Abstract
One of the most important but as yet unanswered questions in inflammation research is not why inflammation occurs (we all get episodes of self limiting inflammation during the course of our lives) but why it does not resolve. Current models of inflammation stress the role of antigen-specific lymphocyte responses and attempt to address the causative agent. However, recent studies have begun to challenge the primacy of the leukocyte and have instead focused on an extended immune system in which stromal cells, such as fibroblasts play a role in the persistence of the inflammatory lesion.
In this review I will illustrate how fibroblasts help regulate the switch from acute resolving to chronic persistent inflammation and provide positional memory during inflammatory responses. In chronic inflammation the normal physiological process of the removal of unwanted inflammatory effector cells becomes disordered, leading to the accumulation of leucocytes within lymphoid aggregates that resemble those seen in lymphoid tissue. I will describe how fibroblasts provide survival and retention signals for leukocytes leading to their inappropriate and persistent accumulation within inflamed tissue.
1. Background
Two characteristic features of chronic inflammatory reactions are their persistence and predilection for certain sites. Why for example do some forms of inflammation resolve, yet others do not? Why does arthritis localize predominantly to the joints compared to psoriasis which localizes predominantly to the skin? It has been assumed that inflammation is a stereotyped response that reflects a common or “public” set of shared pathways leading to endothelial cell activation, leukocyte infiltration and tissue repair. However, there are features of the inflammatory response that remain unique or “private” to the tissue where inflammation occurs. The molecular and cellular basis for such tissue tropism has remained elusive. There is now accumulating evidence that fibroblasts help define tissue topography, provide positional memory and regulate the switch from resolving to persistent inflammation.
The architecture of organs is very closely adapted to their function. Tissue resident cells such as fibroblasts help define the microanatomy and architecture of organs and provide the appropriate microenvironment in which specialized functions can occur. For example, lymphoid tissues have defined architectural features, such as conduits, that differ from those found in non lymphoid tissues. Until quite recently fibroblasts were thought to predominately provide physical structure to tissues. However, it has become increasingly clear that in addition to their landscaping properties, fibroblasts are not just passive players in immune responses, but play an active role in governing the persistence of inflammatory disease, as well as enabling immunological memory to become established. The response of the immune system to tissue damage involves a carefully choreographed series of cellular interactions between immune and non-immune cells. As all inflammatory reactions take place within a defined background of specialized stromal cells, understanding the biology of fibroblasts in lymphoid and non lymphoid tissues is important in order to understand how immune cell infiltrates become established and persistent in chronic immune mediated inflammatory diseases.
During immune responses, peripheral blood leukocyte numbers are tightly regulated. However, little is known about how leukocyte cell numbers within tissues are regulated during inflammatory responses. Although there is considerable evidence that preferential recruitment of cells to inflammatory lesions occurs during the initial stages of an inflammatory response, the mechanisms regulating their survival and retention within inflamed tissue remain largely unexplored. The interaction between leukocytes and stromal cells during an acute inflammatory response ultimately leads to resolution of the inflammatory focus. However, leukocyte interactions with stromal cells at sites of chronic inflammation appear to lead to sustained leukocyte survival and retention with persistence of the inflammatory lesion. Aberrant temporal and spatial expression of adhesion molecules, chemokines/cytokines and their receptors has been shown to lead to persistent leukocyte retention and survival in these inappropriately stable stromal cell microenvironments. While normal homeostasis and resolution of acute inflammation depends on the right cell being in the right place at the right time, it is likely that chronic inflammation involves immune cells being positioned in the wrong place at the wrong time.
During the development of an immune response, immune cells have to be appropriately positioned within lymph nodes and tissues, such that cognate interactions can occur. This movement of cells to appropriate niches within immune organs is driven by chemokines and their receptors. A longstanding observation has been that tissues undergoing chronic inflammatory reactions contain infiltrates of distinct subsets of leukocytes that are often organized into well defined lymphoid like structures (tertiary lymphoid tissue). For example, within the rheumatoid synovium, B cells organized in clusters are in close contact with macrophages, synoviocytes and CD8 T cells at the periphery of a lymphocyte-rich area composed predominantly of CD4 T cells aggregated around post-capillary venules. Why this characteristic pattern of leukocyte accumulation develops outside lymphoid tissues remains obscure, but it turns out that cytokines such as lymphotoxin and constitutive chemokines such as CXCL12/CXCL13 and CCL19/CCL21 play an important role in defining these ectopic lymphoid structures.
In a series of papers over the last decade, our group has demonstrated that tissue-resident fibroblasts are much more important than originally thought in determining both the switch to persistence as well as the site at which inflammation occurs. In chronic inflammation, the resolution phase is prolonged and disordered leading to the persistent accumulation of the inflammatory infiltrate. We have found that this occurs because of the inappropriate production of survival factors such as type I interferon [1] as well as the ectopic expression and function of constitutive chemokines implicated in the retention of lymphocytes within lymphoid tissues such as the bone marrow and lymph node [2-7]. We have shown that fibroblasts isolated from different anatomical sites (synovium, skin, bone marrow, lymph nodes) and from the same anatomical site (synovium) but different diseases (rheumatoid versus osteoarthritis), display topographic differentiation and positional memory. Unlike the case for endothelial cells, this fibroblast phenotype appears to be remarkably stable during in vitro cell culture [8,9]. However by changing the inflammatory stimuli, it is possible to make the transcriptional profile of synovial fibroblasts resemble that of lymphoid fibroblasts, implying that fibroblast regional identity can be modified by inflammation [8-11]. We have explored the functional consequences of these anatomical differences using models of leukocyte–stromal co-culture which have allowed us to determine the effects of fibroblasts in regulating the behaviour (survival) and distribution (retention or emigration) of different leukocyte subsets in tissues [12,13].
In order to better understand the biology of fibroblasts in inflammation and repair, we have screened for fibroblast cell surface proteins that are differentially expressed in different tissues using a monoclonal antibody strategy. This led to the identification of CD248 (Endosialin) as a novel, fibroblast and pericyte-specific transmembrane receptor [14] that is specifically expressed in inflamed, but not normal adult tissue. On structural grounds CD248 is a member of an emerging family (which includes CD93 and CD141) that share the ability to regulate innate immunity as well as tissue remodelling and repair. Whereas CD248 expression is high in many tissues throughout embryogenesis, it largely disappears in the adult. However it is strongly re-expressed on interstitial fibroblasts and pericytes in inflamed tissue and in a number of cancers [15].
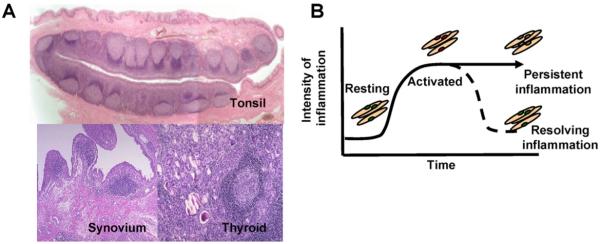
Our work has allowed us to propose that a stromal area post code, predominantly defined by fibroblasts, exists within tissues [11]. Our hypothesis predicts that components of this stromal area post code become disordered during inflammation, leading to the accumulation of lymphocytes in structures that resemble lymphoid tissues (Fig. 1). We have proposed that inflammation is not generic, but contextual and therefore differences in the response of different inflammatory diseases to therapy are likely to be due to intrinsic differences in the behaviour of stromal cells within different microenvironments. Ignoring the contribution of stromal cells to the pathogenesis of chronic inflammatory disease may account for the failure of current therapies to affect a permanent cure. We suggest that stromal cells in general and fibroblasts in particular offer a new family of organ specific targets to treat chronic immune mediated inflammatory diseases such as rheumatoid arthritis, psoriasis and inflammatory bowel disease.
Fig. 1.
A. Tertiary lymphoid structures, found in diseased tissues such as the rheumatoid synovium and thyroid gland share many features suggesting that similar subsets of fibroblasts may be involved in inflammation and immunity. B. During inflammation in peripheral tissues, fibroblasts become activated. Once inflammation resolves, these activated fibroblasts revert back to a resting state. It is suggested that in some cases fibroblasts in tissues may take on features of fibroblasts found in lymphoid tissues, and that these “ectopic” fibroblasts contribute to the formation of tertiary lymphoid structures.
2. Concluding remarks
Immune cells need to interact with a wide variety of cell types both within immune (lymphoid) and peripheral tissues. During inflammation, stromal cells such as fibroblasts, become activated and interact with infiltrating immune cells in a dynamic and site-specific manner. Populations of leukocytes recruited to sites of inflammation should not be considered in isolation, but in conjunction with the non-immune cells that help provide survival, differentiation and positional cues upon which the formation and persistence of leukocyte infiltrates depend. Our work suggests that targeting the stromal microenvironment is likely to be an important strategy for future anti-inflammatory therapies.
References
- [1].Pilling D, Akbar AN, Girdlestone J, Orteu CH, Borthwick NJ, Amft N, et al. Eur J Immunol. 1999;29:1041. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- [2].Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, et al. J Immunol. 2000;165:3423. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- [3].Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Trends Immunol. 2001;22:199. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- [4].Amft N, Curnow SJ, Scheel-Toellner D, Devadas A, Oates J, Crocker J, et al. Arthritis Rheum. 2001;44:2633. doi: 10.1002/1529-0131(200111)44:11<2633::aid-art443>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- [5].Bradfield PF, Amft N, Vernon-Wilson E, Exley AE, Parsonage G, Rainger GE, et al. Arthritis Rheum. 2003;48:2472. doi: 10.1002/art.11219. [DOI] [PubMed] [Google Scholar]
- [6].Buckley CD. Rheumatology. 2003;42:1533. [Google Scholar]
- [7].Buckley CD. Clin Med. 2003;3:361. doi: 10.7861/clinmedicine.3-4-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Parsonage G, Falciani F, Burman A, Filer A, Ross E, Bofill M, et al. Thrombosis Haemo. 2003;90:688. doi: 10.1160/TH03-04-0208. [DOI] [PubMed] [Google Scholar]
- [9].Scaife S, Brown R, Kellie S, Filer A, Martin S, Thomas, et al. Rheumatology. 2004;43:1346. doi: 10.1093/rheumatology/keh347. [DOI] [PubMed] [Google Scholar]
- [10].Burman A, Haworth O, Hardie DL, Amft EN, Siewert C, Jackson, et al. J Immunol. 2005;174:1693. doi: 10.4049/jimmunol.174.3.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, Salmon M, et al. Trends Immunol. 2005;26:150. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lally F, Smith E, Filer A, Stone MA, Shaw JS, Nash GB, et al. Arthritis Rheum. 2005;52:3460. doi: 10.1002/art.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Filer A, Parsonage G, Smith E, Osborne C, Thomas AM, Curnow SJ, et al. Arthritis Rheum. 2006;54:2096. doi: 10.1002/art.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lax S, Hou TZ, Jenkinson E, Salmon M, MacFadyen JR, Isacke CM, et al. FEBS Lett. 2005;581:3550–6. doi: 10.1016/j.febslet.2007.06.063. [DOI] [PubMed] [Google Scholar]
- [15].MacFadyen JR, Haworth O, Roberston D, Hardie D, Webster MT, Morris HR, et al. FEBS Lett. 2007;579:2569–75. doi: 10.1016/j.febslet.2005.03.071. [DOI] [PubMed] [Google Scholar]