience, we refer to IDUs who began injecting in New York in 1995 or later as combined program environment (CPE) IDUs.
We used the statistical software SAS version 9 (SAS Institute, Cary, NC) and Stata version 11 (StataCorp LP, College Station, TX) for analyses. We conducted regression tree analysis, a common procedure in public health and clinical analysis,26–28 by recursive partitioning using the RPART routines in R (R Development Core Team). The regression tree model included HSV-2 serostatus, HCV serostatus, years injecting, gender and MSM behavior, age, and race/ethnicity to predict HIV infection. At each node, the model applied a decision rule (e.g., HSV-2 seropositive individuals vs HSV-2 seronegative individuals) to split observations into mutually exclusive categories that corresponded to specific numbers of estimated and observed HIV-infected IDUs. The segmentation procedure selected the variable most highly predictive of HIV infection and segmented the sample on this variable. We repeated the segmentation procedure until further subgroupings did not produce differences in HIV infection rates.
RESULTS
Table 1 presents selected demographic characteristics and drug use behaviors of the 337 IDUs who began injecting in 1995 or later and whom we recruited into the study between July 19, 2005 and August 31, 2009. The participants primarily were men, were members of ethnic minority groups, had a mean age of 34 years, and had been injecting for a mean of 5 years. Almost all (97%) reported injecting heroin, either by itself or in combination with cocaine (speedball); 51% reported injecting cocaine by itself; and 79% reported injecting daily or more frequently in the 6 months before the interview.
TABLE 1.
Demographics and Drug Use Behaviors Among IDUs (n = 337) Who Started Injecting in 1995 or Later (Combined Prevention Environment): Risk Factors Study, New York City, 2005–2010
| Variable | No. (%) or Mean (SD) |
| Gender | |
| Men | 274 (81) |
| Women | 63 (19) |
| Race/ethnicity | |
| White | 105 (31) |
| Black | 41 (12) |
| Hispanic | 175 (52) |
| Other or mixed | 16 (5) |
| MSM | 25 (9) |
| Daily or more often injection | 267 (79) |
| Injection drug use | |
| Heroin | 326 (97) |
| Cocaine | 173 (51) |
| Smoking crack cocaine | 123 (36) |
| Age at time of study, y | 34 (7) |
| Age at first injection, y | 28 (8) |
| Years injecting | 5 (4) |
| HIV+ | 27 (8) |
| HSV-2+ | 133 (39) |
| HCV+a | 179 (55) |
Note. HSV-2 = herpes simplex virus type 2; IDU = injecting drug user; MSM = men who have sex with men.
HCV+ percentage is relative to the 327 IDUs for which HCV results were available.
Table 2 presents HCV, HSV-2, and HIV seroprevalence among the participants, both for the total group and by demographic and drug use characteristics. HCV prevalence was 55%, HSV-2 prevalence 39%, and HIV prevalence 8%. The patterns for HSV-2 and HIV by demographic characteristics were quite similar, with prevalence for both viruses higher among female IDUs and MSM IDUs compared with non-MSM male IDUs among Blacks, persons older than 40 years, and persons smoking crack cocaine in the 6 months before the interview. By contrast, HCV was associated with recent frequency of injection (daily or more frequent injection in the 6 months before the interview), injecting cocaine in the 6 months before the interview, years injecting, and race/ethnicity. Blacks had the lowest HCV prevalence.
TABLE 2.
HCV, HSV-2, and HIV Prevalence by Demographic Characteristics Among IDUs Who Began Injecting in 1995 or Later: Risk Factors Study, New York City, 2005–2010
| Variable | Total No. | HCV+, No. (%) | HSV-2+, No. (%) | HIV+, No. (%) |
| Total | 337 | 179 (55)a | 133 (39) | 27 (8) |
| Gender/MSM statusb | ||||
| Men (non-MSM) IDUs | 249 | 125 (52) | 70 (28)*** | 9 (4)*** |
| MSM IDUs | 25 | 16 (64) | 13 (52) | 7 (28) |
| Female IDUs | 62 | 38 (63) | 50 (81) | 11 (18) |
| Race/ethnicity | ||||
| White | 105 | 47 (47)** | 28 (27)*** | 3 (3)** |
| Black | 41 | 14 (34) | 31 (76) | 10 (24) |
| Hispanic | 175 | 108 (64) | 70 (40) | 14 (8) |
| Other or mixed | 16 | 10 (67) | 4 (25) | 0 (0) |
| Frequency of injection | ||||
| Daily or less often | 70 | 26 (37)*** | 27 (39) | 5 (7) |
| Daily or more often | 267 | 153 (60) | 106 (40) | 22 (8) |
| Heroin injection | ||||
| No | 11 | 5 (45) | 3 (27) | 1 (9) |
| Yes | 326 | 174 (55) | 130 (40) | 26 (8) |
| Cocaine injection | ||||
| No | 164 | 71 (45)*** | 68 (42) | 17 (10) |
| Yes | 173 | 108 (64) | 65 (38) | 10 (6) |
| Years injecting | ||||
| < 5 | 162 | 68 (43)*** | 68 (42) | 10 (6) |
| ≥ 5 | 175 | 111 (65) | 65 (37) | 17 (10) |
| Smoking crack cocaine | ||||
| No | 214 | 120 (58) | 78 (36) | 8 (4)*** |
| Yes | 123 | 59 (49) | 55 (45) | 19 (15) |
| Age, y | ||||
| < 40 | 259 | 138 (55) | 90 (35)** | 15 (6)** |
| ≥ 40 | 78 | 41 (55) | 43 (55) | 12 (15) |
Note. HSV-2 = herpes simplex virus type 2; IDU = injecting drug user; MSM = men who have sex with men.
HCV results were not available for 10 (3%) IDUs, including 1 HIV seropositive participant. HCV percentages are relative to the 327 IDUs or their demographic subsets, from which HCV results were available.
Gender or MSM and race/ethnicity are used as single, multicategory variables.
*P < .05; **P < .01; ***P < .001, χ2 test for comparison with demographic group.
Almost all the HIV seropositive participants were seropositive to at least 1 of the other viruses: 22/27 (81%) were seropositive for HSV-2, 15/26 (58%) for HCV, 13/26 (50%) for both HSV-2 and HCV, and only 3/26 (12%) for both HSV-2 and HCV. Among the 26 HIV seropositive individuals for whom we had serostatus data for both HSV-2 and HCV, 8 were HSV-2 seropositive and HCV seronegative, and 2 were HSV-2 seronegative and HCV seropositive. HCV results were not obtained for 1 HIV seropositive individual.
The seroprevalence patterns in Table 2 and coinfection data suggest a positive association between HSV-2 and HIV and a lack of association between HCV and HIV. This was indeed the case. The odds ratio (OR) for HSV-2 as a predictor of HIV serostatus was significant (OR = 7.9; 95% confidence interval [CI] = 2.9, 21.4). HCV status was not associated with HIV status (OR = 1.14; 95% CI = 0.5, 2.6). HCV was also not associated with HSV-2 status (OR = 1.4; 95% CI = 0.9, 2.1).
There were some extremely skewed distributions in factors associated with HIV serostatus, e.g., all HIV seropositive women were also HSV-2 seropositive, making it problematic to use conventional multiple logistic regression to identify independent predictors of HIV serostatus. We therefore used regression tree analysis29 to examine the relative strength of different predictors of HIV seropositive status among these IDUs who began injecting in an environment with large-scale syringe exchange or combined HIV prevention programs. The regression tree analysis progressively identified variables that divided the sample into subgroups with relatively high versus relatively low HIV seroprevalence. We also used the Fisher exact test to aid our interpretation of the regression tree. The potential predictors of HIV infection using the regression tree model were HSV-2 status, HCV status, the demographic characteristics in Tables 1 and 2, and the length of an individual's drug-injecting career. (We did not include risk behaviors in the 6 months before the interview in the regression tree analysis, as we believe that the great majority of the HIV infections would have occurred before this period.)
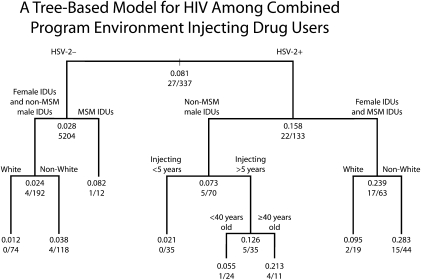
Results of the regression tree analysis are presented in Figure 1. Subgroups with relatively high HIV prevalence are on the right branches of the tree, and subgroups with relatively low HIV prevalence are on the left branches of the tree. The estimated HIV prevalence for each subgroup is represented by the decimal value at each node. The fraction at the bottom of each node represents the number of observed events (HIV seropositive IDUs) as the numerator and the total number of observations in that node (total number of IDUs) as the denominator. As actual participants (either HIV positive IDUs in numerators or total IDUs in denominators in each node) necessarily occur in whole numbers, it is extremely unlikely that there would be a perfect match between the prevalence estimated by the regression tree model and the actual seroprevalence in each node. However, there was clearly a close correspondence between the estimated HIV seroprevalence (given in decimals) and the observed HIV seroprevalence (given in fractions) across all the nodes, indicating a good fit for the regression tree estimates.
FIGURE 1.
A tree-based model for HIV among combined program environment injecting drug users: Risk Factors Study, New York City, 2005–2010.
Note. HSV-2 = herpes simplex virus type 2; IDU = injecting drug user; MSM = men who have sex with men. Non-White includes Black, Hispanic, and Other or mixed. Decimal value represent estimated HIV prevalence. Fractions represent the number of observed events (HIV seropositive IDUs) as the numerator and total number of observations (total number of IDUs) as the denominator.
The first variable distinguishing high versus low HIV prevalence subgroups was HSV-2 status. HIV prevalence was 17% among HSV-2 seropositive individuals and 3% among HSV-2 seronegative individuals (P ≤ .001, Fisher exact test). Among both the HSV-2 seronegative individuals and the HSV-2 seropositive individuals, the next predictor variable was gender and MSM behavior. Among HSV-2 seronegative individuals, HIV prevalence was 6% among MSM IDUs and 2% among the combined female IDUs and non-MSM male IDUs (P = .26). Among the HSV-2 seropositive individuals, HIV seroprevalence was 27% among the combined female IDUs and MSM IDUs and 7% among non-MSM male IDUs (P < .01). Race/ethnicity and years injecting were third-level predictors, but the numbers of HIV seropositive individuals at the third and fourth levels of prediction were relatively modest, so great caution is required in interpreting these results.
The regression tree analysis was generally quite consistent with the univariate associations of different variables with HIV. HIV was most strongly predicted by factors associated with sexual risk: HSV-2, and female gender and MSM sexual behavior. Factors associated with injecting-related risk—HCV infection and years injecting—either had no predictive value (HCV) or entered the prediction only at the later levels (injecting for 5 years or more).
DISCUSSION
HCV and HIV are both transmitted through multiperson use of injecting equipment, with HCV being considerably more transmissible. An important finding of this study is the relatively low HCV prevalence (58%) among the HIV seropositive CPE IDUs. In an international meta-analysis of HCV prevalence among IDUs, the median HCV seroprevalence among HIV seropositive IDUs was 90%.30 The relatively low HCV prevalence among the CPE IDUs in this study represents a historical change in New York City. In a previous study of IDUs recruited from the Beth Israel detoxification program, with serum collected in 1990–1991, 44 of 44 (100%) HIV seropositive individuals were also HCV seropositive. Almost half (19 of 44) of the HIV seropositive individuals in that study (before combined programs) were new injectors (injecting for 6 years or less), so the high HCV seroprevalence (100%) among the HIV seropositive individuals was not a result of long injecting histories.31
In another previous study of IDUs, who began injecting before 1995 and were recruited from the Beth Israel detoxification program between 2005 and 2007, 51 of 52 (98%) HIV seropositive individuals were also HCV seropositive. Thus, among IDUs who began injecting before the large-scale expansion of syringe exchange in New York, almost all HIV seropositive individuals were also HCV seropositive. A χ2 test of HCV seroprevalence among HIV seropositive IDUs who began injecting before 1995 (95 of 96) versus HCV prevalence among HIV seropositive IDUs who began injecting in 1995 or later (14 of 27) was highly significant (P ≤ .001). Combined HIV programs have been more effective for reducing injecting-related HIV transmission than for injecting-related HCV transmission. This epidemiological finding is common for IDUs in the United States and is the result of the much more efficient transmission of HCV (10 times more efficient) than that of HIV through multiperson use of injecting equipment15 and is not a reflection of differences in time in syringe exchange coverage.
A moderate number of studies of HIV and HSV-2 among injecting and noninjecting drug users.32 To our knowledge, the relationship found between HSV-2 and HIV among the CPE IDUs in this study (OR = 7.9; 95% CI = 2.9, 21.4) is the strongest relationship between these 2 viruses in a study of IDUs with at least a moderately large sample size.
The relationship between HSV-2 among these CPE IDUs may be best understood in the context of noninjecting use of heroin and cocaine, particularly crack cocaine, in New York City. Beginning in the mid-1980s, New York also experienced increases in noninjecting drug use, including smoking crack cocaine and intranasal heroin use.33,34 Crack use in particular was associated with exchange of sex for drugs and heterosexual transmission of HIV, particularly to women.8 In our recent study of HSV-2 and HIV among noninjecting drug users in New York, we found moderately high HIV prevalence (16%) and high HSV-2 prevalence (61%).35 HSV-2 was associated with HIV (OR = 3.2; 95% CI = 2.3, 4.5), and 80% of the HIV seropositive noninjecting drug users were also seropositive for HSV-2. Many heroin and cocaine injection users in New York City engage in noninjecting drug use for years before they begin injecting.36 (The average age of first injection among the participants in this study was 28 years.) The participants would thus have been at considerable risk for sexual acquisition of HIV before they began injecting.
The population attributable risk (PAR) percentage is often used to assess the importance of a risk factor.37 Briefly, the PAR is the proportion of the total disease in a population that can be attributed to a specific risk factor. It includes consideration of both the strength of the relationship between the risk factor and the disease and the prevalence of the risk factor in the population. If the specific risk factor was to be eliminated in the population, the disease in the population would be reduced by the PAR percentage.38 For example, a meta-analysis of studies on the relationship of smoking to lung cancer in China concluded that the PAR of smoking for squamous cell carcinoma was 65.44%.39
We did not find a significant association between HCV and HIV, so it is not meaningful to calculate a PAR percentage for HCV infection. In our study, a strong association (OR = 7.9) existed between HSV-2 and HIV, and 39% of participants were HSV-2 seropositive, corresponding to a PAR percentage of 71% of HSV-2 for HIV. There are at least 2 causal pathways linking HSV-2 infection and HIV infection: HSV-2 infection creates a biological vulnerability to HIV infection through recruiting target cells for HIV infection to the genital mucosa,40,41 and the behaviors that put one at risk for HSV-2 infection would also put one at risk for sexual acquisition of HIV. Two meta-analyses12,14 suggest that the HSV-2 biological mechanism increases risk for HIV acquisition by a factor of 2 to 3. This would correspond to a PAR of 40% to 54% among the participants in this study. The additional PAR percentage above the biological mechanism might then be attributed to common risk behaviors. Deconstructing the PAR percentage for HSV-2 to HIV infection in this population should be considered very tentative because it is derived from cross-sectional data and a relatively wide CI, but at 71%, the PAR percentage is clearly quite substantial.
Several limitations of this study should be noted. First, this is a cross-sectional study, so it was not possible to determine the order of HIV, HCV, and HSV-2 infections among the participants. It also was not possible to determine risk behavior before infection with the 3 viruses. However, the participants in this study had a mean age of 34 years and would have been at risk for infection with HSV-2 and HIV since sexual debut and at high risk for HCV since initiation of drug injecting. It would be extremely expensive to conduct a study to observe seroconversions for all 3 viruses in the same participants. Drawing inferences from cross-sectional studies may be the only practical method for studying relationships among the 3 viruses in IDU populations in most geographic areas. Second, the data in this report are also from drug users seen at a single institution. However, trends in HIV among IDUs seen at Beth Israel Medical Center do closely track trends in HIV among drug users in multiple other studies in New York City.42–44
HSV-2 is transmitted sexually, and HCV is transmitted primarily through multiperson use of drug-injecting equipment. Thus, different patterns in the prevalence of HSV-2 and HCV in the same population are not surprising. In this study of persons who began injecting drugs after large-scale implementation of syringe exchange programs in New York City, HSV-2 prevalence was associated with gender and MSM sexual behavior and race/ethnicity, with Blacks having the highest prevalence. By contrast, HCV was associated with length of injecting career and race/ethnicity, with Blacks having the lowest prevalence. The pattern of factors associated with HIV was very similar to that for HSV-2 and different from that associated with HCV. HIV was strongly associated with HSV-2 and not associated with HCV. All these results suggest that the majority of HIV infections among the IDUs in this study occurred through sexual transmission.
Three characteristics of the environment in which these persons injected drugs should be emphasized. (1) There is relatively good access to sterile injection equipment. (2) There is a substantial amount of unsafe sex and HIV-associated infection with noninjecting drug use (particularly crack cocaine use). (3) The high prevalence of HSV-2 would facilitate both sexual acquisition and sexual transmission of HIV.
The current hierarchical classification system for HIV transmission places injection drug use above heterosexual transmission.3 All HIV seropositive individuals who report both injecting risk behavior and heterosexual risk behavior are placed only in the injecting drug use category. This classification system may be misclassifying many HIV infections that occur in risk environments similar to that of New York City. Such misclassification would underestimate both the effectiveness of injecting-related prevention programs and the need for improved prevention programs to address sexual transmission among drug users.
An example of possible misclassification may be helpful. A simple examination of the differences in HIV prevalence by race/ethnicity in Table 2, in which Blacks have the highest HIV prevalence, could lead to a working conclusion that the syringe exchange programs are not providing adequate sterile injecting equipment to that group. Inspection of the HCV and HSV-2 columns in Table 2, however, leads to a working conclusion that the greatest need is preventing sexual transmission of HIV to persons who are already HSV-2 seropositive or are at high risk for acquiring HSV-2.
The HIV epidemic among IDUs is now more than 30 years old, and it is evolving in New York City and other areas. A great need still exists for reducing HIV infection among IDUs, but interventions should incorporate the evolving nature of the epidemic, particularly the increasing importance of sexual transmission.
Acknowledgments
This research was supported by the National Institutes of Health (grants DA 03574 and 2 P30 DA 11041).
Note. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institute on Drug Abuse or the Centers for Disease Control and Prevention.
Human Participant Protection
The Beth Israel institutional review board approved the study.
References
- 1.Bayoumi AM, Zaric GS. The cost-effectiveness of Vancouver's supervised injection facility. CMAJ. 2008;179(11):1143–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–1153 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention CDC HIV/AIDS. Atlanta, GA: Department of Health and Human Services; 2009 [Google Scholar]
- 4.Donoghoe M, Verster A, Mathers B. WHO, UNODC, UNAIDS Technical Guide for Countries to Set Targets for Universal Access to HIV Prevention, Treatment and Care for Injecting Drug Users. Geneva: World Health Organization; 2009 [Google Scholar]
- 5.Lindenburg CE, Krol A, Smit C, Buster MC, Coutinho RA, Prins M. Decline in HIV incidence and injecting, but not in sexual risk behaviour, seen in drug users in Amsterdam: a 19-year prospective cohort study. AIDS. 2006;20(13):1771–1775 [DOI] [PubMed] [Google Scholar]
- 6.van den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Amsterdam cohort. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102(9):1454–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouellet L, Huo D. Declines in HIV prevalence and incidence among injection drug users in Chicago, 1988–2007. : 2009 National HIV Prevention Conference; Atlanta, GA [Google Scholar]
- 8.Edlin BR, Irwin KL, Faruque S, et al. Intersecting epidemics—crack cocaine use and HIV infection among inner-city young adults. Multicenter Crack Cocaine and HIV Infection Study Team. N Engl J Med. 1994;331(21):1422–1427 [DOI] [PubMed] [Google Scholar]
- 9.Edlin BR, Irwin KL, Ludwig DD, et al. High-risk sexual behavior among young street-recruited crack cocaine smokers in three American cities: an interim report. The Multicenter Crack Cocaine and HIV Infection Study Team. J Psychoactive Drugs. 1992;24(4):363–371 [DOI] [PubMed] [Google Scholar]
- 10.Des Jarlais D, Semaan S, Arasteh A. At 30 years: HIV and other STDs among persons who use psychoactive drugs. : Hall B, Hall J, Cockerell C, HIV/AIDS in the Post-HAART Era: Manifestations, Treatment, and Epidemiology. Sudbury, MA: Jones and Barlett; 2011:753–778 [Google Scholar]
- 11.Semaan S, Steele B, Des Jarlais D, Malow R. Methamphetamine Use and HIV Risk Behaviors Among Heterosexual Adolescents and Adults in the United States. : XVI International AIDS Conference; 2006; Toronto, Canada [Google Scholar]
- 12.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Yayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83 [DOI] [PubMed] [Google Scholar]
- 13.Tobian A, Quinn T. Herpes simplex virus type 2 and syphilis infections with HIV: an evolving synergy in transmission and prevention. Curr Opin HIV AIDS. 2009;4(4):294–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185(1):45–52 [DOI] [PubMed] [Google Scholar]
- 15.Des Jarlais D, Semaan S. HIV and other sexually transmitted infections in injection drug users and crack cocaine smokers. : Holmes K, Sparling P, Stamm W, Piot P, Sexually Transmitted Diseases. New York: McGraw Hill; 2008:237–255 [Google Scholar]
- 16.Des Jarlais D, Arasteh K, McKnight C, Hagan H, Perlman D, Friedman S. Using hepatitis C virus and herpes simplex virus-2 to track HIV among injecting drug users in New York City. Drug Alcohol Depend. 2009;101(1–2):88–91 [DOI] [PubMed] [Google Scholar]
- 17.Coates T, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372(9639):669–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Des Jarlais DC, Arasteh K, McKnight C, et al. HIV infection during limited versus combined HIV prevention programs for IDUs in New York City: the importance of transmission behaviors. Drug Alcohol Depend. 2010;109(1–3):154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donoghoe MC, Verster A, Pervilhac C, Williams P. Setting targets for universal access to HIV prevention, treatment and care for injecting drug users (IDUs): towards consensus and improved guidance. Int J Drug Policy. 2008;19(suppl 1):S5–S14 [DOI] [PubMed] [Google Scholar]
- 20.Des Jarlais DC, Perlis T, Arasteh K, et al. HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services. Am J Public Health. 2005;95(8):1439–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Des Jarlais DC, Arasteh K, Perlis T, et al. Convergence of HIV seroprevalence among injecting and non-injecting drug users in New York City. AIDS. 2007;21(2):231–235 [DOI] [PubMed] [Google Scholar]
- 22.Des Jarlais DC, Friedman SR, Sotheran JL, et al. Continuity and change within an HIV epidemic. Injecting drug users in New York City, 1984 through 1992. JAMA. 1994;271(2):121–127 [PubMed] [Google Scholar]
- 23.Stimson GV, Des Jarlais DC, Ball A, Drug Injecting and HIV Infection: Global Dimensions and Local Responses. London: UCL Press; 1998 [Google Scholar]
- 24.Amon JJ, Garfein RS, Ahdieh-Grant L, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994–2004. Clin Infect Dis. 2008;46(12):1852–1858 [DOI] [PubMed] [Google Scholar]
- 25.Garfein RS, Sweartzendruber A, Ouellet LJ, et al. Methods to recruit and retain a cohort of young-adult injection drug users for the Third Collaborative Injection Drug Users Study/Drug Users Intervention Trial (CIDUS III/DUIT). Drug Alcohol Depend. 2007;91(suppl 1):S4–S17 [DOI] [PubMed] [Google Scholar]
- 26.Avila PC, Segal MR, Wong HH, Boushey HA, Fahy JV. Predictors of late asthmatic response. Logistic regression and classification tree analysis. Am J Public Health. 2000;161(6):2092–2095 [DOI] [PubMed] [Google Scholar]
- 27.Lauby JL, Semaan S, O'Connell A, Person B, Vogel A. Factors related to self-efficacy for use of condoms and birth control among women at risk for HIV infection. Women Health. 2001;34(3):71–91 [DOI] [PubMed] [Google Scholar]
- 28.Loh W, Logistic Regression Tree Analysis. New York: Springer; 2006 [Google Scholar]
- 29.Breiman L, Friedman J, Stone C, Olshen R. Classification and Regression Trees. New York: Chapman and Hall/CRC; 1984 [Google Scholar]
- 30.Hagan H, Scheinmann R, Des Jarlais DC, et al. Meta-analysis of HIV/HCV co-infection in injecting drug users. : National HIV Prevention Conference; 2006; Atlanta, GA [Google Scholar]
- 31.Des Jarlais DC, Perlis T, Arasteh K, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. AIDS. 2005;19(suppl 3):S20–S25 [DOI] [PubMed] [Google Scholar]
- 32.Semaan S, Des Jarlais D, Malow R. Sexually transmitted diseases among illicit drug users in the United States: the need for interventions. : Aral S, Douglas J, Behavioral Interventions for Prevention and Control of Sexually Transmitted Diseases, Including HIV. New York: Springer-SBM; 2007:397–430 [Google Scholar]
- 33.Des Jarlais DC, Arasteh K, Perlis T, et al. The transition from injection to non-injection drug use: long-term outcomes among heroin and cocaine users in New York City. Addiction. 2007;102(5):778–785 [DOI] [PubMed] [Google Scholar]
- 34.Frank B. An overview of heroin trends in New York City: past, present and future. Mt Sinai J Med. 2000;67(5–6):340–346 [PubMed] [Google Scholar]
- 35.Des Jarlais DC, Arasteh K, McKnight C, et al. Gender and age patterns in HSV-2 and HIV infection among non-injecting drug users in New York City. Sex Transm Dis. 2010;37(10):637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neaigus A, Atillasoy A, Friedman SR, et al. Trends in the non-injected use of heroin and factors associated with the transition to injecting. : Inciardi J, Harrison LD, Heroin in the Age of Crack Cocaine. Thousand Oaks, CA: Sage; 1998:131–159 [Google Scholar]
- 37.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins; 1998 [Google Scholar]
- 38.Greenland S. Meta-analysis. : Rothman K, Greenland S, Modern Epidemiology. Philadelphia: Raven-Lippincott; 1998:643–673 [Google Scholar]
- 39.Yu SZ, Zhao N. Combined analysis of case-control studies of smoking and lung cancer in China. Lung Cancer. 1996;14(suppl 1):S161–S170 [DOI] [PubMed] [Google Scholar]
- 40.Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6(1):20–28 [DOI] [PubMed] [Google Scholar]
- 41.Kapiga SH, Sam NE, Bang H, et al. The role of herpes simplex virus type 2 and other genital infections in the acquisition of HIV-1 among high-risk women in northern Tanzania. J Infect Dis. 2007;195(9):1260–1269 [DOI] [PubMed] [Google Scholar]
- 42.Des Jarlais DC, Marmor M, Friedmann P, et al. HIV incidence among injecting drug users in New York City, 1992–1997: evidence for a declining epidemic. Am J Public Health. 2000;90(3):352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Des Jarlais DC, Perlis T, Friedman SR, et al. Behavioral risk reduction in a declining HIV epidemic: injection drug users in New York City, 1990–1997. Am J Public Health. 2000;90(7):1112–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Des Jarlais DC, Perlis T, Friedman SR, et al. Declining seroprevalence in a very large HIV epidemic: injecting drug users in New York City, 1991 to 1996. Am J Public Health. 1998;88(12):1801–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]