Abstract
We used a validated smoking simulation model and data from the 2003 Tobacco Use Supplement to the Current Population Survey to project the impact that a US menthol ban would have on smoking prevalence and smoking-attributable deaths. In a scenario in which 30% of menthol smokers quit and 30% of those who would have initiated as menthol smokers do not initiate, by 2050 the relative reduction in smoking prevalence would be 9.7% overall and 24.8% for Blacks; deaths averted would be 633 252 overall and 237 317 for Blacks.
The Family Smoking Prevention and Tobacco Control Act1 authorized the Food and Drug Administration to establish the Center for Tobacco Products to regulate tobacco for the protection of the public health. The Center for Tobacco Products is charged with considering a ban on the menthol flavoring in cigarettes (menthols). The act specifies that in considering the impact of a ban, a broad public health standard is to be applied rather than a narrow individual standard of whether there is more or less harm to individual users of menthols. Although there is evidence that menthol plays a role in smoking initiation and cessation,2–6 little is known about the anticipated impact of such a ban on population-level smoking behavior and subsequent deaths that may be averted. Of particular interest is the effect of a ban on the Black population, which has substantially higher rates of menthol use than do other racial/ethnic groups.7
In the absence of an experimental or actual ban on menthols, simulation modeling can be a useful tool to understand the potential pathways and predict the anticipated effect of such a policy intervention.8 In the current study, we used a validated smoking simulation model, SimSmoke,9–14 in conjunction with plausible ranges of change in patterns of smoking behavior, to examine the potential impact of a menthol ban on future smoking prevalence and smoking-attributable deaths.
METHODS
We extended previous versions of the SimSmoke model to explicitly distinguish menthol and nonmenthol smokers. Separate models were developed for males and females, both for the total population and for Blacks. The model uses self-reported data from the 2003 Tobacco Use Supplement to the Current Population Survey (TUS-CPS) as well as initial population data for the year 2003.
We first distinguished among never, current, and former smokers. Current smokers were those who had smoked at least 100 cigarettes in their lifetime and smoked some or all days. Former smokers were those who had smoked at least 100 cigarettes in their lifetime but did not currently smoke, further distinguished by how many years ago they had quit smoking. Current and former smokers were also differentiated by cigarette type into menthol, nonmenthol, and no usual type, as defined by the TUS-CPS.15 We averaged data over 3-age-year groups (e.g., people aged 18–20 years) and then smoothed.
The smoking model simulates groups of individuals as they transition into and out of smoking through initiation, cessation, and relapse rates, following a discrete first-order Markov process. We measured initiation for each cigarette type through age 24 years as the change in smoking prevalence between successive age-year groups; this figure thus represents initiation net of cessation and switching between types for each age. We applied cessation rates after age 24 years in the model, measured as smokers who had quit in the past year but not in the past 3 months as a percentage of smokers 1 year ago.16 We constructed separate cessation rates by gender and type for 3-age-year groups and then smoothed. We applied the same relapse rates to former smokers by type, distinguished by age and gender on the basis of various sources.17–20
The influence of tobacco-control policies on initiation and cessation through the year 2010 were incorporated into the model by using measures of price, smoke-free air, and expenditure policies obtained from the Impacteen Web site (http://www.impacteen.org). We calibrated the model by comparing smoking rates from the model predicted for 2006 to smoking rates from the 2006 TUS-CPS.
We used the calibrated model to estimate the effect of banning menthol cigarettes as of the year 2011. A ban on menthol cigarettes may have 3 types of effects. First, some former menthol smokers may simply switch to smoking nonmenthol cigarettes (switching effect). However, in a recent preliminary analysis of 2010 TUS-CPS data, only 36.2% of all menthol smokers and 25.7% of Black menthol smokers predicted that they would switch to a nonmenthol brand if menthol cigarettes were no longer available.21 A second effect is that some menthol smokers may quit soon after the ban as a response to the unavailability of their preferred cigarette, that is, the cigarette viewed as more safe or less harsh (cessation effect). Tauras et al.22 did not find close substitutability of the 2 products; in fact, they found that nonmenthol cigarettes were less of a substitute for menthol cigarettes than was the reverse. Indeed, in 2010 TUS-CPS data, 39.0% of all menthol smokers and 46.8% of Black menthol smokers reported that they would quit if menthol cigarettes were not available.21 Although intentions do not always translate into actual behavior, this suggests that menthol smokers are dedicated to menthol flavoring and do not see nonmenthol cigarettes as a suitable substitute.
Finally, some individuals who would have initiated smoking menthol cigarettes may not initiate (initiation effect). Studies have not directly considered the effects of a menthol ban on smoking initiation, but the proportion of menthol smokers is inversely related to age, suggesting that menthol cigarettes are the preferred starter cigarette and that they facilitate initiation.7
Former menthol smokers who remain smokers in the switching effect are assumed to take on the cessation rate of nonmenthol smokers. This rate is directly estimated from the TUS-CPS and has been found to be relatively stable for the years 2003 and 2006.15 Direct estimates were not available for the cessation and initiation effects. On the basis of the studies cited above, we considered 3 conservative, plausible scenarios: (1) 10% of the menthol smokers permanently quit, and 10% of those who would have initiated as menthol smokers do not initiate; (2) 20% quit, and 20% do not initiate; and (3) 30% quit, and 30% do not initiate.
For each scenario, we projected the effect on smoking prevalence, the absolute number of smokers, and the number of smoking-attributable deaths 40 years forward, to the year 2050. We calculated the percentage change in smoking prevalence relative to the baseline case (status quo scenario, i.e., no ban is enacted) and the deaths averted because of a menthol ban as the difference between smoking-attributable deaths in the baseline case and those under a ban. Previous studies do not clearly distinguish mortality risks of menthol and nonmenthol smokers, so we applied the same relative risks to menthol and nonmenthol that have been applied to all smokers in previous SimSmoke models.10,11,23,24
In the baseline scenario, the model incorporates switching between menthols and nonmenthols up through age 24 years through our measure of net initiation by type, but the model does not consider switching after age 24 years. The few studies that examine switching yield mixed results.4,25,26 In the model, those smokers maintaining no preference for either menthol or nonmenthol—who are probably most likely to switch—are conservatively assumed to continue as nonmenthol smokers after the ban.
RESULTS
In the absence of a menthol ban, the model predicts a slow downward trend in overall smoking prevalence from 18.1% (20.3% for males and 16.1% for females) in 2003 to 8.2% in 2050. Smoking rates decline, but the percentage of those smoking menthols is projected to increase. From 2003 to 2050, menthol use increases from about 23% to 27% among all males and from 65% to 77% among Black males. For females, the menthol rate stays flat for all smokers, but it increases from 76% in 2003 to 83% in 2050 among Blacks (results not shown).
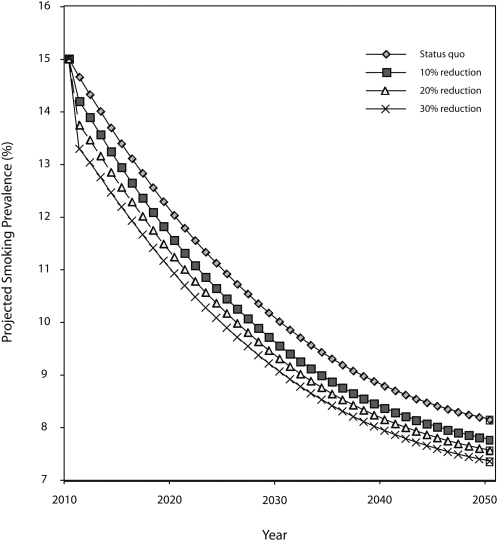
Figure 1 presents the projected smoking prevalence of all smokers under the status quo and the projected changes in population prevalence under a scenario of 10% change (10% reduction in initiation and 10% increase in cessation), a scenario of 20% change, and a scenario of 30% change. At 10 years following the hypothetical ban on menthol in cigarettes, the model projects a 4% relative reduction in smoking prevalence compared with the status quo under the 10% scenario, increasing to 4.6% at 20 years and 4.8% at 40 years. At 40 years, the model projects a 7.2% decrease under the 20% scenario and a 9.7% decrease under the 30% scenario. For Blacks in 2050, the projected relative reduction is a 9.1% decrease under the 10% scenario, a 17.0% decrease under the 20% scenario, and a 24.8% decrease under the 30% scenario.
FIGURE 1.
Smoking prevalence if menthol is banned under 3 scenarios (10%, 20%, and 30% change in initiation and cessation), projected from 2010 to 2050: United States.
Table 1 presents the projected number of smoking-attributable deaths at 10-year intervals through 2050 for each scenario and computes deaths averted at 2050 relative to status quo estimates. In 2020, the menthol ban results in 1.06 million fewer smokers under the most conservative scenario, increasing slightly through 2030 and then declining (results not shown). In 2020 alone, there are 4764 smoking-attributable deaths averted, increasing to 11 355 in 2040. From 2011 to 2050, a total of 323 107 deaths are averted under the 10% scenario, 478 154 under the 20% scenario, and 633 252 under the 30% scenario. Almost one third of the deaths averted are among Blacks, for whom 91 744 deaths are averted under the 10% scenario, 164 465 under the 20% scenario, and 237 317 under the 30% scenario.
TABLE 1.
Smoking-Attributable Deaths (SADs) and Deaths Averted if Menthol is Banned Under 3 Scenarios (10%, 20%, and 30% Change in Initiation and Cessation), Projected From 2010 to 2050: Total Population and Black Population, United States
| Menthol Ban Scenarios | SADs, 2010 | SADs, 2020 | SADs, 2030 | SADs, 2040 | SADs, 2050 | Total SADs | Total SADs Averted Compared With Status Quo |
| Total population | |||||||
| Status quo | 386 732 | 410 809 | 399 028 | 342 472 | 272 424 | 17 923 889 | – |
| 10% change | 386 732 | 406 046 | 388 347 | 331 117 | 262 574 | 17 600 782 | 323 107 |
| 20% change | 386 732 | 402 568 | 382 621 | 326 799 | 259 002 | 17 445 735 | 478 154 |
| 30% change | 386 732 | 399 091 | 376 893 | 322 478 | 255 424 | 17 290 637 | 633 252 |
| Black population | |||||||
| Status quo | 53 836 | 57 056 | 53 382 | 45 022 | 37 475 | 2 433 536 | – |
| 10% change | 53 836 | 55 234 | 50 086 | 42 175 | 35 320 | 2 341 792 | 91 744 |
| 20% change | 53 836 | 53 706 | 47 562 | 40 044 | 33 340 | 2 269 071 | 164 465 |
| 30% change | 53 836 | 52 177 | 45 036 | 37 908 | 31 347 | 2 196 219 | 237 317 |
Note. Total SADs averted include all years from 2010 through 2050 and therefore include years not represented in the table.
DISCUSSION
This application of SimSmoke modeling suggests that a menthol ban would have large population-level benefits in reducing smoking prevalence, the number of smokers, and the number of smoking-attributable deaths in the United States over a 40-year period. We have provided 3 plausible scenarios to address the lack of data on the proportion of menthol smokers who would quit or never start smoking in the case of a ban on menthol, and our results suggest that somewhere between 323 000 and 633 000 deaths could be avoided under a ban, almost one third of which would be among Blacks. Even under the most conservative scenario, the model predicts a substantial public health benefit of a ban on menthols consistent with the broad public health standard specified by the Family Smoking Prevention and Tobacco Control Act of 2009.1
As is typically the case with simulated projections, the models are limited by current evidence regarding switching and initiation behaviors, assumptions inherent in the model, and the reliability of the data. The model uses data from the 2003 TUS-CPS, which yields smoking prevalence rates below those from the National Health Interview Survey (NHIS). We used TUS-CPS data to calibrate our model to predict well between 2003 and 2006. The 2009 TUS-CPS data were not yet available, but the model overpredicts the percentage change in smoking rates from 2006 to 2009 implied by NHIS data. Still, the lower initial smoking level seen in TUS-CPS data relative to NHIS data and the greater projected change in smoking prevalence than is observed in the NHIS data between 2006 and 2009 can both be expected to reduce the estimated number of smoking-attributable deaths and consequently increase the number of deaths averted as a result of the ban. Therefore, the estimate of deaths averted is likely to be conservative.
The immediate effects of a ban are simulated as occurring through cessation in the first year of the ban. The results of a gradual change, either because the ban is implemented in steps or because reactions to the ban occur over a longer period than 1 year, would yield slightly different results in the earlier years but almost identical results by 2020 and certainly identical results by 2050.
SimSmoke incorporates the effect of tobacco-control policies through 2010, assuming that policies have the same percentage effects on menthol and nonmenthol smokers. Evidence on these effects is limited, but some evidence suggests that price and clean-air policies may be less effective among menthol smokers. In the absence of a ban, the percentage of menthol smokers might be expected to increase with stricter tobacco-control policies.22 We have assumed that relative mortality risks are equal for menthol and nonmenthol smokers, and for Black smokers relative to other racial/ethnic groups. Although the higher lung cancer risk among Black smokers suggests a link to menthol use,27,28 studies fail to find a clear association between menthol smoking and increased risk for lung cancer or other disease.29–31 If a menthol ban increases smoking cessation and reduces initiation, Blacks would experience even greater health benefits, which could serve to reduce health disparities.
Given the tremendous harms associated with smoking,32 public health efforts are needed to positively influence population-level smoking behavior and reinvigorate the stalled decline in adult smoking prevalence in the United States.33 Such efforts are especially important for populations at increased risk, such as Blacks, who disproportionately smoke menthols. If a menthol ban were accompanied by effective mass-media campaigns and increased access to evidence-based cessation services, additional reductions in smoking prevalence would be likely, further contributing to the public health impact of this policy intervention.
Acknowledgments
We would like to thank the American Legacy Foundation for the funds that supported the preparation of this brief.
Human Participant Protection
No protocol approval was necessary because the study used secondary data from a public-use data set.
References
- 1. Family Smoking Prevention and Tobacco Control Act, 21 USC §301 (2009)
- 2.Okuyemi KS, Ahluwalia JS, Ebersole-Robinson M, Catley D, Mayo MS, Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers? Addiction. 2003;98(10):1387–1393 [DOI] [PubMed] [Google Scholar]
- 3.Hersey JC, Ng SW, Nonnemaker JM, et al. Are menthol cigarettes a starter product for youth? Nicotine Tob Res. 2006;8(3):403–413 [DOI] [PubMed] [Google Scholar]
- 4.Pletcher MJ, Hulley BJ, Houston T, Kiefe CI, Benowitz N, Sidney S. Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arch Intern Med. 2006;166(17):1915–1922 [DOI] [PubMed] [Google Scholar]
- 5.Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102(12):1979–1986 [DOI] [PubMed] [Google Scholar]
- 6.Gandhi KK, Foulds J, Steinberg MB, Lu SE, Williams JM. Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract. 2009;63(3):360–367 [DOI] [PubMed] [Google Scholar]
- 7.Rock VJ, Davis SP, Thorne SL, Asman KJ, Caraballo RS. Menthol cigarette use among racial and ethnic groups in the United States, 2004–2008. Nicotine Tob Res. 2010;12(suppl 2):S117–S124 [DOI] [PubMed] [Google Scholar]
- 8.Levy DT, Chaloupka F, Gitchell J, Mendez D, Warner KE. The use of simulation models for the surveillance, justification and understanding of tobacco control policies. Health Care Manage Sci. 2002;5(2):113–120 [DOI] [PubMed] [Google Scholar]
- 9.Levy DT, Nikolayev L, Mumford E. Recent trends in smoking and the role of public policies: results from the SimSmoke tobacco control policy simulation model. Addiction. 2005;100(10):1526–1536 [DOI] [PubMed] [Google Scholar]
- 10.Levy DT, Hyland A, Higbee C, Remer L, Compton C. The role of public policies in reducing smoking prevalence in California: results from the California tobacco policy simulation model. Health Policy. 2007;82(2):167–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy DT, Ross H, Powell L, Bauer JE, Lee HR. The role of public policies in reducing smoking prevalence and deaths caused by smoking in Arizona: results from the Arizona tobacco policy simulation model. J Public Health Manag Pract. 2007;13(1):59–67 [DOI] [PubMed] [Google Scholar]
- 12.Levy DT, Tworek C, Hahn EJ, Davis RE. The Kentucky SimSmoke tobacco policy simulation model: reaching Healthy People 2010 goals through policy change. South Med J. 2008;101(5):503–507 [DOI] [PubMed] [Google Scholar]
- 13.Levy DT, Benjakul S, Ross H, Ritthiphakdee B. The role of tobacco control policies in reducing smoking and deaths in a middle income nation: results from the Thailand SimSmoke simulation model. Tob Control. 2008;17(1):53–59 [DOI] [PubMed] [Google Scholar]
- 14.Levy DT, Cho SI, Kim YM, Park S, Suh MK, Kam S. SimSmoke model evaluation of the effect of tobacco control policies in Korea: the unknown success story. Am J Public Health. 2010;100(7):1267–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy D, Blackman K, Tauras J, et al. Quit attempts and quit rates among menthol and non-menthol smokers: findings from a national population survey. Am J Public Health. 2011;101(7):1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns D, Anderson C, Johnson M, et al. Cessation and cessation measures among daily adult smokers: national- and state-specific data. : National Cancer Institute, Population-Based Smoking Cessation: A Conference on What Works to Influence Smoking in the General Population. Bethesda, MD: National Cancer Institute; 2000:113–304 Smoking and Tobacco Control Monograph No. 12 [Google Scholar]
- 17.US Dept of Health and Human Services The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Atlanta, GA: US Dept of Health and Human Services; 1990 [Google Scholar]
- 18.Gilpin EA, Pierce JP, Farkas AJ. Duration of smoking abstinence and success in quitting. J Natl Cancer Inst. 1997;89(8):572–576 [DOI] [PubMed] [Google Scholar]
- 19.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38 [DOI] [PubMed] [Google Scholar]
- 20.Hughes JR, Peters EN, Naud S. Relapse to smoking after 1+ year of abstinence: a meta-analysis. Addict Behav. 2008;33(12):1516–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. US Food and Drug Administration January 10–11, 2011-TPSAC meeting webcast. Available at: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/TobaccoProductsScientificAdvisoryCommittee/ucm239227.htm. Accessed April 12, 2011
- 22.Tauras JA, Levy D, Chaloupka FJ, et al. Menthol and non-menthol smoking: the impact of prices and smoke-free air laws. Addiction. 2010;105(suppl 1):115–123 [DOI] [PubMed] [Google Scholar]
- 23.Levy DT, Friend K. Examining the effects of tobacco treatment policies on smoking rates and smoking related deaths using the SimSmoke computer simulation model. Tob Control. 2002;11(1):47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy DT, Mumford EA, Pesin B. Tobacco control policies and reductions in smoking rates and smoking-related deaths. Expert Rev Pharmacoecon Outcomes Res. 2003;3(4):457–468 [DOI] [PubMed] [Google Scholar]
- 25.Sidney S, Tekawa I, Friedman GD. Mentholated cigarette use among multiphasic examinees, 1979–86. Am J Public Health. 1989;79(10):1415–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter P, Beistle D, Pederson L, O'Hegarty M. Small-group discussions on menthol cigarettes: listening to adult African American smokers in Atlanta, Georgia. Ethn Health. 2008;13(2):171–182 [DOI] [PubMed] [Google Scholar]
- 27.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–342 [DOI] [PubMed] [Google Scholar]
- 28.Abidoye O, Ferguson MK, Salgia R. Lung carcinoma in African Americans. Nat Clin Pract Oncol. 2007;4(2):118–129 [DOI] [PubMed] [Google Scholar]
- 29.Sidney S, Tekawa IS, Friedman GD, Sadler MC, Tashkin DP. Mentholated cigarette use and lung cancer. Arch Intern Med. 1995;155(7):727–732 [PubMed] [Google Scholar]
- 30.Carpenter CL, Jarvik ME, Morgenstern H, McCarthy WJ, London SJ. Mentholated cigarette smoking and lung-cancer risk. Ann Epidemiol. 1999;9(2):114–120 [DOI] [PubMed] [Google Scholar]
- 31.Brooks DR, Palmer JR, Strom BL, Rosenberg L. Menthol cigarettes and risk of lung cancer. Am J Epidemiol. 2003;158(7):609–616 [DOI] [PubMed] [Google Scholar]
- 32.US Dept of Health and Human Services The Health Consequences of Smoking: A Report of the Surgeon General. Washington, DC: US Dept of Health and Human Services; 2004 [Google Scholar]
- 33.Centers for Disease Control and Prevention Vital signs: current cigarette smoking among adults aged > or = 18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(35):1135–1140 [PubMed] [Google Scholar]