Abstract
Background
Recent studies suggest that the hematopoietic and cardiac lineages have close ontogenic origins, and that an early mesodermal cell population has the potential to differentiate into both lineages. Studies also suggest that specification of these lineages is inversely regulated. However, the transcriptional networks that govern the cell fate specification of these progenitors are incompletely defined.
Methods and Results
Here, we show that Nkx2-5 regulates the hematopoietic/erythroid fate of the mesoderm precursors early during cardiac morphogenesis. Utilizing transgenic technologies to isolate Nkx2-5 expressing cells, we observed an induction of the erythroid molecular program, including Gata1, in the Nkx2-5 null embryos. We further observed that overexpression of Nkx2-5 using an Nkx2-5-inducible embryonic stem (ES) cell system significantly repressed Gata1 gene expression and suppressed the hematopoietic/erythroid potential but not the endothelial potential of the ES cells. This suppression was cell-autonomous and was partially rescued by overexpressing Gata1. In addition, we demonstrated that Nkx2-5 binds to the Gata1 gene enhancer and represses the transcriptional activity of the Gata1 gene.
Conclusions
Our results demonstrate that the hematopoietic/erythroid cell fate is suppressed via Nkx2-5 during mesodermal fate determination and that the Gata1 gene is one of the targets that are suppressed by Nkx2-5.
Keywords: Nkx2-5, Gata1, cardiac progenitors, gene regulation
Introduction
Previous studies support the hypothesis that multipotent progenitors give rise to diverse lineages in the developing embryo. Examples of multipotent progenitors include the mesoangioblast (which parents hematopoietic, smooth muscle, endothelial and cardiomyocyte lineages1), hemangioblasts (a common progenitor of hematopoietic and endothelial lineages2) and multipotent cardiac progenitor cells (CPCs), which have the potential to differentiate into all three major cell types of the heart including cardiac myocytes, endothelial cells and smooth muscle cells3. While the multipotent CPCs express Nkx2-5, the transcriptional networks that govern the fate of these multipotent progenitors remain incompletely defined. Our previous analysis of the cardiac cell lineage demonstrated that the Nkx2-5 expressing progenitors transiently express hematopoietic markers, supporting the notion that there is a bipotential stage in early mesoderm during which cardiac and hematopoietic markers are coexpressed4.
Nkx2-5 is one of the earliest transcription factors expressed in multipotent CPCs during vertebrate heart development. Global elimination of Nkx2-5 in mice results in severe growth retardation, perturbed cardiac morphogenesis and embryonic lethality at approximately E9.5–E10.05, 6. However, conditional elimination of Nkx2-5 in cardiac myocytes results in viable neonates that have progressive cardiac dysfunction7. Although these studies highlight the significance of Nkx2-5 transcriptional activity during cardiac morphogenesis, they also support the notion that the embryonic lethality in mice with global disruption of Nkx2-5 is due, in part, to perturbations of other lineages as these null embryos have severe anemia, angiogenic defect and the absence of endocardial cushion5, 6. These results further indicate that the functional role of Nkx2-5 during embryogenesis is not restricted to promoting cardiac muscle development.
Gata factors have been grouped based on their expression and role during development. Gata-1/2/3 are involved in hematopoietic commitment, erythrocyte differentiation, progenitor cell proliferation and T-cell differentiation, respectively, while Gata-4/5/6 play important roles during cardiac morphogenesis8. Gata1 is the first transcription factor shown to be important for the genesis of the erythroid lineage early during embryogenesis9. Targeted disruption or overexpression of Gata1 results in embryonic lethality due to anemia, suggesting that Gata1 dosage levels are critically regulated during erythropoiesis. Previous studies have identified the essential regulatory elements for Gata1 expression in erythroid lineages and have also established that Gata1 gene expression is partly regulated by Gata1 itself 9. Recent fate mapping studies in zebrafish defined that the hematopoietic and cardiac fates from lateral plate mesoderm are inversely regulated10. While this latter study describes SCL and ER71 as transcription factors that promote the hematopoietic fate and represses the cardiac fate, the cardiac transcription factor that functions in a reciprocal fashion is yet to be defined.
In the present study, we define an Nkx2-5 mediated mechanism that coordinately regulates the cellular fate of mesoderm progenitors. Utilizing genetic mouse models and molecular analyses of Nkx2-5 expressing cells, we observed an induction of the erythroid molecular program including Gata1 in the progenitors isolated from the developing Nkx2-5−/− hearts. We also demonstrated that overexpression of Nkx2-5, using an inducible ES/EB system, significantly repressed the hematopoietic/erythroid program but not the endothelial program in a cell-autonomous fashion. This repression was partially rescued by Gata1 overexpression. Using a reporter gene assay, we observed that Nkx2-5 represses the Gata1 promoter activity by binding to the Gata1 gene enhancer. Our studies support a model for Nkx2-5-dependent regulation of Gata1 gene expression in the multipotent progenitors. This novel and previously undefined functional role for Nkx2-5 will complement and extend our understanding of the mechanisms by which cardiac lineages are determined in mesodermal precursors.
Methods
Transcriptional assays
The 3.9 kb Gata1 hematopoietic enhancer and minigene was previously described11. All transcriptional assays were performed in the K562 cell line. Specific conditions can be found in Online Data Supplements.
Generation of Nkx2-5-inducible ES/EB system (iNkx2-5 cells)
Generation and maintenance of iNkx2-5 cells, formation and differentiation of embryoid bodies (EBs) were carried out as described12, 13. Doxycycline (0.5 μg/ml) was added to induce Nkx2-5 expression for 24 hours at the start of Day 3. EBs were washed and fed fresh medium on Day 4 and further cultured for an additional two days to evaluate the effect of Nkx2-5 on hematopoietic commitment. To monitor the effect of Nkx2-5 on hematopoietic/erythroid differentiation, EBs were treated with Doxycycline for 48 hours beginning on Day 4 of EB formation. EBs were collected on Day 6 and processed for isolation of RNA and FACS analyses or for methylcellulose assays.
Statistical analysis
Data represent average of more than three replicates (replicate numbers are indicated in the text) and standard deviation. Significance was tested by the Mann-Whiney U test for two groups and Kruskal-Wallis test with Dunn’s Multiple Comparison Test for more than two groups. For colony counts, normalizing transformation (square root) was done prior to statistical analysis. The above analyses were done with Prism 4.0 software (GraphPad Software). Quantitative RT-PCR data were analyzed by the RQ analysis algorithm (ABI). Error bars in indicate the 99% confidence interval.
Results
Induction of the erythroid molecular program in the Nkx2-5 null heart
We have previously generated a transgenic mouse model using an Nkx2-5 cardiac specific enhancer to direct EYFP reporter expression to the developing heart. This transgenic model recapitulates endogenous Nkx2-5 expression early during heart development4. Transcriptome analyses from EYFP positive embryonic cells revealed that the cardiac transcriptional regulators are enriched in this population4. The analysis further revealed that the progenitors isolated from the E7.75 cardiac crescent have transient expression of genes that are essential for erythroid differentiation. These genes were downregulated at later stages of heart development (E9.5)4. These data suggested that cardiac progenitors co-express erythroid genes, but cardiac transcriptional regulators promote cardiac differentiation by negatively regulating the gene expression of the erythroid program.
To address the functional role of Nkx2-5 in this regulatory pathway, we examined the molecular signature of the EYFP expressing cells in the presence or absence of Nkx2-5. We made crosses of Nkx2-5+/− and Nkx2-5-EYFP transgenic;Nkx2-5+/− mice, and isolated EYFP positive cells from the developing hearts of wild-type (Nkx2-5+/+) or Nkx2-5 null (Nkx2-5−/−) progeny. Persistent EYFP expression was observed in the heart field of both Nkx2-5+/+ and Nkx2-5−/− embryos (Figure 1A).
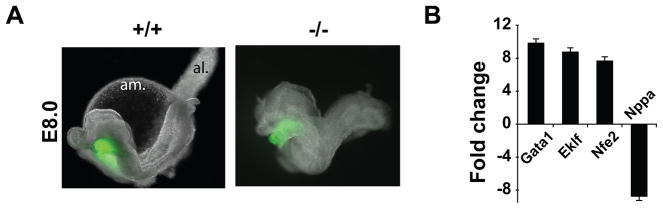
Figure 1. Upregulation of Gata1 expression in Nkx2-5−/− progenitors.
(A) Age-matched Nkx2-5-EYFPTg/+: Nkx2-5+/+ and Nkx2-5-EYFPTg/+: Nkx2-5−/− littermate embryos at E8.0. Al: allantois, am: amnion (B) qRT-PCR shows that hematopoietic transcripts were significantly upregulated in the GFP+ population in the Nkx2-5−/− embryos. Note upregulation of Gata1 and downregulation of Nppa, a known downstream target of Nkx2-5 in the absence of Nkx2-5. Bars indicate 99% confidence interval (n = 3).
The EYFP positive (EYFP+) cells from individual WT and null littermate embryos at E8.0 were sorted using flow cytometry and processed for gene expression analysis by quantitative RT-PCR (qRT-PCR) (Figure 1B). We observed that Nppa, a direct downstream target of Nkx2-55, 14, was downregulated in the Nkx2-5−/− CPCs whereas the hematopoietic markers, Gata1, Eklf, and Nfe2, were upregulated (Figure 1B). Thus, our analysis demonstrated increased expression of hematopoietic markers in Nkx2-5−/−progenitors.
Overexpression of Nkx2-5 represses erythroid differentiation in ES cells
We utilized the embryonic stem cells/embryoid body (ES/EB) system to enhance our understanding of the role of Nkx2-5 regulating hematopoietic fate as this is a powerful model system for the study of cellular fate specification and lineage commitment15. Previous studies have demonstrated that the commitment of EBs to a hematopoietic fate occurs between days 2.75 and 3.75, whereas hematopoietic differentiation occurs between days 4 and 616. Moreover, our laboratory and others have demonstrated that Nkx2-5 expression is observed as early as 3.5 days of EB differentiation and is robustly expressed by day 5 17–19. As the onset of Nkx2-5 expression (day 3.5) coincides with the divergence of hematopoietic and cardiac fate in the ES/EB system, we hypothesized that Nkx2-5 has a functional role in this process. To test this hypothesis, we generated an iNkx2-5 cell line, an ES cell line that expresses Nkx2-5 in response to doxycycline12. Nkx2-5 was induced for 24 hours from Day 3 to Day 4 EBs to examine the effect on hematopoietic commitment, or for 48 hours from Day 4 to Day 6 EBs to evaluate the effect on hematopoietic differentiation and the respective EBs were collected on Day 6 (Figure 2A).
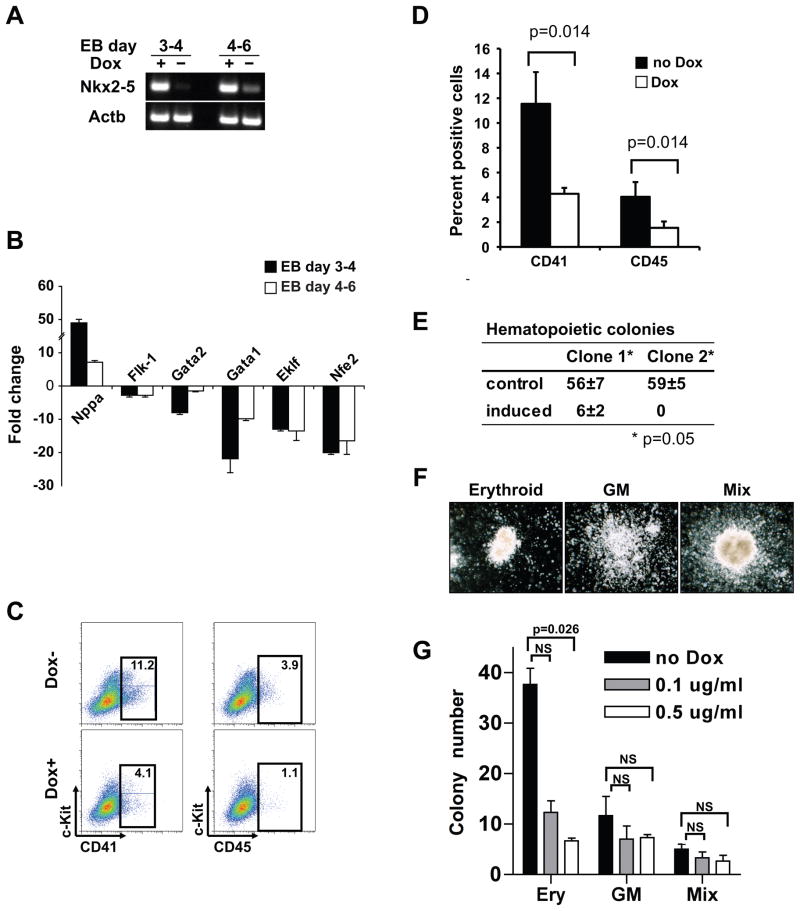
Figure 2. Overexpression of Nkx2-5 results in repression of the hematopoietic/erythroid lineages.
(A) Induction of the Nkx2-5 transcript in iNkx2-5 cells was verified by RT-PCR. Beta-actin (Actb) is a loading control. (B) qRT-PCR analysis of Dox-treated and untreated EBs (n=6) revealed that overexpression of Nkx2-5 significantly suppressed erythroid genes including Gata1. In contrast, an Nkx2-5 downstream target gene Nppa was significantly upregulated. Bars represent 99% confidence interval (n=3). (C) FACS analysis of cell surface markers of differentiating EB cells. EBs were treated (Dox+) or left untreated (Dox-) from day 4 to day 6 and analyzed for hematopoietic markers on day 6. For early and late hematopoietic markers, combinations of c-kit/CD41 and c-kit/CD45 were used, respectively. Experiment was repeated 5 times and representative data are shown. (D) Quantification of the FACS data in C (analyzed by Mann-Whitney U test; n=5). (E & F) Induction of Nkx2-5 expression suppresses hematopoietic/erythroid colony formation. (E) Day 6 EBs from two different clones of iNkx2-5 cells were isolated and plated in complete methylcellulose in the absence (control) or presence (induced) of doxycycline (analyzed by Mann-Whitney U test after normalizing transformation; n=3). Note that little or no colony formation was observed in the presence of doxycycline, while erythroid, GM (granulocyte-macrophage) and mixed colonies were detected when EBs were cultured without doxycycline. (G) Induction of Nkx2-5 expression from day 4 to day 6 with lower concentrations of doxycycline preferentially repressed erythroid colony formation. Day 4 EBs with or without 48 hour induction with the specified concentration of doxycycline were dissociated and equal numbers of cells were plated in methylcellulose without doxycycline. Note the significant repression of erythroid (ery) colony formation compared to the GM and mixed colonies (analyzed by Mann-Whitney U test after normalizing transformation; n=3. NS: not significant).
Using qRT-PCR, we observed that genes involved in erythroid differentiation are downregulated in doxycycline-induced EBs with both treatment schedules (Figure 2B). In contrast, a downstream target gene of Nkx2-5 in cardiac development, Nppa, was significantly upregulated (Figure 2B). Therefore, Nkx2-5 does not act as a nonspecific repressor of gene expression but specifically inhibits the erythroid program in doxycycline-induced EBs. The reciprocal response of these genes to overexpression and loss-of-function of Nkx2-5 supports the notion that Nkx2-5 inhibits the erythroid program while promoting cardiac development (Figures 1B and 2B).
Using FACS analysis, we then examined the cell surface markers of iNkx2-5 cells. Control and doxycycline induced EBs were dissociated at day 6 of differentiation and analyzed for c-kit and CD41 (hematopoietic progenitor markers) vs. c-kit and CD45 (more committed hematopoietic cell markers) expression. When Nkx2-5 was induced from day 3 to day 4, we identified no hematopoietic progenitors (c-kit+/CD41+) or mature hematopoietic cells (c-kit+/CD45+) (data not shown). When Nkx2-5 was overexpressed between days 4 and 6 of differentiation, both early and late hematopoietic progenitors (c-kit+/CD41+ and c-kit+/CD45+) were detectable, but significantly reduced in the presence of Nkx2-5 (Figures 2C and 2D).
To further evaluate whether Nkx2-5 may also play a role in hematopoietic/erythroid differentiation of ES cells, we utilized colony-forming cell assays. Untreated day 6 EBs were trypsinized and 5 × 104 cells were plated on methylcellulose plates for hematopoietic colony formation in the presence or absence of doxycycline. Colonies were quantified after six days of culture. Few or no colonies were formed from two independent clones of iNkx2-5 cells when doxycycline was added to the methylcellulose, while erythroid, granulocyte-macrophage and mixed colonies were detected when cultured in the absence of doxycycline (Figures 2E and 2F). To determine whether Nkx2-5 overexpression specifically affected the erythroid program or has a broader role in hematopoiesis, day 4 EBs were induced for 48 hours (with variable concentrations of doxycycline) and then plated on methylcellulose in the absence of doxycycline (Figure 2G). Induction of Nkx2-5 expression in EBs at this earlier time of hematopoietic development resulted in a preferential repression of erythroid colony formation in a dose dependent fashion (Figure 2G). The reduction of erythroid colonies was readily observed at the lowest dose of doxycycline (0.1 μg/ml) examined, whereas formation of other colonies was less affected. Collectively, our data support the hypothesis that Nkx2-5 either directly or indirectly represses the expression of genes that are essential for erythroid specification and differentiation during development.
Overexpression of Nkx2-5 does not repress endothelial differentiation in ES cells
Since previous studies have suggested that hematopoietic and endothelial cells, and cardiomyocyte and endothelial cells, respectively, are derived from common progenitors16, 17, 20, we examined whether overexpression of Nkx2-5 also repressed the endothelial specification/differentiation of EBs. Day 4 EBs were induced with doxycycline or left uninduced for 48 hours, dissociated on day 6 and analyzed using FACS. Cells were doubly stained with combinations of antibodies against Flk-1, VE-cadherin and CD31 (Pecam) to identify endothelial lineages. We observed that the induction of Nkx2-5 expression did not repress the endothelial program (Figures 3A and 3B), in contrast to the strong repression of hematopoietic/erythroid potential (Figure 2). These results indicated that overexpression of Nkx2-5 specifically repressed hematopoietic/erythroid potential, but not the endothelial/vascular potential of the differentiating EBs.
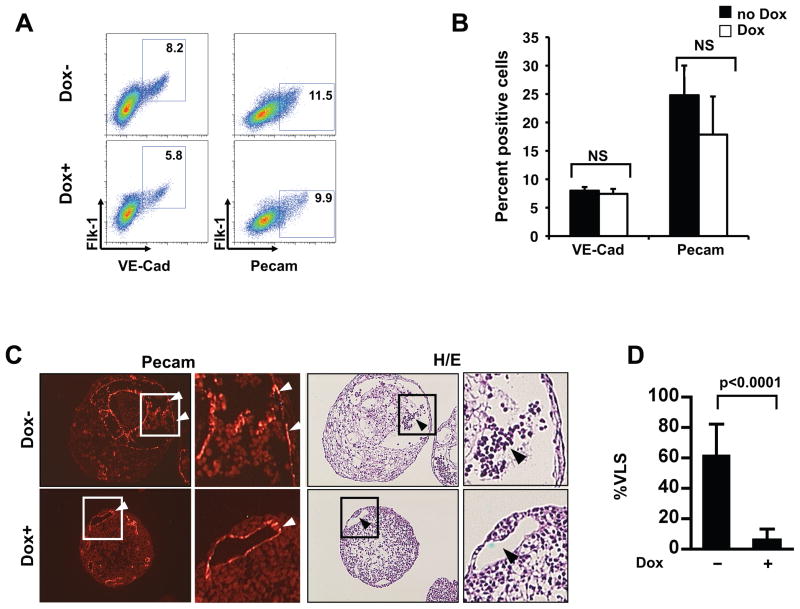
Figure 3. Overexpression of Nkx2-5 does not affect endothelial differentiation of ES/EBs.
(A and B) Cells from day 6 EBs treated (+Dox) or untreated (−Dox) with doxycycline for 48 hrs were analyzed for expression of indicated markers using FACS. Note that overexpression of Nkx2-5 did not affect the endothelial progenitors in the differentiating EBs. These FACS data are quantified in panel B (analyzed by one-tailed Mann-Whitney U test; n=3. NS: not significant). (C & D) Adjacent sections of treated (+Dox) and untreated (−Dox) day 6 EBs were stained with anti CD31/Pecam antibody (left) or hematoxylin and eosin (H/E; right) to analyze the formation of vessel like structures (VLS; white arrow heads) and blood cells (black arrow heads). Boxed areas are enlarged. Three hundred randomly selected VLS from 20 EBs were scored for the presence of blood cells in panel D (analyzed by one-tailed Mann-Whitney U test; n=33). Bars show SD.
To further confirm these results, we utilized morphological techniques of serial sections. An antibody against CD31/Pecam and Hematoxylin & Eosin staining were used to identify vessel-like structures (VLS) and blood cells, respectively. As shown in Figure 3C, CD31/Pecam positive VLS were detected in both induced and non-induced EBs, but VLS containing blood-like cells were detected almost exclusively in the non-induced EBs. Quantitation of VLS from both doxycycline-treated and untreated EBs revealed that only 7% of the VLS contained blood-like cells in the Nkx2-5 induced EBs, while approximately 60% of the VLS had blood components in the non-induced EBs (Figure 3D). These data further confirmed that the overexpression of Nkx2-5 negatively regulated the hematopoietic/erythroid program without affecting endothelial differentiation of progenitor cells.
Nkx2-5 represses hematopoietic differentiation of ES cells cell-autonomously
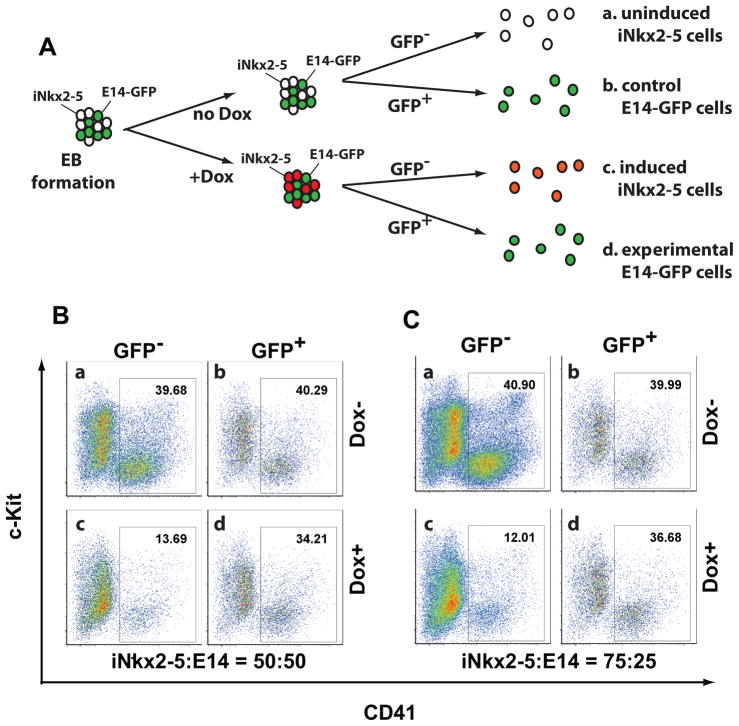
We examined whether repression of hematopoietic differentiation by Nkx2-5 is mediated by secreted factors or is a cell-autonomous effect. We mixed iNkx2-5 cells with E14-GFP cells, an ES cell line that constitutively expressed the GFP protein21, and differentiated EBs in the presence or absence of doxycycline (Figure 4A). We analyzed the EBs on day 6 of induction. GFP positive and negative cells were isolated and analyzed for CD41 expression (as a measure of hematopoiesis) using FACS. When iNkx2-5 cells and E14-GFP cells were mixed at a 50:50 ratio, the hematopoietic potential of iNkx2-5 cells (GFP negative population) was suppressed after treatment with doxycycline (Figure 4B, compare panels a and c). In contrast, the hematopoietic potential of E14-GFP cells was not significantly altered in the same culture (Figure 4B, compare panels b and d). To address whether a putative soluble factor is limiting in the culture, we mixed the cells at a 75:25 ratio, but the hematopoietic potential of E14-GFP cells was still not affected (Figure. 4C). Thus, these data demonstrated that Nkx2-5 repressed hematopoietic differentiation of iNkx2-5 cells in a cell-autonomous fashion.
Figure 4. Nkx2-5 suppresses hematopoiesis in a cell-autonomous fashion.
(A) iNkx2-5 cells were mixed with wild-type E14-GFP cells and induced to form EBs. Cells were either treated with doxycycline from day 2 to day 4, or left untreated. On day 6, cells were dissociated and analyzed by FACS. (B, C) Representative FACS profiles from experiments in which iNkx2-5 cells and E14-GFP cells were mixed at a 50:50 ratio (B) or a 75:25 ratio (C). Note that in both conditions, the CD41+ cells originated from iNkx2-5 cells (CD41+, GFP− population) were reduced upon Nkx2-5 induction (compare panels a and c), whereas those that originated from E14-GFP cells (CD41+, GFP+ population) remained unchanged (compare panels b and d).
Nkx2-5 represses Gata1 transcriptional activity
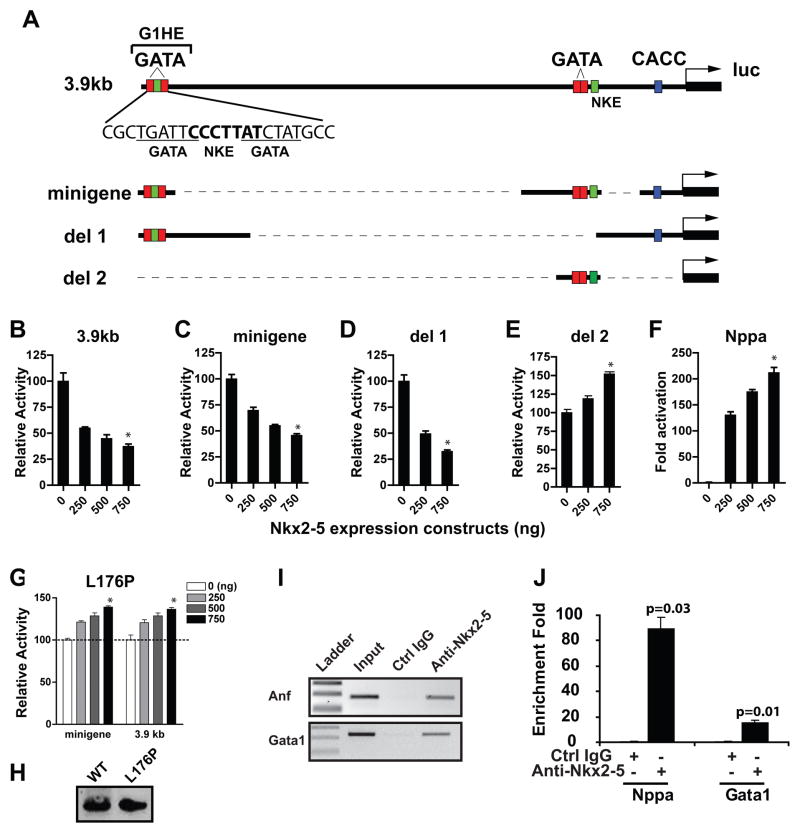
Gata1 is an essential transcription factor for the genesis of the erythroid lineage early during embryogenesis22. Since Gata1 and its downstream targets (e.g. Eklf1 and Nfe2)23, were dysregulated with loss of function or overexpression of Nkx2-5, we hypothesized that the Gata1 gene is regulated by Nkx2-5. Previous studies established that the 3.9 kb upstream sequence is essential for Gata1 expression in both primitive and definitive erythroid cells (Figure 5A)11, 24, 25. Four elements including the Gata-1 hematopoietic enhancer (G1HE) region, two proximal GATA-binding sites and the CACCC motif, are necessary for Gata1 expression, and the minigene construct (Figure 5A) that contains these four elements recapitulates Gata1 gene expression in primitive erythroid cells11.
Figure 5. Nkx2-5 represses Gata1 promoter activity.
(A) Schematic of the 3.9 kb upstream region of the Gata1 gene and reporter plasmids used in the transcriptional assays. Two GATA binding sequences (underlined) and an Nkx2-5 consensus sequence (bolded) within the G1HE are indicated. (B–G) Transcriptional assay in K562 cells reveal a dose-dependent repression of luciferase activity by Nkx2-5 using the 3.9 kb erythroid enhancer (B) or the minigene (C). Deletion 2 (E) but not deletion 1 (D) abolished the response to Nkx2-5. (F) Nppa, a downstream target of Nkx2-5, was activated under the same condition. (G) The L176P mutation of Nkx2-5 in the homeodomain impairs its activity to repress Gata1 transcription. Panels B–G were analyzed by Kruskal-Wallis test (n=3). *:p<0.05 compared to the sample without the Nkx2-5 expression vector (0ng). Bars without an asterisk were not significant. (H) Western blot analysis from the transfected samples (500ng vector) shows that wild type protein and the L175P mutant are expressed at equivalent levels. (I, J) Chromatin was isolated from EBs of iNkx2-5 cells induced for 24 hours with doxycycline and analyzed by ChIP. Nkx2-5 antibody, but not control IgG precipitated genomic fragments containing the Gata1 and the Nppa promoter region (I). J shows quantification of the band intensity by q-PCR (analyzed by Wilcoxon signed rank test; n=4).
When the 3.9 kb reporter plasmid was co-transfected with increasing amounts of the Nkx2-5 expression vector, the luciferase activity was repressed in a dose-dependent fashion (Figure 5B). The minigene construct responded similarly to Nkx2-5 (Figure 5C). There are two sequences that show homology to the Nkx binding site (NKE) within the minigene construct (Figure 5A). Deletion of the proximal double GATA site and NKE (del1) reduced the baseline activity of the promoter, but did not change its response to Nkx2-5 (Supplemental Figure 1, Figure 5D). In contrast, when G1HE was deleted (del2), the repression by Nkx2-5 was abolished (Figure 5E). Similarly, the point mutation of the distal NKE (mut1) but not the proximal NKE (mut2) siginificantly reduced the response to Nkx2-5 (Supplemental Figure 2). Thus, our results demonstrated that the 233bp fragment harboring the G1HE and the NKE therein mediate the repressive activity of Nkx2-5. The repressive activity of Nkx2-5 was not a general inhibition of transcription, as the promoter of Nppa, a known target of Nkx2-5, was activated over 100 fold in the same system (Figure 5F).
Next, we determined whether Nkx2-5 directly binds to the Gata1 gene. We generated a DNA binding mutant of Nkx2-5 (leucine 176 to proline;L176P) that functions as a dominant negative inhibitor of Nkx2-526. Transient transfection assay demonstrated that the L176P DNA binding mutant did not inhibit the activity of 3.9 kb upstream region or the Gata1 minigene although the protein was expressed at similar levels as the wild-type (Figure 5G, H). Next, we examined whether Nkx2-5 binds to the G1HE region using chromatin immunoprecipitation assay (ChIP). A specific band corresponding to G1HE as well as Nppa regulatory region was amplified from immunoprecipitates using the Nkx2-5 antibody, but not the control immunoglobulin (Figure 5I, J). Furthermore, electrophoretic mobility shift assay (EMSA) using nuclear extracts prepared from iNkx2-5 cells induced for 6 hrs (to ensure that there is no secondarily induced genes) identified a specific shift that appears only in the induced extract (Supplemental Figure 3). Collectively, our results support the conclusion that Nkx2-5 binds to the distal NKE and represses Gata1 gene transcription.
Repression of hematopoietic genes by Nkx2-5 is rescued by overexpression of Gata1
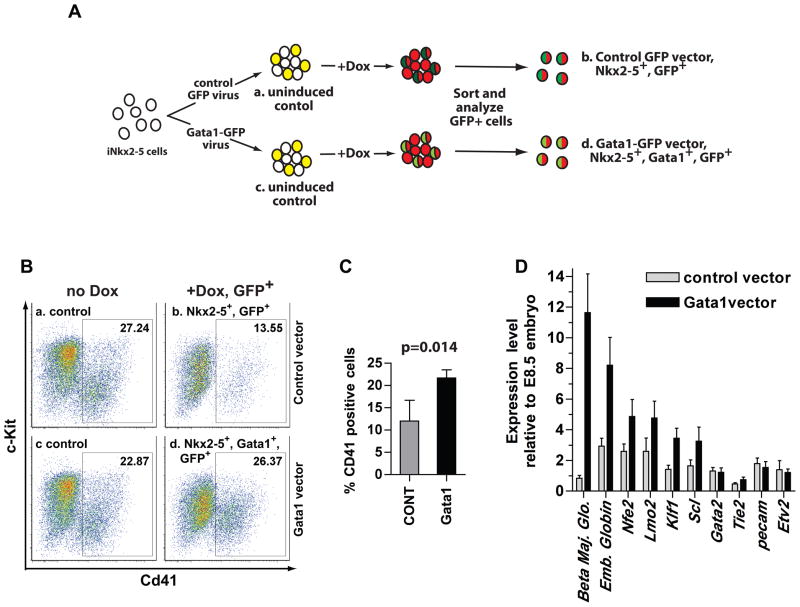
To determine whether Gata1 is a direct downstream target of Nkx2-5, we performed a rescue experiment (Figure 6A). We utilized an inducible lentiviral vector to express GFP alone (control) or the Gata1 and GFP proteins in response to doxycycline. The same promoter controlled expression of Nkx2-5 and Gata1. iNkx2-5 cells were infected, expanded, sorted for GFP positive cells, and were differentiated to form EBs. EBs were treated with 1.0 μg/ml doxycycline from day 4 to day 6 of induction and analyzed on day 6 for expression of CD41. In this experimental paradigm, all cells treated with doxycycline expressed Nkx2-5 (Figure 6A, groups b and d; depicted in red). Sorting and analyzing GFP positive cells allowed us to examine only the infected cells with the control GFP virus (group b, represented in dark green), or the Gata1-GFP virus (Group d, indicated in light green). FACS analysis demonstrated that 22–26% of uninduced groups (a and c) were positive for CD41, whereas group b, which expressed Nkx2-5 and GFP, contained on average 12.0% of CD41 positive cells (Figure. 6C). Group d, which expressed Nkx2-5, GFP and Gata1 contained 21.7% of CD41 positive cells, indicating that Gata1 partially rescued the Nkx2-5 mediated repression (as measured by CD41 expression) (Figure 6C). Congruent with the FACS profile, gene expression profile of the sorted cells demonstrated a 15 fold increase of Gata1 transcript, and between 2 and 12 fold increase of erythroid specific genes such as beta major globin, embryonic globin, Nfe2, Lmo2, Klf1 and Scl (Figure 6D). Thus, our data demonstrated that overexpression of Gata1 at least partially rescued the inhibitory effect of Nkx2-5 on hematopoiesis.
Figure 6. Gata1 partially rescues repression of hematopoiesis by Nkx2-5.
(A) iNkx2-5 cells were infected with a lentiviral vector that expresses IRES-GFP, or a vector that expresses Gata1-IRES-GFP in response to doxycycline. Infected cells were enriched by sorting for GFP positive cells, and induced to form EBs. After inducing from day 4 to day 6, cells were dissociated and analyzed for CD41 expression. The GFP positive cells in the control group express Nkx2-5, and GFP (group b), whereas those in the experimental group express Nkx2-5, Gata1 and GFP (group d). (B) Representative FACS profile of groups a–d. (C) Quantification of the FACS analyses. Mean number and standard deviation from four independent experiments are shown. Data were analyzed by one-tailed Mann-Whitney U test (n=4). (D) Cells from groups b and d were collected and gene expression was analyzed by qRT-PCR. Note upregulation of hematopoietic/erythroid markers by Gata1 overexpression. Bars indicate 99% confidence interval (n=3).
Discussion
Multipotent cardiac progenitors express Nkx2-5 and generate diverse lineages that contribute to heart formation during embryogenesis. While intense interest has focused on the genesis of the cardiac progenitors, the molecular networks that govern cellular fate specification remain incompletely defined. In the present study, we utilized an array of technologies to define the functional role of Nkx2-5 and fate specification. Our studies have advanced this field by making several significant findings. First, using a genetic marking method we observed that genes involved in erythroid differentiation are upregulated in the Nkx2-5−/− progenitors. This finding led us to hypothesize that an undescribed function of Nkx2-5 is to suppress non-cardiac lineages during embryogenesis. This hypothesis was tested using the ES/EB system, which mirrors embryonic development and has been used as a model system to analyze the fate of cell lineages. We showed that overexpression of Nkx2-5 represses hematopoietic/erythroid differentiation in ES/EBs as assessed by three independent measures, 1) gene expression pattern, 2) expression of cell surface markers and morphology, and 3) colony forming activity. We further demonstrated that repression of hematopoietic/erythroid differentiation is a cell-autonomous effect. Interestingly, our cellular and immunohistochemical analyses revealed that overexpression of Nkx2-5 specifically and significantly repressed the hematopoietic/erythroid program but not the endothelial potential of the differentiating EBs. Based on this observation, we hypothesize that Nkx2-5 does not function at the level of the hemangioblast, the putative common precursor for hematopoietic and endothelial cells. Rather, our studies suggest that Nkx2-5 may function distinctly to maintain one lineage (endothelial or cardiomyocyte) by repressing gene expression associated with other (i.e. hematopoietic) lineages.
Whether a common progenitor for cardiac and hematopoietic lineages exists has been controversial. Although analysis based on colony forming assays show that hematopoietic and cardiac lineages arise from distinct mesodermal progenitors17, 27, several lines of evidence suggest that there is a stage during development when mesodermal cells have multilineage potential and can generate blood or cardiac cells depending on the environment4, 10, 28. For example, chick tissues from posterior primitive streak, an area outside the heart field, can be induced to differentiate into cardiac myocytes by coculturing with anterior endoderm tissue. This takes place at the expense of the blood lineage28. In the zebrafish cloche mutant, specification of endothelial and hematopoietic lineages is impaired in the anterior lateral plate mesoderm, and in turn the cardiac lineage is expanded10, 29. Similar findings have been obtained in the ER71−/− embryos, in which blood and vessel development was severely repressed whereas cardiac markers were overexpressed30. Furthermore, in mouse embryos, it has been shown that the epiblast tissue maintains the hematopoietic potential during gastrulation, even after the period of hematopoietic differentiation is over and cardiac specification is initiated31. These observations support the notion that there is a bi- or multi- potential stage in early mesoderm and that activation of the cardiac program antagonizes the hematopoietic program. In the present study, we propose that Nkx2-5 has a dual role of promoting a cardiac fate and repressing hematopoietic fate in multipotent mesodermal progenitors. There is an apparent paradox with the previous report that hematopoiesis is impaired in Nkx2-5 mutant embryos5. We speculate that Nkx2-5 function is required in multiple stages of mesoderm development and the apparent lack of yolk sac hematopoiesis reflects an earlier function of Nkx2-55, 32. With the ability to identify progenitor cell populations earlier during embryogenesis (E6.5–E7.5), future studies will be aimed at defining the roles of Nkx2-5 in multiple stages of mesoderm development.
Using transcriptional assays, we showed that one of the targets of Nkx2-5 is the Gata1 gene, an essential transcription factor in erythroid differentiation. Gata1 expression is strongly suppressed in Nkx2-5 overexpressing ES cells, and overexpression of Gata1 rescued the inhibitory effect of Nkx2-5 on hematopoietic development. The region in the Gata1 minigene, which is responsible for Nkx2-5 mediated repression coincided with the Gata1 hematopoietic enhancer (G1HE). Although the absolute requirement of G1HE in erythroid transcription remains controversial33, G1HE maps with an erythroid specific DNaseI hypersensitive site and is required for expression of the Gata1 gene in primitive and definitive erythroid cells9. Mutation of the Gata binding site within G1HE severely impairs the promoter activity. Our ChIP assay and EMSA demonstrated that Nkx2-5 binds to the G1HE region of the Gata1 gene. This region contained an evolutionarily conserved sequence that shows homology to the Nkx2-5 recognition sequence (Figure 5A). Further work is warranted to investigate the molecular nature of Nkx2-5 mediated transcriptional repression. It is interesting to note that Nkx2-5 not only activates transcription but can also inhibit transcription34. Our findings suggest that hematopoietic genes are additional targets of Nkx2-5’s suppressive activity.
In summary, our data have uncovered a novel biological function for Nkx2-5 that represses hematopoietic/erythroid but not the vascular potential of the mesodermal progenitors. These studies further enhance our understanding of the regulatory mechanisms that govern cardiac morphogenesis and may contribute to regenerative pathways involving cardiac progenitor cell populations following injury of the adult heart.
Supplementary Material
Acknowledgments
The authors acknowledge Alicia Wallis, Camille Walter and Danielle Rux for technical assistance. We thank Masayuki Yamamoto for the Gata1 promoter construct and Benoit Bruneau for the Nppa/ANF reporter plasmid.
Funding sources
We acknowledge the funding support from the NIH (U01 HL100407, R01 HL085729) and the American Heart Association (Jon Holden DeHaan Foundation, 0970499N). DJG is an Established Investigator of the American Heart Association.
Footnotes
Disclosures
None
References
- 1.Cossu G, Bianco P. Mesoangioblasts–vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Mikkola HK, Orkin SH. The search for the hemangioblast. J Hematother Stem Cell Res. 2002;11:9–17. doi: 10.1089/152581602753448504. [DOI] [PubMed] [Google Scholar]
- 3.Garry DJ, Olson EN. A common progenitor at the heart of development. Cell. 2006;127:1101–1104. doi: 10.1016/j.cell.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Masino AM, Gallardo TD, Wilcox CA, Olson EN, Williams RS, Garry DJ. Transcriptional regulation of cardiac progenitor cell populations. Circ Res. 2004;95:389–397. doi: 10.1161/01.RES.0000138302.02691.be. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–1280. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- 6.Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 7.Pashmforoush M, Lu JT, Chen H, Amand TS, Kondo R, Pradervand S, Evans SM, Clark B, Feramisco JR, Giles W, Ho SY, Benson DW, Silberbach M, Shou W, Chien KR. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117:373–386. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- 8.Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–422. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko H, Shimizu R, Yamamoto M. GATA factor switching during erythroid differentiation. Curr Opin Hematol. 2010;17:163–168. doi: 10.1097/MOH.0b013e32833800b8. [DOI] [PubMed] [Google Scholar]
- 10.Schoenebeck JJ, Keegan BR, Yelon D. Vessel and blood specification override cardiac potential in anterior mesoderm. Dev Cell. 2007;13:254–267. doi: 10.1016/j.devcel.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohneda K, Shimizu R, Nishimura S, Muraosa Y, Takahashi S, Engel JD, Yamamoto M. A minigene containing four discrete cis elements recapitulates GATA-1 gene expression in vivo. Genes Cells. 2002;7:1243–1254. doi: 10.1046/j.1365-2443.2002.00595.x. [DOI] [PubMed] [Google Scholar]
- 12.Iacovino M, Hernandez C, Xu Z, Bajwa G, Prather M, Kyba M. A conserved role for Hox paralog group 4 in regulation of hematopoietic progenitors. Stem Cells Dev. 2009;18:783–792. doi: 10.1089/scd.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78:442–453. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 14.Durocher D, Chen CY, Ardati A, Schwartz RJ, Nemer M. The atrial natriuretic factor promoter is a downstream target for Nkx-2.5 in the myocardium. Mol Cell Biol. 1996;16:4648–4655. doi: 10.1128/mcb.16.9.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 16.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 17.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 21.Ismailoglu I, Yeamans G, Daley GQ, Perlingeiro RC, Kyba M. Mesodermal patterning activity of SCL. Exp Hematol. 2008;36:1593–1603. doi: 10.1016/j.exphem.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Harigae H. GATA transcription factors and hematological diseases. Tohoku J Exp Med. 2006;210:1–9. doi: 10.1620/tjem.210.1. [DOI] [PubMed] [Google Scholar]
- 23.Layon ME, Ackley CJ, West RJ, Lowrey CH. Expression of GATA-1 in a non-hematopoietic cell line induces beta-globin locus control region chromatin structure remodeling and an erythroid pattern of gene expression. J Mol Biol. 2007;366:737–744. doi: 10.1016/j.jmb.2006.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onodera K, Takahashi S, Nishimura S, Ohta J, Motohashi H, Yomogida K, Hayashi N, Engel JD, Yamamoto M. GATA-1 transcription is controlled by distinct regulatory mechanisms during primitive and definitive erythropoiesis. Proc Natl Acad Sci U S A. 1997;94:4487–4492. doi: 10.1073/pnas.94.9.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vyas P, McDevitt MA, Cantor AB, Katz SG, Fujiwara Y, Orkin SH. Different sequence requirements for expression in erythroid and megakaryocytic cells within a regulatory element upstream of the GATA-1 gene. Development. 1999;126:2799–2811. doi: 10.1242/dev.126.12.2799. [DOI] [PubMed] [Google Scholar]
- 26.Toko H, Zhu W, Takimoto E, Shiojima I, Hiroi Y, Zou Y, Oka T, Akazawa H, Mizukami M, Sakamoto M, Terasaki F, Kitaura Y, Takano H, Nagai T, Nagai R, Komuro I. Csx/Nkx2-5 is required for homeostasis and survival of cardiac myocytes in the adult heart. J Biol Chem. 2002;277:24735–24743. doi: 10.1074/jbc.M107669200. [DOI] [PubMed] [Google Scholar]
- 27.Kouskoff V, Lacaud G, Schwantz S, Fehling HJ, Keller G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc Natl Acad Sci U S A. 2005;102:13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultheiss TM, Xydas S, Lassar AB. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121:4203–4214. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- 29.Gering M, Yamada Y, Rabbitts TH, Patient RK. Lmo2 and Scl/Tal1 convert non-axial mesoderm into haemangioblasts which differentiate into endothelial cells in the absence of Gata1. Development. 2003;130:6187–6199. doi: 10.1242/dev.00875. [DOI] [PubMed] [Google Scholar]
- 30.Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanatsu M, Nishikawa SI. In vitro analysis of epiblast tissue potency for hematopoietic cell differentiation. Development. 1996;122:823–830. doi: 10.1242/dev.122.3.823. [DOI] [PubMed] [Google Scholar]
- 32.Stanley EG, Biben C, Elefanty A, Barnett L, Koentgen F, Robb L, Harvey RP. Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3′UTR-ires-Cre allele of the homeobox gene Nkx2-5. Int J Dev Biol. 2002;46:431–439. [PubMed] [Google Scholar]
- 33.McDevitt MA, Shivdasani RA, Fujiwara Y, Yang H, Orkin SH. A “knockdown” mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc Natl Acad Sci U S A. 1997;94:6781–6785. doi: 10.1073/pnas.94.13.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nimura K, Ura K, Shiratori H, Ikawa M, Okabe M, Schwartz RJ, Kaneda Y. A histone H3 lysine 36 trimethyltransferase links Nkx2-5 to Wolf-Hirschhorn syndrome. Nature. 2009;460:287–291. doi: 10.1038/nature08086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.